Abstract
Background
The clinical and neurobiological underpinnings of transient non-motor (TNM) psychiatric symptoms during the optimization of stimulation parameters in the course of STN-DBS remain under intense investigation.
Methods
Forty-nine patients with refractory PD underwent bilateral STN-DBS implants and were enrolled in a 24-week prospective, naturalistic follow-up study. Patients who exhibited transient non-motor (TNM) psychiatric manifestations during DBS parameter optimization were evaluated for potential associations with clinical outcome measures.
Results
Twenty nine TNM(+) episodes were reported by 15 patients. No differences between TNM(+) and TNM(−) groups were found in motor outcome. However, unlike the TNM(−) group, TNM(+) patients did not report improvement in subsyndromal depression or quality of life. TNM(+) episodes were more likely to emerge during bilateral monopolar stimulation of the medial STN.
Conclusions
The occurrence of TNM psychiatric symptoms during optimization of stimulation parameters was associated with the persistence of subsyndromal depression and with lower quality of life ratings at 6 months. The neurobiological underpinnings of TNM are investigated yet remain difficult to explain.
Introduction
The subthalamic nucleus (STN) is a key node in the basal ganglia-thalamocortical circuitry, integrating information flowing through four functionally independent parallel loops (motor, limbic, associative and oculomotor) [1]. The integrative function of the STN is critical in merging individual goal-directed motor behavior with its appropriate emotional component [2]. Furthermore, the STN regulates the limbic function through influencing neuronal activity in the ventral pallidum. Transient perturbations in the regulatory function of the STN during optimization of stimulation parameters in patients undergoing STN-DBS for Parkinson’s disease (PD) have been identified in the literature as transient non-motor (TNM) psychiatric manifestations. Single case reports of various TNM symptoms include abrupt sadness, happiness, laughter, and anger [3, 4]; elevated mood [5]; pleasant sensations [6]; hypomania and overt manic episodes [7–10]; and brief psychosis [11].
In this study, we examined whether the experience of TNM psychiatric symptoms during optimization of stimulation parameters in PD patients with bilateral STN-DBS has long-term impact on motor, mood, or quality of life outcomes, and evaluated the neuroanatomical correlates underlying these symptoms. To our knowledge, this is the largest study of TNM psychiatric symptoms in STN-DBS.
Materials and Methods
Patients
After obtaining approval from the Mayo Clinic Institutional Review Board, a series of 49 consecutive patients (15 females) with medically-refractory PD were enrolled after the nature of the experimental procedures was explained. The cognitive capacity to understand the risks, benefits, and alternative treatment for STN-DBS was carefully verified for every patient before enrollment. All patients provided a written informed consent before any study procedures took place. All subjects were diagnosed with PD by a board certified neurologist and evaluated by a board certified psychiatrist at the Mayo Clinic. Patients were approved for DBS surgery by the Mayo Clinic Neuromodulation Committee, a multidisciplinary team of neurologists, neurosurgeons, neuroradiologists, psychiatrists, neuropsychologists, neuroethicist, and support personnel.
Motor assessment
Clinical assessment of motor symptoms was performed using the Unified Parkinson’s Disease Rating Scale (UPDRS) part III [12] and the levodopa equivalent daily dose (LEDD) [13]. Clinical evaluation was performed at preoperative baseline and 2-, 12-, 16-, and 24-week postoperative visits. All motor ratings were evaluated by a board-certified neurologist. Furthermore, DBS stimulation parameters (active contact, stimulation polarity, voltage, frequency, and pulse-width) were recorded at each postoperative visit.
Quality of life assessment
The Quality of Life Enjoyment and Satisfaction scale (Q-LES) [14] was used to assess self-reported quality of life and health status both at baseline and at the last study follow-up visit (24-weeks).
Psychiatric assessment
Clinical interview by a board-certified psychiatrist was conducted to evaluate for Axis-I diagnoses. Screening for the presence of depressive symptoms was done using the 17-item Hamilton Rating Scale (HAM-D-17) [15] and the Beck Depression Inventory (BDI-II) [16]. The Young Mania Rating Scale (YMRS) [17] was used to assess hypomanic and manic symptoms at baseline and each follow-up visit.
TNM psychiatric symptom assessment
Optimization of stimulation parameters was systematically performed by the neurology team. Patients were informed that they receive active stimulation. However, they were blinded to the specific parameters or timing a stimulation train is delivered. Each stimulation setting was applied for one minute. Immediately following the application of each stimulation setting, the patient was asked in a nondirective question to describe his/her emotional state. Patients’ prompted and spontaneous utterances and behaviors regarding their emotional experiences and feelings were carefully documented every time a new stimulation parameter was applied. Settings that evoked psychiatric symptoms were not replicated. A wash-out period of one to two minutes was allowed before changing settings. Simultaneous documentation of stimulation parameters (laterality, active contact, polarity, voltage, frequency, and pulse-width) that resulted in TNM symptoms and those associated with best motor responses was performed at each setting.
For operative approach, postoperative programming, and localization of stimulating electrode active contact, refer to a previous report by our group [18].
Statistical analysis
The changes in clinical variables (motor (UPDRS and LEDD), mood (HAM-D-17, BDI, and YMRS), and quality of life (Q-LES)) from baseline to the 24-week visit; were presented as mean ± SEM. Two-tailed paired t-test was used to examine for differences between the TNM(+) and TNM(−) groups, low and high LEDD and between younger and older than 60 years old patient groups. Fischer’s exact test was used to compare differences in the frequencies of monopolar configuration between TNM(+) and TNM(−) groups. Pearson correlation analysis between LEDD and depressive symptoms and between LEDD and quality of life in all patients and in TNM(+) patients was performed. Results were considered significant where p < 0.05.
Results
Patient characteristics
Forty nine patients (15 females) with PD underwent bilateral STN-DBS surgery. The mean age at the time of surgery was 61.7±8.4 years, and the mean duration between identification of parkinsonian symptoms and DBS surgery was 11±5.6 years. Twenty-six patients (53%) had history of comorbid psychiatric conditions (depression, n=19; bipolar disorder or impulse control symptoms secondary to dopaminergic medications, n=14; 7 patients had both conditions).
TNM psychiatric symptoms
Fifteen patients (30%) (7 with psychiatric history: depression, n=5; bipolar/impulse control symptoms, n=2) experienced 29 episodes of TNM symptoms during the optimization of stimulation parameters: crying (n=4); feeling relaxed, more at ease, or less anxious (n=4); sense of euphoria, feeling as if drunk, or “punch drunk” (n=4); feeling anxious, increased apprehension, “messed up”, “out of sorts”, sense of doom, uncertainty, or pained look (n=13); and experiencing cognitive changes, feeling “confused or spaced out”, unable to focus, or difficulty concentrating (n=4). Nine out of fifteen patients experienced more than one TNM episode. Patient #1 experienced 4 episodes of crying, while patients #3 and #8 had four and three episodes of anxiety respectively. Patient #9 had two episodes of euphoria. Different episodes in the same patient were also observed. For instance, patient #2 experienced anxiety at 2.5 volts and relaxation at slightly lower (2.0) volts. Similarly, in patient #10 the anxiety evoked by 2.8 volts dissipated and was replaced with a feeling of relaxation by reducing the voltage to 1.5 volts. Interesting however, reducing the stimulation voltage from 3.0 to 2.2 volts in patient #14 changed the anxiety feeling to confusion while increasing the voltage in patient #6 from 3.3 volts to 3.5 volts was associated with remarkable change from feeling spaced to feeling relaxed. In general, it seems that a spectrum of TNM could be noticed with lower stimulation voltages associated with a sense of relaxation (1.53±0.34 V), and higher stimulation amplitudes were associated with euphoria (2.35 ± 0.23 V), crying (2.35 ± 0.81 V), and anxiety (2.78 ± 0.86 V). Two episodes of confusion occurred at comparatively high amplitudes (3.4 ± 0.66 V). (Table-1)
Table-1. Individual TNM episodes, contact location, stimulation parameters and differential regional activation maps.
List of patients (n=15) and individual TNM episodes and associated active contact location, current configuration, and stimulation voltage during the occurrence of TNM symptoms (C = case, Zi = zona incerta, IC = internal capsule, Ni = substantia nigra).
| Patients (n=15) | TNM episodes (n=29) | Stimulating Electrode Contact | ||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Episode number | Description | Visit (week) | Active (negative) contact | Contact location | Positive contact | Voltage | |
| 1 | F | 1 | Crying | 2 | Left 3 | IC | C | 3.4 |
| 2 | Crying | 2 | Right 1 | Medial STN/Ni | C | 2.5 | ||
| 3 | Crying | 12 | Left 1 | Medial STN/Ni | C | 2.0 | ||
| 4 | Crying | 12 | Left 1 | Medial STN/Ni | C | 1.5 | ||
| 2 | M | 1 | Feeling anxious | 2 | Right 3 | IC | C | 2.5 |
| 2 | Feeling relaxed | 12 | Right 2 | Medial STN | Right 3 (Zi) | 2.0 | ||
| 3 | M | 1 | Feeling anxious | 2 | Left 0 | Medial STN/Ni | C | 2.5 |
| 2 | Feeling anxious | 2 | Left 1 | Medial STN | C | 4.0 | ||
| 3 | Feeling anxious | 2 | Right 0 | Medial STN/Ni | C | 4.0 | ||
| 4 | Feeling anxious | Right 0 | Medial STN/Ni | C | 3.7 | |||
| 4 | M | 1 | Feeling anxious | 2 | Right 1 | Medial STN | C | 2.8 |
| 5 | M | 1 | Feeling confused | 2 | Right 0 | Lateral STN | C | 3.4 |
| 6 | M | 1 | Feeling spaced out | 2 | Right 3 | IC | C | 3.3 |
| 2 | Feeling relaxed | 2 | Right 1 | Lateral STN | C | 3.8 | ||
| 7 | F | 1 | Feeling relaxed | 2 | Left 0 | Zi | C | 1.15 |
| 8 | M | 1 | Feeling anxious | 2 | Left 0 | Ni | C | 2.0 |
| 2 | Feeling anxious | 2 | Left 1 | Ni | C | 2.0 | ||
| 3 | Feeling anxious | 2 | Left 2 | Lateral STN | C | 1.0 | ||
| 9 | M | 1 | Sense of euphoria | 2 | Left 1 | Medial STN | C | 2.5 |
| 2 | Sense of euphoria | 2 | Right 3 | IC | C | 2.0 | ||
| 10 | F | 1 | Feeling anxious | 2 | Left 0 | Medial STN | Left 1 (Lateral STN) | 2.8 |
| 2 | Feeling relaxed | 2 | Left 2 | Lateral STN | C | 1.5 | ||
| 11 | M | 1 | Feeling relaxed | 2 | Right 0 | Lateral STN | C | 1.5 |
| 12 | M | 1 | Sense of euphoria | 2 | Left 0 | Medial STN | C | 2.5 |
| 2 | Sense of euphoria | 2 | Left 1 | Medial STN | C | 2.7 | ||
| 13 | M | 1 | Feeling spaced out/confused | 2 | Left 2 | Lateral STN | C | 4.0 |
| 14 | M | 1 | Feeling anxious | 2 | Left 1 | Lateral STN | C | 3.0 |
| 2 | Feeling spaced out/confused | 2 | Left 0 | Medial STN | C | 2.5 | ||
| 15 | M | 1 | Feeling anxious | 2 | Right 2 | Medial STN | C | 2.5 |
Clinical outcome
Both TNM(+) and TNM(−) groups showed significant improvement in motor outcomes as measured by the reduction in UPDRS with no significant differences between the two groups: mean change (24-week-baseline) for TNM(+) vs. TNM(−): −14.08±2.09 vs. −17.47±1.92, t=1.376, p=0.1 (figure-1A). A similar reduction in LEDD between the two groups was evident: TNM(+) vs. TNM(−): −564±129 vs. −820±133, t=0.386, p=0.7 (figure-1B).
Figure-1.
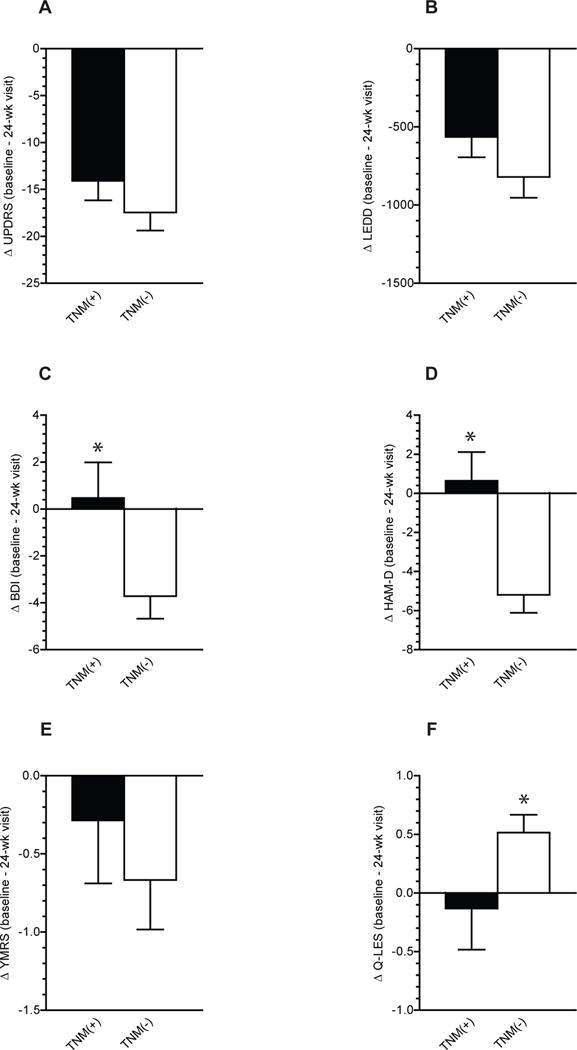
Change (baseline to 24-week visit) in (a) UPDRS, (b) LEDD, (c) BDI, (d) HAM-D, (e) YMRS and (f) Q0LES scores in TNM (+) and TNM (−) patients. Data presented as means ± SEM. (*) = P<0.05 by two-tailed paired t-test.
Since previous studies have alluded to potential influence of age at time of surgery on DBS effectiveness [19–21], we stratified patients into two groups, younger and older than 60. Among younger patients 7/19 (37%) reported TNM(+) psychiatric symptoms compared to 8/30 (27%) in the older age group. This difference was not statistically significant (X2 = 0.06, P=0.8). Younger patients had higher UPDRS scores and used more LEDD at baseline, and had more reduction in UPRS and LEDD by 24-week visit compared to older patients. However none of these differences reached significance. UPDRS at baseline [6o years old or younger (n=19) vs. older than 60 (n=30): 36.1±3 vs 34.7±1.5, P=0.6)], change from baseline to 24-week visit, ΔUPDRS (−19.3±2.7 vs. −14.7±1.6, P=0.13), baseline LEDD (1985±277.6 vs. 1578±150.9, P=0.16), and ΔLEDD (−856.2±221.1 vs. −527±95.5, P=0.12).
Furthermore, depressive symptoms and quality of life scores were not significantly different between the two groups at baseline; BDI (9.1±1.8 vs. 9.1±1.4, P=0.9), Q-LES (3.4±0.2 vs. 3.3±0.1, P=0.5) or ΔBDI (−2.7±2.0 vs. −2.6±1.0, P=0.9), and ΔQ-LES (0.06±0.3 vs. 0.4±0.1, P=0.2). On the other hand, the changes in depression rating scales were more pronounced in the TNM(−) group: change in BDI scores (24-week – baseline) in TNM(+) vs. TNM(−)was 0.4±1.5 vs. −4.4±0.9, t=2.166, p=0.04 (figure 1C). Similar findings were identified with HAM-D scores: TNM(+) vs. TNM(−); 0.4±1.5 vs. −5.5±0.8, t=2.188, p=0.04 (figure-1D). However, no significant differences were detected in the change in YMRS scores between the groups: TNM(+) vs. TNM(−)were 0.3±0.5 vs. −1.0±0.4, t=0.74, p=0.4, (figure-1E).
Interestingly, the TNM(+) group had a minimal increase (0.1±0.3) in the quality of life score as measured by Q-LES which was slightly, but significantly, less than the increase (0.5 ± 0.1) in the TNM(−) (t=2.128, df=14, p=0.05, figure-1F).
Given that TNM(+) patients were taking 30% less levodopa, we looked at the relationship between levodopa dose, depressive symptoms and quality of life in all patients and in TNM(+) patients. A significant positive correlation between the ΔLEDD and ΔBDI scores was evident in all patients (r= 0.3062, n= 42, P= 0.0486), but not in TNM(+) subgroup (r= −0.1972, n= 15, P= 0.4). No significant correlation was found between LEDD and Q-LES neither in all patients (r= −0.1956, n= 42, P= 0.2), nor in TNM(+) subgroup (r= 0.1350, n= 14, P= 0.6). We then compared patients taking lower levodopa dose; less than 1500 mg (n=22) with those who were taking 1500 mg or more (n=27) at baseline. No significant differences were found between the two groups in the frequency of TNM(+) patients [(7/22 (32%) vs. 8/27 (30%), P=0.9], mean baseline BDI scores (8.4±1.6 vs. 9.7±1.7, P = 0.5), ΔBDI (−2.7±1.4 vs. −2.6±1.4. P=0.9), baseline Q-LES (3.5±0.2 vs. 3.3±0.16, P = 0.2) or Δ Q-LES (0.14±0.2 vs. 0.57±0.19, P = 0.12).
Neuroanatomical correlates of TNM
Localization of active contacts
Active contacts were found to be localized within the STN bilaterally in 16 cases and in the surrounding fiber tracts zona incerta (Zi), internal capsule (IC), or neighboring structures such as substantia nigra (SN) in 13 cases. In six cases, active contacts were localized at the borders between STN and surrounding fibers, and in 13 cases one active contact was located in the STN and the other in the surrounding fibers (figure-2A).
Figure-2. Neuroanatomical localization of active contacts.
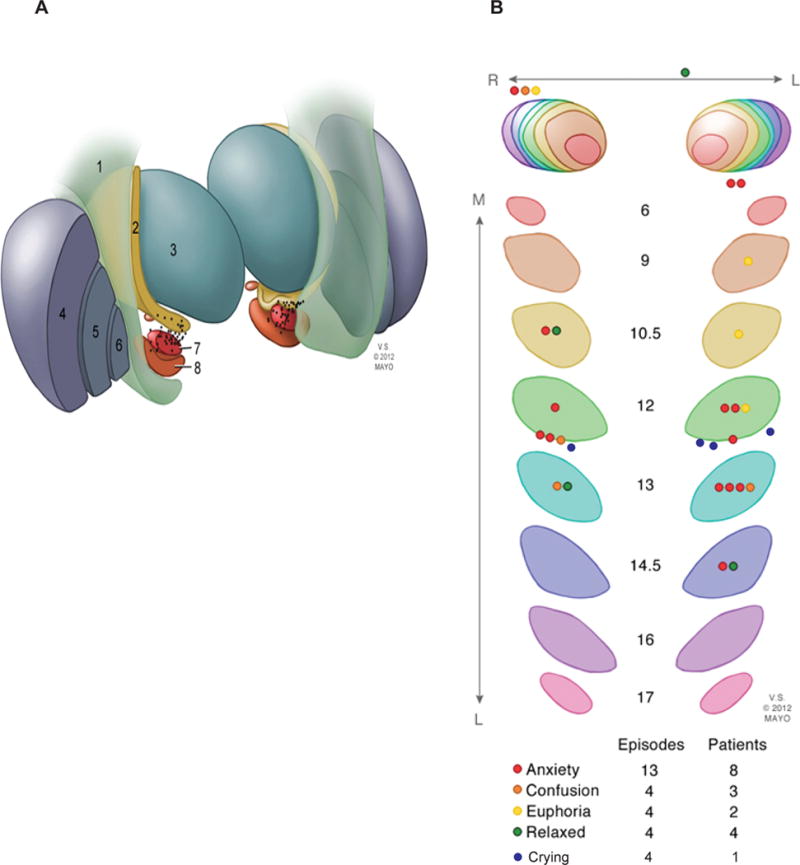
The spatial location of the active contacts on postoperative MR images fused with the Schaltenbrand and Wahren human brain atlas. (A) Illustration demonstrating the anatomic location of the active contacts in relation to surrounding structures: 1. corticospinal tract, 2. zona incerta, 3. thalamus, 4. putamen, 5. globus pallidus external segment, 6. globus pallidus internal segment, 7. subthalamic nucleus, 8. substantia nigra. (B) Serial sections of the STN corresponding to slices in the Schaltenbrand and Wahren human brain atlas on the left (L6–L17) and right (R6–R17) showing the locations of active contacts and corresponding TNM categories.
The relationship between the anatomical location of active contact, stimulation polarity, voltage and the occurrence of TNM symptoms
Active contacts stimulated during the TNM episodes were located within the STN in 22/29 episodes (figure-2B); the majority (n=15) lied within the medial subregion. The remainder were located in the lateral STN (n=7), within the surrounding fibers (n=5), and in the SN (n=2). In the 4 episodes of crying, all but one were localized within the medial STN bordering the SN (figure-2B).
As detailed in table-1, nearly all TNM episodes (93%, 27/29) occurred during monopolar stimulation of either left (n=17) or right (n=12) contacts. At the last study follow-up visit (week 24), all TNM(+) patients (n=15) had bilateral monopolar stimulation compared with only 52% (18/34) of TNM(−) patients (p<0.0001).
Discussion
Our understanding of the role of STN continues to evolve. The observed TNM psychiatric manifestations in a subgroup of patients in the present study point to the function of the STN in regulating the activity of the limbic circuit. Turner and colleagues [22] demonstrated that the STN can impact limbic activity through influencing the neuronal firing amplitude at the ventral pallidum. Neuroanatomically, the limbic and associative loops cross the ventromedial STN [23] where processing of emotional information takes place [24] through integrating the limbic and associative loops [1] and modulation of limbic activation occurs in order to formulate the appropriate emotional component associated with specific motor tasks [22]. Transient overstimulation of the STN ventromedial subregion in certain patients could lead to overactivation of the limbic circuits which manifests as difficulties in emotional recognition [25] or, as in our subjects, as TNM psychiatric symptoms. This is consistent with our localization results where the majority of TNM episodes occurred during monopolar configuration of active contacts located within the medial STN. Furthermore, case reports of transient laughter were observed during monopolar stimulation [5]. Along the same lines, transient difficulty in emotional recognition [25], abrupt pleasant sensations [6], hypomania [9], and mania [7] were reproducible with bilateral stimulation of the medial STN. Monopolar, in contrast with bipolar, configuration is known to produce larger charge density. This suggests that the limbic circuitry engages normally in motivational behaviors, and hyperactivation of that network disrupts its regulatory function manifesting as TNM symptoms. Studies employing functional imaging modalities in patients with STN-DBS are needed to test this hypothesis.
The escalation of the stimulation voltage was found to be associated with change in TNM symptom category. At lower mean voltages, patients experienced a relaxing effect. By increasing the stimulating voltage, patients reported feelings of euphoria followed by crying, anxiety, and finally feeling cognitively impaired. Before we discuss this interesting observation further, we need to acknowledge that because the overall number of episodes was small, we pooled all episodes together as if they were independent even though some patients had more than one episode. Despite this methodological limitation, it seems that voltage-dependent emergence of TNM symptoms is consistent with our previous case report of voltage-dependent mania [7] and with preclinical data from our group utilizing fMRI in anesthetized pigs [26]. Clinical case report showed remarkable increase in manic-like symptoms as measured by YMRS corresponding with increase in stimulation voltage in a PD patient with no past psychiatric history. Also, along the same lines with our current neuroanatomical results, in this case report, active contacts were located in the medial STN. Our preclinical results demonstrated that STN-DBS was uniquely associated with activation in the ipsilateral thalamus, the somatosensory association cortex, prepyriform area, hippocampus, lateral geniculate nuclei, pedunculopontine nucleus (PPN), the contralateral temporal cortex, parahippocampal gyrus, and cerebellum. Moreover, increasing stimulation amplitude (from 1 to 2 volts) was associated with a corresponding increase in the size of the activated areas to include non-motor limbic structures such as the cingulate cortex, caudate, and putamen. Furthermore, previous results from our group show that STN stimulation evokes dopamine release from the striatum in a voltage-dependent manner [27]. Taken together, it could be postulated that, during optimization of stimulation parameters, the escalation in amplitude causes abrupt release of striatal dopamine [28] and excites distal axons activating the limbic and associative loops [26], which presents as TNM symptoms. An intriguing clinical corollary is that individual variability in PD pathology leads to potentially vulnerable limbic circuits in certain patients. The dopamine hypothesis for TNM remains a mere speculation [29] waiting for supportive evidence that may be achieved through simultaneous intraoperative measurement of dopamine release using our recently developed Wireless Instantaneous Neurotransmitter Concentration System (WINCS) device [30] during testing of stimulating electrodes.
Of clinical relevance, over half of the study cohort had comorbid psychiatric conditions and about a third experienced TNM symptoms. Individual variations in basal ganglia pathology in patients with PD could be speculated as reason why only certain patients experienced TNM. In their seminal study, Turner and colleagues [22] showed that the effect of STN on limbic function depends on ventral pallidal neuronal activity status. STN-evoked excitatory post-synaptic potential amplitude was markedly larger when evoked in spontaneously firing neurons. This limbic vulnerability to over-activation and eventually the development of TNM symptoms does not seem to be related to the presence or absence of comorbid psychiatric conditions. Among the fifteen TNM (+) patients, seven had psychiatric history: depression, n=5; bipolar/impulse control symptoms, n=2) and 8 did not have history of comorbid psychiatric conditions. Noteworthy, patients with comorbid psychiatric conditions are often excluded by various DBS selection committees under the notion that they may be at higher risk for developing postoperative psychiatric complications [31]. Our results underscore the necessity of further studies to systematically examine the relationship between psychiatric comorbidity and long-term outcome in PD patients who undergo STN-DBS.
Remarkably, the experience of TNM symptoms was not associated with negative impact on motor outcome. This is consistent with our previous report that motor outcome is not significantly different between STN-DBS PD patients who had active contacts within the medial versus lateral STN subregions [18]. On the other hand, mood and quality of life outcomes varied significantly between the two groups. Compared to TNM(+) patients, those who did not experience TNM symptoms reported about 4-point reduction in BDI and HAM-D scores and about half a point increase in Q-LES scores over the study interval. These differences, albeit small, were significant. It is not entirely clear whether the persistence of subsyndromal depression in the TNM(+) group could have contributed to the lack of improvement in the quality of life measure. Troster et al [32] found significant improvement in QOL and depression (BDI) after STN DBS in 26 patients and a significant correlation between the changes in QOL and changes in BDI scores. Daniels and colleagues [33] found a strong correlation between changes in depression scores from baseline to 6-months postoperatively and quality of life scores in a cohort of 59 PD patients who underwent bilateral STN-DBS. Similar findings were reported in non-STN-DBS bipolar patients, where a significant negative correlation was observed between Global Assessment of Functioning scores and HAM-D scores despite the fact that no patient had a HAM-D score high enough to be considered clinically depressed [34].
The current results concur with a rich body of literature demonstrating that the frequency of TNM psychiatric symptoms are more likely to manifest in STN compared to GPi patients [35]. In a recent meta-analysis of 6 randomized controlled trials with a total of 563 PD patients comparing motor and psychiatric outcomes between STN and GPi DBS, no significant difference was found between the two targets in UPDRS II score changes. However, significant reduction in LEDD was found in STN and significantly lower BDI scores in GPi patients. These results are consistent with earlier reports. One study found transient anxiety and hallucinations in 30% of patients following STN (n=10), but not GPi DBS (n=10) [36]. Another study demonstrated that 53% of patients who had STN DBS (n=49) reported psychiatric adverse events compared with 35% of GPi patients (n=20) at 4 year post operatively [37]. More reduction in depressive symptoms and more improvement in QOL were reported at 6 month post GPi (n=20) compared to STN DBS (n=22) [38] while transient depression, anhedonia, and abulia were reported in patients after STN (n=16) but not GPi DBS (n=11) [39]. TNM psychiatric manifestations were attributed to the larger reduction of LEDD with STN compared to GPi as all symptoms improved by increasing levodopa and not by changing stimulation parameters [39]. Taken together, these observations suggest that the aggressive medication reduction after STN DBS can lead to levodopa agonist withdrawal which could unmask an underlying mesolimbic hypodopaminergic vulnerability state [40] and manifest as psychiatric symptoms (reviewed in [41, 42]. To test this intriguing concept, Okun et al [43] compared the effect of slow reduction in LEDD on psychiatric side effects following unilateral STN (n=16) and GPi DBS (n=14). No significant differences were found between the two targets in mood, anxiety or motor outcomes. However there was steady worsening in mood and anxiety at 2, 4, 6 and 12 months compared to baseline when combining the STN and GPi groups. Furthermore, the changes in LEDD did not correlate with changes in mood or UPDRS II scores in both groups [43] or with QOL at 1 year post STN DBS in another study [44]. Similarly we did not find a correlation between LEDD and Q-LES in our cohort, but we observed significant positive correlation between the change in LEDD and change in BDI scores in all patients but not in TNM(+) subgroup. Further studies designed specifically to probe the relationship between levodopa dose and psychiatric adverse events and quality of life is warranted.
The intriguing observation of objective motor improvement without corresponding subjective improvement of quality of life remains unexplained. Routine use of standard rating scales in clinical settings and large-scale, long-term follow-up studies designed to explore the presence and impact of subsyndromal depression on outcome measures following STN-DBS are warranted.
Equally important, these TNM psychiatric manifestations that took place briefly at the second week of the optimization process had a significant long term impact on mood and quality of life. While a clear understanding of the underlying neurobiology of how such a transient over-activation of the limbic circuitry could leave a profound effect on mood or quality of life is lacking, one explanation could relate TNM and TNM-related effect on mood and quality of life to individual limbic circuitry vulnerability.
The results of this study must be interpreted with caution. We have made significant effort to standardize our method in collecting TNM responses from patients over different visits. However, spontaneous responses over time are hard to quantitate across a group and it is possible that we have missed some responses. It is also possible that some relaxation feelings reported as TNM are not directly stimulation related but reflect motor effect. In addition, only one patient exhibited crying. This makes it impossible to distinguish electrode-location effects from other patient-specific effects with respect to that symptom. Furthermore, in order to determine the location of the active contact relative to the MRI, the center of the MRI artifact was assumed to represent the center of the DBS electrode as previously described [45]. However, it is recognized that these artifacts are subject to paramagnetic inhomogeneity [46]. Despite these limitations, our study presents that largest number of patients reporting TNM psychiatric symptoms in the course of STN DBS and adds an important insight into the association between TNM and stimulation voltage and electrode location.
Acknowledgments
We would like to thank Ms. Veneliza Salcedo for making the illustration.
Funding Source
This work is supported by a grant support from NIH/NCRR CTSA KL2 (RR024151) (OAA) and Mayo Foundation (MAF).
References
- 1.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in brain research. 1990;85:119–46. [PubMed] [Google Scholar]
- 2.Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain research bulletin. 2009;78(2–3):69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bejjani BP, et al. Transient acute depression induced by high-frequency deep-brain stimulation. The New England journal of medicine. 1999;340(19):1476–80. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- 4.Sensi M, et al. Explosive-aggressive behavior related to bilateral subthalamic stimulation. Parkinsonism & related disorders. 2004;10(4):247–51. doi: 10.1016/j.parkreldis.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Krack P, et al. Mirthful laughter induced by subthalamic nucleus stimulation. Movement disorders : official journal of the Movement Disorder Society. 2001;16(5):867–75. doi: 10.1002/mds.1174. [DOI] [PubMed] [Google Scholar]
- 6.Benedetti F, et al. Autonomic and emotional responses to open and hidden stimulations of the human subthalamic region. Brain research bulletin. 2004;63(3):203–11. doi: 10.1016/j.brainresbull.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Chopra A, et al. Voltage-dependent mania after subthalamic nucleus deep brain stimulation in Parkinson’s disease: a case report. Biological psychiatry. 2011;70(2):e5–7. doi: 10.1016/j.biopsych.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Kulisevsky J, et al. Mania following deep brain stimulation for Parkinson’s disease. Neurology. 2002;59(9):1421–4. doi: 10.1212/wnl.59.9.1421. [DOI] [PubMed] [Google Scholar]
- 9.Mallet L, et al. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(25):10661–6. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raucher-Chene D, et al. Manic episode with psychotic symptoms in a patient with Parkinson’s disease treated by subthalamic nucleus stimulation: improvement on switching the target. Journal of the neurological sciences. 2008;273(1–2):116–7. doi: 10.1016/j.jns.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Chen SY, Lin SZ, Lee TW. Subthalamic nucleus stimulation and the development of delusion. Journal of psychiatric research. 2004;38(6):637–8. doi: 10.1016/j.jpsychires.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Fahn S, Elton RL, Committee U. Unified Parkinson’s Disease Rating Scale, in Recent Developments in Parkinson’s Disease. In: Fahn S, et al., editors. McMillan Healthcare Information. Florham Park, NJ: 1987. [Google Scholar]
- 13.Lozano AM. Deep brain stimulation for Parkinson’s disease. Parkinsonism & related disorders. 2001;7(3):199–203. doi: 10.1016/s1353-8020(00)00057-2. [DOI] [PubMed] [Google Scholar]
- 14.Endicott J, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacology bulletin. 1993;29(2):321–6. [PubMed] [Google Scholar]
- 15.Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AT, et al. An inventory for measuring depression. Archives of general psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 17.Young RC, et al. A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry: the journal of mental science. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 18.Kasasbeh A, et al. Lack of differential motor outcome with subthalamic nucleus region stimulation in Parkinson’s disease. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2013;20(11):1520–6. doi: 10.1016/j.jocn.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Tavella A, et al. Deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: long-term follow-up. Neurol Sci. 2002;23(Suppl 2):S111–2. doi: 10.1007/s100720200094. [DOI] [PubMed] [Google Scholar]
- 20.Erola T, et al. Bilateral subthalamic nucleus stimulation improves health-related quality of life in Parkinsonian patients. Parkinsonism Relat Disord. 2005;11(2):89–94. doi: 10.1016/j.parkreldis.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Rothlind JC, et al. Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for Parkinson’s disease. J Int Neuropsychol Soc. 2007;13(1):68–79. doi: 10.1017/S1355617707070105. [DOI] [PubMed] [Google Scholar]
- 22.Turner MS, et al. Regulation of limbic information outflow by the subthalamic nucleus: excitatory amino acid projections to the ventral pallidum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(8):2820–32. doi: 10.1523/JNEUROSCI.21-08-02820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamani C, Neimat J, Lozano AM. Deep brain stimulation for the treatment of Parkinson’s disease. Journal of neural transmission. Supplementum. 2006;(70):393–9. doi: 10.1007/978-3-211-45295-0_59. [DOI] [PubMed] [Google Scholar]
- 24.Brucke C, et al. The subthalamic region is activated during valence-related emotional processing in patients with Parkinson’s disease. The European journal of neuroscience. 2007;26(3):767–74. doi: 10.1111/j.1460-9568.2007.05683.x. [DOI] [PubMed] [Google Scholar]
- 25.Dujardin K, et al. Subthalamic nucleus stimulation induces deficits in decoding emotional facial expressions in Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 2004;75(2):202–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Min HK, et al. Deep brain stimulation induces BOLD activation in motor and non-motor networks: an fMRI comparison study of STN and EN/GPi DBS in large animals. NeuroImage. 2012;63(3):1408–20. doi: 10.1016/j.neuroimage.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shon YM, et al. High frequency stimulation of the subthalamic nucleus evokes striatal dopamine release in a large animal model of human DBS neurosurgery. Neuroscience letters. 2010;475(3):136–40. doi: 10.1016/j.neulet.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KH, et al. Evolution of Deep Brain Stimulation: Human Electrometer and Smart Devices Supporting the Next Generation of Therapy. Neuromodulation : journal of the International Neuromodulation Society. 2009;12(2):85–103. doi: 10.1111/j.1525-1403.2009.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilker R, et al. Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov Disord. 2003;18(1):41–8. doi: 10.1002/mds.10297. [DOI] [PubMed] [Google Scholar]
- 30.Kasasbeh A, et al. Wireless neurochemical monitoring in humans. Stereotactic and functional neurosurgery. 2013;91(3):141–7. doi: 10.1159/000345111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Machado AG, Deogaonkar M, Cooper S. Deep brain stimulation for movement disorders: patient selection and technical options. Cleveland Clinic journal of medicine. 2012;79(Suppl 2):S19–24. doi: 10.3949/ccjm.79.s2a.04. [DOI] [PubMed] [Google Scholar]
- 32.Troster AI, et al. Effect of motor improvement on quality of life following subthalamic stimulation is mediated by changes in depressive symptomatology. Stereotact Funct Neurosurg. 2003;80(1–4):43–7. doi: 10.1159/000075159. [DOI] [PubMed] [Google Scholar]
- 33.Daniels C, et al. Is improvement in the quality of life after subthalamic nucleus stimulation in Parkinson’s disease predictable? Movement disorders: official journal of the Movement Disorder Society. 2011;26(14):2516–21. doi: 10.1002/mds.23907. [DOI] [PubMed] [Google Scholar]
- 34.Altshuler LL, et al. Subsyndromal depression is associated with functional impairment in patients with bipolar disorder. The Journal of clinical psychiatry. 2002;63(9):807–11. doi: 10.4088/jcp.v63n0910. [DOI] [PubMed] [Google Scholar]
- 35.Volkmann J, et al. Long-term effects of pallidal or subthalamic deep brain stimulation on quality of life in Parkinson’s disease. Mov Disord. 2009;24(8):1154–61. doi: 10.1002/mds.22496. [DOI] [PubMed] [Google Scholar]
- 36.Anderson VC, et al. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62(4):554–60. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- 37.Hariz MI, et al. Multicenter study on deep brain stimulation in Parkinson’s disease: an independent assessment of reported adverse events at 4 years. Mov Disord. 2008;23(3):416–21. doi: 10.1002/mds.21888. [DOI] [PubMed] [Google Scholar]
- 38.Zahodne LB, et al. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. J Neurol. 2009;256(8):1321–9. doi: 10.1007/s00415-009-5121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volkmann J, et al. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. 2001;56(4):548–51. doi: 10.1212/wnl.56.4.548. [DOI] [PubMed] [Google Scholar]
- 40.Thobois S, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain. 2010;133(Pt 4):1111–27. doi: 10.1093/brain/awq032. [DOI] [PubMed] [Google Scholar]
- 41.Voon V, Howell NA, Krack P. Psychiatric considerations in deep brain stimulation for Parkinson’s disease. Handb Clin Neurol. 2013;116:147–54. doi: 10.1016/B978-0-444-53497-2.00012-7. [DOI] [PubMed] [Google Scholar]
- 42.Williams NR, Foote KD, Okun MS. STN vs. GPi Deep Brain Stimulation: Translating the Rematch into Clinical Practice. Mov Disord Clin Pract. 2014;1(1):24–35. doi: 10.1002/mdc3.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okun MS, et al. Acute and Chronic Mood and Apathy Outcomes from a randomized study of unilateral STN and GPi DBS. PLoS One. 2014;9(12):e114140. doi: 10.1371/journal.pone.0114140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sobstyl M, et al. Quality of life in advanced Parkinson’s disease after bilateral subthalamic stimulation: 2 years follow-up study. Clin Neurol Neurosurg. 2014;124:161–5. doi: 10.1016/j.clineuro.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Starr PA, Vitek JL, Bakay RA. Ablative surgery and deep brain stimulation for Parkinson’s disease. Neurosurgery. 1998;43(5):989–1013. doi: 10.1097/00006123-199811000-00001. discussion 1013–5. [DOI] [PubMed] [Google Scholar]
- 46.Paek SH, et al. Electrode position determined by fused images of preoperative and postoperative magnetic resonance imaging and surgical outcome after subthalamic nucleus deep brain stimulation. Neurosurgery. 2008;63(5):925–36. doi: 10.1227/01.NEU.0000334045.43940.FB. discussion 936–7. [DOI] [PubMed] [Google Scholar]


