We describe clinical and imaging findings for 8 patients with Powassan virus (POWV) encephalitis in New England from 2013 to 2015, demonstrating that POWV is an emerging diagnosis in this region and may be more prevalent than is currently appreciated.
Keywords: Powassan, deer tick virus, encephalitis, New England
Abstract
Background. Powassan virus (POWV) is a rarely diagnosed cause of encephalitis in the United States. In the Northeast, it is transmitted by Ixodes scapularis, the same vector that transmits Lyme disease. The prevalence of POWV among animal hosts and vectors has been increasing. We present 8 cases of POWV encephalitis from Massachusetts and New Hampshire in 2013–2015.
Methods. We abstracted clinical and epidemiological information for patients with POWV encephalitis diagnosed at 2 hospitals in Massachusetts from 2013 to 2015. We compared their brain imaging with those in published findings from Powassan and other viral encephalitides.
Results. The patients ranged in age from 21 to 82 years, were, for the most part, previously healthy, and presented with syndromes of fever, headache, and altered consciousness. Infections occurred from May to September and were often associated with known tick exposures. In all patients, cerebrospinal fluid analyses showed pleocytosis with elevated protein. In 7 of 8 patients, brain magnetic resonance imaging demonstrated deep foci of increased T2/fluid-attenuation inversion recovery signal intensity.
Conclusions. We describe 8 cases of POWV encephalitis in Massachusetts and New Hampshire in 2013–2015. Prior to this, there had been only 2 cases of POWV encephalitis identified in Massachusetts. These cases may represent emergence of this virus in a region where its vector, I. scapularis, is known to be prevalent or may represent the emerging diagnosis of an underappreciated pathogen. We recommend testing for POWV in patients who present with encephalitis in the spring to fall in New England.
Powassan virus (POWV) encephalitis is an arthropod-borne infection caused by a flavivirus that, unlike most arboviruses, is transmitted by ticks rather than mosquitoes. It has been infrequently reported in North America, with approximately 100 cases since 1958 [1]. In the Great Lakes region, Ixodes cookei ticks transmit the traditional lineage of POWV. More recently, cases have been identified in the Northeast, where POWV lineage II (also known as deer tick virus) is transmitted by Ixodes scapularis, which also transmits the causative agent of Lyme disease. POWV encephalitis has been reported in New York State, with 14 cases from 2004 to 2012 and 4 cases in 2013. In 2013, 1 case was reported in Massachusetts and 1 in New Hampshire [2]; previously there had been only 1 case identified in Massachusetts in 1994. It is likely that POWV is more prevalent in New England than is currently appreciated, given the prevalence of other I. scapularis–borne infections. Here, we describe the clinical features and brain imaging of 8 patients with POWV encephalitis from Massachusetts and New Hampshire from 2013 to 2015. The study was reviewed and approved by the Lahey Clinic, Inc., institutional review board.
CASES
Here, we briefly summarize the 8 cases of POWV encephalitis, with further demographic and clinical information presented in Table 1. For all patients, there were no abnormalities on basic laboratory tests (eg, elevated transaminases, cytopenias), except as noted below. All patients initially received empiric treatment for bacterial meningitis as well as empiric acyclovir. Results of initial cerebrospinal fluid (CSF) analysis for each patient are shown in Table 2, and all patients had negative Gram stain and bacterial culture from the CSF. The results of diagnostic testing for Powassan encephalitis are summarized in Table 1; all patients additionally had negative tests for herpes simplex virus, West Nile virus, Eastern equine encephalitis virus, and Lyme (from serology and, in some cases, CSF).
Table 1.
Demographic and Clinical Features of 8 Patients With Powassan Virus Encephalitis
| Patient No., Age, and Gender | Comorbidity | Presentation | Exposure | Signs and Symptoms |
Powassan Diagnosticsa | Immunomodulatory Treatment | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| Fever | Rash | Gastrointestinal | |||||||
| Patient 1 82 M |
Coronary artery disease | September 2015 | Outdoors | Y | N | Vomiting | Serum IgM + PRNT 1:80 Cerebrospinal fluid IgM + PRNT 1:8 | None | Death |
| Patient 2 74 M |
Psoriatic arthritis, on methotrexate | June 2015 | Outdoors, gardening | Y | N | None | Cerebrospinal fluid IgM + PRNT 1:512 | IVIG | Residual deficits |
| Patient 3 21 M |
None | September 2014 | Outdoors | Y | Y | Vomiting | Serum IgM + PRNT 1:320 | Steroids, IVIG | Improved |
| Patient 4 67 M |
DLBCL, in remission | September 2014 | Outdoors | Y | Y | Vomiting, diarrhea | Serum IgM + PRNT 1:160 | None | Improved |
| Patient 5 65 F |
Migraine headache | June 2014 | Tick bites | Y | N | Vomiting | Serum IgM + PRNT 1:160 | None | Improved |
| Patient 6 52 M |
None | May 2014 | Tick bites | Y | N | None | Serum IgM/IgG + PRNT 1:160b | None | Residual deficits |
| Patient 7 49 M |
None | May 2014 | Tick bites | Y | N | None | Serum IgM + PRNT Negativec | None | Death |
| Patient 8 44 M |
Hypertension | May 2013 | Outdoors, hunting | N | Y | None | Serum IgM/IgG + PRNT 1:10240d | None | Improved |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; IgG, immunoglobulin-G; IgM, immunoglobulin-M; IVIG, intravenous immunoglobulin; PRNT, plaque reduction neutralization test.
a All testing was performed by the Centers for Disease and Prevention, Control Arboviral Diseases Branch, Fort Collins, Colorado. Testing for other arboviruses was negative except as noted below.
b This patient also had evidence of a California group orthobunyavirus on enzyme-linked immunosorbent assay but negative PRNT.
c Although confirmatory PRNT was negative, the sample was sent only 6 days into the patient's illness. Testing was otherwise negative for herpes simplex virus, enterovirus, Lyme, West Nile virus, equine encephalitis virus, St. Louis encephalitis virus, LaCrosse virus, Jamestown Canyon virus, and lymphocytic choriomeningitis virus.
d This patient also had positive serology for LaCrosse and Jamestown Canyon viruses but at lower levels, suggesting possible cross-reactivity or coinfection.
Table 2.
Cerebrospinal Fluid Measurements
| Patient No. | Glucose (mg/dL) |
Protein (mg/dL) | Red Blood Cells/µL | White Blood Cells/µL | White Blood Cell Differential (%) |
||
|---|---|---|---|---|---|---|---|
| Cerebrospinal Fluid | Plasma | Neutrophils | Lymphocytes | ||||
| 1 | 45 | 96 | 230 | 1 | 169 | 0 | 83 |
| 2 | 76 | NA | 201 | 0 | 108 | 31 | 51 |
| 3 | 63 | 108 | 85 | 3 | 93 | 75 | 20 |
| 4 | 49 | NA | 89 | 17 | 557 | 2 | 75 |
| 5 | 44 | 91 | 84 | 2 | 220 | 14 | 73 |
| 6 | 53 | 93 | 113 | 30 | 420 | 2 | 80 |
| 7 | 54 | 118 | 107 | 35 | 146 | NA | 93 |
| 8 | 91 | 145 | 58 | 7 | 720 | 40 | 47 |
Abbreviation: NA, not available.
In all cases, Gram stain and bacterial culture were negative.
Patient 1
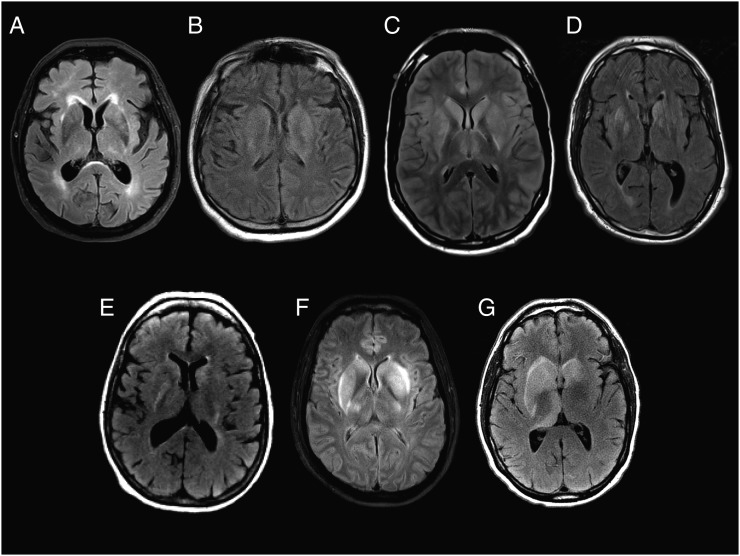
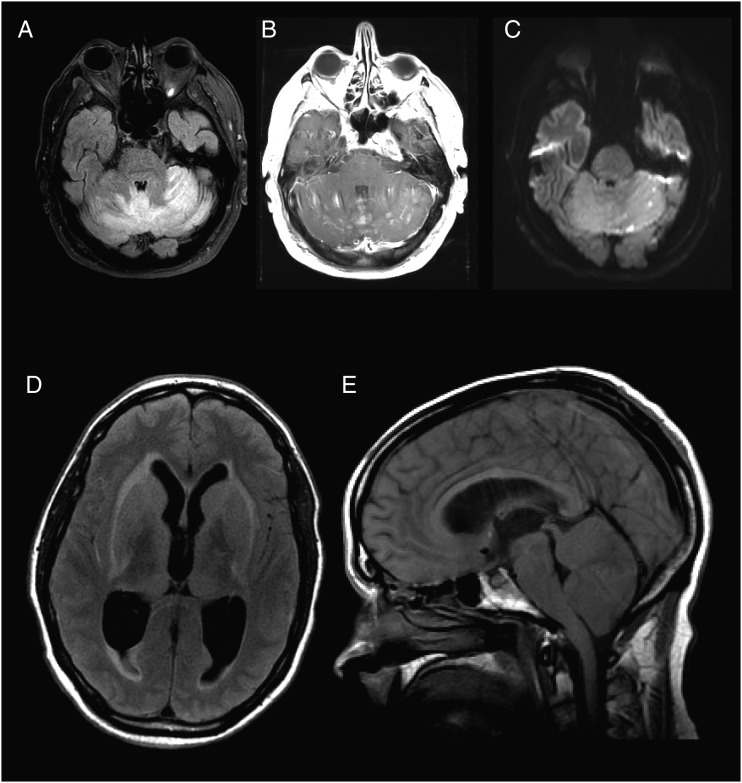
An 82-year-old man from Massachusetts presented with sudden onset of dizziness followed by nausea and vomiting (Table 1). On initial exam, he was afebrile with a normal neurologic examination; initial lab data were remarkable only for a platelet count of 140 000 cells/µL. Over the next few days he developed an elevated temperature of 102 F as well as an upward gaze deviation, direction-changing nystagmus, severe axial rigidity, and bilateral Babinski signs. Brain magnetic resonance imaging (MRI) on day 5 of illness showed subtle, diffuse, high T2 signal intensity in the cerebellum and subtle enhancement of the vermis (not shown). He required intubation for airway protection. CSF analysis demonstrated lymphocytic pleocytosis (Table 2). Repeat brain MRI on day 10 showed significant worsening (Figure 1A, Figure 2A–C). The patient continued to decline and expired on day 18 of illness.
Figure 1.
Initial T2/fluid-attenuation inversion recovery magnetic resonance images from patients 1 (A), 2 (B), 3 (C), 4 (D), 5 (E), 6 (F), and 7 (G) show varying degrees of hyperintensity in the bilateral caudate heads, lenticular nuclei, and thalami, with mass effect on the lateral ventricles seen in patients 3, 4, 6, and 7. Patient 3 (C) and patient 6 (F) also demonstrate hyperintensity throughout the cortical ribbon with sulcal effacement, which for patient 3 correlated with reversible diffusion restriction (Figure 3). No abnormal enhancement was found for any of the patients. Patient 8 had normal imaging.
Figure 2.
Selected images from 2 fatal cases of Powassan virus encephalitis. Axial T2/fluid-attenuation inversion recovery (FLAIR) (A), T1 post-contrast (B), and diffusion weighted imaging (C) images obtained from patient 1 ten days after symptom onset demonstrate bilateral cerebellar edema (A), diffuse cerebellar parenchymal and leptomeningeal enhancement (B), and multiple foci of restricted diffusion (C). Susceptibility weighted imaging demonstrated multiethnic areas of blooming artifact consistent with hemorrhage (not shown). Axial T2/FLAIR (D) and sagittal T1 (E) images obtained from patient 7 three days after prior imaging (Figure 1G) show extensive sulcal effacement and ventricular enlargement (D) with cerebellar edema and tonsillar herniation through the foramen magnum (E).
Patient 2
A 74-year-old man from Massachusetts with a history of psoriatic arthritis on methotrexate presented with 2 weeks of upper respiratory symptoms, right eye pain, and visual blurring (Table 1). On initial exam, he was afebrile with a normal neurologic examination; however, over the next day, he developed an elevated temperature of 102 F and became increasingly obtunded, requiring intubation. MRI of the brain showed T2/fluid-attenuation inversion recovery (FLAIR) hyperintensities throughout the brainstem and basal ganglia bilaterally, extending to the right anterior frontal subcortical white matter (Figure 1B) without diffusion restriction or areas of abnormal enhancement. CSF analysis showed lymphocytic pleocytosis (Table 2). He was treated with intravenous immunoglobulin (IVIG) 2 g/kg 7 days after initial symptom onset. He improved over his hospitalization but had notable residual deficits at the time of discharge. He could track, regard, and intermittently follow 1 simple command and had purposeful movement only in the left upper extremity.
Patient 3
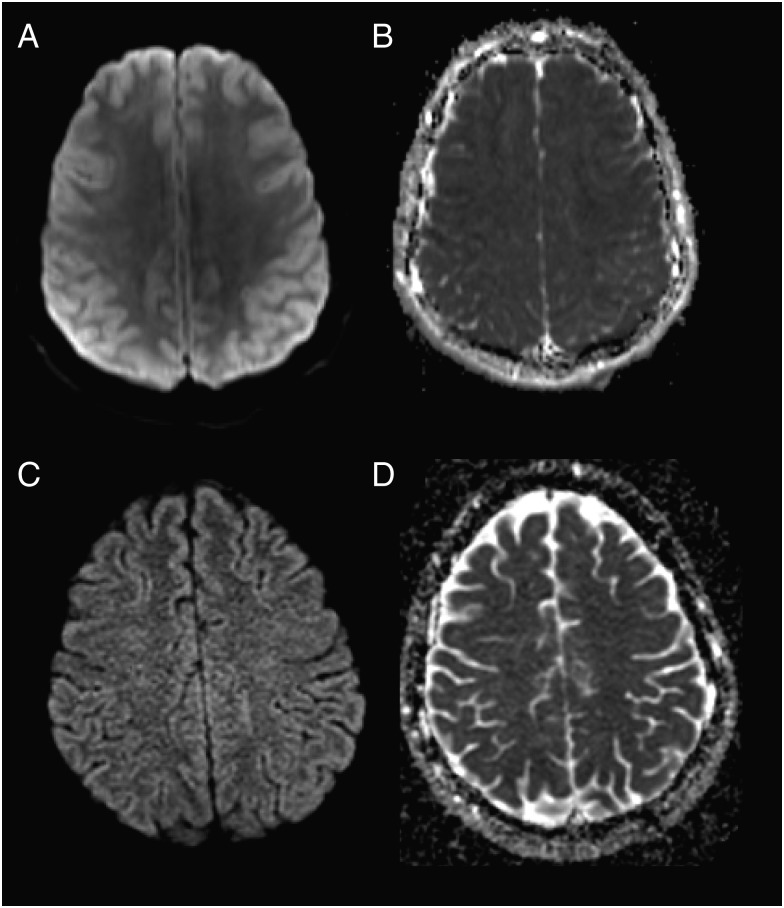
A 21-year-old man from Massachusetts presented with 3 days of vomiting, confusion, and fever (Table 1). On exam, he was somnolent but arousable and followed simple commands. He had a faint maculopapular rash on his trunk. Brain MRI showed T2/FLAIR hyperintensities in the basal ganglia and thalami bilaterally and diffusion restriction throughout the cortex (Figure 1C, Figure 3). Initial CSF cells were primarily polymorphonuclear (Table 2). He became progressively more obtunded, requiring intubation. Repeat MRI showed progression of the T2/FLAIR hyperintensities in the insula, caudate heads, and putamen bilaterally (Supplementary Figure 1). A repeat CSF analysis performed 3 days after his initial lumbar puncture demonstrated 156 white blood cells (WBCs; 30% polymorphonuclear cells, 51% lymphocytes). He was treated with methylprednisolone 1 g on days 7–11 after symptom onset and IVIG on days 8–12 after symptom onset. He clinically improved and by discharge was alert, oriented, and speaking in short sentences. Seven months later, the patient had returned to work and his brain MRI was normal (Figure 3, Supplementary Figure 1).
Figure 3.
Initial imaging from patient 3 shows increased signal intensity throughout the cortical ribbon on diffusion weighted imaging (DWI) (A) and decreased signal intensity on apparent diffusion coefficient (ADC) (B), demonstrating restricted diffusion. On follow-up DWI (C) and ADC (D) 9 months later, this had resolved, as had fluid-attenuation inversion recovery hyperintensities (Supplementary Figure 1).
Patient 4
A 67-year-old man from Massachusetts presented with 4 days of confusion, vomiting, diarrhea, and fever (Table 1). General physical exam was notable only for a faint maculopapular rash over the chest and upper back. Neurologic exam was notable for marked encephalopathy (inattention and decreased level of arousal) but preserved memory, orientation, and language. He had a forehead-sparing right facial droop, diffusely hyperactive reflexes, and extensor plantar responses. An MRI of the brain showed T2/FLAIR hyperintensities in the caudate heads, putamina, and thalami (Figure 1D). He was treated supportively and improved; 3 months later he was living independently, had returned to work, and had a normal neurological exam.
Patient 5
A 65-year-old woman from Massachusetts presented with 7 days of fever, confusion, headache, and vomiting (Table 1). On exam, she was oriented to place and year but distractible and had slow speech. She also had mild right nasolabial fold flattening and a mild postural and action tremor in the upper extremities. Brain MRI was severely limited by patient motion, but subtle evidence of abnormal T2/FLAIR hyperintensity was visible involving the basal ganglia bilaterally (Figure 1E). She recovered within 6 days, was discharged, and did not return for follow-up.
Patient 6
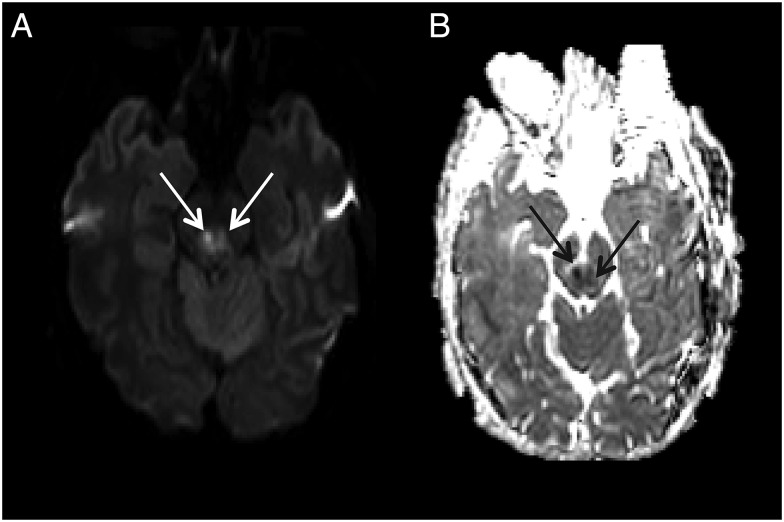
A 52-year-old man from Massachusetts presented with 2 days of fever and myalgias (Table 1). He lived outside of Boston but had recently traveled to Cape Cod, New York, Pennsylvania, and Wisconsin. At the time of symptom onset, he was camping in a lake area of New Hampshire. Although his initial neurological exam was normal, several days later he developed inattention, somnolence, and left upper extremity dysmetria. Brain MRI showed evidence of bilateral basal ganglia and thalamic T2/FLAIR hyperintensity and diffusion restriction in the dorsal midbrain (Figure 1F and Figure 4). The patient improved incompletely and was discharged to an acute rehabilitation facility. Fifteen months later, he had persistent headaches as well as cerebellar dysarthria, delayed motor function, and incoordination.
Figure 4.
Axial diffusion weighted imaging (A) and apparent diffusion coefficient (B) imaging from patient 6 demonstrates bilateral midbrain diffusion restriction (arrows).
Patient 7
A 49-year-old man from Massachusetts presented with 4 days of fever and headache. The day of admission he was found to be the unrestrained driver in a motor vehicle accident and could not recall the details (Table 1). On presentation, he was fully oriented and moved all extremities purposefully. The day after admission he was slow to respond to commands and did not know his name or date of birth. An electroencephalogram (EEG) showed diffuse slowing. MRI of the brain showed evidence of asymmetric T2/FLAIR hyperintensities in the basal ganglia and thalami (Figure 1G). Repeat MRI 3 days later showed severe mass effect with sulcal effacement and cerebellar swelling with hydrocephalus and tonsillar herniation (Figure 2D–E). The patient developed acute herniation symptoms and was transitioned to comfort care. He expired in the hospital on day 10. No autopsy was performed.
Patient 8
A 44-year-old man from New Hampshire presented with 3 days of headache, fatigue, diplopia, and a diffuse rash over his trunk and extremities (Table 1). His initial exam was notable for word-finding difficulty, and he subsequently had a generalized tonic–clonic seizure. A brain MRI was normal (not shown). EEG showed moderate generalized slowing as well as intermittent right frontoparietal slowing. The patient was treated with supportive measures, clinically recovered, and was discharged.
DISCUSSION
Epidemiology
POWV is a flavivirus that is related to West Nile virus, St. Louis encephalitis virus, tick-borne encephalitis virus (TBEV), and Japanese encephalitis virus. Ticks transmit TBEV and POWV, whereas mosquitoes transmit other types of arboviral encephalitis . There are 2 lineages of Powassan, which differ by approximately 15% [3] but are not distinguishable by serologic testing. The traditional lineage (POWV) is transmitted by the I. cookei tick, found primarily near the Great Lakes. In that region, Powassan encephalitis is fairly uncommon, perhaps because its vector, I. cookei, has a relatively high degree of specificity for its primary hosts, woodchucks and rodents [4]. By contrast, Powassan lineage II (also known as deer tick virus) is transmitted by I. scapularis ticks, a very common vector of tick-borne infections in the Northeast. Unlike other infectious agents transmitted by I. scapularis, POWV can be transmitted within approximately 15 minutes of attachment to the host [5]. Surveys of ticks in New York and Connecticut have identified POWV II in approximately 3% of ticks [6–8], a frequency similar to that of Babesia microti but much lower than Borrelia burgdorferi, which is found in up to 55% of ticks. A study of deer in Connecticut, Vermont, and Maine demonstrated an increasing prevalence of POWV seropositivity, from <25% prior to 1996, to 80%–90% in 2005–2009 [9]. Not surprisingly, there has been an increase in cases of POWV encephalitis reported in humans in the Northeast, most notably 14 cases identified in the Lower Hudson Valley of New York State from 2004 to 2012 [10]. The first case of POWV encephalitis in Massachusetts was reported in 1994 [11], and there were no further reported cases until 2013, when a single case was reported [2]. In 2014, there were 4 cases of POWV encephalitis reported from Massachusetts, out of 7 total in the United States [12]. From New Hampshire, 1 case was reported in 2013, and none in 2014. [2, 12]. As with other tick-borne infections, POWV encephalitis is most common in the spring through fall; our cases occurred from May through September.
Clinical Features and Diagnosis
The incubation period for POWV ranges from approximately 1 to 5 weeks [13, 14]. As for other arboviruses, presenting symptoms can include fever and features of brain involvement, including confusion, depressed level of consciousness, seizures, and focal neurological deficits. Rash [10, 15–17] and gastrointestinal symptoms [18] are also fairly common. In one case series of 14 patients, all had fever, 8 had confusion, 6 had seizure, 6 had headache, and 5 had focal neurologic deficits [10]. Similarly, among our patients, 7 had fever, 7 had altered sensorium, and 7 had focal neurologic deficits. Some patients may have mild thrombocytopenia [19], as was the case for 1 of our patients; otherwise, POWV encephalitis is not commonly associated with laboratory abnormalities. Consistent with the outcomes in our patients, the reported mortality rate for POWV encephalitis is 10%–15%, and even among survivors, memory loss and focal weakness can persist [4]. At the other end of the spectrum, asymptomatic infection may occur [18].
CSF analysis usually shows pleocytosis (up to 200 WBCs/µL in prior cases and up to 700 WBCs/µL among our patients), which can be either lymphocyte or polymorphonuclear predominant. As was found in our patients, most prior cases reported elevated CSF protein and normal glucose [10, 19, 20]; however, CSF findings can be nearly normal [20].
POWV infection is diagnosed by a compatible clinical syndrome plus 1 of the following: virus isolation, detection of immunoglobulin-M (IgM) antibodies by enzyme-linked immunosorbent assay (ELISA) with a confirmatory plaque neutralization test (which measures the ability of patient serum to reduce virus infectivity in an experimental setting), detection of IgM in the CSF with negative studies for other causes of encephalitis, or documented 4-fold rise in antibody titer [15]. Seven of the 8 patients in this series met these diagnostic criteria; in all patients, serum or CSF was positive for Powassan IgM. However, in patient 7, the confirmatory plaque reduction neutralization test was negative. The sample had been sent only 6 days into his illness, however, and no follow-up testing was possible. In the absence of an alternative diagnosis, we regard this as a probable case of Powassan encephalitis. Among our patients, patients 6 and 8 also tested positive for other viral encephalitides by ELISA but negative or low by confirmatory plaque reduction neutralization, suggesting either cross-reactivity between the tests or coinfection.
Imaging
Imaging descriptions of POWV encephalitis are sparse, though there have been previous reports of T2/FLAIR hyperintensities similar to those seen in our patients. For example, 1 patient had T2/FLAIR hyperintensities in the left putamen, bilateral caudate nuclei, and scattered throughout the hemispheric and cerebellar white matter without enhancement or restricted diffusion [21]. Another had asymmetric bilateral thalamic involvement with increased T2/FLAIR signal intensity as well as regions of restricted diffusion; follow-up imaging 2 days later demonstrated microhemorrhages [16]. Both of these patients recovered substantially. MRI in a fatal case [17] demonstrated T2/FLAIR hyperintensities throughout the superior cerebellum, left pons, and bilateral basal ganglia, as well as a large focus of restricted diffusion in the superior cerebellum. MRI in 2 additional cases demonstrated nonspecific white matter changes (in the parietal lobes bilaterally in one, in the temporal lobes bilaterally in the other), without any foci of restricted diffusion or abnormal contrast enhancement [14].
By contrast, MRI scans were normal in 2 other patients [20], one who presented after a generalized tonic–clonic seizure but rapidly improved and had no further seizures or neurologic deficits and the other who experienced hemodynamic instability but no focal neurologic deficits. In another case with normal MRI, the patient initially improved but died of unrelated causes approximately 1 month later. Autopsy showed an inflammatory infiltrate predominately in the medial temporal lobes, ventral midbrain, and basal ganglia, with relative sparing of the white matter [13].
In the cases presented here, MRI findings were largely consistent with previous reports. However, in patients 1, 3, 6, and 7, there was evidence of restricted diffusion acutely. In patient 3, this finding was seen throughout the cortex and had completely resolved on follow-up imaging. To our knowledge, this is the first report of reversible restricted diffusion of the cortical ribbon in POWV encephalitis. Although this finding is sometimes seen in seizure, hypoxic ischemic injury, or metabolic insult, patient 3 had no evidence of these complications. The significance of this finding is unknown. It is notable that the other 3 cases with diffusion restriction, including midbrain infarction in patient 6 and cerebellar findings in patients 1 and 7, had poor outcomes. This suggests that diffusion restriction of the brainstem and posterior fossa may impart some negative prognostic significance.
Overall, POWV encephalitis shares many imaging characteristics with other arboviral encephalitides, especially Eastern equine encephalitis (EEE) and TBE. A review of 36 patients with EEE [22] revealed T2/FLAIR abnormalities in almost all, most commonly affecting the basal ganglia and thalamus, with noncontiguous lesions observed in the brainstem, cortex, and periventricular white matter. The midbrain was the most commonly affected brainstem region, whereas cortical lesions were relatively uncommon. By contrast, in a large review of TBE [23], only 18 of 102 patients imaged had abnormalities on MRI, most commonly confined to the thalami.
Treatment
As with other arboviral encephalitides, treatment for POWV encephalitis is supportive. There is a reported fatality rate of 10%–15% with residual neurologic deficits in 50% of survivors [4]. In some case reports, patients have been treated with high-dose corticosteroids [10, 19], and all of these patients survived. However, the relationship of corticosteroids to outcome remains entirely unclear. IVIG has been given with possible benefit in EEE and West Nile virus encephalitis [24–27] but has not previously been reported with POWV encephalitis. IVIG was used in 1 of our patients whose outcome was excellent, despite severe MRI changes, and in another patient who improved but had significant residual neurological deficits at discharge.
SUMMARY
We describe 8 recent cases of POWV encephalitis in Massachusetts and New Hampshire. Prior to these, there had been only 2 other cases identified in Massachusetts (in 1994 and 2013) and none in New Hampshire. In New England, POWV lineage II is transmitted by I. scapularis, the same tick vector that transmits B. burgdorferi, Borrelia miyamotoi, Anaplasma phagocytophilum, and B. microti. Given the relatively high prevalence of these infections in New England, we suspect that POWV infection may be more common than is currently appreciated. The recent increase in cases may be due to an increase in testing or may be due to true emergence of the disease in this region. The increasing seropositivity among deer in New England supports the latter [9]. A high index of suspicion is needed for diagnosis because the clinical features and laboratory findings in POWV encephalitis resemble those of other arboviruses. As reviewed here, the MRI findings are also nonspecific but characteristically demonstrate extensive T2/FLAIR hyperintensities within the brainstem that extend to the deep gray structures and cortex. We recommend testing for POWV in the evaluation of patients who reside in New England and present with symptoms of encephalitis. Further studies are needed to evaluate the extent to which POWV causes less severe disease or even asymptomatic infection.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Brian J. Scott, MD, for contributing to the neurological care of our patients.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J 1959; 80:708–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Lindsey NP, Lehman J, Staples JE, Fischer M. West Nile virus and other arboviral diseases—United States, 2013. MMWR Morb Mortal Wkly Rep 2014; 63:521–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Ebel GD, Spielman A, Telford SR. Phylogeny of North American Powassan virus. J Gen Virol 2001; 82:1657–65. [DOI] [PubMed] [Google Scholar]
- 4.Ebel GD. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 2010; 55:95–110. [DOI] [PubMed] [Google Scholar]
- 5.Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg 2004; 71:268–71. [PubMed] [Google Scholar]
- 6.Aliota MT, Dupuis AP, Wilczek MP, Peters RJ, Ostfeld RS, Kramer LD. The prevalence of zoonotic tick-borne pathogens in Ixodes scapularis collected in the Hudson Valley, New York State. Vector Borne Zoonotic Dis 2014; 14:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupuis AP, Peters RJ, Prusinski MA, Falco RC, Ostfeld RS, Kramer LD. Isolation of deer tick virus (Powassan virus, lineage II) from Ixodes scapularis and detection of antibody in vertebrate hosts sampled in the Hudson Valley, New York State. Parasit Vectors 2013; 6:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JF, Armstrong PM. Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. Am J Trop Med Hyg 2012; 87:754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nofchissey RA, Deardorff ER, Blevins TM et al. . Seroprevalence of Powassan virus in New England deer, 1979–2010. Am J Trop Med Hyg 2013; 88:1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Khoury MY, Camargo JF, White JL et al. . Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York, U.S.A. Emerg Infect Dis 2013; 19:1926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arboviral disease—United States, 1994. MMWR Morb Mortal Wkly Rep 1995; 44:641–4. [PubMed] [Google Scholar]
- 12.Lindsey NP, Lehman J, Staples JE, Fischer M. West Nile virus and other nationally notifiable arboviral diseases—United States, 2014. MMWR Morb Mortal Wkly Rep 2015; 64:929–34. [DOI] [PubMed] [Google Scholar]
- 13.Gholam BI, Puksa S, Proviast JP. Powassan encephalitis: a case report with neuropathology and literature review. Can Med Assoc J 1999; 161:1419–22. [PMC free article] [PubMed] [Google Scholar]
- 14.Outbreak of Powassan Encephalitis—Maine and Vermont, 1999. MMWR 09/07/2001. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5035a4.htm. Accessed 22 December 2015. [PubMed]
- 15.CDC Arboviral Disease Case Definition. 2015. Available at: http://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/. Accessed 22 December 2015.
- 16.Choi E, Taylor R. A case of Powassan viral hemorrhagic encephalitis involving bilateral thalami. Clin Neurol Neurosurg 2012; 114:172–5. [DOI] [PubMed] [Google Scholar]
- 17.Tavakoli NP, Wang H, Dupuis M et al. . Fatal case of deer tick virus encephalitis. N Engl J Med 2009:2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artsob H. Powassan Encephalitis. In: Monath TP, ed. The arboviruses: epidemiology and ecology. Vol IV Boca Raton, FL: CRC Press, Inc, 1988:29–49. [Google Scholar]
- 19.Sung S, Wurcel AG, Whittier S et al. . Powassan meningoencephalitis, New York, New York, USA. Emerg Infect Dis 2013; 19:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raval M, Singhal M, Guerrero D, Alonto A. Powassan virus infection: case series and literature review from a single institution. BMC Res: Notes 2012; 5:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hicar M, Edwards K, Bloch K. Powassan virus infection presenting as acute disseminated encephalomyelitis in Tennessee. Pediatr Infect Dis J 2011; 30:86–8. [DOI] [PubMed] [Google Scholar]
- 22.Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of Eastern equine encephalitis. N Engl J Med 1997; 336:1867–74. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser R. The clinical and epidemiological profile of tick-borne encephalitis in southern Germany 1994–98. Brain 1999; 122:2067–78. [DOI] [PubMed] [Google Scholar]
- 24.Golomb MR, Durand ML, Schaefer PW, McDonald CT, Maia M, Schwamm LH. A case of immunotherapy-responsive Eastern equine encephalitis with diffusion-weighted imaging. Neurology 2001; 56:420–1. [DOI] [PubMed] [Google Scholar]
- 25.Mukerji SS, Lam AD, Wilson MR. Eastern equine encephalitis treated with intravenous immunoglobulins. Neurohospitalist 2015:28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haley M, Retter AS, Fowler D, Gea-Banacloche J, O'Grady NP. The role for intravenous immunoglobulin in the treatment of West Nile virus encephalitis. Clin Infect Dis 2003; 37:e88–90. [DOI] [PubMed] [Google Scholar]
- 27.Makhoul B, Braun E, Herskovitz M, Ramadan R, Hadad S, Krivoy N. Hyperimmune gammaglobulin for the treatment of West Nile virus encephalitis. Isr Med Assoc J 2009; 11:151–3. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.