Abstract
Animal studies have shown that olfactory sensitivity is greater when fasted than when fed. However, human research has generated inconsistent results. One possible explanation for these conflicting findings is metabolic health. Many metabolic peptides, including ghrelin, are moderated by adiposity and influence olfaction and olfactory-guided behaviors. We tested whether the effect of a meal on the perceived intensity of suprathreshold chemosensory stimuli is influenced by body mass index and/or metabolic response to a meal. We found that overweight or obese (n = 13), but not healthy weight (n = 20) subjects perceived odors, but not flavored solutions, as more intense when hungry than when sated. This effect was correlated with reduced postprandial total ghrelin suppression (n = 23) and differential brain response to odors in the cerebellum, as measured with functional magnetic resonance imaging. In contrast, it was unrelated to circulating leptin, glucose, insulin, triglycerides, or free fatty acids; or to odor pleasantness or sniffing (n = 24). These findings demonstrate that the effect of a meal on suprathreshold odor intensity perception is associated with metabolic measures such as body weight and total ghrelin reactivity, supporting endocrine influences on olfactory perception.
Key words: cerebellum, fMRI, metabolism, olfaction, psychophysics, sniffing
Introduction
Olfaction provides critical information about the presence of food in the external environment in order to facilitate the procurement of nutrients. Alterations in metabolic state may in turn regulate this behavior by producing changes in olfactory sensitivity. Research in animals consistently shows that olfactory sensitivity is enhanced in a fasted state. Starvation increases olfactory discrimination in Caenorhabditis elegans (Colbert and Bargmann 1997), facilitates transmission from olfactory sensory neurons in Drosophila (Root et al. 2011), and enhances mitral cell food odor reactivity (Pager et al. 1972), olfactory bulb Fos expression (Prud’Homme et al. 2009), and the ability to detect minute concentrations of an aversive odorant in rodents (Aimé et al. 2007). Surprisingly, human studies have generated conflicting results with reports of increased sensitivity, decreased sensitivity, or no change as a function of internal state (Langfeld 1914; Goetzl and Stone 1947; Koelega 1994; Albrecht et al. 2009; Stafford and Welbeck 2011; Cameron et al. 2012).
One possible contributor to these inconsistent findings may be variability in body weight and metabolic function in human samples. Increased body weight has been associated with decreased olfactory acuity (reviewed in Palouzier-Paulignan et al. 2012) and there is evidence that body weight and feeding states interact to influence olfactory sensitivity. For instance, differences in odor detection (Cameron et al. 2012) and discrimination threshold (Stafford and Welbeck 2011) between fasted and fed conditions are more pronounced in those of higher body weights. It is important to note, however, that obesity is not simply a state of having increased body weight. It is also associated with disturbances in peripherally circulating markers of energy balance. Injections of some of these markers are sufficient to mimic the differential effects of physiological hunger and satiety states on olfactory perception (Pager et al. 1972; Julliard et al. 2007; Marks et al. 2009). In particular, the orexigenic gastric peptide ghrelin is well poised to be a modulator of olfaction at the interface of body weight and feeding status. Its receptor is expressed in the olfactory bulb and central ghrelin infusions enhance odor detection in rodents (Tong et al. 2011). At the same time, obesity is associated with lower circulating levels of ghrelin (Tschöp et al. 2001) and may blunt the phasic suppression of ghrelin concentrations after a meal (English et al. 2002; Perreault et al. 2004; Engström et al. 2007; Mittelman et al. 2010; Meyer-Gerspach et al. 2014), though the reliability of the latter effect varies based on factors such as insulin sensitivity and macronutrient composition of the meal (Tentolouris et al. 2004; Bacha and Arslanian 2005; Baldelli et al. 2006; Foster-Schubert et al. 2008; Misra et al. 2009). Although it has been demonstrated that peripheral ghrelin infusion increases sniff magnitude in healthy humans (Tong et al. 2011), its interaction with body weight and perceived odor intensity has not been investigated.
The aim of this study was therefore to determine whether the effect of feeding state on olfactory sensitivity is influenced by excess body weight and/or changes in circulating satiety peptides, including ghrelin. We measured olfactory and flavor perception in hungry and sated individuals concomitant to performing functional magnetic resonance imaging (fMRI) and pneumotachograph recording to measure brain response and sniffing. We predicted that body mass index (BMI), plasma total ghrelin concentrations and feeding state would interact to influence odor intensity perception, and that this effect would be associated with increased central responses to the olfactory stimuli in chemosensory regions.
Materials and methods
Subjects
Thirty-three right-handed subjects (16 male; BMI [kg/m2]: M = 24.2, SD = 3.7, range = 19.5–33.6; Age [years]: M = 26.9, SD = 6.2, range = 18–40) were recruited from the greater New Haven area through the Yale University Interdisciplinary Research Consortium on Stress, Self-Control and Addiction (IRCSSA) P30 Subject’s core as well as via flyer advertisement. Three were Hispanic, 26 were non-Hispanic, and 4 did not report their ethnicity. Additionally, 19 were White, 7 were Asian, 5 were Black, and 2 did not report their race. Twenty of the 33 subjects were healthy weight (HW), as defined by having a BMI <25.0kg/m2 (9 male; BMI: M = 21.7, SD = 1.4, range = 19.5–24.7; Age: M = 26.0, SD = 5.9, range = 18–37). Of the HW group, 1 was Hispanic, 16 were non-Hispanic, and 3 did not report their ethnicity. Also of the HW group, 13 were White, 4 were Asian, 2 were Black, and 1 did not report their ethnicity. Thirteen of the 33 subjects were overweight or obese (OW), as defined by having a BMI >25.0kg/m2 (7 male; BMI: M = 28.1, SD = 2.5, range = 25.2–33.6; Age: M = 28.2, SD = 6.6, range = 19–40). Of the OW group, 2 were Hispanic, 10 were non-Hispanic, and 1 did not report their ethnicity. Also of the OW group, 6 were White, 3 were Asian, 3 were Black, and 1 did not report their race. Two subjects in the OW group were obese, as defined by having a BMI >30.0kg/m2; 1 male and 1 female, and their BMIs were 30.6 and 33.6, respectively.
Subjects were initially screened over the phone to be 40 years of age or younger, free of psychiatric disorders, eating disorders, current dieting behavior, alcoholism, use of tobacco or drugs other than alcohol, history of head injury with loss of consciousness, use of daily medication other than monophasic birth control, chemosensory impairments, lactose intolerance, or food allergies. Weight history was not obtained. In order to avoid scanning females during menstruation or ovulation, they self-reported the start date of their last menstruation, which was then designated day 1 of a 28-day cycle. Scans were not scheduled on days 1–4 or 10–14 of the cycle. All subjects provided written informed consent at their first visit. The study complied with the Declaration of Helsinki for Medical Research involving Human Subjects and was approved by the Yale Human Investigations Committee.
Stimuli
Food odors were “chocolate cookie” and “strawberry and cream” odors (6002335, 6106524 from Bell Labs Flavors and Fragrances, Inc.). Nonfood odors were “honeysuckle” and “lilac” odors (039831 Chey N-3 from Firmenich, Inc.; 31731066 Lilac 71 from International Flavors and Fragrances, Inc.). Flavors consisted of chocolate and strawberry milkshakes prepared from whole milk, commercially available bottled milkshakes and flavored syrup. One participant disliked the chocolate milkshake, so a vanilla milkshake was substituted in lieu of the chocolate for this participant only. Two different flavors of milkshake were presented in an interleaved fashion during the scan to minimize the potential for sensory adaptation.
The standardized breakfast consisted of prepackaged commercially available granola bars (Nature Valley brand Crunchy granola bars, per package: 190 kcal, 6–8g fat, 28–29g carbohydrate, 4g protein). Subjects were instructed to select from 4 varieties (Roasted Almond, Pecan Crunch, Oats and Honey, Maple Brown Sugar), and their selection was held constant across sessions. The lunch consisted of sliced apples and deli style sandwiches prepared in the laboratory on the morning of each session (Apples, approximate per serving: 25 kcal, 0g fat, 7g carbohydrates, 0g protein; Sandwiches, approximate per sandwich: 395–415 kcal, 16–23g fat, 29–35g carbohydrates, 14–31g protein). Subjects were instructed to select from 4 varieties of sandwich (tuna, ham, turkey, avocado).
Stimulus delivery
Odors were presented by a custom built, MRI compatible olfactometer programmed in Labview (National Instruments). A detailed description of the olfactory stimulation system can be found in a previous publication (Small et al. 2008). In brief, mass flow controllers (MKS Instruments) adjust the flow of humidified and temperature-controlled air over stainless steel wells containing an odorant, allowing the air to pick up vaporized odor molecules. The independent odor channels converge into a mixing manifold and exit through 1 of 2 Teflon tubes where the first is dedicated to odors and the second is dedicated to clean air. The trunk terminates in a Teflon manifold (Teqcom) resting on the participant’s chest. A vacuum line connected to this manifold creates a closed loop to evacuate odorized as well as odorless (OL) air, preventing head space contamination. The subjects receive OL and odor stimuli embedded in a continuous stream of clean, OL air from the manifold through a nasal mask (Philips Respironics). As air exits the mask, it is drawn out through a final Teflon tube by another vacuum line. The mask is also coupled to a pneumotachograph to measure airflow in the nose (Johnson and Sobel 2007), which is in turn connected to a spirometer (AD Instruments). The signal from the spirometer is fed into an amplifier (AD Instruments, PowerLab 4SP) and digitally recorded at 100 Hz using Chart 5.5.6 (AD Instruments).
Flavors were presented by a portable gustometer system, a detailed description of which can be found in a previous publication (Veldhuizen et al. 2007). In brief, flavored liquids are held in 60mL syringes loaded into BS-8000 syringe pumps (Braintree Scientific). Each syringe infuses its contents into 25 feet of Tygon beverage tubing (Saint-Gobain Performance Plastics) connected to a custom designed Teflon gustatory manifold mounted on the MRI headcoil. The flavors flow from the tubing into individually machined channels in the gustatory manifold that in turn converge into a silicon tube that rests in the mouth. Each infusion delivers 0.5mL of liquid over 4s.
Experimental procedures
Subjects took part in 1 fMRI training session, 3 fMRI scanning sessions (Hungry, Sated, and Ad libitum conditions), and 1 behavioral test session. All sessions were conducted on separate days and scan order was counterbalanced. Data from the Ad libitum condition will not be reported here, as in this condition subjects were instructed before the scan to eat “as much as you’d like” from a large quantity of sandwiches and apples. These instructions resulted in extreme variability in caloric intake (range of 113–1395 kcal) that would confound interpretations. A schematic of Hungry and Sated sessions can be found in Figure 1A.
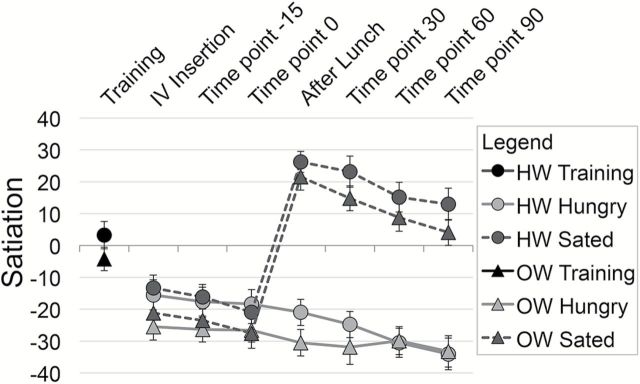
Figure 1.
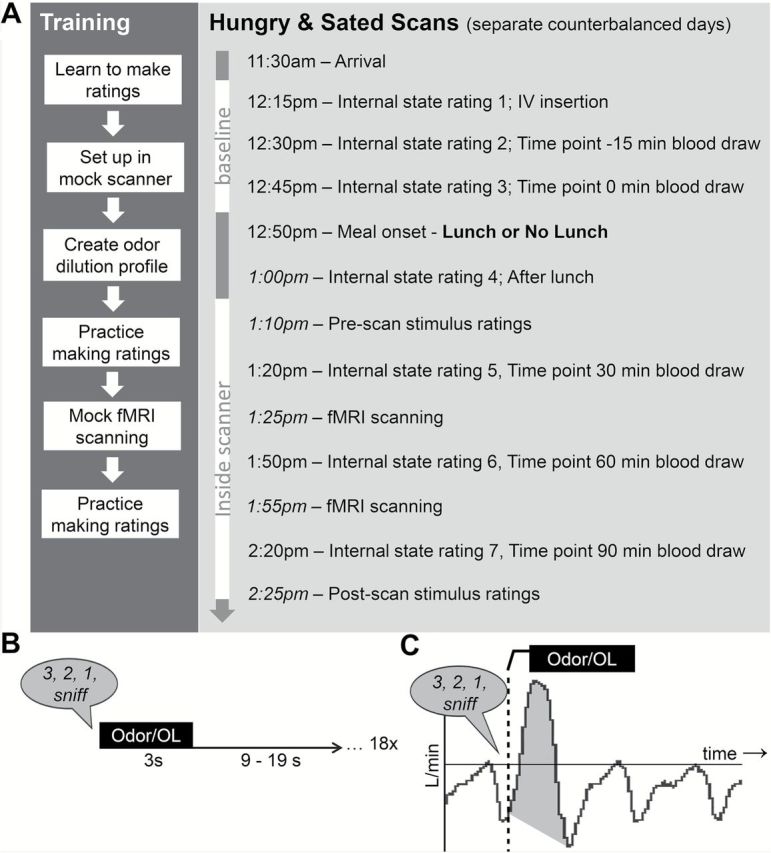
Experiment design. (A) Schematic of training and fMRI scan sessions. Times provided in italics are approximate. (B) Odor and OL stimulus delivery during an odor run. (C) Diagram of sample spirometer data with an odor or OL presentation event. Event onset is marked by the dashed vertical line. Shaded area represents the area under the curve calculated for sniff volume.
fMRI training session
Subjects were instructed to arrive neither hungry nor full and refrain from eating or drinking anything other than water for at least 1h before the training session. Upon arrival, subjects were trained to rate their internal state and the perceptual qualities of flavor and odor stimuli on computerized scales. Internal state ratings consisted of a series of adapted cross-modal general Labeled Magnitude Scales (gLMS) consisting of a 100mm vertical line scale. Considering the scale to be 100 units with a range from 0 to 100, the labels were placed at no sensation, 0; barely detectable, 1.4; weak, 6; moderate, 17; strong, 34.7; very strong, 52.5; and strongest imaginable, 100 (Green et al. 1993, 1996; Bartoshuk et al. 2004). Subjects were instructed to rate the intensity of their hunger, fullness, thirst, anxiety, and need to urinate. Perceptual quality ratings consisted of ratings of stimulus intensity, liking, familiarity, edibility, and wanting to eat. Stimulus intensity was measured with the gLMS as described above. Stimulus liking was measured using a labeled hedonic scale (LHS) consisting of a 100mm vertical line scale. Considering the scale to be 200 units with a range of −100 to 100, the labels were placed at most disliked imaginable, −100; dislike extremely, −65.72; dislike very much, −44.43; dislike moderately, −17.82; dislike slightly, −6.25; neutral, 0; like slightly, 6.25; like moderately, 17.82; like very much, 44.43; like extremely, 65.72; and most liked imaginable, 100 (Lim et al. 2009). Stimulus edibility, familiarity, and wanting to eat were rated on 200mm visual analogue scales labeled at the left (−10), center (0), and right (10) anchor points. Edibility labels were “not edible at all,” −10; neutral, 0; and “very edible,” 10. Familiarity labels were “not familiar at all,” −10; “neutral,” 0; and “very familiar,” 10. Wanting to eat labels were “I would never want to consume this,” −10; “neutral,” 0; and “I would want to consume this more than anything,” 10.
Subjects were then brought to a mock fMRI scanner and outfitted with the flavor and odor delivery systems. First, each odor was delivered one at a time and subjects verbally rated the intensity of each presentation by telling the experimenter approximately where on the gLMS the odor’s intensity fell in relation to the scale’s labels (e.g., “Very Strong,” “A little less than Weak,” “Halfway between Moderate and Strong”). An experimenter then manually adjusted the odorant concentration settings on the olfactometer so that each odor was rated moderate in intensity. This resulted in the creation of a personalized dilution profile for each odor and each participant, allowing us to control for individual differences in sensory acuity. Next, subjects practiced making internal state ratings as well as perceptual ratings about each of the odors and flavors using a mouse on a computer monitor viewed via back projection on a headcoil-mounted mirror.
After completing the ratings, subjects were inserted into the bore of the mock scanner and underwent simulated odor and flavor runs. The flavor runs have been described in previous publications from our lab (Sun et al. 2014, 2015). The odor run was 5min 54s long, and subjects were instructed to breathe in through their nose after receiving the verbal cue “3, 2, 1, sniff” through headphones. Odor or OL delivery always occurred immediately following the auditory cue so that delivery was time-locked to sniff onset. Olfactory stimulation lasted for 3s followed by a 9–19s rest period before the next trial. There were 6 repetitions each of food odors, nonfood odors, and OL stimuli. A schematic of the odor run is illustrated in Figure 1B. Subjects were asked to keep their eyes closed during the functional runs, and no behavioral task was required other than to experience the stimuli.
Following the mock scans, subjects were removed from the bore and asked to make a second round of internal state and stimulus perception ratings. They were then provided with the standardized breakfast bars and told to keep them until their next session.
fMRI scanning sessions
Subjects were instructed to eat the breakfast bars (1 package for females, 1.5 packages for males) in the morning at home and then refrain from eating or drinking, with the exception of water, until their session which began at 11:30 AM. Subjects were not told a specific time to eat the breakfast bars. Upon arrival, subjects filled out paperwork, including a food diary for that morning as well as the previous day. If the food diary at the first scan revealed that the subject did not consume the breakfast bars that morning as instructed, then breakfast bars were not provided for the remaining sessions in order to match AM intake across sessions. Starting at 12:15 PM, a catheter was inserted into an antecubital vein for blood sampling and a series of 3 baseline internal state ratings were obtained at 15min increments. Baseline blood samples were obtained concomitant to the second and third internal state ratings. About 5min after the third internal state rating, subjects ate either a fixed portion meal titrated for gender and designed to constitute approximately 25% of daily caloric need (Sated condition, consisting of 1 sandwich and 1 serving of apple slices for females, 1.5 sandwiches and 1 serving of apple slices for males) or nothing (Hungry condition). One participant removed the cheese from the sandwich and one participant did not eat the skins of the apple slices; otherwise, subjects consumed the entire fixed meal. After making a fourth set of internal state ratings, the subjects were taken to the scanner, outfitted with the odor and flavor delivery devices, and inserted into the bore of the scanner. Perceptual ratings of each of the odors (using each subject’s personalized dilution profile) and flavors were measured as in the training session.
Imaging data were acquired on a Siemens 3 Tesla TIM Trio Scanner at the Yale University Magnetic Resonance Research Center. High resolution T1-weighted structural scans were acquired for each participant with TR = 2230ms, TE = 1.73ms, flip angle = 9º, matrix = 256×256, slice thickness = 1mm, FOV = 250×250, 176 slices. A susceptibility-weighted single shot echo planar sequence was used to image regional distribution of the blood oxygenation level dependent (BOLD) signal. At the beginning of each functional run, the magnetic resonance signal was allowed to equilibrate over 6 scans for a total of 12s, which were subsequently excluded from the analyses. Acquisition parameters were: TR = 2000ms, TE = 20ms, flip angle = 80°, FOV = 220, matrix = 64×64, slice thickness = 3mm. Forty contiguous slices were acquired in an interleaved manner to reduce the crosstalk of the slice selection pulse.
Four odor runs and 2 flavor runs were collected at each scan. Internal state ratings and further blood samples were collected at 30, 60, and 90min from time of meal (or no meal) onset. After all runs were completed, subjects made a final set of stimulus ratings before being removed from the scanner.
Data analyses
Planned comparisons of behavioral, biochemical, and spirometer data, as well as psychophysical ratings were analyzed in PASW Statistics 18 (SPSS, Inc.). Main effects and interactions were tested using repeated measures analyses of variance (ANOVA). Significant interactions were further examined using post hoc pairwise t-tests. Where post hoc analyses required that multiple timepoints be tested independently, a Bonferonni correction was applied where the alpha level of 0.05 was divided by the number of tests made (e.g., insulin was tested at t = −15, 0, 30, 60, and 90; thus, adjusted alpha = 0.05 ÷ 5 = 0.01). Pearson correlation, partial correlation, and stepwise regression were used to test for associations between individual differences in perceptual ratings and metabolic measures. For analysis, log transformations were calculated for gLMS ratings by replacing zero values with 0.17 prior to transformation. Internal state ratings were not obtained due to equipment malfunction for 1 HW female at all sessions and 1 additional HW female at the training session only. In the event of missing values, cases were excluded pairwise (on a comparison-by-comparison basis).
Sufficient quantities of blood for biochemical analysis were obtained from a subset of 23 subjects (10 male; BMI: M = 24.4, SD = 3.9, range = 19.5–33.6; Age: M = 26.0, SD = 5.6, range = 18–39). Of these, 2 were Hispanic, 18 were non-Hispanic, and 3 did not report their ethnicity. Additionally, 15 were White, 3 were Black, 4 were Asian, and 1 did not report their race. Nine of the 23 subjects with blood available were in the OW group (5 male; BMI: M = 28.4, SD = 2.8, range = 25.2–33.6; Age: M = 26.2, SD = 5.9, range = 19–36). Of the OW, 1 was Hispanic and 8 were non-Hispanic. Also of the OW, 4 were White, 3 were Black, and 2 were Asian. Both obese subjects previously described in the OW group were able to give blood. Fourteen of the 23 subjects with blood available were in the HW group (5 male; BMI: M = 21.9, SD = 3.9, range = 19.5–24.7; Age: M = 25.9, SD = 5.7, range = 18–39). Of the HW, 1 was Hispanic, 10 were non-Hispanic, and 3 did not report their ethnicity. Also of the HW, 11 were White, none were Black, 2 were Asian, and 1 did not report their race.
Blood samples were collected in vacutainer tubes treated with EDTA (glucose, total ghrelin, insulin at time points −15, 0, 30, 60, and 90min) and lithium heparin/plasma separator gel (FFA, triglycerides at time points −15, 0, and 60min) and centrifuged immediately. The plasma component was then transferred to a clean polypropelene cryovial and kept on ice until the end of the session, at which time they were frozen at −80 °C (FFA, total ghrelin, insulin) or −20 °C (glucose, triglycerides). Plasma levels of feeding-related nutrients and hormones were measured with commercially available materials. Insulin and total ghrelin levels were measured with radioimmunoassay kits that utilize the double antibody technique with 125I-labeled hormone and hormone antiserum (Total ghrelin: Cat. # GHRT-89HK; Insulin: Cat. #HI-14K; Leptin: Cat. #HL-81K, EMD Millipore Corporation). Glucose, free fatty acids (FFA), and triglyceride concentrations were measured using enzymatic colorimetric techniques (FFA: Cat. #999–34691, 991034891, 993–35191, Wako Pure Chemical Industries, Ltd.; Triglycerides: Cat. #SA1023, RX1023; Glucose: Cat. #SA1014, RX1014, Alfa Wassermann Diagnostic Techniques).
Neuroimaging data were analyzed using the SPM8 software (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience) in MATLAB R2010b 7.11.0 (Mathworks, Inc.). Functional images were time-acquisition corrected to the slice obtained at 50% of the TR and realigned to the mean image. Anatomical and functional images were normalized to the standard MNI template brain implemented in SPM8, resulting in voxel sizes of 3 and 1mm3, respectively. Functional timeseries data were detrended and then smoothed using a 6mm FWHM isotropic Gaussian kernel. Motion parameters were included as regressors in the design matrix at the single subject level.
As the neuroimaging analyses in this manuscript were informed by an olfaction-specific psychophysical effect, only odor fMRI data are reported here. The main effects of internal state on brain response to the milkshake flavors were presented in a previous manuscript (Sun et al. 2014). A design matrix was created for each participant for odor runs across all scan days that produced 2 events of interest: 1) Odors and 2) OL. Based upon the psychophysical results we did not divide odor trials into food and nonfood odors. Event onsets were defined as the beginning of odor onset and event durations were defined as the 3s of odor delivery. A 270s high pass filter was applied to the time series data to remove low frequency noise and slow signal drifts. The general linear model was employed to estimate condition-specific effects at each model. A canonical hemodynamic response function was used to model neural response to events of interest.
One-sample t-test random effects analyses were used to examine the fMRI results. To investigate the main effect of smelling odors versus OL, brain response to Odors > OL were compared at Hungry and Sated scans together [(Odor Hungry + Odor Sated) − (OL Hungry + OL Sated)]. To investigate the main effect of metabolic state, brain response to Odors > OL were compared under Hungry > Sated and Hungry < Sated scan conditions [(Odor Hungry − OL Hungry) vs. (Odor Sated − OL Sated)]. To investigate the association between BOLD response to odors, between-condition change in odor intensity perception, and the extent of postprandial plasma total ghrelin change, one-sample t-test random effects analyses were performed in which the contrast of Odor > OL under Hungry > Sated scan conditions [(Odor Hungry − OL Hungry) − (Odor Sated − OL Sated)] was regressed separately against the difference in average odor intensity ratings (i.e., average of pre- and post-scan ratings) between Hungry and Sated conditions and absolute change in plasma total ghrelin levels from baseline to 90min post-lunch. The t-map threshold was set at P uncorrected < 0.005 and a 5 voxel cluster size. Unpredicted responses were considered significant at a clusterwise P < 0.05 family wise error (FWE) corrected across the entire brain for multiple comparisons. Predicted responses using a region of interest (ROI) approach were considered significant at a peakwise P < 0.05 FWE corrected across the individual ROI. Functional ROIs of the piriform (primary olfactory cortex) and orbitofrontal cortex (OFC; secondary olfactory cortex) derived from olfactory activation likelihood estimates were downloaded from the website of Johann Lundström (Seubert et al. 2013). Anatomical ROIs of the amygdala and insula were also selected as these areas have been previously shown to code chemosensory intensity (Anderson et al. 2003; Small et al. 2003); These ROIs were defined using masks from the WFU PickAtlas software for SPM (ANSIR Laboratory, Wake Forest University).
Spirometer data were analyzed in Chart 5.5.6. The start of each sniff was defined as the time of odor onset, and the end of each sniff was defined as when the spirometry trace reached a nadir prior to the beginning of the next inhalation. A sample sniff is shown in Figure 1C. Sniff volume was defined as the area under the curve of the sniff minus a baseline drawn from sniff start to sniff end (shaded area). This allowed us to use the same criteria to define a sniff across all subjects regardless of sniffing “style” (e.g., deeper inhalation vs. prolonged inhalation). Sniff vigor was defined as the maximum slope of the sniff. Sniff volume and vigor to all odors per scan condition was calculated by first averaging sniff volume and vigor in response to odor presentations during each scan, and then dividing them by the average volume and vigor of 20 randomly selected nonsniff breaths from the same scan day. Nine of the 33 subjects’ spirometer datasets were unusable due to technical issues during scanning; therefore, the spirometer analysis includes 24 subjects (13 male; BMI: M = 24.0, SD = 3.7, range = 19.5–33.6; Age: M = 27.0, SD = 6.0, range = 18–39). Of these, 3 were Hispanic, 17 were non-Hispanic, and 4 did not report their ethnicity. Additionally, 14 were White, 4 were Black, 5 were Asian, and 1 did not report their race. Eight of 24 subjects with sniff data available were in the OW group (5 male; BMI: M = 28.4, SD = 2.7, range = 25.3–33.6; Age: M = 27.9, SD = 7.7, range = 19–40). Of the OW, 2 were Hispanic, 5 were non-Hispanic, and 1 did not report their ethnicity. Also of the OW, 3 were White, 3 were Black, and 2 were Asian. Both obese subjects previously described in the OW group had sniff data. Sixteen of the 24 subjects with sniff data available were in the HW group (8 male; BMI: M = 21.8, SD = 1.5 range = 19.5–24.7; Age: M = 26.5, SD = 5.2, range = 18–39). Of the HW, 1 was Hispanic, 12 were non-Hispanic, and 3 did not report their ethnicity. Also of the HW, 11 were White, 1 was Black, 3 were Asian, and 1 did not report their race.
Results
Internal state
A satiation index was created by subtracting hunger ratings from fullness ratings at each time point that internal state ratings were collected (Figure 2). Satiation was rated as neutral (neither hungry nor full) at the training session (satiation M = 0.01, SD = 18.3). A 2 (condition) × 7 (time) repeated measures ANOVA was performed on satiation with BMI group as a between subjects variable. The ANOVA revealed a main effect of condition [F(1, 30) = 122.11, P < 0.001], where satiation was higher at the Sated scan than at the Hungry scan, as well as a main effect of time [F(1, 30) = 43.62, P < 0.001]. This is qualified by a significant condition by time interaction [F(1, 30) = 76.11, P < 0.001]. Pairwise post hoc comparisons of this interaction (alpha = 0.007) showed that there were no significant differences in satiation between Hungry and Sated sessions during the three baseline internal state ratings (Internal state [IS] rating 1: t = −0.051, P = 0.301; IS rating 2, t = −0.811, P = 0.424; IS rating 3: t = 0.684, P = 0.499), while satiation was greater following lunch at the Sated session than following no lunch at the Hungry session (IS rating 5: t = −12.62, P < 0.001; IS rating 6: t = −13.11, P < 0.001; IS rating 7: t = −11.05, P < 0.001; IS rating 7: t = −11.25, P < 0.001). There was no main effect or significant interactions with BMI group.
Figure 2.
Subjective ratings of internal state. Ratings were analyzed using repeated measures ANOVA with pairwise post hoc investigation of significant interactions. Error bars represent standard error of the mean. Subjects were in a neutral satiation state (neither hungry nor full) at training and were equivalently hungry before the lunch manipulation at Hungry and Sated fMRI scans. The lunch provided at the Sated scan effectively induced a satiated state. Internal state ratings did not differ between HW and OW groups.
Plasma measures
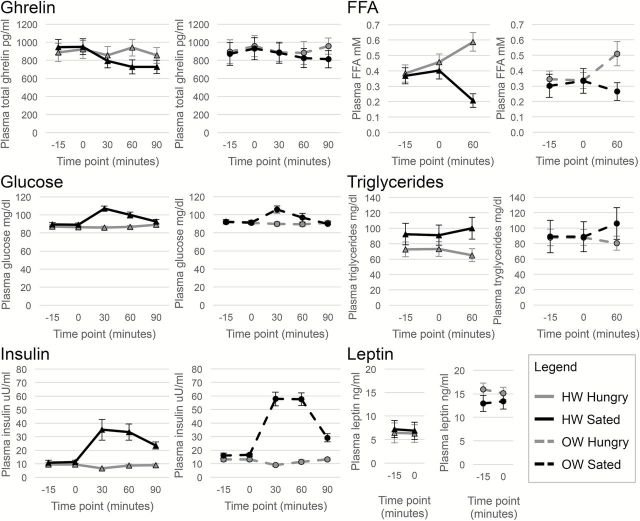
Plasma concentrations of total ghrelin, glucose, insulin, FFA, triglycerides, and leptin for HW and OW groups across the time course of Hungry and Sated scans can be found in Figure 3. A 2 (condition) × 5 (time) repeated measures ANOVA was performed on plasma concentrations of total ghrelin, glucose, and insulin with BMI group as a between subjects variable. For total ghrelin, there was a significant main effect of time [F(4, 84) = 3.78, P = 0.018] and a condition by time interaction [F(4, 84) = 5.68, P = 0.002]. Pairwise post hoc comparisons of this interaction (alpha = 0.01) showed that plasma total ghrelin concentration was greater at Hungry than Sated sessions at 60 (t = 4.02, P = 0.001) and 90min (t = 3.86, P = 0.001) after meal onset. For glucose, there was a significant main effect of condition [F(1, 21) = 13.98, P = 0.001], time [F(4, 84) = 9.96, P < 0.001], and a condition by time interaction [F(4, 84) = 19.05, P < 0.001]. Pairwise post hoc comparisons of this interaction (alpha = 0.01) showed that plasma glucose concentration was greater at Sated than Hungry sessions at 30 (t = −7.57, P < 0.001) and 60min (t = −3.39, P = 0.003) after meal onset. For insulin, there was a significant main effect of condition [F(1, 31) = 87.32, P < 0.001], time [F(4, 84) = 17.19, P < 0.001], and a trend toward a main effect of BMI group [F(1, 21) = 4.23, P = 0.052]. There was also a significant condition by time interaction [F(4, 84) = 25.19, P < 0.001]. Pairwise post hoc comparisons of this interaction (alpha = 0.01) showed that plasma insulin concentration was greater at Sated than Hungry sessions at 30 (t = −5.35, P < 0.001), 60 (t = −5.35, P < 0.001), and 90 (t = −7.64, P < 0.001) after meal onset. Plasma insulin levels were also slightly greater at Sated than Hungry sessions immediately prior to meal onset (time point 0; t = −2.27, P = 0.033), but this difference did not meet our adjusted threshold for significance with Bonferonni correction. A 2 (condition) × 3 (time) repeated measures ANOVA was performed on plasma concentrations of FFA and triglycerides. For FFA, there was a significant main effect of condition [F(1, 21) = 7.97, P = 0.010], as well as a condition by time interaction [F(2, 42) = 23.14, P < 0.001]. Pairwise post hoc comparisons of this interaction (alpha = 0.017) showed that plasma FFA concentration was greater at Hungry than Sated sessions at 60min (t = 5.72, P < 0.001) after meal onset. For triglycerides, there was a significant main effect of condition [F(1, 21) = 5.48, P = 0.029], as well as a condition by time interaction [F(2, 42) = 16.13, P < 0.001]. Pairwise post hoc comparisons of this interaction (alpha = 0.017) showed that plasma triglyceride concentration was greater at Sated than Hungry sessions at 60min (t = −3.79, P = 0.001) after meal onset. A 2 (condition) × 2 (time) repeated measures ANOVA was performed on plasma concentrations of leptin. There was a significant main effect of BMI group [F(1, 21) = 5.75, P = 0.026], as well as a condition by BMI group interaction [F(21) = 4.41, P = 0.048]. However, this interaction was driven by an outlier. After excluding the outlier, only the main effect of BMI group for leptin remained significant [F(20) = 4.50, P = 0.047].
Figure 3.
Plasma measures. Plasma concentrations of total ghrelin, glucose, insulin, FFAs, triglycerides, and leptin for HW and OW groups across the time course of Hungry and Sated scans were analyzed using repeated measures ANOVA with pairwise post hoc investigation of significant interactions. Error bars represent error of the mean. Plasma total ghrelin levels were higher at Hungry than Sated scans at 30 and 60min after meal onset. Plasma glucose levels were higher at Sated than Hungry sessions at 30 and 60min after meal onset. Plasma insulin levels were higher at Sated than Hungry sessions at 30, 60, and 90min after meal onset as well as, on average, immediately prior to meal onset (time point 0). Plasma FFA levels were greater at Hungry than Sated sessions 60min after meal onset. Plasma triglycerides levels were greater at Sated than Hungry sessions at 60min after meal onset. Plasma leptin levels were greater for the OW versus the HW group. No other effects or interactions with weight group were observed, other than a putative BMI by condition interaction for leptin that was driven by an outlier.
To determine whether these plasma measures were correlated with BMI, time points −15 and 0 at Hungry and Sated sessions were averaged for every subject to obtain a baseline measurement of each nutrient or hormone (calculation 1). BMI was positively correlated with baseline leptin [r(1, 21) =0.52, P = 0.011], where individuals with higher BMIs had greater plasma leptin concentrations. No correlations between BMI and baseline measurements of ghrelin, insulin, glucose, triglycerides, or FFA were found. To determine whether BMI was correlated with the reactivity of plasma measures with a meal (with the exception of leptin, as only baseline leptin measurements were obtained), the extent of postprandial change in concentration was calculated by taking the change from baseline at each post-manipulation time point at Sated scan and subtracting it from the change from baseline at each equivalent post-manipulation time point at Hungry scan (i.e., for ghrelin at time point 30: Hungry [(ghrelin at 30min) − (ghrelin at baseline)] − Sated [ghrelin at 30min) − (ghrelin at baseline)]; calculation 2). The inclusion of the Hungry scan in the calculation of postprandial reactivity was necessary in order to account for fluctuations caused by time as well as ingesting milkshake in the scanner. BMI was negatively correlated with the extent of postprandial plasma total ghrelin change at the 60min time point (r(1, 21) = −0.57, P = 0.004), where individuals with higher BMIs had lesser meal-induced suppression of plasma total ghrelin. We also expressed the extent of postprandial change in concentration as a percentage of each subject’s baseline concentration [i.e., (calculation 2)/(calculation 1) × 100]. Again we found that BMI was negatively correlated with the percentage change of plasma total ghrelin at the 60min time point [r(1, 21) = −0.51, P = 0.014]. No correlations between BMI and reactivity of other plasma measures or at other time points were found.
Psychophysics
Edibility
To ensure that subjects were able to distinguish between food and nonfood odor categories, average edibility ratings were collapsed across time (pre- and post-scan) and condition (Hungry and Sated) and examined using paired sample t-test. Edibility ratings were indeed higher for food odors (M = 43.8, SD = 28.4) than nonfood odors (M = −7.8, SD = 43.4) [t(1, 32) = 6.24, P < 0.001]. Food versus nonfood odor intensity ratings were then entered into a 2 (stimulus) × 2 (condition) × 2 (time) repeated measures ANOVA with BMI group as a between subjects variable in order to search for a main effect of, or significant interactions with, stimulus. None were identified. Thus, food and nonfood odors were collapsed in all subsequent analyses.
Intensity
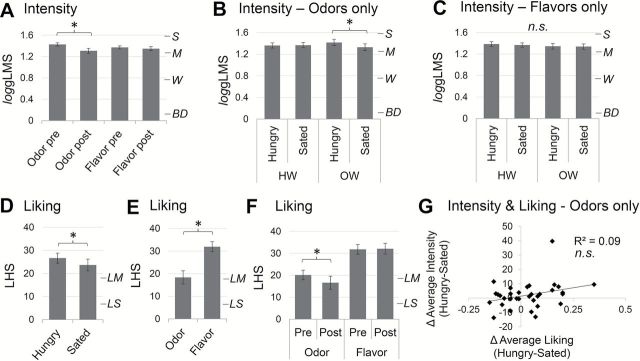
Next, intensity ratings of odors and flavors were compared using a 2 (stimulus) × 2 (condition) × 2 (time) repeated measures ANOVA with BMI group as a between subjects variable. There was a main effect of time [F(1, 31) = 19.05, P < 0.001] where all stimuli were perceived as more intense pre-versus post-fMRI scanning. This was qualified by a significant stimulus by time interaction [F(1, 31) = 9.74, P = 0.004; Figure 4A], which revealed that the perceived intensity of odors, but not flavors, decreased post-versus pre-fMRI irrespective of condition (i.e., Hunger/Sated). Finally, there was a 3-way interaction between stimulus, condition and BMI [F(1, 31) = 4.88, P = 0.035] where OW, but not HW, subjects perceived the odors to be more intense when Hungry than when Sated irrespective of time [Main effect of condition for OW F(1, 12) = 5.57, P = 0.036; Figure 4B]. No such difference in intensity appeared for flavors (Figure 4C).
Figure 4.
Intensity (gLMS) and liking (LHS) ratings of chemosensory stimuli. Ratings were analyzed using repeated measures ANOVA. *P < 0.05; n.s., not significant. Error bars represent standard error of the mean. Vertical marks on the right side of intensity graphs correspond to BD = “barely detectable,” W = “weak,” M = “moderate,” S = “strong.” Vertical marks on the right side of liking graphs correspond to LS = “like slighty”, LM = “like moderately.” (A) Perceived intensity of odors, but not flavors, was greater pre- versus post-fMRI scanning. (B) OW, but not HW, subjects found odors more intense when Hungry than when Sated. (C) Flavor intensity perception was not influenced by feeding or body weight status. (D) All stimuli (odors and flavors) were more liked when hungry than when sated. (E) Flavors were more liked than odors. (F) Odors, but not flavors, were more liked pre- versus post-fMRI scanning. (G) The change in average odor liking between sessions was not correlated with the change in average odor intensity perception between sessions as assessed with Pearson correlation.
Pleasantness
Since intensity and pleasantness are known to be related (Wundt 1896; Henion 1971), we next examined liking ratings of the chemosensory stimuli. Again a 2 (stimulus) × 2 (condition) × 2 (time) repeated measures ANOVA with BMI group as a between subjects variable was performed. This time, we observed a main effect of condition [F(1, 31) = 5.62, P = 0.024; Figure 4D] where all stimuli (odors and flavors) were found to be more pleasant at Hungry than at Sated scans, and a main effect of stimulus [F(1, 31) = 38.02, P < 0.001; Figure 4E] where flavors were more liked than odors. There was also a significant stimulus by time interaction [F(1, 31) = 5.79, P = 0.022; Figure 4F], where odor, but not flavor pleasantness decreased from pre- to post-scan [main effect of time for odors: F(1, 32) = 6.66, P = 0.015]. However, in contrast to the intensity ratings, there was no significant interaction among stimulus, condition, and BMI group. Additionally, the perceived change in average odor intensity between Hungry and Sated sessions was not correlated with the perceived change in average odor liking as assessed with Pearson correlation (Figure 4G). We also correlated average odor liking with the perceived change in average odor intensity using a Pearson correlation to test whether stimulus pleasantness influenced potential effects of internal state on olfactory sensitivity. The correlation was not significant (P = 0.61; data not shown).
Relationship with plasma measures and BMI
The change in average perceived odor intensity (average of pre- and post-scan intensity ratings) in Hungry-Sated sessions—hereafter referred to simply as “change in odor intensity perception”—was significantly correlated with markers of metabolic status. As assessed with Pearson correlation, change in odor intensity perception was positively correlated with BMI [r(31) = 0.40, P = 0.038; Figure 5A] and negatively correlated with in the extent of postprandial plasma total ghrelin suppression—hereafter referred to simply as “postprandial ghrelin reactivity”—at 90min from baseline between the Hungry and the Sated scans [r(21) = −0.45, P = 0.030; Figure 5B]. Greater change in odor intensity perception was associated with higher BMI and lesser postprandial ghrelin reactivity. A similar effect was observed for percent change from baseline between the Hungry and the Sated scans at 60 [r(21) = −0.48, P = 0.020; Figure 5C] and 90min [r(21) = −0.56, P = 0.006; Figure 5D]. No relationships were observed between change in odor intensity perception and baseline glucose, insulin, triglycerides, or FFA; or their reactivity at any time point. Given the correspondence between percent change and raw change in ghrelin levels, we focus subsequent analyses on the raw change in ghrelin levels from baseline between Hungry and Sated scans at 90min.
Figure 5.
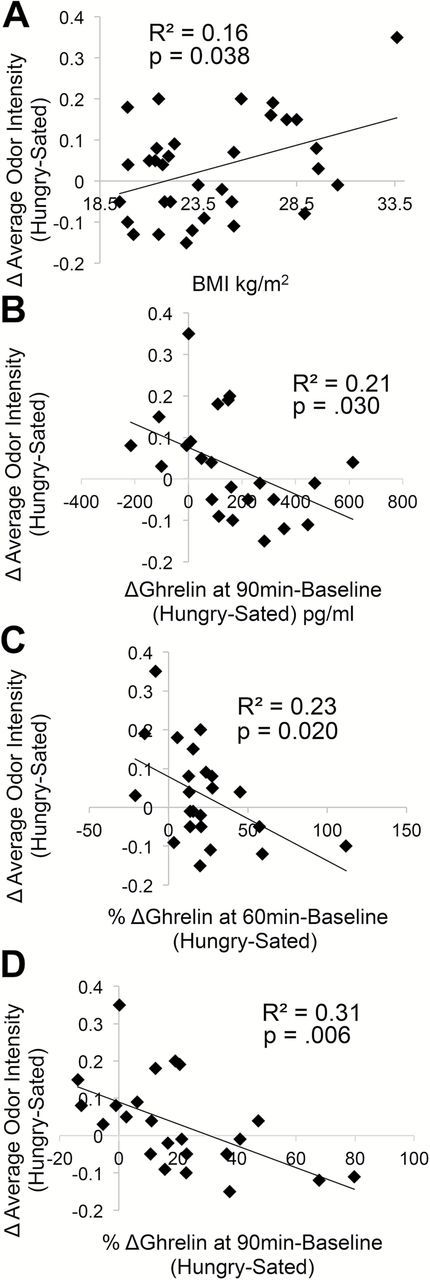
Metabolic measures are related to odor intensity perception as assessed with Pearson correlation. Greater between-session (Hungry-Sated) changes in average odor intensity perception occurred in individuals with (A) higher BMIs and (B–D) lesser postprandial ghrelin suppression (absolute or percentage change from baseline, Hungry-Sated).
To determine the relative contributions of BMI and postprandial ghrelin reactivity to change in odor intensity perception, we performed a stepwise regression analysis where postprandial ghrelin reactivity and BMI were entered as independent variables, and change in odor intensity perception as the dependent variable. Postprandial ghrelin reactivity significantly predicted change in odor intensity perception [F(1, 21) = 5.46, P = 0.030]. The multiple correlation coefficient was 0.45, indicating that 20.6% of the variance in odor intensity change could be accounted for by postprandial ghrelin reactivity. BMI did not enter (i.e., significantly improve) the model (t = 2.04, P = 0.055), indicating that BMI does not underlie the relationship between postprandial ghrelin reactivity and change in odor intensity perception. The regression equation was:
Odor intensity change = 0.076 − 0.0003 (ghrelin reactivity)
As this finding suggests that postprandial ghrelin reactivity may account for the effect of BMI on change in odor intensity perception, we included it as a covariate in the previously described ANOVAs of stimulus intensity. When postprandial ghrelin reactivity is accounted for, the 3-way interaction between stimulus, condition, and BMI group is no longer significant (P = 0.156); however, the effect of condition on odor intensity perception for the OW group alone survives [F(1, 7) = 7.62, P = 0.028].
Neuroimaging
Main effect of odor
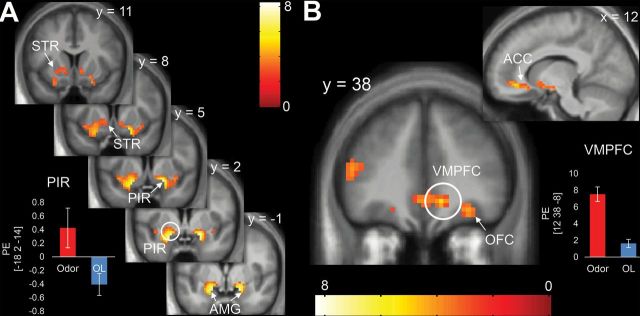
To verify that our stimuli activated olfactory cortex, we compared response to Odor > OL irrespective of odor category (food/nonfood) and condition (Hungry/Sated, Tables 1 and 2). This revealed whole brain-corrected activations in the ventromedial prefrontal cortex (VMPFC) extending into anterior cingulate cortex (Figure 6A), as well as in the piriform primary olfactory cortex extending into the amygdala and striatum (Figure 6B). Activation was also observed in the OFC that was significant when corrected for multiple comparisons across the ROI.
Table 1.
Results of whole brain-corrected showing brain regions where significantly greater BOLD response to Odor > OL was observed across both Hungry and Sated sessions
| Odor > Odorless: Whole brain-corrected | |||||||
|---|---|---|---|---|---|---|---|
| Size (voxels) | pFWE-cluster | MNI coordinates | Region | L/R | Z | ||
| x | y | z | |||||
| 194 | 0.010 | 12 | 38 | −8 | Frontal medial orbital gyrus | R | 4.32 |
| 9 | 26 | −11 | Anterior cingulate cortex | R | 3.98 | ||
| 21 | 29 | −14 | Frontal superior orbital gyrus | R | 3.86 | ||
| 30 | 35 | −14 | Frontal inferior orbital gyrus | R | 3.77 | ||
| 24 | 32 | −11 | Inferior frontal gyrus | R | 3.73 | ||
| −3 | 38 | −8 | Anterior cingulate cortex | L | 3.52 | ||
| 3 | 41 | −5 | Frontal medial orbital gyrus | R | 3.40 | ||
| 3 | 50 | −8 | Frontal medial orbital gyrus | R | 2.99 | ||
| 0 | 44 | 7 | Anterior cingulate cortex | — | 2.81 | ||
| 184 | 0.013 | −18 | 2 | −14 | Piriform | L | 5.58 |
| −36 | 5 | −11 | Piriform | L | 3.73 | ||
| −12 | 8 | −5 | Nucleus accumbens | L | 3.04 | ||
| −18 | 14 | −5 | Putamen | L | 2.88 | ||
Italics denote subpeaks within the cluster.
Table 2.
Results of ROI analyses showing brain regions where significantly greater BOLD response to Odor>OL was observed across both Hungry and Sated sessions.
| Odor > Odorless: ROI analyses | ||||||
|---|---|---|---|---|---|---|
| Size (voxels) | pFWE-peak | MNI coordinates | L/R | Z | ||
| x | y | z | ||||
| Amygdala | ||||||
| 14 | <0.001 | 21 | −1 | −14 | R | 5.33 |
| 23 | <0.001 | −21 | −1 | −17 | L | 5.27 |
| Piriform | ||||||
| 45 | <0.001 | 21 | 5 | −14 | R | 6.04 |
| 59 | <0.001 | −18 | 2 | −14 | L | 5.58 |
| OFC | ||||||
| 31 | <.001 | −24 | 32 | −17 | L | 4.99 |
| 48 | 0.008 | 21 | 29 | −14 | R | 3.86 |
| 0.011 | 30 | 35 | −14 | R | 3.77 | |
Figure 6.
Brain response to odors (Odor–OL Hungry + Sated). Sniffing odors versus OL stimuli led to significant activations in the (A) piriform (PIR; primary olfactory cortex) extending into amygdala (AMG) and striatum (STR) as well as (B) ventrolateral prefrontal cortex (VMPFC) extending into the anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC; secondary olfactory cortex). Color bars represent t-values. Bar graphs show parameter estimates (PE) to Odor and OL at the peak voxel within each cluster. Error bars represent standard error. Circled areas indicate location of whole brain-corrected peaks.
Main effect of internal state
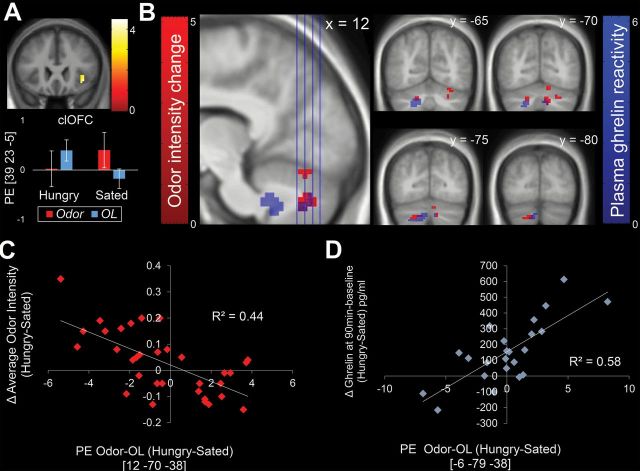
To test for an effect of internal state on brain response to odor, we compared Odor > OL in the Hungry versus the Sated session. We observed greater response in the OFC in the Sated compared to Hungry condition (Figure 7A; Table 3).
Figure 7.
Neural correlates of feeding status, changes in odor intensity perception, and postprandial ghrelin reactivity. Color bars represent t-values. (A) BOLD response to Odor > OL was greater when Sated than Hungry in the caudolateral region of the orbitofrontal cortex (clOFC) ROI. Error bars are standard error. (B) Whole-brain corrected regions where BOLD responses to Odor > OL and Hungry > Sated are negatively associated with change in odor intensity perception (red) overlap with regions positively associated with postprandial ghrelin reactivity (blue). Scatterplots show parameter estimates (PE) from the peak voxel from the regions associated with (C) change in odor intensity perception Hungry–Sated and (D) postprandial ghrelin reactivity Hungry–Sated.
Table 3.
Results of ROI analyses showing brain regions where BOLD response to Odor > OL Hungry > Sated was significantly different.
| Odor > Odorless Hungry > Sated: ROI analyses | ||||||
|---|---|---|---|---|---|---|
| Size (voxels) | pFWE-peak | MNI coordinates | L/R | Z | ||
| x | y | z | ||||
| OFC | ||||||
| 14 | 0.007 | 39 | 23 | −5 | R | 3.93 |
Associations with perception
To examine the neural correlates of the observed change in odor intensity perception, we regressed odor intensity change against the change in BOLD response to odors in the Hungry versus Sated sessions, (Average odor intensity Hungry − Average odor intensity Sated) vs. [(BOLD response to odors Hungry − BOLD response to OL Hungry) − (BOLD response to odors Sated − BOLD response to OL Sated)]. No correlations were observed in our ROIs (amygdala, piriform, OFC, and insula). However, strong whole brain-corrected negative associations in the cerebellum emerged with greater change in odor intensity perception associated with lesser change in cerebellar response (Figure 7B,C; Table 4). To determine if this effect was related to ghrelin signaling, we regressed postprandial ghrelin reactivity against the change in BOLD response to Odors > OL ([(Ghrelin at 90min Hungry − ghrelin baseline Hungry) − (Ghrelin at 90min Sated − ghrelin baseline Sated)] vs. [(BOLD response to odors Hungry − BOLD response to OL Hungry) − (BOLD response to odors Sated − BOLD response to OL Sated)]. Note that positive values represent greater postprandial ghrelin reactivity after a meal. Again whole brain-corrected correlations were observed in the cerebellum, but not in our ROIs (Figure 7B,D; Table 4). Lastly, we repeated the analyses with BMI since BMI is also related to change in odor intensity perception. No whole brain-corrected or ROI relationships were observed.
Table 4.
Results of whole brain-corrected analyses showing brain regions where BOLD response to Odor > OL Hungry > Sated was correlated with change in odor intensity perception Hungry > Sated (top) and ghrelin change at 90min after baseline Hungry > Sated (bottom).
| Negative correlation with odor intensity change: Whole brain-corrected | |||||||
|---|---|---|---|---|---|---|---|
| Size (voxels) | pFWE-cluster | MNI coordinates | Region | L/R | Z | ||
| x | y | z | |||||
| 240 | <0.001 | 12 | −70 | −38 | Cerebellar lobule VIII | R | 4.19 |
| 15 | −70 | −17 | Cerebellar lobule VI | R | 3.98 | ||
| 27 | −70 | −32 | Cerebellar crus I | R | 3.59 | ||
| 30 | −73 | −38 | Cerebellar crus I | R | 3.52 | ||
| 9 | −76 | −26 | Cerebellar crus I | R | 3.43 | ||
| 27 | −64 | −20 | Cerebellar lobule VI | R | 3.37 | ||
| 45 | −67 | −32 | Cerebellar crus I | R | 3.18 | ||
| 42 | −52 | −38 | Cerebellar crus I | R | 3.12 | ||
| Positive correlation with postprandial ghrelin reactivity: Whole brain-corrected | |||||||
| Size (voxels) | pFWE-cluster | MNI coordinates | Region | L/R | Z | ||
| x | y | z | |||||
| 779 | <0.001 | −6 | −79 | −38 | Cerebellar crus II | L | 4.42 |
| −18 | −64 | −38 | Cerebellar lobule VIII | L | 4.20 | ||
| 6 | −49 | −35 | Cerebellar lobule IX | R | 3.93 | ||
| 27 | −55 | −35 | Cerebellar lobule VI | R | 3.91 | ||
| −24 | −73 | −44 | Cerebellar lobule VIIb | L | 3.62 | ||
| 24 | −67 | −32 | Cerebellar lobule VI | R | 3.47 | ||
| 9 | −73 | −38 | Cerebellar lobule VIII | R | 3.45 | ||
| −9 | −46 | −38 | Cerebellar lobule IX | L | 3.40 | ||
| −24 | −52 | −29 | Cerebellar lobule VI | L | 3.36 | ||
| −36 | −61 | −32 | Cerebellar crus I | L | 2.90 | ||
| 12 | −31 | −38 | Pons | R | 2.75 | ||
| 6 | −28 | −41 | Pons | R | 2.69 | ||
Italics denote subpeaks within the cluster.
Sniffing behavior
To determine if change in odor intensity perception was related to sniffing behavior, we examined sniffing behavior using a spirometer to measure airflow simultaneous to fMRI scanning. We performed a 1-way repeated measure ANOVA examining the effect of condition on the volume and vigor of sniffing. Sniff volume in response to odors did not differ between Hungry and Sated scans (Figure 8A). Sniff vigor in response to odors was greater during the Hungry scan than during the Sated scan [F(1, 23) = 4.65, P ≤ 0.0425; Figure 8B]. However, the difference in sniff vigor between Hungry and Sated scans was not correlated with change in odor intensity perception or cerebellar response between scans, BMI, or postprandial ghrelin reactivity as assessed with Pearson correlation (Figure 8C–E).
Figure 8.
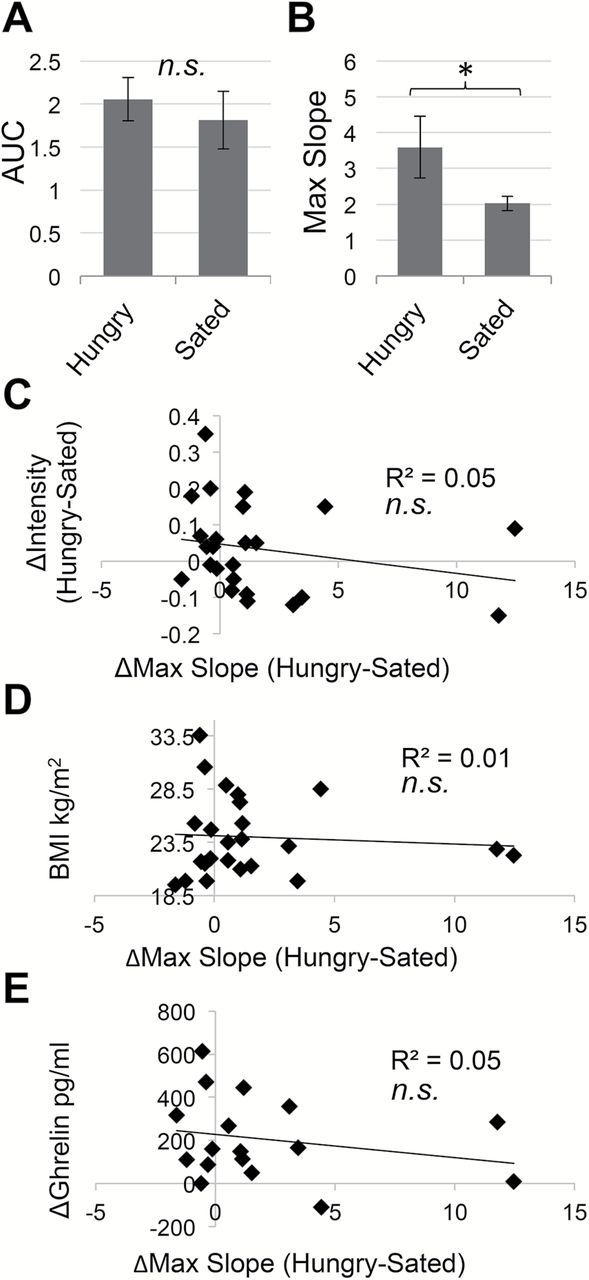
Sniffing behavior. Sniff parameters were assessed using repeated measures ANOVA. Error bars represent standard error of the mean. *P < 0.05; n.s., not significant. (A) Sniff volume (area under the curve, AUC) in response to odors did not differ between Hungry and Sated scans. (B) Sniff vigor (max slope) in response to odors was greater during Hungry versus Sated scans. Individual differences in sniff vigor did not correlate with (C) change in odor intensity perception Hungry–Sated, (D) BMI, or (E) raw postprandial ghrelin reactivity at 90min from baseline Hungry–Sated, as assessed using Pearson correlation.
Discussion
In keeping with emerging literature highlighting the importance of metabolism in olfaction (Palouzier-Paulignan et al. 2012), we report that meal consumption differentially influences the perceived intensity, but not the liking of suprathreshold odors in OW compared to HW individuals. This effect does not generalize to flavored milkshakes, which contain a retronasal olfactory component, and it cannot be accounted for by differences in sniffing. However, it is associated with lesser postprandial ghrelin reactivity, which is a better predictor of changes in odor intensity perception than BMI. We also found that response in the cerebellum, but not in the olfactory cortex, correlated with change in odor intensity perception and ghrelin reactivity. Together the findings suggest that compromised phasic ghrelin suppression, often observed in obesity, is associated with increased influence of internal state on olfactory perception and decreased satiety-induced attenuation of cerebellar response to odors.
Reports of the effect of internal state on olfactory sensitivity in humans have been peppered with conflicting results (reviewed in Koelega 1994; and Palouzier-Paulignan et al. 2012). Three recent well-controlled studies using Sniffin Sticks (Hummel et al. 1997) to test olfactory sensitivity found, using the same nonfood odor (n-butanol), no influence of internal state (Albrecht et al. 2009) or greater sensitivity when fasted than when fed (Stafford and Welbeck 2011; Cameron et al. 2012). Both of the latter studies reported lower odor detection thresholds, while Cameron et al. (2012) also observed a sensitizing effect of fasting on olfactory discrimination. In contrast, Albrecht et al. and Stafford and Welbeck found greater sensitivity when fed than when fasted for food odors assessed using detection thresholds (Albrecht et al. 2009) and discrimination (Stafford and Welbeck 2011).
There are several possible explanations for the discrepant findings. First, the effect of internal state may differ for food and nonfood odors. However, the current results do not support this possibility since our subjects reliably identified food odors as edible and nonfood odors as inedible, yet we did not observe an effect of odor category on changes in intensity perception. Alternately, it is possible that sensory-specific satiety moderates the effect of odor category. Albrecht et al. used a banana odor (isoamyl acetate) and bananas were a component of the satiating breakfast. Likewise, the satiating lunch provided by Stafford and Welbeck consisted mostly of savory foods and their food odor was a savory herb-based odorant. In comparison, our food odors were sweet and dessert-like strawberry and chocolate aromas that were not included in the satiating lunch. Thus, it is possible that the effect of odor category on modulation of intensity perception by internal state depends on sensory-specific satiety. However, the direction of the observed effects are difficult to reconcile with this explanation since sensitivity increases for aromas associated with the satiating meal, which should be devalued and produce weaker brain responses (Rolls et al. 1981; Gottfried et al. 2003). Furthermore, obese and HW individuals do not show differences in sensory-specific satiety (Snoek et al. 2004; Brondel et al. 2006; Havermans et al. 2012), yet in the current study effects are dependent upon BMI.
Another possibility is that internal state differentially influences olfactory detection thresholds (the minimum amount of odorant needed to detect its presence) and suprathreshold intensity perception (the strength of perceived odors). Prior work shows that threshold and suprathreshold measures of olfactory sensitivity reflect different dimensions of olfaction (Koskinen et al. 2004). Future work comparing these two dimensions in food and nonfood odors is needed. For example, it is possible that the effect of category appears for low concentration but not higher concentrations of odorants. Another possibility is that pleasantness plays a role, as prior studies have used n-butanol, a chemical smell, as the nonfood stimulus, versus presumably pleasant food stimuli. In contrast, our food (chocolate and strawberry) and nonfood (honeysuckle and lilac) odors were selected to be pleasant. It has been suggested that the relationship between pleasantness and intensity is somewhat independent for negative versus positive components of a stimulus, in that negative components increase faster and contribute more to the overall perception of a stimulus than its positive components (Coombs and Avrunin 1977; Lawless 1977; Cacioppo and Bernston 1999; Rozin and Royzman 2001). Therefore, perhaps odor category only contributes to differential effects of internal state on olfactory sensitivity when the categories are associated with distinctly positive or negative valenced odors. Finally, since odor category did not influence findings it is possible that the interaction between ghrelin and odor coding occurs prior to coding odor objects (e.g., in the olfactory bulb).
Ghrelin reactivity and olfactory perception
In keeping with prior work, we found that higher BMI is associated with an increased influence of internal state on olfactory sensitivity (Stafford and Welbeck 2011; Cameron et al. 2012). We also extend these findings by providing evidence that the effect of BMI may be partly explained by postprandial ghrelin reactivity, which has been found to be altered in obesity (Tschöp et al. 2001; English et al. 2002). This is in line with prior work highlighting a critical role for ghrelin in modulating reactivity to food cues. First, baseline levels of ghrelin correlate positively with response to food images in visual, limbic and paralimbic areas (Kroemer et al. 2013). Second, ghrelin reactivity is associated with resting state neural activity (Li et al. 2012) and with the effect of a meal on brain response to a palatable and energy dense milkshake (Sun et al. 2014). Moreover, intravenous administration of ghrelin in sated individuals increases brain response to food cues in feeding and reward-related areas, potentially contributing to the revaluation of food by mimicing a fasted state (Malik et al. 2008; Goldstone et al. 2014). Finally, Tong et al. (2011) found that infusing ghrelin in humans as well as animals leads to increased exploratory sniffing.
Within the context of this literature, it was surprising that ghrelin reactivity was not associated with response in the amygdala or the olfactory cortex. This is in contrast to previous findings from our lab showing that ghrelin reactivity moderates the satiety-induced attenuation in amygdala and mOFC response to milkshake (Sun et al. 2014), suggesting differences in the metabolic modulation of brain response to odors (signaling food availability in the external environment) versus flavors (signaling the imminent delivery of nutrients to the gut). Here, ghrelin reactivity as well as changes in odor intensity perception were strongly correlated with changes in BOLD response to Odors > OL in the cerebellum. Thus, a failure of the meal to induce changes in circulating ghrelin and cerebellar response to odors is associated with larger effects of the meal on perception. This suggests that ghrelin may influence perception in part by acting on the cerebellum.
A role for the cerebellum in olfaction has been previously described. Cerebellar responses to odors have been found to be concentration-dependent (Sobel et al. 1998) and cerebellar lesions can produce olfactory impairments by influencing sniff size (Mainland et al. 2005). This supports the notion that cerebellar circuits are critical for reflexively adjusting sniff size in response to odorant concentration (Teghtsoonian et al. 1978; Laing 1983; Sobel et al. 1998). Our data show that this circuit may include an inhibitory component since we found that weaker cerebellar responses correspond to greater changes in odor intensity. This suggestion is in line with prior observations that the cerebellum tonically inhibits brainstem respiratory centers (Moruzzi 1940; Glasser et al. 1966; Decima and von Euler 1969).
Notably, dopaminergic signaling may be implicated in cerebellar influences on olfaction. The ventral tegmental area (VTA), a major dopaminergic hub, directly connects the primary olfactory cortex with the cerebellum (Oades and Halliday 1987; Ikai et al. 1992, 1994; Sobel et al. 1998). This pathway bypasses other olfactory brain regions and may explain why we did not observe correlations between odor intensity perception and brain response to Odors > OL in the amygdala or insula, two regions that have been shown to encode intensity of chemosensory stimuli (Anderson et al. 2003; Small et al. 2003). Ghrelin receptors are localized on dopamine neurons (Abizaid et al. 2006), and a subpopulation of dopaminergic neurons in the VTA may have long-range projections to both the piriform and the cerebellum (Ikai et al. 1992, 1994). Pharmacological blockade of the dopamine receptor D2 (DRD2) improves olfactory performance in rats (Yue et al. 2004; Wei et al. 2006) and DRD2 signaling within the mesolimbic reward pathway has been shown to be altered by diet induced obesity (Wang et al. 2001; Johnson and Kenny 2010; de Weijer et al. 2011). Furthermore, DRD2 is expressed in olfactory receptor neuron terminals in the rodent olfactory bulb and may be involved in the presynaptic inhibition of olfactory information (Nickell et al. 1991; Koster et al. 1999; Ennis et al. 2001). It is thus possible that differential effects of ghrelin on DRD2 signaling in OW or obese versus lean individuals gives rise to alterations in olfactory perception by influencing cerebellar circuits. An important caveat with this interpretation is that we did not observe an association between sniffing behavior and ghrelin reactivity or intensity perception. Nevertheless, our study was not optimized for detailed analysis of sniff parameters (i.e., sniffs were overlaid on the respiratory cycle) and sniff measurements were only collected for a subset of our subjects, limiting power.
Interspecies differences
Animal studies consistently show heightened olfactory sensitivity during the fasted state, an effect that is not consistently observed in humans. Our results suggest that the influence of internal state on odor sensitivity may be related to metabolic health, since the effect is only observed in OW or obese individuals. Relatedly, it has been argued that most commonly used strains of laboratory rodents are in fact metabolically compromised (Martin et al. 2010), and calorie restriction in a lab setting has a beneficial effect on the life span of vertebrates and invertebrates alike (McCay et al. 1935; reviewed in Koubova and Guarente 2003). This raises the interesting possibility that effects in animal studies are also influenced by metabolic health. However, it is also possible that the interspecies difference reflects brain evolution. The human olfactory bulb contains 8 times more glomeruli as would be predicted from models of olfactory receptor neuron convergence derived from rodent studies (Maresh et al. 2008). Humans also have a much expanded neocortex compared to rodents (Shepherd 2010), which may serve to maintain olfactory constancy despite a changing internal milieu (de Araujo et al. 2005; Zelano et al. 2005).
Flavors
We did not observe changes in the perceived intensity of flavored milkshake between hungry and sated states. This was surprising, as intensity ratings of retronasal and orthonasal odors have been shown to be highly correlated (Koskinen et al. 2004). However, the intensity of flavor is determined primarily by taste (Green et al. 2012), raising the possibility that differences in retronasal olfactory intensity perception were masked by the regulation of flavor intensity perception by taste, which is not influenced by internal state (Pangborn 1959; Pasquet et al. 2006).
Another possibility is that the constraints on head movement imposed by fMRI scanning may have restricted the degree to which subjects could move their tongue, oral cavity, and/or pharynx, thus limiting their ability to perceive the milkshake stimuli. It would therefore be interesting to measure the volume of “swallow breaths” (i.e., exhaled air that travels from the throat to the nasal cavity after swallowing), as these have been posited to be the retronasal correlate of sniffs in orthonasal olfaction (Burdach and Doty 1987) and exhibit inter-individual variation (Rabe et al. 2004; Mishellany-Dutour et al. 2012). Testing whether swallow breath size or tongue movement magnitude are related to retronasal odor intensity perception or metabolism may be a fruitful, albeit technically challenging, avenue for future research.
Brain response to odors and the influence of internal state
Comparison of odor to odorless trials revealed strong responses in regions of the brain that are sensitive to internal state (O’Doherty et al. 2000; Small et al. 2001; Gottfried et al. 2003; Kringelbach et al. 2003). However, the only effect of meal observed in the current study was in the caudolateral OFC, which responded more when sated than when hungry. Although this result is consistent with prior reports (Small et al. 2001) it was nevertheless surprising that the meal did not influence response to odors in the medial OFC, amygdala, or piriform cortex. It is possible that the lack of effect relates to differences in design since most prior studies measure sensory specific satiety (O’Doherty et al. 2000; Gottfried et al. 2003).
Limitations
Our study had several limitations. First, blood and sniff measures were only collected on a subset of subjects, limiting power. Second, we only measured total ghrelin rather than distinguishing between acyl, or active, ghrelin and desacyl ghrelin. The vast majority of the literature on the effects of ghrelin on feeding is thought to be due to the specific action of acyl ghrelin, regardless of whether acyl or total ghrelin is measured. However while traditionally thought of as metabolically inert, desacyl ghrelin levels are suggested to differ between HW and OW, and may have physiologically relevant effects on appetite and metabolism that are independent from those of acyl ghrelin and not mediated by the known ghrelin receptor (Toshinai et al. 2006; Barazzoni et al. 2007; Liu et al. 2008; Delhanty et al. 2012). Third, we did not measure other important peptides that regulate feeding such as cholecystokinin (CCK) (Duca et al. 2013). We also did not measure insulin sensitivity, which is known to effect ghrelin signaling (Flanagan et al. 2003; Bacha and Arslanian 2005). Therefore, we cannot rule out the possibility that the correlation between ghrelin reactivity and change in odor intensity perception is mediated by an unmeasured factor such as insulin sensitivity or CCK. Finally, recent weight change can influence ghrelin signaling (Hansen et al. 2002; Leidy et al. 2004) and we did not collect the weight history. It is therefore possible that weight change prior to the study influenced the metabolic effects that we observed.
Conclusions
In summary, the present study found that body weight and the integrity of the postprandial ghrelin response influence the effect of a meal on odor intensity perception. This effect is in turn associated with cerebellar response to odors. These findings highlight the interaction between the endocrine and olfactory systems and may help to explain conflicting findings of the effect of a meal on olfaction.
Funding
This work was funded by the National Institutes of Health [R01 DK085579 and PL1 DA024859].
Acknowledgments
We would like to thank Drs Ivan de Araujo and Robert Sherwin for their thoughtful discussions and insight in the design of this study. We would also like to thank Yuko Nakamura, Paul Geha, Sarah Nolan-Poupart, Francois Chouinard-Decorte, Xanna D’Agostino, Gina Lombardi, and Mary Kurjanowicz with their help in acquiring the data.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, et al. 2006. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 116(12):3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimé P, Duchamp-Viret P, Chaput MA, Savigner A, Mahfouz M, Julliard AK. 2007. Fasting increases and satiation decreases olfactory detection for a neutral odor in rats. Behav Brain Res. 179:258–264. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Schreder T, Kleemann AM, Schöpf V, Kopietz R, Anzinger A, Demmel M, Linn J, Kettenmann B, Wiesmann M. 2009. Olfactory detection thresholds and pleasantness of a food-related and a non-food odour in hunger and satiety. Rhinology. 47(2):160–165. [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. 2003. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 6(2):196–202. [DOI] [PubMed] [Google Scholar]
- Bacha F, Arslanian SA. 2005. Ghrelin suppression in overweight children: a manifestation of insulin resistance? J Clin Endocrinol Metab. 90(5):2725–2730. [DOI] [PubMed] [Google Scholar]
- Baldelli R, Bellone S, Castellino N, Petri A, Rapa A, Vivenza D, Bellone J, Broglio F, Ghigo E, Bona G. 2006. Oral glucose load inhibits circulating ghrelin levels to the same extent in normal and obese children. Clin Endocrinol (Oxf). 64(3):255–259. [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, Dore F, Fonda M, Ciocchi B, Cattin L, et al. 2007. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab. 92(10):3935–3940. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. 2004. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 82(1):109–114. [DOI] [PubMed] [Google Scholar]
- Brondel L, Romer M, Van Wymelbeke V, Walla P, Jiang T, Deecke L, Rigaud D. 2006. Sensory-specific satiety with simple foods in humans: no influence of BMI? Int J Obes. 31(6):987–995. [DOI] [PubMed] [Google Scholar]
- Burdach KJ, Doty RL. 1987. The effects of mouth movements, swallowing, and spitting on retronasal odor perception. Physiol Behav. 41(4):353–356. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Bernston GG. 1999. The affect system: architecture and operating characteristics. Curr Dir Psychol Sci. 8:133–137. [Google Scholar]
- Cameron JD, Goldfield GS, Doucet É. 2012. Fasting for 24h improves nasal chemosensory performance and food palatability in a related manner. Appetite. 58(3):978–981. [DOI] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI. 1997. Environmental signals modulate olfactory acuity, discrimination, and memory in Caenorhabditis elegans . Learn Mem. 4(2):179–191. [DOI] [PubMed] [Google Scholar]
- Coombs CH, Avrunin G. 1977. A theorem on single-peaked preference in one dimension. J Math Psychol. 16:261–266. [Google Scholar]
- de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. 2005. Cognitive modulation of olfactory processing. Neuron. 46:671–679. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C. 1969. Intercostal and cerebellar influences on efferent phrenic activity in the decerebrate cat. Acta Physiol Scand. 76:148–58. [DOI] [PubMed] [Google Scholar]
- Delhanty PJD, Neggers SJ, van der Lely AJ. 2012. Ghrelin: the differences between acyl- and des-acyl ghrelin. Eur J Endocrinol. 167:601–608. [DOI] [PubMed] [Google Scholar]
- de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, van de Laar A, Fliers E, Serlie MJ, Booij J. 2011. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca FA, Zhong L, Covasa M. 2013. Reduced CCK signaling in obese-prone rats fed a high fat diet. Horm Behav. 64:1–6. [DOI] [PubMed] [Google Scholar]
- English P, Ghatei M, Malik I, Bloom S, Wilding J. 2002. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 87:2984–2987. [DOI] [PubMed] [Google Scholar]
- Engström BE, Ohrvall M, Sundbom M, Lind L, Karlsson FA. 2007. Meal suppression of circulating ghrelin is normalized in obese individuals following gastric bypass surgery. Int J Obes (Lond). 31(3):476–480. [DOI] [PubMed] [Google Scholar]
- Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT. 2001. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol. 86(6):2986–2997. [DOI] [PubMed] [Google Scholar]
- Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS. 2003. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab. 284(2):E313–E316. [DOI] [PubMed] [Google Scholar]
- Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE. 2008. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab. 93(5):1971–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser R, Tippett J, Davidian VA., Jr 1966. Cerebellar activity, apneustic breathing, and the neural control of respiration. Nature. 209:810–812. [DOI] [PubMed] [Google Scholar]
- Goetzl F, Stone F. 1947. Diurnal variations in acuity of olfaction and food intake. Gastroenterology. 9:444–453. [PubMed] [Google Scholar]
- Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G, Deliran SS, Beckmann C, Ghatei MA, Ashby DR, et al. 2014. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr. 99(6):1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. 2003. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 301:1104–1107. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. 1996. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 21(3):323–334. [DOI] [PubMed] [Google Scholar]
- Green BG, Nachtigal D, Hammond S, Lim J. 2012. Enhancement of retronasal odors by taste. Chem Senses. 37(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM. 1993. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 18:683–702. [Google Scholar]
- Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, Jørgensen JO. 2002. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf). 56(2):203–206. [DOI] [PubMed] [Google Scholar]
- Havermans RC, Roefs A, Nederkoorn C, Jansen A. 2012. No rapid recovery of sensory-specific satiety in obese women. Flavour. 1:5. [Google Scholar]
- Henion KE. 1971. Odor pleasantness and intensity: a single dimension? J Exp Psychol. 90(2):275–279. [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 1997. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 22(1):39–52. [DOI] [PubMed] [Google Scholar]
- Ikai Y, Takada M, Mizuno N. 1994. Single neurons in the ventral tegmental area that project to both the cerebral and cerebellar cortical areas by way of axon collaterals. Neuroscience. 61(4):925–934. [DOI] [PubMed] [Google Scholar]
- Ikai Y, Takada M, Shinonaga Y, Mizuno N. 1992. Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience. 51(3):719–728. [DOI] [PubMed] [Google Scholar]
- Johnson BN, Sobel N. 2007. Methods for building an olfactometer with known concentration outcomes. J Neurosci Methods. 160(2):231–245. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. 2010. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 13(5):635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julliard AK, Chaput MA, Apelbaum A, Aim P. 2007. Changes in rat olfactory detection performance induced by orexin and leptin mimicking fasting and satiation. Behav Brain Res. 183:123–129. [DOI] [PubMed] [Google Scholar]
- Koelega HS. 1994. Diurnal variations in olfactory sensitivity and the relationship to food intake. Percept Mot Skills. 78(1):215–226. [DOI] [PubMed] [Google Scholar]
- Koskinen S, Vento S, Malmberg H, Tuorila H. 2004. Correspondence between three olfactory tests and suprathreshold odor intensity ratings. Acta Otolaryngol. 124(9):1072–1077. [DOI] [PubMed] [Google Scholar]
- Koster NL, Norman AB, Richtand NM, Nickell WT, Puche AC, Pixley SK, Shipley MT. 1999. Olfactory receptor neurons express D2 dopamine receptors. J Comp Neurol. 411(4):666–673. [DOI] [PubMed] [Google Scholar]
- Koubova J, Guarente L. 2003. How does calorie restriction work? Genes Dev. 17:313–321. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. 2003. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 13(10):1064–1071. [DOI] [PubMed] [Google Scholar]
- Kroemer NB, Krebs L, Kobiella A, Grimm O, Pilhatsch M, Bidlingmaier M, Zimmermann US, Smolka MN. 2013. Fasting levels of ghrelin covary with the brain response to food pictures. Addict Biol. 18(5):855–862. [DOI] [PubMed] [Google Scholar]
- Laing DG. 1983. Natural sniffing gives optimum odour perception for humans. Perception. 12(2):99–117. [DOI] [PubMed] [Google Scholar]
- Langfeld HS. 1914. On the psychophysiology of a prolonged fast. Psychol Monogr. 16:1–14. [Google Scholar]
- Lawless HT. 1977. The pleasantness of mixtures in taste and olfaction. Sens Processes. 1(3):227–237. [PubMed] [Google Scholar]
- Leidy HJ, Gardner JK, Frye BR, Snook ML, Schuchert MK, Richard EL, Williams NI. 2004. Circulating ghrelin is sensitive to changes in body weight during a diet and exercise program in normal-weight young women. Journal of Clinical Endocrinology and Metabolism. 89:2659–2664. [DOI] [PubMed] [Google Scholar]
- Li J, An R, Zhang Y, Li X, Wang S. 2012. Correlations of macronutrient-induced functional magnetic resonance imaging signal changes in human brain and gut hormone responses. Am J Clin Nutr. 96(2):275–282. [DOI] [PubMed] [Google Scholar]
- Lim J, Wood A, Green BG. 2009. Derivation and evaluation of a labeled hedonic scale. Chem Senses. 34(9):739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, Veldhuis P, Gordon DA, Howard AD, Witcher DR, et al. 2008. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 93(5):1980–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainland JD, Johnson BN, Khan R, Ivry RB, Sobel N. 2005. Olfactory impairments in patients with unilateral cerebellar lesions are selective to inputs from the contralesional nostril. J Neurosci. 25(27):6362–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. 2008. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 7(5):400–409. [DOI] [PubMed] [Google Scholar]
- Maresh A, Rodriguez Gil D, Whitman MC, Greer CA. 2008. Principles of glomerular organization in the human olfactory bulb—implications for odor processing. PLoS One. 3(7):e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. 2009. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci. 29(20):6734–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. 2010. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A. 107(14):6127–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard L. 1935. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 10:63–79. [PubMed] [Google Scholar]
- Meyer-Gerspach AC, Wölnerhanssen B, Beglinger B, Nessenius F, Napitupulu M, Schulte FH, Steinert RE, Beglinger C. 2014. Gastric and intestinal satiation in obese and normal weight healthy people. Physiol Behav. 129:265–271. [DOI] [PubMed] [Google Scholar]
- Mishellany-Dutour A, Woda A, Labouré H, Bourdiol P, Lachaze P, Guichard E, Feron G. 2012. Retro-nasal aroma release is correlated with variations in the in-mouth air cavity volume after empty deglutition. PLoS One. 7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Tsai PM, Mendes N, Miller KK, Klibanski A. 2009. Increased carbohydrate induced ghrelin secretion in obese vs. normal-weight adolescent girls. Obesity (Silver Spring). 17(9):1689–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman SD, Klier K, Braun S, Azen C, Geffner ME, Buchanan TA. 2010. Obese adolescents show impaired meal responses of the appetite-regulating hormones ghrelin and PYY. Obesity (Silver Spring). 18(5):918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi G. 1940. Paleocerebellar inhibition of vasomotor and respiratory carotid sinus reflexes. J Neurophysiol. 3:20–32. [Google Scholar]
- Nickell WT, Norman AB, Wyatt LM, Shipley MT. 1991. Olfactory bulb DA receptors may be located on the terminals of the olfactory nerve. Neuroreport. 2:9–12. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. 1987. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 434(2):117–165. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. 2000. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 11(4):893–897. [DOI] [PubMed] [Google Scholar]
- Pager J, Giachetti I, Holley A, Le Magnen J. 1972. A selective control of olfactory bulb electrical activity in relation to food deprivation and satiety in rats. Physiol Behav. 9(4):573–579. [DOI] [PubMed] [Google Scholar]
- Palouzier-Paulignan B, Lacroix MC, Aimé P, Baly C, Caillol M, Congar P, Julliard AK, Tucker K, Fadool DA. 2012. Olfaction under metabolic influences. Chem Senses. 37(9):769–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangborn RM. 1959. Influence of hunger on sweetness preferences and taste thresholds. Am J Clin Nutr. 7(3):280–287. [DOI] [PubMed] [Google Scholar]
- Pasquet P, Monneuse MO, Simmen B, Marez A, Hladik CM. 2006. Relationship between taste thresholds and hunger under debate. Appetite. 46(1):63–66. [DOI] [PubMed] [Google Scholar]
- Perreault M, Istrate N, Wang L, Nichols AJ, Tozzo E, Stricker-Krongrad A. 2004. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. Int J Obes Relat Metab Disord. 28(7):879–885. [DOI] [PubMed] [Google Scholar]
- Prud’homme MJ, Lacroix MC, Badonnel K, Gougis S, Baly C, Salesse R, Caillol M. 2009. Nutritional status modulates behavioural and olfactory bulb Fos responses to isoamyl acetate or food odour in rats: roles of orexins and leptin. Neuroscience. 162(4):1287–1298. [DOI] [PubMed] [Google Scholar]
- Rabe S, Linforth RS, Krings U, Taylor AJ, Berger RG. 2004. Volatile release from liquids: a comparison of in vivo APCI-MS, in-mouth headspace trapping and in vitro mouth model data. Chem Senses. 29(2):163–173. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Rolls ET, Rowe EA, Sweeney K. 1981. Sensory specific satiety in man. Physiol Behav. 27(1):137–142. [DOI] [PubMed] [Google Scholar]
- Root CM, Ko KI, Jafari A, Wang JW. 2011. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 145(1):133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P, Royzman EB. 2001. Negativity bias, negativity dominance, and contagion. Pers Soc Psychol Rev. 5:296–320. [Google Scholar]
- Seubert J, Freiherr J, Frasnelli J, Hummel T, Lundström JN. 2013. Orbitofrontal cortex and olfactory bulb volume predict distinct aspects of olfactory performance in healthy subjects. Cereb Cortex. 23(10):2448–2456. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. 2010. New perspectives on olfactory processing and human smell. Neurobiol Olfaction. 395–405. [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. 2003. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 39(4):701–711. [DOI] [PubMed] [Google Scholar]
- Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. 2008. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 57(5):786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. 2001. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 124(Pt 9):1720–1733. [DOI] [PubMed] [Google Scholar]
- Snoek HM, Huntjens L, Van Gemert LJ, De Graaf C, Weenen H. 2004. Sensory-specific satiety in obese and normal-weight women. Am J Clin Nutr. 80(4):823–831. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Gabrieli JD, Sullivan EV. 1998. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. 18(21):8990–9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford LD, Welbeck K. 2011. High hunger state increases olfactory sensitivity to neutral but not food odors. Chem Senses. 36(2):189–198. [DOI] [PubMed] [Google Scholar]
- Sun X, Kroemer NB, Veldhuizen MG, Babbs AE, de Araujo IE, Gitelman DR, Sherwin RS, Sinha R, Small DM. 2015. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci. 35(20):7964–7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Veldhuizen MG, Wray AE, de Araujo IE, Sherwin RS, Sinha R, Small DM. 2014. The neural signature of satiation is associated with ghrelin response and triglyceride metabolism. Physiol Behav. 136:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teghtsoonian R, Teghtsoonian M, Berglund B, Berglund U. 1978. Invariance of odor strength with sniff vigor: an olfactory analogue to size constancy. J Exp Psychol Hum Percept Perform. 4(1):144–152. [DOI] [PubMed] [Google Scholar]
- Tentolouris N, Kokkinos A, Tsigos C, Kyriaki D, Doupis J, Raptis SA, Katsilambros N. 2004. Differential effects of high-fat and high-carbohydrate content isoenergetic meals on plasma active ghrelin concentrations in lean and obese women. Horm Metab Res. 36(8):559–563. [DOI] [PubMed] [Google Scholar]
- Tong J, Mannea E, Aimé P, Pfluger PT, Yi CX, Castaneda TR, Davis HW, Ren X, Pixley S, Benoit S, et al. 2011. Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J Neurosci. 31(15):5841–5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, Date Y, Mondal MS, Shimbara T, Kawagoe T, et al. 2006. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 147(5):2306–2314. [DOI] [PubMed] [Google Scholar]
- Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. 2001. Circulating ghrelin levels are decreased in human obesity. Diabetes. 50(4):707–709. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM. 2007. Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem Senses. 32(6):569–581. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusll N, Fowler JS. 2001. Brain dopamine and obesity. Lancet. 357:354–357. [DOI] [PubMed] [Google Scholar]
- Wei CJ, Linster C, Cleland TA. 2006. Dopamine D(2) receptor activation modulates perceived odor intensity. Behav Neurosci. 120(2):393–400. [DOI] [PubMed] [Google Scholar]
- Wundt WM. 1896. Grundriß der Psychologie. Leipzig: Engelmann. [Google Scholar]
- Yue EL, Cleland TA, Pavlis M, Linster C. 2004. Opposing effects of D1 and D2 receptor activation on odor discrimination learning. Behav Neurosci. 118(1):184–190. [DOI] [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N. 2005. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 8(1):114–120. [DOI] [PubMed] [Google Scholar]