In 55% of forearm arteriovenous fistulas (AVFs) (68 of 124) and 83% of upper-arm AVFs (341 of 411), at least 50% of the 6-week blood flow rate was achieved at 1 day; both 1-day and 2-week diameters and blood flow measurements are predictive of 6-week measurements, but the association is higher with later US measures.
Abstract
Purpose
To assess the anatomic development of native arteriovenous fistula (AVF) during the first 6 weeks after creation by using ultrasonographic (US) measurements in a multicenter hemodialysis fistula maturation study.
Materials and Methods
Each institutional review board approved the prospective study protocol, and written informed consent was obtained. Six hundred and two participants (180 women and 422 men, 459 with upper-arm AVF and 143 with forearm AVF) from seven clinical centers underwent preoperative artery and vein US mapping. AVF draining vein diameter and blood flow rate were assessed postoperatively after 1 day, 2 weeks, and 6 weeks. Relationships among US measurements were summarized after using multiple imputation for missing measurements.
Results
In 55% of forearm AVFs (68 of 124) and 83% of upper-arm AVFs (341 of 411) in surviving patients without thrombosis or AVF intervention prior to 6 weeks, at least 50% of their 6-week blood flow rate measurement was achieved at 1 day. Among surviving patients without thrombosis or AVF intervention prior to week 2, 70% with upper-arm AVFs (302 of 433) and 77% with forearm AVFs (99 of 128) maintained at least 85% of their week 2 flow rate at week 6. Mean AVF diameters of at least 0.40 cm were seen in 85% (389 of 459), 91% (419 of 459), and 87% (401 of 459) of upper-arm AVFs and in 40% (58 of 143), 73% (104 of 143), and 77% (110 of 143) of forearm AVFs at 1 day, 2 weeks, and 6 weeks, respectively. One-day and 2-week AVF flow rates and diameters were used to predict 6-week levels, with 2-week prediction of 6-week measures more accurate than those of 1 day (flow rates, R2 = 0.47 and 0.61, respectively; diameters, R2 = 0.49 and 0.82, respectively).
Conclusion
AVF blood flow rate at 1 day is usually more than 50% of the 6-week blood flow rate. Two-week measurements are more predictive of 6-week diameter and blood flow than those of 1 day. US measurements at 2 weeks may be of value in the early identification of fistulas that are unlikely to develop optimally.
© RSNA, 2015
Introduction
Marked local hemodynamic changes occur when a hemodialysis arteriovenous fistula (AVF) is created. Connecting a high-pressure artery to a low-pressure vein often causes arterial and venous dilation and substantially increased blood flow in the new circuit. Early AVF development by using ultrasonographic (US) diameter and flow measurements has only been described in small, single-center studies (1–4). Publications have focused on forearm AVF, with relatively little quantitative data reported on upper-arm AVFs, a common site of AVF placement in the United States. AVF longevity is improved, and infection rate is better than that with synthetic grafts or central venous catheters, but failure to mature is a common problem, occurring in 20%–60% of patients (5–7). Detailed information about early anatomic changes may be useful in assessing the progression of an individual AVF toward maturity. US assessment performed in the weeks prior to usual initial cannulation time may help determine the need for early interventional strategies to promote AVF maturation and thereby limit catheter use.
The Hemodialysis Fistula Maturation (HFM) study was a large, multicenter prospective observational cohort study of 602 U.S. patients in whom new AVFs were created. Participants underwent scheduled, standardized US examinations to map the upper-extremity vessels preoperatively, and their AVFs were examined with US at about 1 day, 2 weeks, and 6 weeks postoperatively. The purpose of this analysis was to assess the anatomic development of native AVFs during the first 6 weeks after AVF creation by using US measurements in a multicenter hemodialysis fistula maturation study.
Materials and Methods
Overview
This prospective study was institutional review board approved and compliant with the Health Insurance Portability and Accountability Act at each institution. Written informed consent was obtained from all participants. The HFM study design has been detailed previously (6). Participants were recruited from seven clinical centers. Patients undergoing maintenance hemodialysis or projected to begin hemodialysis within 3 months were eligible for enrollment if scheduled for a new upper-extremity AVF created in a single-stage surgery. Baseline demographic and clinical data, including age, sex, race, prior coronary artery or peripheral vascular disease, and body mass index, were ascertained by means of direct patient interview and chart review. Standardized preoperative mapping US was performed before AVF surgery. US evaluation of various AVF parameters has been proposed as a quantitative AVF maturation surrogate (8,9). Therefore, postoperative US images were obtained about 1 day after surgery (from immediately after surgery to 2 days after surgery, subsequently referred to as the 1-day visit) and at 2 weeks and 6 weeks. US preoperative mapping and postoperative AVF evaluation were performed by using modified standardized protocols (8,10–12) (see Tables E1 and E2 [online]). All presented anatomic data were obtained preoperatively and in the first 6 postoperative weeks, prior to AVF intervention, and prior to determination of clinical maturation.
HFM US Core Facility
Training and performance.—Fifty-five sonographers from seven clinical centers were trained at intensive 1.5-day sessions at the University of Alabama at Birmingham (UAB). Blood flow rate acquisition standardization and methods to minimize errors during scanning and vessel measurement were intensively addressed in sonographer training. Sonographers were certified to perform HFM US examinations on the basis of observed HFM US examinations performed in their own institutions, in addition to those performed during training at the HFM core facility. US examinations were performed by using iU22 (Philips Healthcare, Andover, Mass), ATL HDI5000 (Philips Healthcare), Logic E9 (GE Medical Systems, Milwaukee, Wis), or Xario (Toshiba America Medical Systems, Tustin, Calif) equipment. US scanners that matched the equipment used clinically in this study in the seven clinical centers were loaned solely for use during the training sessions by Philips Healthcare, GE Medical Systems, and Toshiba America Medical Systems. All US personnel were credentialed as registered diagnostic medical sonographers, registered vascular technologists, registered vascular specialists, or registered cardiac sonographers.
Core facility interpretations.—US examination data were sent to the US core facility at UAB electronically via secure virtual private network tunnels, with compact disk backup as needed, and stored electronically in a US MiniPACS system (Imorgon Medical, Redwood City, Calif) with secure server backup. One of two registered diagnostic medical sonographers (nonauthors), each with 10 years of experience, assessed all images from every US examination and the accompanying study worksheet for protocol adherence and measurement accuracy. Diameters were remeasured by using the MiniPACS (Imorgon Medical) measurement tool if incorrectly measured by means of visual image inspection. Evaluation of all US images and worksheets was then performed by one of three radiologists who specialized in US vascular studies with 22 years (M.L.R.), 3 years (H.R.U.), and 4 years (a nonauthor) of experience, without knowledge of the participants’ clinical information, and final measurements were electronically entered into the HFM database. Quality control evaluations were performed for each US examination.
Statistics
Baseline characteristics were summarized and compared between participants with upper-arm and forearm AVFs by using all available data for each characteristic. All longitudinal analyses described were performed after generating multiple imputations (13) for missing measurements (see Appendix E1 [online]). The proportions of participants categorized as having discrete states were defined by (a) participant death, (b) AVF thrombosis, (c) AVF intervention without thrombosis, and (d) designated ranges of either AVF venous diameters or flow rates, which were enumerated at each follow-up visit. The AVF venous diameter categories were less than 0.30, 0.30–0.40, 0.40–0.50, 0.50–0.60, and at least 0.60 cm. The flow rate categories were less than 250, 250–500, 500–750, 750–1000, and at least 1000 mL/min. Because of the use of multiple imputation, estimates of the numbers of patients that met designated conditions on the basis of the follow-up US examinations often included a fractional component; these estimates have been rounded to the nearest integer. In some cases, rounded integer values have been jiggered up or down by 1 to improve the consistency of the arithmetic after rounding.
We similarly tabulated the proportions of participants that simultaneously met both AVF flow and venous diameter criteria for maturation (8,9) at each visit. For each pair of visits (1 day and 2 weeks, 1 day and 6 weeks, and 2 and 6 weeks), enumeration was also used to estimate transition probabilities between each pair of states designated earlier. For example, we estimated the probabilities that a participant with the state defined by an AVF blood flow between 500 and 750 mL/min at the 2-week visit would by 6 weeks have first experienced AVF thrombosis, undergone a surgical intervention for some other cause, died, or, without having had an intervention, attained a flow within the 500–750-mL/min category or within each of the other flow rate categories at 6 weeks. For each follow-up time, the 10th, 25th, 50th, 75th, and 90th percentiles were used to describe the distributions of AVF flow and draining vein diameter averaged over several measured locations along its length. All summaries were stratified by upper-arm versus forearm AVF location.
Squared Pearson correlations were used to summarize the fractions by which the variation in the 6-week AVF flow and diameters were reduced by predicting them from their 1-day and 2-week respective flows and diameters. The R2 values for the 1-day flow and diameter also incorporated the time in hours from the fistula placement surgery to the 1-day US measurement. Separate multivariable logistic regressions with cubic spline terms for AVF flow and mean venous internal diameter were used to model the probabilities that AVF flow and venous diameter criteria for maturation would be simultaneously satisfied at 6 weeks, as a function of either the 1-day or 2-week AVF flows and diameters.
P values for comparisons between upper-arm and forearm fistulas were computed by using Wilcoxon rank sum tests for continuous variables and χ2 tests for categorical variables. All analyses were performed in SAS version 9.4 software (SAS Institute, Cary, NC).
Results
Six hundred and two participants were enrolled between March 2010 and September 2013. Thirty percent were female (180 of 602) and 44% were black (264 of 594), with a median age of 56 years at AVF surgery (age range, 18–88 years). Sixty-four percent of patients (383 of 602) were undergoing hemodialysis at the time of surgery. Seventy-six percent of patients (459 of 602) had an upper-arm AVF (cephalic vein in 285, basilic vein transposition in 162, and other in 12), and 24% (143 of 602) had a forearm AVF (cephalic vein in 133). The placement of forearm AVFs was less likely with female sex, diabetes, a history of coronary artery disease, and older age (P < .05). AVF placement (forearm vs upper arm) was not significantly associated with body mass index, race, or history of peripheral arterial disease (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics

Note.—Unless indicated otherwise, data are number of patients with percentages in parentheses.
*Data are medians, with 10th–90th percentiles in parentheses.
†Self-reported race was missing for eight patients, all with upper-arm AVFs.
The overall median preoperative feeding artery internal diameter (brachial artery or radial or ulnar artery in the case of a high brachial artery bifurcation [14]) for upper-arm AVFs was 0.42 cm, approximately 80% larger than the forearm AVF median distal radial artery diameter of 0.23 cm. Similarly, the median vein internal diameter used for AVF creation in upper-arm AVFs was approximately 25% larger than the median forearm vein internal diameter. Table 2 lists preoperative diameters of feeding arteries and minimum draining vein internal diameters, categorized by AVF location and patient sex.
Table 2.
Preoperative Diameter Measurements at US

Note.—Unless indicated otherwise, data are medians, with 10th–90th percentiles in parentheses.
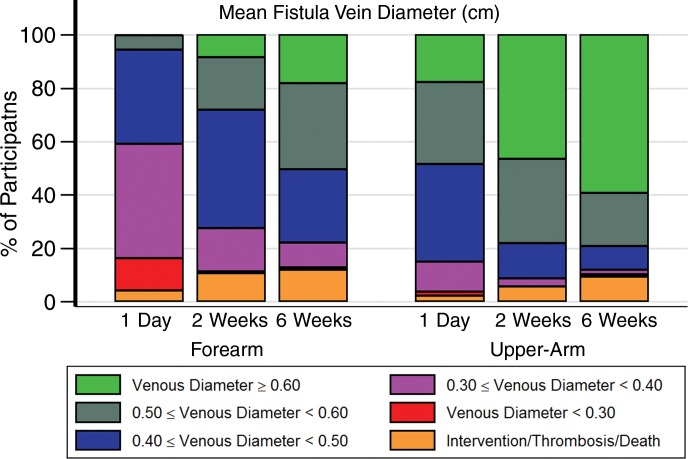
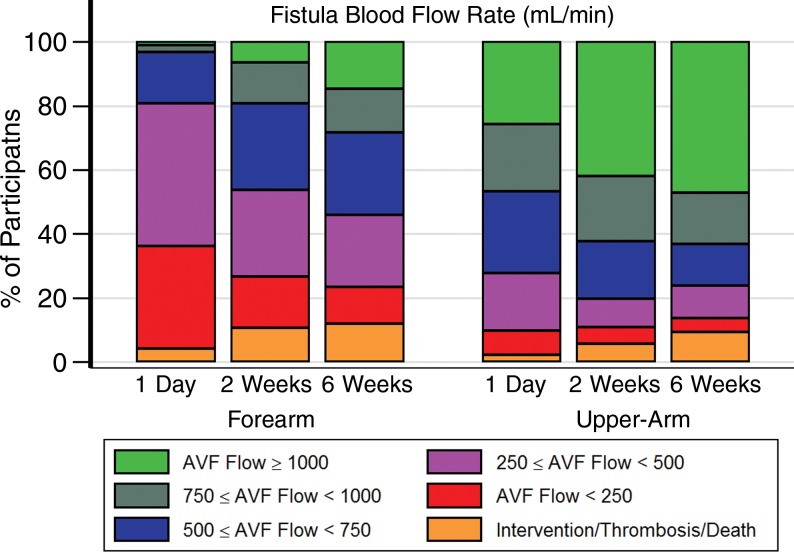
Figure 1 displays mean inner vein diameter preoperatively through 6 weeks and AVF blood flow rate from 1 day through 6 weeks. Larger absolute values observed preoperatively in upper-arm versus forearm diameters persisted at all time periods. The 1-day US measurements were performed at a median of 19.4 hours (5th and 95th percentiles, 0.7 and 49.1 hours, respectively) after surgery. Fifty-five percent of forearm AVFs (68 of 124) and 83% of upper-arm AVFs (341 of 411) in surviving participants without thrombosis or an AVF intervention at 6 weeks had reached at least 50% of their 6-week blood flow rate measurement at 1 day. Figure 2 displays the percentages of participants categorized as having a state defined by an AVF intervention, thrombosis, or death or having a state defined by designated ranges for mean venous diameter and AVF flow rate for patients without one of these events. While substantial diameter and AVF flow rate heterogeneity is evident, the percentages in the upper ranges of diameter and flow rate increase over time. Overall, 85% (389 of 459), 91% (419 of 459), and 87% (401 of 459) of participants with upper-arm AVFs had mean vein diameters of at least 0.40 cm at the 1-day, 2-week, and 6-week assessments, respectively. The corresponding percentages for participants with forearm AVFs were 40% (58.0 of 143), 73% (104 of 143), and 77% (110 of 143), respectively. Similarly, 72% (331 of 459), 80% (369 of 459), and 76% (348 of 459) of participants with upper-arm AVFs and 20% (28 of 143), 46% (66 of 143), and 54% (77 of 143) of participants with forearm AVFs had AVF flows of at least 500 mL/min at the three US examinations. Relatively few participants died (six patients), experienced thrombosis of the AVF (41 patients), or underwent AVF intervention without thrombosis (20 patients) prior to the 6-week US assessment (Table E3 [online]).
Figure 1a:
Box plots of AVF trends over time. (a) The mean AVF inner vein diameter was averaged over the length of the vein from the preoperative to 6-week postoperative time points. The plots indicate the mean and the 10th, 25th, 50th, 75th, and 90th percentiles of the mean fistula vein diameter. The blue boxes represent forearm fistulas, and the orange boxes represent upper-arm fistulas. Multiple imputation was used to impute missing values prior to AVF intervention, thrombosis, or death. (b) The AVF blood flow rate is shown for the 1-day to 6-week postoperative time points. The plots indicate the mean and the 10th, 25th, 50th, 75th, and 90th percentiles of the fistula flow. The blue boxes represent forearm fistulas, and the orange boxes represent upper-arm fistulas. Multiple imputation was used to impute missing values prior to AVF intervention, thrombosis, or death.
Figure 2a:
Graphs show percentages of AVFs categorized as having designated anatomic states at postoperative visits at 1 day, 2 weeks, and 6 weeks. Indicated are percentages of patients with upper-arm (right) or forearm (left) AVFs categorized as having designated states defined by death, AVF thrombosis, or AVF intervention (orange shading) or who remained alive without thrombosis or intervention with (a) mean AVF draining vein internal diameter (in centimeters) or (b) AVF blood flow rate (in milliliters per minute) within the designated ranges. Multiple imputation was used to impute missing flow or diameter measurements that occurred prior to death, thrombosis, or AVF intervention.
Figure 1b:
Box plots of AVF trends over time. (a) The mean AVF inner vein diameter was averaged over the length of the vein from the preoperative to 6-week postoperative time points. The plots indicate the mean and the 10th, 25th, 50th, 75th, and 90th percentiles of the mean fistula vein diameter. The blue boxes represent forearm fistulas, and the orange boxes represent upper-arm fistulas. Multiple imputation was used to impute missing values prior to AVF intervention, thrombosis, or death. (b) The AVF blood flow rate is shown for the 1-day to 6-week postoperative time points. The plots indicate the mean and the 10th, 25th, 50th, 75th, and 90th percentiles of the fistula flow. The blue boxes represent forearm fistulas, and the orange boxes represent upper-arm fistulas. Multiple imputation was used to impute missing values prior to AVF intervention, thrombosis, or death.
Figure 2b:
Graphs show percentages of AVFs categorized as having designated anatomic states at postoperative visits at 1 day, 2 weeks, and 6 weeks. Indicated are percentages of patients with upper-arm (right) or forearm (left) AVFs categorized as having designated states defined by death, AVF thrombosis, or AVF intervention (orange shading) or who remained alive without thrombosis or intervention with (a) mean AVF draining vein internal diameter (in centimeters) or (b) AVF blood flow rate (in milliliters per minute) within the designated ranges. Multiple imputation was used to impute missing flow or diameter measurements that occurred prior to death, thrombosis, or AVF intervention.
Tables 3 and 4 display the percentages of participants who transitioned between states defined in Figure 2 between 2-week and 6-week visits. Most AVF veins reached at least 0.4 cm at 2 weeks, even in the forearm. Approximately 94% of AVF veins (394 of 419) that reached a diameter of at least 0.4 cm in the upper arm at 2 weeks maintained a diameter of at least 0.4 cm at 6 weeks, and 96% of AVF veins (122 of 127) that reached a diameter of at least 0.3 cm in the forearm at 2 weeks maintained a diameter of at least 0.3 cm at 6 weeks. Among participants with upper-arm AVFs, 69% (58 of 84), 89% (82 of 92), and 97% (186 of 192) of those that reached AVF blood flows of 500–750, 750–1000, and at least 1000 mL/min at 2 weeks, respectively, maintained an AVF flow of at least 500 mL/min at 6 weeks. Similarly, 85% (33 of 39), 83% (15 of 18), and 100% (nine of nine) of forearm AVFs that achieved an AVF blood flow of 500–750, 750–1000, and at least 1000 mL/min at 2 weeks maintained an AVF flow of at least 500 mL/min at 6 weeks. Overall, 77% (335 of 433) of participants with upper-arm AVFs and 84% (108 of 128) with forearm AVFs either remained in the same state or transitioned to a more favorable AVF flow rate state between 2 and 6 weeks. Tables E4 and E5 (online) provide similar summaries of fractions of participants transitioning from 1 day to 2 weeks and from 1 day to 6 weeks. Table E6 (online) lists the 2-week AVF flow and vein diameters for those with clinical events between 2 weeks and 6 weeks, and Table E7 (online) lists the 1-day AVF flow and vein diameters for clinical events between 1 day and 2 weeks.
Table 3.
Transition Probabilities based on Fistula Vein Diameter from 2 Weeks to 6 Weeks
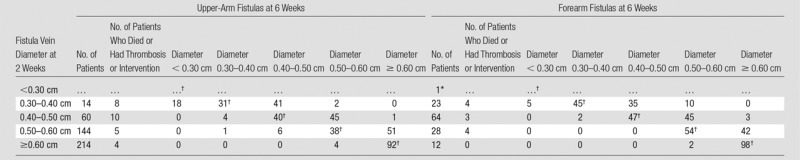
Note.—Shown are the transition probabilities (expressed as percentages) from the 2-week states indicated by the AVF vein diameter to the 6-week states. Twenty-six participants with upper-arm AVFs and 15 additional participants with forearm AVFs died, experienced thrombosis, or had an AVF intervention prior to the 2-week visit and were excluded from this summary. Multiple imputation was used to impute missing US measurements.
*Transition probabilities are not provided if the total number of patients was less than five.
†The AVF vein diameter states were the same at 2 weeks and 6 weeks.
Table 4.
Transition Probabilities based on Fistula Flow Rate from 2 Weeks to 6 Weeks

Note.—Shown are the transition probabilities (expressed as percentages) from the 2-week states indicated by the blood flow rate to the 6-week states. Twenty-six participants with upper-arm AVFs and 15 additional participants with forearm AVFs died, experienced thrombosis, or had an AVF intervention prior to the 2-week visit and were excluded from this summary. Multiple imputation was used to impute missing US measurements.
*The blood flow rate states were the same at 2 weeks and 6 weeks.
One-day AVF flow rate and diameter were moderately predictive of their respective 6-week levels after accounting for the time interval between each patient’s AVF creation surgery and 1-day examination (R2 = 0.47 and 0.49 for flow rate and diameter, respectively). Two-week values of the same measures had higher associations with the 6-week values (R2 = 0.61 for flow rate and 0.82 for diameter).
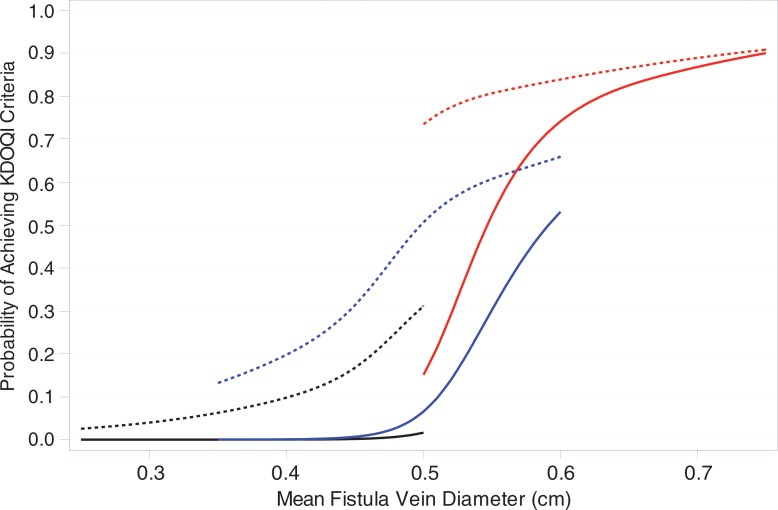
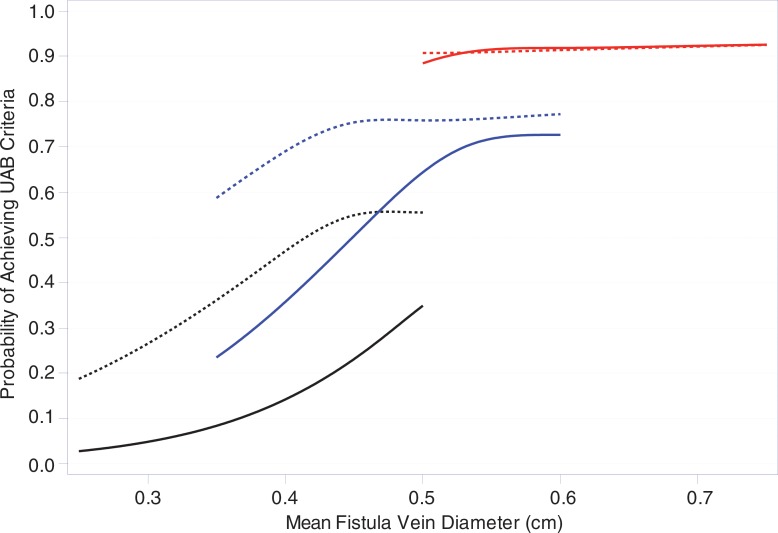
Percentages of participants who met 1-day, 2-week, and 6-week criteria for Kidney Disease Outcomes Quality Initiative (KDOQI) maturation criteria and alternate UAB criteria are shown in Table 5. Figure 3 displays estimates of the proportions of participants who met the KDOQI and UAB criteria at 6 weeks as a function of the mean vein diameter and fistula flow at 1 day or 2 weeks. Larger vein diameter and higher AVF blood flow at 1 day or 2 weeks are predictive of higher probabilities of meeting both the KDOQI and UAB maturation criteria. However, the predictive relationships are steeper for the 2-week measurements than for the 1-day measurements of diameter and blood flow, and the probability of meeting the UAB criteria is substantially higher than that for meeting the KDOQI criteria.
Table 5.
Percentage of Fistulas that Met Surrogate US Maturation Criteria

Note.—Numbers in parentheses are the number of patients used to calculate the percentages. The UAB criteria were fistula vein flow of at least 500 mL/min and mean fistula vein diameter of at least 0.4 cm (11). The KDOQI criteria were fistula vein flow of at least 600 mL/min and mean fistula vein diameter of at least 0.6 cm (13). Multiple imputation was used to impute values for missing fistula blood flow rates and mean fistula inner vein diameter prior to participant death or fistula thrombosis. Participants who died or experienced thrombosis prior to the scheduled US measurements were classified as failing to meet the designated flow or diameter criteria.
Figure 3a:
Graphs show the estimated proportions of participants who met anatomic maturation criteria on the basis of 1-day and 2-week AVF flow and vein diameter. Shown are the predicted probabilities that the (a) KDOQI and (b) UAB criteria will be reached at 6 weeks for different levels of either 1-day (dashed curves) or 2-week (solid curves) mean vein diameter (horizontal axis) and AVF blood flow with the black, blue, and red curves representing AVF blood flows of 250, 500, and 1000 mL/min, respectively. The curves were fit by using logistic regression with cubic splines for both AVF flow and vein diameters. The different ranges in mean vein diameter in the predicted probability curves for different AVF flows reflect the positive association of AVF flow and mean vein diameter, with higher vein diameter ranges for higher AVF flows. Fistula location was not a significant predictor of meeting the anatomic maturation criteria after accounting for the 1-day or 2-week AVF flows and was thus not included in the models.
Figure 3b:
Graphs show the estimated proportions of participants who met anatomic maturation criteria on the basis of 1-day and 2-week AVF flow and vein diameter. Shown are the predicted probabilities that the (a) KDOQI and (b) UAB criteria will be reached at 6 weeks for different levels of either 1-day (dashed curves) or 2-week (solid curves) mean vein diameter (horizontal axis) and AVF blood flow with the black, blue, and red curves representing AVF blood flows of 250, 500, and 1000 mL/min, respectively. The curves were fit by using logistic regression with cubic splines for both AVF flow and vein diameters. The different ranges in mean vein diameter in the predicted probability curves for different AVF flows reflect the positive association of AVF flow and mean vein diameter, with higher vein diameter ranges for higher AVF flows. Fistula location was not a significant predictor of meeting the anatomic maturation criteria after accounting for the 1-day or 2-week AVF flows and was thus not included in the models.
Discussion
AVF maturation trajectory evaluation is important, as early postoperative identification of AVF that is unlikely to be suitable for hemodialysis may call for alternative plans to establish vascular access, therefore decreasing the duration of catheter dependence. The 1-day and 2-week US examinations in this study were performed to determine whether this trajectory can be ascertained early after placement. The mean AVF vein diameter and blood flow rate increased progressively over 6 weeks, which is similar to findings from other small single-center studies in forearm (1–3,15,16) and upper-arm (17) AVFs. Interestingly, 55% of forearm AVFs and 83% of upper-arm AVFs reached at least 50% of the 6-week blood flow rate measurements at 1 day, which is similar to findings in three small previous studies (4,18,19), only one including upper-arm AVF. Collectively, our large data set and the literature indicate that substantial increases in blood flow and dilation of the vein occur within a day of AVF creation.
Analysis of 1-day and 2-week US vein diameters and AVF blood flow rate show positive correlations with 6-week measurements, although the 2-week relationship is stronger. Postoperative vessel spasm and hematoma or soft-tissue swelling that influences blood flow should be decreased by 2 weeks, which potentially explains the lower correlation with 1-day measurements versus the 2-week and 6-week measurements. A 2-week postoperative check of the participant and surgical site is routine clinical practice. US examination at that time may be useful to ascertain if the AVF is maturing adequately if not apparent at physical examination. However, substantial heterogeneity in AVF development among individuals is clearly demonstrated, with some AVFs remaining small and others rapidly increasing in size and blood flow. Moreover, not all AVFs that reached a given diameter and blood flow rate sustained those levels at the next time point, indicating that AVFs did not progress uniformly. Potential causes of poor AVF development include abnormal vein wall (20) and lack of arterial distention (5,21,22) and will be further explored in this consortium study.
With the KDOQI guidelines, a minimum AVF vein diameter of 0.6 cm and AVF vein blood flow rate of 600 mL/min are considered to be US surrogates for maturation, but these criteria are opinion based and have not been formally validated (9). Smaller diameter (0.4 cm) and lower blood flow rate (500 mL/min) cutoffs are proposed in the UAB criteria, as validated with clinical AVF maturation and adequacy for hemodialysis in two single-center studies (8,23). As expected, the number of AVFs that meet these lower thresholds at 6 weeks is higher than the corresponding number that meets the KDOQI thresholds. A noninvasive, quantifiable surrogate maturation end point could be useful in clinical trials of intervention strategies designed to promote AVF maturation.
This large, multicenter, prospective cohort study provides a detailed assessment of both preoperative and postoperative AVF vein diameters and blood flow rates during the first 6 weeks of AVF maturation. With regard to AVF placement, the associations with participant factors, such as age, sex, race, obesity, diabetes, and coronary or peripheral artery disease, have been extensively evaluated previously (8,24–28). In the present cohort, placement of upper-arm AVFs was more common in women, older patients, and those with coronary artery disease and diabetes. However, we observed no significant associations of upper-arm and forearm AVF placement with black race, body mass index, or peripheral arterial disease.
An upper-arm AVF was placed in 76% of participants (459 of 602), 89% (159 of 179) women versus 71% (300 of 423) men. A higher proportion of upper-arm AVF placement in women than in men (64% vs 36%) has been shown previously, even when using preoperative mapping US (29). The percentages of upper-arm AVF placement in both women and men in this study are higher than those reported previously (29,30) and likely reflect continued evolution of practice patterns in response to higher reported forearm AVF nonmaturation (5). This highlights the need for upper-arm AVF longitudinal development data, which are relatively scarce in the literature.
Preoperative US vessel mapping results were available to surgeons to plan AVF placement location. Recent recommendations include minimum arterial diameter of at least 0.20 cm and venous diameter of at least 0.20–0.25 cm for both forearm and upper-arm AVF (9,31). In this study, median preoperative forearm radial artery diameter was 0.23 cm, and the median of the smallest preoperative diameter measured along the forearm cephalic vein was 0.25 cm, reflecting current surgical practice.
Our study has several strengths. It is the largest multicenter observational study of hemodialysis fistula maturation to date, with intensive standardized US evaluation of vascular anatomy before and after AVF creation. It adds substantial anatomic data to a paucity of literature regarding AVFs in the upper arm, a common placement site in the United States. It focuses on AVF development during the first 6 weeks in a cohort in which very few patients were lost to death, AVF intervention, or thrombosis. The creation of a standardized core US protocol, standardized central US training, careful quality control of US examinations, and central radiologist interpretation of the nearly 2800 US examinations performed throughout this 6-year study optimized US technique across the seven institutions.
Limitations of this study include the lack of artery and vein measurement intraoperatively prior to surgical anastomosis. Intraoperative US was not used because of concerns regarding logistics and intraoperative vessel spasm. Measurements in the approximate area of the AVF obtained during the preoperative mapping US were used instead and could vary several centimeters from the actual section of vessel used for anastomosis. US measurement of the vessel diameter may be affected by transducer pressure, a potential problem addressed specifically during centralized training of the sonographers. Blood flow rates determined with US are based on a model of laminar flow, which may not exist in the AVF draining vein (19,32,33). At least one planned US measurement was missing for 14% of patients across the three postoperative US assessments. Finally, observational outcome data presented are based on the natural history of anatomic AVF development prior to AVF intervention, without clinical assessment of maturation of each AVF and ability to use it for dialysis. In future analysis, we will assess US surrogate criteria with regard to clinical AVF maturation in this group.
In conclusion, 55% of forearm AVFs (68 of 124) and 83% of upper-arm AVFs (341 of 411) achieved at least 50% of their 6-week blood flow rate at 1 day. Both 1-day and 2-week diameter and blood flow measurements are predictive of 6-week measurements, but the association is higher with later US measures.
Advances in Knowledge
■ In 55% of forearm arteriovenous fistulas (AVFs) (68 of 124) and 83% of upper-arm AVFs (341 of 411), the AVF achieved at least 50% of the 6-week blood flow rates at 1 day.
■ Both 1-day and 2-week AVF flow rates (R2 = 0.47 and 0.61, respectively) and diameters (R2 = 0.49 and 0.82, respectively) were associated with the 6-week values.
Implication for Patient Care
■ Early US evaluation of AVF (within the first 2 weeks) may be useful in predicting AVF development.
APPENDIX
Acknowledgments
Acknowledgments
We acknowledge Lin Belt, RDMS, Carl Abts, RDMS, and Lauren Alexander, MD, for their invaluable expertise at the HFM US core facility; Lance Hamff, MSHI, for his information technology support; Philips Healthcare, (Andover, Mass), GE Medical Systems (Milwaukee, Wis), and Toshiba America Medical Systems (Tustin, Calif), for the generous loan of US scanners for UAB training sessions; and Imorgon Medical, Redwood City, Calif, for providing US picture archiving and communication system software that matched software in use clinically within the UAB radiology department to facilitate HFM US core interpretations.
The members of the Hemodialysis Fistula Maturation Study Group are as follows: Chair, Steering Committee, University of Pennsylvania: H. Feldman; Clinical Centers, Boston University: L. Dember (principal investigator [PI]), A. Farber, J. Kaufman, L. Stern, P. LeSage, C. Kivork, D. Soares, M. Malikova; University of Alabama: M. Allon (PI), C. Young, M. Taylor, L. Woodard, K. Mangadi; University of Cincinnati: P. Roy-Chaudhury (PI), R. Munda, T. Lee, R. Alloway, M. El-Khatib, T. Canaan, A. Pflum, L. Thieken, B. Campos-Naciff; University of Florida: T. Huber (PI), S. Berceli, M. Jansen, G. McCaslin, Y. Trahan; University of Texas Southwestern: M. Vazquez (PI), W. Vongpatanasin, I. Davidson, C. Hwang, T. Lightfoot, C. Livingston, A. Valencia, B. Dolmatch, A. Fenves, N. Hawkins; University of Utah: A. Cheung (PI), L. Kraiss, D. Kinikini, G. Treiman, D. Ihnat, M. Sarfati, I. Lavasani, M. Maloney, L. Schlotfeldt; University of Washington: J. Himmelfarb (PI), C. Buchanan, C. Clark, C. Crawford, J. Hamlett, J. Kundzins, L. Manahan, J. Wise; Data Coordinating Center, Cleveland Clinic: G. Beck (PI), J. Gassman, T. Greene, P. Imrey, L. Li, J. Alster, M. Li, J. MacKrell, M. Radeva, B. Weiss, K. Wiggins; histology core facility, University of Washington: C. Alpers (PI), K. Hudkins, T. Wietecha; US core facility, University of Alabama at Birmingham: M. Robbin (PI), H. Umphrey, L. Alexander, C. Abts, L. Belt; vascular function core facility, Boston University: J. Vita (PI, deceased), N. Hamburg (PI). M. Duess, A. Levit; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) biosample repository, Fisher BioServices: H. Higgins, S. Ke, O. Mandaci, C. Snell; NIDDK DNA repository, Fred Hutchinson Cancer Research Center: J. Gravley, S. Behnken, R. Mortensen; External Expert Panel: G. Chertow (Chair), A. Besarab, K. Brayman, M. Diener-West, D. Harrison, L. Inker, T. Louis, W. McClellan, J. Rubin; NIDDK: J. Kusek, R. Star.
Received February 14, 2015; revision requested March 25; revision received July 4; accepted July 21; final version received and accepted October 23.
Funding: This research was supported by the National Institutes of Health (grants U01DK066597, U01DK082179, U01DK082189, U01DK082218, U01DK082222, U01DK082236, and U01DK082240).
Members of the Hemodialysis Fistula Maturation Study Group are listed in the acknowledgments.
Disclosures of Conflicts of Interest: M.L.R. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received a grant from Philips Medical. Other relationships: disclosed no relevant relationships. T.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received grants from Keryx, Jansen Pharmaceuticals, and Genkyotech. Other relationships: disclosed no relevant relationships. A.K.C. disclosed no relevant relationships. M. Allon Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received payment from CorMedix and Gore for consulting. Other relationships: disclosed no relevant relationships. S.A.B. disclosed no relevant relationships. J.S.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received payment from Proteon for board membership. Other relationships: disclosed no relevant relationships. M. Allen disclosed no relevant relationships. P.B.I. disclosed no relevant relationships. M.K.R. disclosed no relevant relationships. Y.T.S. disclosed no relevant relationships. H.R.U. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received a GERRAF fellowship grant from GE Healthcare. Other relationships: disclosed no relevant relationships. C.J.Y. disclosed no relevant relationships.
Abbreviations:
- AVF
- arteriovenous fistula
- HFM
- Hemodialysis Fistula Maturation
- KDOQI
- Kidney Disease Outcomes Quality Initiative
- UAB
- University of Alabama at Birmingham
References
- 1.Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A. Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg 1996;12(2):207–213. [DOI] [PubMed] [Google Scholar]
- 2.Mahmutyazicioğlu K, Kesenci M, Fitöz S, Büyükberber S, Sencan O, Erden I. Hemodynamic changes in the early phase of artificially created arteriovenous fistula: color Doppler ultrasonographic findings. J Ultrasound Med 1997;16(12):813–817. [DOI] [PubMed] [Google Scholar]
- 3.Malovrh M. Non-invasive evaluation of vessels by duplex sonography prior to construction of arteriovenous fistulas for haemodialysis. Nephrol Dial Transplant 1998;13(1):125–129. [DOI] [PubMed] [Google Scholar]
- 4.Lomonte C, Casucci F, Antonelli M, et al. Is there a place for duplex screening of the brachial artery in the maturation of arteriovenous fistulas? Semin Dial 2005;18(3):243–246. [DOI] [PubMed] [Google Scholar]
- 5.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int 2002;62(4):1109–1124. [DOI] [PubMed] [Google Scholar]
- 6.Dember LM, Imrey PB, Beck GJ, et al. Objectives and design of the hemodialysis fistula maturation study. Am J Kidney Dis 2014;63(1):104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huijbregts HJ, Bots ML, Wittens CH, et al. Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol 2008;3(3):714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbin ML, Chamberlain NE, Lockhart ME, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 2002;225(1):59–64. [DOI] [PubMed] [Google Scholar]
- 9.Vascular Access 2006 Work Group . Clinical practice guidelines for vascular access. Am J Kidney Dis 2006;48(Suppl 1):S176–S247. [DOI] [PubMed] [Google Scholar]
- 10.Robbin ML, Gallichio MH, Deierhoi MH, Young CJ, Weber TM, Allon M. US vascular mapping before hemodialysis access placement. Radiology 2000;217(1):83–88. [DOI] [PubMed] [Google Scholar]
- 11.Umphrey HR, Lockhart ME, Abts CA, Robbin ML. Dialysis grafts and fistulae: planning and assessment. Ultrasound Clin 2011;6(4):477–489. [Google Scholar]
- 12.Silva MB, Jr, Hobson RW, 2nd, Pappas PJ, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg 1998;27(2):302–307; discussion 307–308. [DOI] [PubMed] [Google Scholar]
- 13.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8(1):3–15. [DOI] [PubMed] [Google Scholar]
- 14.McCormack LJ, Cauldwell EW, Anson BJ. Brachial and antebrachial arterial patterns; a study of 750 extremities. Surg Gynecol Obstet 1953;96(1):43–54. [PubMed] [Google Scholar]
- 15.Tordoir JH, Rooyens P, Dammers R, van der Sande FM, de Haan M, Yo TI. Prospective evaluation of failure modes in autogenous radiocephalic wrist access for haemodialysis. Nephrol Dial Transplant 2003;18(2):378–383. [DOI] [PubMed] [Google Scholar]
- 16.Lin SL, Chen HS, Huang CH, Yen TS. Predicting the outcome of hemodialysis arteriovenous fistulae using duplex ultrasonography. J Formos Med Assoc 1997;96(11):864–868. [PubMed] [Google Scholar]
- 17.Shemesh D, Goldin I, Berelowitz D, Zaghal I, Zigelman C, Olsha O. Blood flow volume changes in the maturing arteriovenous access for hemodialysis. Ultrasound Med Biol 2007;33(5):727–733. [DOI] [PubMed] [Google Scholar]
- 18.Seyahi N, Altiparmak MR, Tascilar K, Pekpak M, Serdengecti K, Erek E. Ultrasonographic maturation of native arteriovenous fistulae: a follow-up study. Ren Fail 2007;29(4):481–486. [DOI] [PubMed] [Google Scholar]
- 19.Caroli A, Manini S, Antiga L, et al. Validation of a patient-specific hemodynamic computational model for surgical planning of vascular access in hemodialysis patients. Kidney Int 2013;84(6):1237–1245. [DOI] [PubMed] [Google Scholar]
- 20.Lee T, Chauhan V, Krishnamoorthy M, et al. Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant 2011;26(7):2264–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lok CE, Allon M, Moist L. Predicting successful arteriovenous fistula creation. Am J Kidney Dis 2012;60(3):498; author reply 498–499. [DOI] [PubMed] [Google Scholar]
- 22.Dixon BS. Why don’t fistulas mature? Kidney Int 2006;70(8):1413–1422. [DOI] [PubMed] [Google Scholar]
- 23.Singh P, Robbin ML, Lockhart ME, Allon M. Clinically immature arteriovenous hemodialysis fistulas: effect of US on salvage. Radiology 2008;246(1):299–305. [DOI] [PubMed] [Google Scholar]
- 24.Smith GE, Gohil R, Chetter IC. Factors affecting the patency of arteriovenous fistulas for dialysis access. J Vasc Surg 2012;55(3):849–855. [DOI] [PubMed] [Google Scholar]
- 25.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 2006;17(11):3204–3212. [DOI] [PubMed] [Google Scholar]
- 26.Vassalotti JA, Falk A, Cohl ED, Uribarri J, Teodorescu V. Obese and non-obese hemodialysis patients have a similar prevalence of functioning arteriovenous fistula using pre-operative vein mapping. Clin Nephrol 2002;58(3):211–214. [DOI] [PubMed] [Google Scholar]
- 27.Konner K, Hulbert-Shearon TE, Roys EC, Port FK. Tailoring the initial vascular access for dialysis patients. Kidney Int 2002;62(1):329–338. [DOI] [PubMed] [Google Scholar]
- 28.Lilly MP, Lynch JR, Wish JB, et al. Prevalence of arteriovenous fistulas in incident hemodialysis patients: correlation with patient factors that may be associated with maturation failure. Am J Kidney Dis 2012;59(4):541–549. [DOI] [PubMed] [Google Scholar]
- 29.Allon M, Lockhart ME, Lilly RZ, et al. Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 2001;60(5):2013–2020. [DOI] [PubMed] [Google Scholar]
- 30.Dixon BS, Novak L, Fangman J. Hemodialysis vascular access survival: upper-arm native arteriovenous fistula. Am J Kidney Dis 2002;39(1):92–101. [DOI] [PubMed] [Google Scholar]
- 31.Tordoir J, Canaud B, Haage P, et al. EBPG on vascular access. Nephrol Dial Transplant 2007;22(Suppl 2):ii88–ii117. [DOI] [PubMed] [Google Scholar]
- 32.Ene-Iordache B, Cattaneo L, Dubini G, Remuzzi A. Effect of anastomosis angle on the localization of disturbed flow in ‘side-to-end’ fistulae for haemodialysis access. Nephrol Dial Transplant 2013;28(4):997–1005. [DOI] [PubMed] [Google Scholar]
- 33.Hoyt K, Hester FA, Bell RL, Lockhart ME, Robbin ML. Accuracy of volumetric flow rate measurements: an in vitro study using modern ultrasound scanners. J Ultrasound Med 2009;28(11):1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.