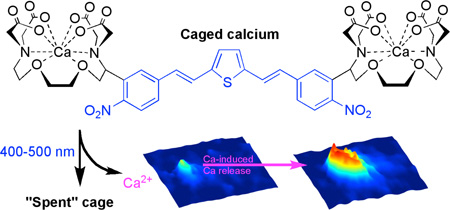
Abstract
We have designed a nitroaromatic photochemical protecting group that absorbs visible light in the violet-blue range. The chromophore is a dinitro derivative of bisstyrylthiophene (or BIST) that absorbs light very effectively (ε440 = 66,000 M−1 cm−1 and two-photon cross section of 350 GM at 775 nm). We developed a “caged calcium” molecule by conjugation of BIST to a Ca2+ chelator that upon laser flash photolysis rapidly releases Ca2+ in less than 0.2 ms. Using the patch-clamp method the optical probe, loaded with Ca2+, was delivered into acutely isolated mouse cardiac myocytes, where either one- and two-photon uncaging of Ca2+ induced highly local or cell-wide physiological Ca2+ signaling events.
Graphical abstract
Introduction
Photochemical uncaging of organic substrates is a valuable optical method that is used in many areas of science1. Invented by chemists for organic synthesis in the 1960s2, it was adopted by physiologists in the 1970s3 to control the concentration of cellular signaling molecules4. Material chemists have also found an important application of this method to construct high-density micro array chips5. Uncaging remains a uniquely powerful way to control the concentration of intracellular calcium ions ([Ca2+]i), but since covalent bonds cannot be made with ionized calcium, photolysis is normally used to decrease in an irreversible manner the affinity of Ca2+ chelators for the cation6,7 (note sodium8 and zinc9 uncaging use this approach and that other approaches for Ca2+ have been suggested recently10,11). We have used the bifurcation of known high affinity tetracarboxylic molecules into known low affinity dicarboxylates for highly efficient, rapid Ca2+ uncaging12–14. Because this is the only optical method for creating large changes in [Ca2+]i of 10–100 µM, our optical probes have been extensively used for many biochemical and physiological experiments15.
The most widely used chromophores for all uncaging experiments are the ortho-nitrobenzyl and ortho-nitroveratryl protecting groups, which absorb light in the near-ultraviolet range but are not efficiently photolyzed by visible light of longer wavelengths. Subsequently several chromophores have been used for uncaging of biological signaling molecules with blue light16–21. Photochemical protecting groups sensitive to green light have also been reported22–24, but to our knowledge no biological studies have appeared with these probes. Some of these probes are very important additions to the arsenal of caged compounds available for use by biologists, however none of them offer the same generality of carbon-heteroatom bond scission that is part of ortho-nitrobenzyl photochemistry.
Extended π-electron systems have been used to tune the absorption maximum of fluorophores for one- and two-photon (or 1P and 2P) excitation25–29. However when these molecules are used for uncaging25,27 they do not preserve the generality of simple ortho-nitrobenzyl groups. Thus, we have taken a step to address this need by creating an ortho-nitrobenzyl caging chromophore with an absorption maximum that is relatively bathochromic compared to the ortho-nitroveratryl chromophore. The new chromophore, a dinitro derivative of bisstyrylthiophene (or BIST, blue substructure of 1 in Fig. 1), has a 1P absorption maximum at 440 nm (Fig. 2a), and is very sensitive to linear excitation across the 400–500 nm range of the electromagnetic spectrum. Further, it has large 2P absorption cross-section, of at least 250 GM, in the 720–830 nm range (Fig. 2b). Here we detail the application of the BIST chromophore to the development of a photosensitive, Ca2+-selective chelator based on ethyleneglycoltetraacetic acid (or EGTA)30,31, which, with the addition of the cation becomes a caged calcium probe (Fig. 1) that is activated very efficiently by visible light.
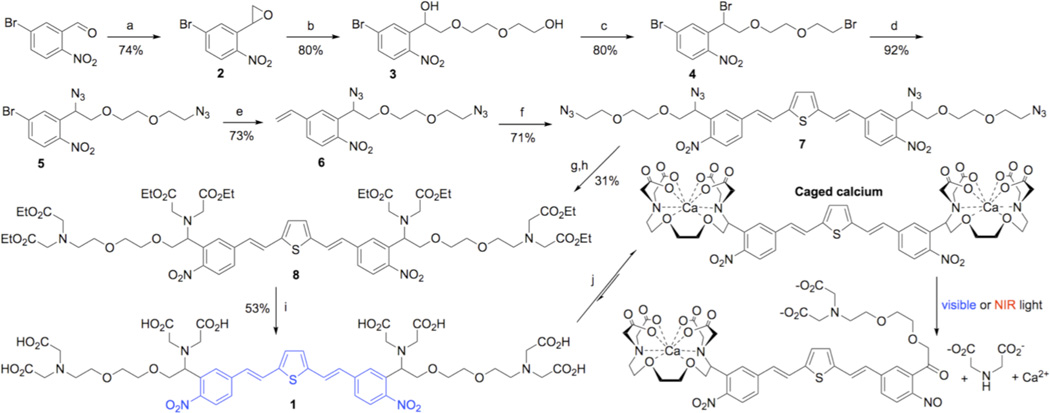
Figure 1. Synthesis of BIST-2EGTA.
Reagents and conditions: (a) Carboxymethyl-4-cyanophenylmethylsulfonium trifluoromethanesulfonate, Cs2CO3, THF; (b) diethylene glycol, NaH; (c) CBr4, PPh3, DCM; (d) NaN3, Nal, DMF; (e) Pd(PPh3)4, 2,4,6-trivinyl-boroxin pyridine complex, K2CO3, DME, H2O; (f) dibromothiophene, Pd(OAc)2, LiCI, TBACI, NaHCO3, DMF; (g) PPh3, dioxane, NaOH (aq); (h) BrCH2COOEt, pentamethylpiperidine, acetonitrile; (i) KOH, MeOH (note for clarity the countercation is not shown); (j) CaCI2 in H2O (note for clarity the ionic valence and counterion are not depicted).
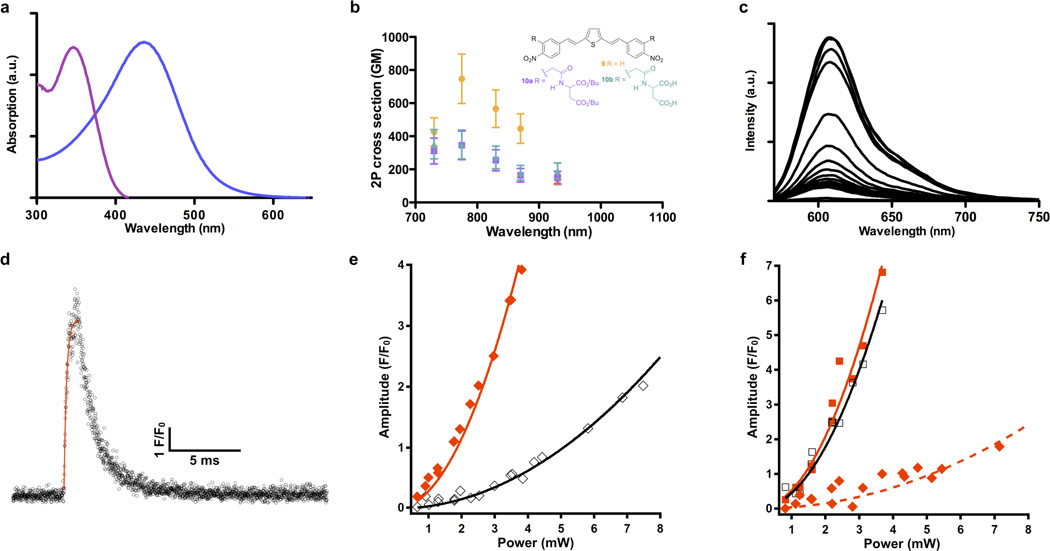
Figure 2. Photochemical characterization of BIST-2EGTA.
Nonlinear absorption properties of BIST derivatives were measured using the z-scan technique or by 2P fluorescence emission. Rapid changes in [Ca2+]free were monitored in point scan mode (10 µs per pixel) or in bidirectional line scanning mode (244 µs per line) using laser-scanning confocal microscopy at 561 or 473 nm after 2P photolysis with a mode-locked Ti:sapphire laser tuned to 810 or 720 nm. (a) Absorption spectra of the BIST-2EGTA (blue) and ortho-nitroveratryl (DM-nitrophen, violet) chromophores showing their relative 1P maxima. (b) 2P absorption spectra of BIST derivatives. Compound 9 had a 2P absorption maximum in DMSO of 740 GM at 775 nm (orange). Each data point is an average of 5 or 6 measurements. Compounds 10a (R purple) and 10b (R green) have a 2P absorption maximum in DMSO of 350 GM at 775 nm. All points are shown ± SD. (c) Example of Ca titration of a solution of BIST-2EGTA with X-rhod-1. Addition of 0.1 mM amount of CaCI2 to a solution of BIST-2EGTA (0.5 mM) at pH 7.2 with KCI (100 mM) showed the chelator had a high prephotolysis affinity for Ca2+. (d) 2P uncaging of BIST-2EGTA produced a rapid increase in Ca monitored by point scan confocal imaging using rhod-FF. The exponential time-constant for the fluorescence increase was 164 µs. (e) The relative efficacy of 2P uncaging of DM-nitrophen at 810 and 720 nm was determined by monitoring the photoreleased Ca2+ during a power train at these two wavelengths. Both wavelengths showed a quadratic dependence on incident power, and release was 7.4 ± 0.23 times more effective at 720 nm (closed red diamonds) compared to 810 nm (open black diamonds). fluo-3 was used to monitor Ca2+ release. (f) Ca2+ release from BIST-2EGTA:Ca2+ complex at 810 and 720 nm was determined by monitoring the fluorescence signal from rhod-FF during a power train at these two wavelengths (810, open black squares; 720 closed red squares). The increase in fluorescence showed a quadratic dependence on incident power and was equally effective at both wavelengths. An identical power train was also used for photolysis of DM-nitrophen (720, closed red diamonds). The resting [Ca2+]free, the Ca2+-bound, and Ca2+-free indicator concentrations were the same for both caged calcium compounds.
Results
Chemical synthesis
Concerned about the lipophilic nature of the BIST chromophore, we chose as our first target a symmetrical molecule bearing two EGTA chelators. The synthesis of this molecule (Fig. 1), which we call BIST-2EGTA, started by conversion of 2-nitro-5-bromobenyzaldehyde into the epoxide with carboxymethyl-4-cyanophenylmethylsulfonium trifluoromethanesulfonate in presence of cesium carbonate to give 2 in a 74% yield. The diether backbone of the Ca2+ coordination sphere was created using base-catalyzed ring opening of the epoxide with ethylene glycol to give the desired diol 3 in 80% yield, along with 5% of the other regiomer. The mixture of diols was converted to their dibromides using Ph3P and CBr4. From this mixture pure dibromide 4 was easily isolated by flash chromatography and then treated with sodium azide to give 5 in 74% overall yield for the two steps. The vinyl unit was added to the chromophore by treatment of 5 with 2,4,6-trivinyl-boroxin pyridine complex and tetrakis(triphenylphosphine)palladium to give 6 in a yield of 73%. The complete chromophore was constructed by Heck coupling of 6 with dibromothiophene to give 7 in 71% yield. The ethyl ester of BIST-2EGTA was made by reduction of the tetraazide 7 to its tetraamine in 70% yield, followed by alkylation with ethyl bromoacetate to give octaester 8 in 31% yield. Finally, the esters were hydrolyzed with an excess of KOH to give the target chelator BIST-2EGTA (1). Importantly, we have found such solutions to be highly stable. Routinely we store all caged compounds at −40°C, and solutions of BIST-2EGTA have be found to be stable for more than one year, as judged by HPLC (same single peak). Furthermore, such solutions are equally efficacious when used for uncaging inside cells.
One- and two-photon absorption properties of BIST
Irradiation of BIST-2EGTA with visible light revealed that the new photosensitive chelator was photolyzed slightly more slowly than DEAC450-Glu (quantum yield 0.3932). Thus, HPLC analysis showed that BIST-2EGTA photolyzed with a quantum yield of photolysis of 0.23 (Supplemental Fig. 1 shows representative HPLC traces). The absorption maximum of BIST was bathochromically shifted when compared to the widely used ortho-nitroveratryl (DM-nitrophen33) photochemical protecting group (Fig. 2a) such that BIST-2EGTA showed peak absorption at 440 nm with an extinction coefficient of 66,000 M−1 cm−1. We used the z-scan method34 and 2P-induced fluorescence to determine the 2P absorption properties of simple BIST derivatives 9 and 10 (Fig. 2b). For simplicity we chose to examine molecules without benzylic substitutions as such functionalities would be photolyzed during such measurements. The simplest dinitro-BIST (9) had a 2P absorption cross section in DMSO of 740 GM at 775 nm. This chromophore absorbed 2 photons strongly across the 720–900 nm range (Fig. 2b). A BIST derivative having substituents ortho to both nitro groups (10) showed similar, large 2P absorption properties, with a maximum 2P cross section of 350 GM at 775 nm (Fig. 2b). Note, the effects of aqueous solvent on the 2P cross section in other reports of similar compounds are modest35. The 2-fold reduction of the 2P cross section of 9 is probably caused by steric clash between the ortho substituents and the nitro groups twisting the latter out of planarity with the aromatic ring system in compound 10.
Calcium binding and release
Calcium titration of BIST-2EGTA in aqueous solution was used to determine the apparent dissociation constant at various pH vales. Fig. 2c shows the increase in fluorescence emission from X-rhod-1 during addition of defined aliquots of Ca2+ (0.1 mM) to a solution of BIST-2EGTA. These data allow the free [Ca2+] to be determined as previously described30, and showed that the chelator bound Ca2+ with high affinity (Kd 84 nM at pH 7.2, 50 nM at pH 7.35 and 19 nM at pH 7.5). In independent experiments we used Ca2+-selective electrodes to measure to the Kd and these data gave identical values. The presence of physiological Mg2+ concentrations (i.e. 1 mM) had no effect on these values. Importantly, Ca2+ was shown to be a photoproduct of the BIST-2EGTA:Ca2+ complex upon irradiation with visible light. Photolysis of a solution of BIST-2EGTA 85% saturated with Ca2+ (1 mM cage with 1.7 mM Ca2+ at pH 7.35) with a blue laser (473 nm) increased the [Ca2+]free from 0.2 µM to 20 µM measured with a Ca2+-selective electrode. HPLC analysis showed 50% of BIST-2EGTA remained in this experiment (Supplemental Fig. 2). The large increase in free [Ca2+] is to be expected as iminodiacetic acids are known to have Ca2+ affinity of about 1 mM in the physiological pH range36. We modeled our reaction with "Patcher's Power Tools" and found that the increase in free [Ca2+] must result from a decrease in affinity of approximately 20,000-fold (i.e. from 50 nM to 1 mM). It should be noted that the affinities of BIST-2EGTA before and after photolysis are, as expected, the same as the UV-light sensitive Ca2+ cage NP-EGTA30,31, a chelator that is structurally identical in terms of Ca2+ binding.
Characterization of dynamic calcium uncaging
The rate of substrate release is important for many applications of caged compounds, especially those concerned with Ca2+ signaling30. Low affinity fluorescent Ca2+ dyes allow the [Ca2+] to be measured with temporal fidelity, as the rate-limiting step for equilibration of the dye:Ca2+ complex is determined by the off-rate of the dye37. Fluorescence imaging with a confocal microscope in point scan mode revealed that rhod-FF (Kd = 19 µM) showed a rapid change in signal (exponential time constant of less than 200 µs) when BIST-2EGTA was photolyzed using 2P excitation at 810 nm (Fig. 2d).
The widely used15 nitroaromatic caged calcium compounds (e.g. DM-nitrophen33 or NP-EGTA31) photolyze much more effectively at relatively short wavelengths (e.g. 2P excitation at 720 nm, or 1P excitation in the UV-C range) when compared to longer wavelengths (i.e. >800 nm for 2P excitation and 400–500 nm for 1P excitation). Thus DM-nitrophen showed a relative 2P-uncaging efficacy of 7.40 for 720 nm versus 810 nm (Fig. 2e). Consistent with the 2P absorption spectrum (Fig. 2b), we found that BIST-2EGTA was equally sensitive to 2P photolysis at these two wavelengths (Fig. 2f). However, when DM-nitrophen was photolyzed under the same resting [Ca2+]free the fluorescence signal from rhod-FF upon Ca2+ release from BIST-2EGTA was about 13.7× larger than after release from DM-nitrophen at 720 nm (Fig. 2f). Figs. 2e,f also imply BIST is about 100× more effective at 810 nm. Taken together these data show that BIST-2EGTA binds Ca2+ with high affinity, absorbs light strongly and is photolyzed efficiently to create changes in [Ca2+] that are potentially useful for physiological studies. We tested such effectiveness in acutely isolated cardiac myocytes.
Two-photon uncaging of Ca2+: photocontrol of local Ca2+ signaling
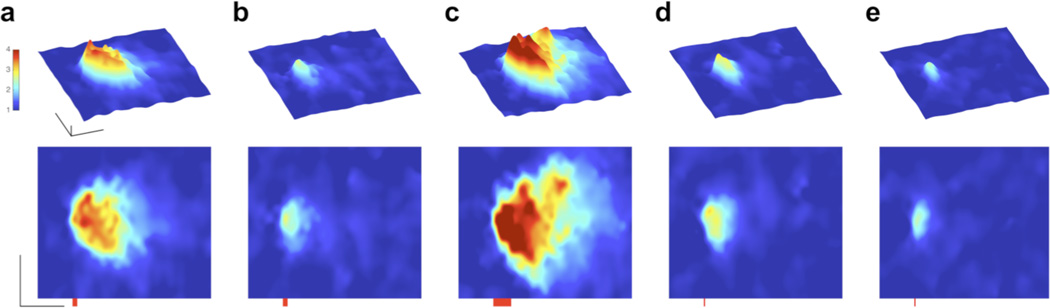
In cardiac myocyte cells a small amount of Ca2+ enters the cytoplasm upon depolarization and initiates Ca2+-induced Ca2+ release from the sarcoplasmic reticulum (SR) store. Such release events can remain highly localized or initiate “Ca2+ waves” that propagate through the cell38. These signals were recorded as confocal line scan (x,t) images, where the vertical axis shows the spatial dimension while the horizontal axis represents time. Individual myocytes were loaded with BIST-2EGTA (0.5 or 1 mM) and rhod-2 (0.1 mM) or X-rhod-5F (0.1 mM) via a patch pipette. 2P excitation at 810 nm produced localized Ca2+ transients that were considerably larger (Fig. 3a) than those produced by BIST-2EGTA photolysis in caffeine-treated cells (Fig. 3b). Caffeine completely unloads Ca2+ from the SR, so allows the pure photolytic Ca2+ signal to be detected. The difference between the two signals therefore reflects the biological Ca2+ release from the SR. Uncaging for longer periods triggered intracellular Ca2+ “mini-waves” that caused separate release events a small distance from the uncaging site (Fig. 3c). In contrast, we also found that very brief irradiation of BIST-2EGTA for 1 ms could initiate highly localized Ca2+-induced Ca2+ release from the SR (Fig. 3d,e). Previously we have found that with DM-nitrophen any Ca2+ release from the SR always required substantially longer flash durations (ca. 50 ms) 13,14,39–41.
Figure 3. Localized control of Ca2+-induced Ca2+ release in cardiac myocytes using 2P photolysis.
Single cardiac myocytes were loaded via a patch pipette with BIST-2EGTA and rhod-2. Changes in [Ca2+]free were monitored in line scan mode (2.116 ms per line) using laser-scanning confocal microscopy at 561 nm after 2P uncaging at the center of the line with a mode-locked Ti:sapphire laser tuned to 810 nm. Line scan data are displayed as 3D surface plots (time in x, space in y and fluorescence as F/F0 on a pseudo-color scale in z) with the corresponding 2D plots (time in x and space in y) below each 3D panel. Scale bars are 1 F/F0, 5 µn and 50 ms. (a) Point 2P irradiation with 5 ms pulse (red bar) triggered local Ca2+-induced Ca2+ release from the SR. (b) Photolysis of BIST-2EGTA produced highly spatially confined Ca2+ release. The cell was treated with caffeine (20 mM) to unload the Ca2+ from the SR. (c) Increasing pulse duration to 20 ms initiated a Ca2+ “mini” wave, with discrete Ca2+ release events apparent beyond the initial uncaging location, (d) Reducing pulse duration to 1 ms produced rapid, efficient and highly localized Ca2+-induced Ca2+ release, (e) Pure photolytic release of Ca2+ from BIST-2EGTA during irradiation for 1 ms (cell treated with caffeine as in b).
Photochemically initiated intracellular Ca2+ waves using visible light and 2P excitation
We found that 2P excitation of BIST-2EGTA could also initiate strong Ca2+-induced Ca2+ release processes that extended considerable distances from the uncaging point. In Fig. 4a we show using line scan fluorescence imaging that 2P excitation can produce such Ca2+ waves that propagate rapidly in both directions Note that such line scan imaging (x,t) has the advantage of a higher imaging rate compare to full frame (x,y,t), but conveys limited spatial information about the entire cardiac cell. Thus we combined visible light uncaging with full frame imaging which allowed us to produce striking Ca2+ waves that propagated throughout the cell (Fig. 4b). These signals are similar to those reported in many physiological studies of cardiac myocytes (reviewed in 38,42). It should be noted there was negligible optical cross talk between the 810-nm 2P uncaging and 561-nm confocal imaging lasers under these conditions. Specifically, imaging at 561 nm was performed with energies 0.1% of those used for photolysis with 405 nm. Further, irradiation with EGTA in place of BIST-2EGTA (Supplementary Fig. 3), or without prior chelator loading with Ca2+, did not produce any Ca2+ waves. Importantly, cells displayed normal excitation-contraction coupling, as seen in the cellular Ca2+ transient preceding photolytic Ca2+ release, implying that BIST-2EGTA is nontoxic inside cells (not shown).
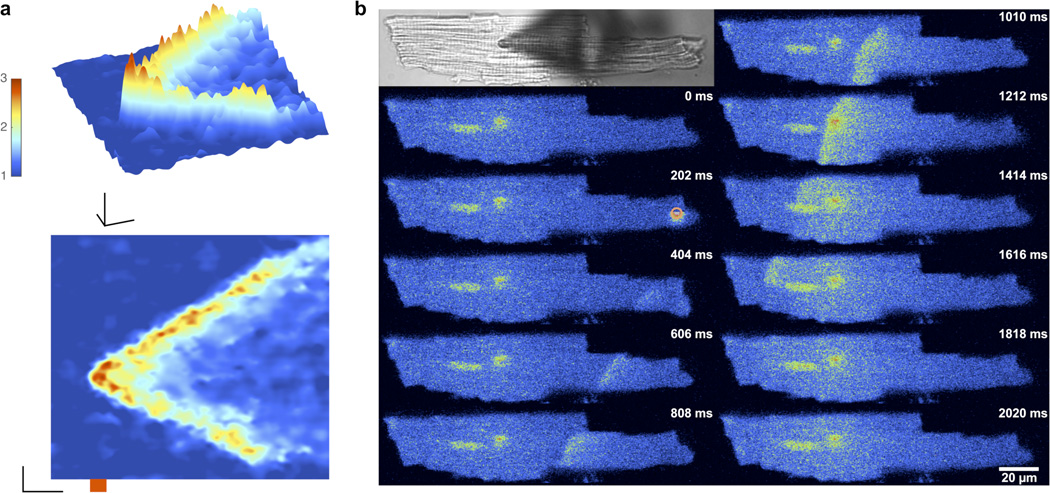
Figure 4. Visible light and 2P excitation of BIST-2EGTA:Ca2+ complex in cardiac myocytes initiates intracellular Ca2+ waves.
Single cardiac myocytes were loaded via a patch pipette with BIST-2EGTA and X-rhod-5F or rhod-2. Changes in [Ca2+]free were monitored in line scan (x,t) mode (2.116 ms per line) using laser-scanning confocal microscopy at 561 nm after 2P uncaging at the center of the line or by whole cell frame scan (202 ms per frame, 406 × 96 pixels) imaging after uncaging with visible light. A mode-locked Ti:sapphire laser tuned to 810 nm was used for 2P excitation (20 mW, 20 ms). A 405-nm continuous-wave laser was used for uncaging with visible light (10 mW, 100 ms). Line scan data are displayed as 3D surface plots (time in x, space in y and fluorescence as F/F0 on a pseudo-color scale in z) with the corresponding 2D plots (time in x and distance in y) below, (a) Rapid line scan confocal imaging revealed 2P excitation (20 ms, red bar) could initiate Ca2+ signals that propagated extensively in both directions away from the initial uncaging position. Scale bars for units of 1 F/F0, 5 µn and 50 ms. (b) Uncaging with visible light (orange circle) initiated a Ca2+ wave that propagated throughout the cell. Top left is a transmitted light image of the cell with the pipette seen as a shadow. Pseudocolor images represent raw fluorescence intensity data. Frame sequence is top left to bottom left, followed by top right to bottom right. The nucleus and patch pipette can be seen as bright structures in the left portion of the cell. The time stamp is from the beginning of each frame.
Discussion
We have developed an extended π–electron nitrobenzyl caging chromophore, which we call “BIST”, that is photolyzed efficiently with visible light in the violet-blue region. Several recently developed caging chromophores are photolyzed in this range1, however these cages are somewhat limited by the photosolvolysis reactions they use32. In contrast, the intramolecular photoredox reaction used by BIST can be used for carbon-amine and carbonether photoscission43 (see Supplementary Fig. 4 where we show that amines and alcohols are uncaged cleanly from BIST). We took advantage of this unique feature by using BIST to cleave a C-N tertiary amine bond at the heart of the Ca2+-selective chelator EGTA to develop a caged Ca2+ probe that is photolyzed with visible light, and thus fabricate a caged Ca2+ probe with distinctive optical properties (Table 1).
Table 1.
Photochemical Properties of Caged Ca2+ Probesa
| probe | ϕ | εmax (nm) | ε405 (nm) | ε473 (nm) | ϕεmax | 2PCS (CM) | τ (µs) |
|---|---|---|---|---|---|---|---|
| nitr-558 | 0.012 | 5500 (350) | 165 | 0 | 66 | 0.01 | 400 |
| DM-nitrophen33 | 0.18 | 4300 (350) | 129 | 0 | 774 | 0.01 | 26 |
| NP-EGTA31 | 0.23 | 975 (350) | 31 | 0 | 224 | <0.001 | 15 |
| azid-159 | 1.0 | 33,000 (342) | 840 | 0 | 33,000 | 1.0 | 2000 |
| NDBF-BGTA14 | 0.7 | 18,400 (330) | 312 | 0 | 12,900 | 0.6 | 50 |
| BIST-2EGTA | 0.23 | 66,000 (440) | 54,000 | 46,000 | 15,200 | 350b | 164 |
Symbols and notes: ϕ, quantum yield; ε, extinction coefficient; 2PCS, 2-photon cross section; τ, Ca2+ release time constant.
Measured in DMSO for 10, aqueous solvents could reduce this value by 20–40%.
Originally ultraviolet lasers were used to photolyze ortho-nitrobenzyl caged compounds rapidly44,45. Subsequently flash lamps with a filtered output were used46,47. However, blue lasers are now much more widely available than either of these light sources, being standard on all confocal microscopes and used extensively for “optogenetics”48. Therefore the development of a new caging chromophore that is sensitive and efficiently photolyzed in this region of the electromagnetic spectrum is a potentially useful addition to the optical toolkit available to chemists and biologists. Having an extinction coefficient of 66,000 M−1 cm−1 (c.f. fluorescein49 that has an extinction coefficient of 77,000 M−1 cm−1), it absorbs light very efficiently in the blue region, a property which is unique compared to other caged Ca2+ probes (Table 1). Furthermore, 2P uncaging is also considerably more efficient than the ortho-nitroveratryl caging chromophore (Fig. 2), suggesting that direct attachment of a large 2P antenna to the caged substrate, rather than relying on resonance transfer50, can be very effective for photorelease. Typically most extended π-electron systems examined by material chemists for 2P absorption are electron rich, with symmetrical diamino derivatives being very popular51. It is interesting that our electron deficient dinitro-BIST derivatives have similar 2P absorption properties to comparable diamino derivatives52, suggesting that polarization per se is the crucial property for chromophores in this context.
Conclusions
BIST is a new photochemical protecting group that absorbs visible light efficiently. Beyond our initial application to Ca2+ uncaging, since BIST uses similar photochemistry to the widely used ortho-nitrobenzyl and ortho-nitroveratryl chromophores, we tentatively suggest BIST could be used to uncage the widest variety of functionalities. Our current data show that BIST-2EGTA is an exceptionally photosensitive caged Ca2+ probe (Table 1), making superb use of both 1P and 2P excitation. Importantly, BIST-2EGTA is photolyzed by blue light and the wide availability of light sources in this region could make Ca2+ uncaging experiments attractive to many more laboratories.
Methods
Chemical synthesis
See supplement for full methods and analytic details.
Calcium affinity
The Ca2+ affinity of BIST-2EGTA was measured as previously described by us for NP-EGTA31. Briefly, a homemade Ca2+-selective electrode was made using ETH-129. To a solution of BIST-2EGTA (0.5 – 1.0 mM) in HEPES (10 mM) and KCl (100 mM) was added incremental amounts (0.1 mM) of CaCl2 and the change in [Ca2+]free was measured. Scatchard analysis of such titrations revealed that at pH 7.2 the Kd was 82 nM, 50 nM at pH 7.35 and 19 nM at pH 7.5. In separate experiments we also used changes in fluorescence from X-rhod-1 to measure the [Ca2+]free. Values for the Kd using these methods were identical. Using homemade electrodes we found that the [Ca2+]free of a solution of BIST-2EGTA (1 mM) with 1.7 mM CaCl2 increased from 0.2 µM to 20 µM when 50% of the caged compound was photolyzed in a quartz cuvette with a 473nm-laser. Patcher's Power Tools (MPI for Biophysical Chemistry, Gottingen web site) allowed us to model this reaction to account for the change if [Ca2+]free showing the affinity for Ca must be 1 mM.
Quantum yield of photolysis
The quantum yield of photolysis of BIST-2EGTA was measured as before31,32. Briefly, solutions of BIST-2EGTA and DEAC450-Glu, each with an optical density of 0.4 at 410 nm, were photolyzed with a 410 nm laser. The time-course of photolysis was followed by HPLC analysis. Each time point was analyzed thrice and the photolysis of each compound was also performed three times. The HPLC showed that the rate of photolysis of BIST-2EGTA was 60% of DEAC450-Glu with a 410-nm laser, implying a quantum yield of 0.23.
2P absorption cross-section
The 2P absorption cross-section, δ (in GM = 1 × 10−50 cm4 s molecules−1 photon−1), of the compounds examined was measured in the 730–930 nm wavelength range. We used the 2P-induced fluorescence (2PF) method53 using 5-ns, 10-Hz pulses (compounds 10a,b) and the z-scan technique using 60-fs, 1-kHz pulses (compound 9). In 2PF experiment, an optical parametric oscillator laser (Spectra Physics, premiScan) pumped by a Q-switched Nd:YAG laser (Spectra Physics, Quanta-Ray Pro-250). The measurements were conducted in a regime where the fluorescence signal showed a quadratic dependence on the intensity of the excitation beam. The fluorescence signal was collected by a lens and transferred via a fiber to a spectrometer (HORIBA Scientific, iHR320) with a CCD camera (HORIBA Scientific, Synapse). Coumarin 485 (ref54, Sigma-Aldrich), rhodamine B, and bisstyrylbenzene (compound 8 in55), whose 2P properties have been well characterized in methanol, were used as the references in 2PF measurements. The samples were prepared in DMSO (Sigma-Aldrich, Spectrophotometric grade) at a concentration of 3–8×10−4 M and the optical pathlength of sample cuvettes for 2PF measurements was 1 cm. For z-scan measurements56, an optical parametric amplified laser (Spectra Physics, TOPAS) pumped by a mode-locked Ti:sapphire regenerative amplifier system (Spectra Physics, Solstice) was used as the excitation source. The excitation beam for z-scan is spatially-filtered as a near Gaussian beam with M2 < 1.1 and waist ω(HW1/e2) ~ 40 µm. The excitation irradiance ranged from 50–150 GW/cm2. The samples were prepared in DMSO (ca. 2 mM) and the optical pathlength of sample cuvettes for z-scan measurements was 1 mm.
Laser flash photolysis in droplets
A mode-locked Ti:sapphire laser (Mira 9000F, Coherent, Santa Clara, CA, USA) pumped by 8 W solid-state Verdi V-8 (Coherent) was used for 2P excitation of BIST-2EGTA or DM-nitrophen. Wavelength of the laser was set to 720 or 810 nm with pulse duration of ~120 fs. Power of the laser was adjusted by neutral density and polarizing filters and was measured at the objective focal plane by a power meter (PM200 with sensor S170C, Thorlabs, Newton, New Jersey, USA). The laser beam was guided to the SIM scanner of the confocal microscope (Fluoview 1000, Olympus, Volketswil, Switzerland) operating in single point excitation mode simultaneously with the main scanner. The photolysis period was 1 or 20 ms and the duration was controlled by an electronic shutter LS3 (Vincent Associates, Rochester, NY, USA) and triggered by the confocal microscope. The main scanner of confocal microscope was operating in line scan mode. To record changes in Ca2+ concentration fluo-3, rhod-2 (both Biotium Inc., Hayward, CA, USA), X-rhod-5F (Life Technologies) or rhod-FF (Teflabs, Austin, TX, USA) were excited at 473 or 561 nm, respectively. Solutions used for droplet experiments were composed of (mM): for Fig. 2d: 1 BIST-2EGTA, 0.1 rhod-FF, 2 CaCl2, 100 KCl, 10 HEPES, pH = 7.80; for Fig. 2f: 1 BIST-2EGTA, 0.1 rhod-FF, 2 CaCl2, 100 KCl, 10 HEPES, pH = 8.0; for Fig. 2e: 2 DM-nitrophen, 0.5 CaCl2, 0.1 fluo-3, 1 GSH, 5 K2ATP, 10 HEPES, 20 TEA-Cl, 120 L-Aspartic acid, 120 CsOH, pH = 7.20; for Fig. 2f: 2 DM-nitrophen, 2 CaCl2, 0.1 rhod-FF, 100 KCl, 10 HEPES, pH=7.20. Recorded images were analyzed in MATLAB (MathWorks, Inc., Natick, MA, USA) and Igor Pro (WaveMetrics, Inc., Portland, OR, USA).
Laser flash photolysis in cardiac myocytes
Cardiac ventricular myocytes were isolated from C57Bl/6 mice as described before57. Myocytes were whole-cell patch-clamped at a resting potential −80 mV. A train of five to ten pre-pulses from −40 to 0 mV in the presence of 100 nM isoproterenol was applied to load the sarcoplasmic reticulum with Ca2+. A photolytic pulse with a duration of 1–100 ms was applied 1–3 s after last conditioning pulse to release Ca2+ from BIST-2EGTA. For 2P photolysis we used a Ti:sapphire laser with a wavelength of 810 nm, and for single photon photolysis we used a UV DPSS laser with wavelength 405 nm. Both laser beams were guided to the SIM scanner and data acquisition was the same as described above. Myocytes were placed in a recording chamber in external bath solution containing (mM): 140 NaCl, 5 KCl, 1 CsCl, 1.8 CaCl2, 0.5 BaCl2, 10 HEPES, 10 glucose, pH = 7.40. Pipettes were filled with internal solution containing (mM): for Figs. 3a–e, 4a: 0.5 BIST-2EGTA, 0.8 CaCl2, 0.1 rhod-2, 1 GSH, 4 K2ATP, 5 MgCl2, 10 HEPES, 20 TEA-Cl, 120 L-Aspartic acid, 120 CsOH, 8 NaCl, pH = 7.50; for Fig. 4b: 1 BIST-2EGTA, 1.5 CaCl2, 0.1 X-rhod-5F, 1 GSH, 5 K2ATP, 10 HEPES, 20 TEA-Cl, 120 L-Aspartic acid, 120 CsOH, 8 NaCl, pH = 7.40. Images in Figs. 3a–e, 4a were normalized, filtered with Gaussian (kernel [5 5]) and Wiener filters (kernel [10 10]) and smoothed by cubic spline (p=0.5 in MATLAB “caps” function). Experiments were performed at room temperature. All recorded images were processed and analyzed in MATLAB, imageJ and Igor Pro.
Supplementary Material
Acknowledgments
This work was supported by grants from the US NIH (GM053395 and NS069720) to GCRE-D, the AFOSR MURI (FA9550-10-10558) to JWP, and the Swiss National Science Foundation (31-156375 and the Microscopy Imaging Center, or “MIC“) to EN. We would like to thank Drs. Simon Langenegger and Robert Häner for their help.
GED conceived of the project, supervised it in its entirety, and wrote the paper. HKA synthesized and characterized the chemical compounds. RJ performed the 2-photon uncaging and physiological studies. SHC measured the 2-photon cross sections. All authors commented on the paper and approved the final submitted version.
Footnotes
Supporting information. NMR spectra and HR-MS for all new compounds.
The authors declare no competing financial interest.
References
- 1.Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A. Angew Chem Int Edit. 2012;51:8446–8476. doi: 10.1002/anie.201202134. [DOI] [PubMed] [Google Scholar]
- 2.Barltrop JA, Plant PJ, Schofield P. Chem. Commun. 1966:822–823. [Google Scholar]
- 3.Kaplan JH, Forbush B, Hoffman JF. Biochemistry. 1978;17:1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- 4.Ellis-Davies GCR. Nat Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGall GH, Barone AD, Diggelmann M, Fodor SPA, Gentalen E, Ngo N. J. Am. Chem. Soc. 1997;119:5081–5090. [Google Scholar]
- 6.Adams SR, Tsien RY. Annu Rev Physiol. 1993;55:755–784. doi: 10.1146/annurev.ph.55.030193.003543. [DOI] [PubMed] [Google Scholar]
- 7.Ellis-Davies GCR. Methods Enzymol. 2003;360:226–238. doi: 10.1016/s0076-6879(03)60112-6. [DOI] [PubMed] [Google Scholar]
- 8.Warmuth R, Grell E, Lehn JM, Bats JW, Quinkert G. Helv Chim Acta. 1991;74:671–681. [Google Scholar]
- 9.Basa PN, Antala S, Dempski RE, Burdette SC. Angew Chem-Int Ed. 2015;54:13027–13031. doi: 10.1002/anie.201505778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu L, Dai Y, Marriott G. Org Lett. 2011;13:2018–2021. doi: 10.1021/ol200408j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D, Hérault K, Isacoff EY, Oheim M, Ropert N. J Physiol (Lond) 2012;590:855–873. doi: 10.1113/jphysiol.2011.219345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis-Davies GCR, Kaplan JH, Barsotti RJ. Biophys J. 1996;70:1006–1016. doi: 10.1016/S0006-3495(96)79644-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DelPrincipe F, Egger M, Ellis-Davies GC, Niggli E. Cell Calcium. 1999;25:85–91. doi: 10.1054/ceca.1998.0009. [DOI] [PubMed] [Google Scholar]
- 14.Momotake A, Lindegger N, Niggli E, Barsotti RJ, Ellis-Davies GC. Nat Methods. 2006;3:35–40. doi: 10.1038/nmeth821. [DOI] [PubMed] [Google Scholar]
- 15.Ellis-Davies GCR. Chem Rev. 2008;108:1603–1613. doi: 10.1021/cr078210i. [DOI] [PubMed] [Google Scholar]
- 16.Amatrudo JM, Olson JP, Agarwal HK, Ellis-Davies GCR. Eur J Neurosci. 2015;41:5–16. doi: 10.1111/ejn.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GCR, Higley MJ. Science. 2013;340:759–762. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rial Verde EM, Zayat L, Etchenique R, Yuste R. Front. Neural Circuits. 2008;2:2. doi: 10.3389/neuro.04.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson JP, Banghart MR, Sabatini BL, Ellis-Davies GCR. J Am Chem Soc. 2013;135:15948–15954. doi: 10.1021/ja408225k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Priestman MA, Shell TA, Sun L, Lee H-M, Lawrence DS. Angew Chem Int Edit. 2012;51:7684–7687. doi: 10.1002/anie.201202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fournier L, Gauron C, Xu L, Aujard I, Le Saux T, Gagey-Eilstein N, Maurin S, Dubruille S, Baudin JB, Bensimon D, Volovitch M, Vriz S, Jullien L. ACS Chem Biol. 2013;8:1528–1536. doi: 10.1021/cb400178m. [DOI] [PubMed] [Google Scholar]
- 22.Goswami PP, Syed A, Beck CL, Albright TR, Mahoney KM, Unash R, Smith EA, Winter AH. J Am Chem Soc. 2015;137:3783–3786. doi: 10.1021/jacs.5b01297. [DOI] [PubMed] [Google Scholar]
- 23.Rubinstein N, Liu P, Miller EW, Weinstain R. Chem Commun. 2015;51:6369–6372. doi: 10.1039/c5cc00550g. [DOI] [PubMed] [Google Scholar]
- 24.Umeda N, Takahashi H, Kamiya M, Ueno T, Komatsu T, Terai T, Hanaoka K, Nagano T, Urano Y. ACS Chem Biol. 2014;9:2242–2246. doi: 10.1021/cb500525p. [DOI] [PubMed] [Google Scholar]
- 25.Zhou W, Kuebler SM, Braun KL, Yu T, Cammack JK, Ober CK, Perry JW, Marder SR. Science. 2002;296:1106–1109. doi: 10.1126/science.296.5570.1106. [DOI] [PubMed] [Google Scholar]
- 26.Hara K, Sato T, Katoh R, Furube A, Ohga Y, Shinpo A, Suga S, Sayama K, Sugihara H, Arakawa H. J. Phys. Chem. B. 2003;107:597–606. [Google Scholar]
- 27.Gug S, Bolze F, Specht A, Bourgogne C, Goeldner M, Nicoud J-F. Angew Chem Int Edit. 2008;47:9525–9529. doi: 10.1002/anie.200803964. [DOI] [PubMed] [Google Scholar]
- 28.Albota M, Beljonne D, Bredas J, Ehrlich J, Fu J, Heikal A, Hess S, Kogej T, Levin M, Marder S, McCord-Maughon D, Perry J, Rockel H, Rumi M, Subramaniam C, Webb W, Wu X, Xu C. Science. 1998;281:1653–1656. doi: 10.1126/science.281.5383.1653. [DOI] [PubMed] [Google Scholar]
- 29.Reinhardt B, Brott L, Clarson S, Dillard A, Bhatt J, Kannan R, Yuan L, He G, Prasad P. Chem Mater. 1998;10:1863–1874. [Google Scholar]
- 30.Ellis-Davies GCR, Barsotti RJ. Cell Calcium. 2006;39:75–83. doi: 10.1016/j.ceca.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Ellis-Davies GCR, Kaplan JH. Proc Natl Acad Sci U S A. 1994;91:187–191. doi: 10.1073/pnas.91.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson JP, Kwon HB, Takasaki KT, Chiu CQ, Higley MJ, Sabatini BL, Ellis-Davies GCR. J Am Chem Soc. 2013;135:5954–5957. doi: 10.1021/ja4019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan JH, Ellis-Davies GCR. Proc Natl Acad Sci U S A. 1988;85:6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorley KJ, Hales JM, Kim H, Ohira S, Bredas JL, Perry JW, Anderson HL. Chemistry-a European Journal. 2013;19:10370–10377. doi: 10.1002/chem.201300609. [DOI] [PubMed] [Google Scholar]
- 35.Woo HY, Liu B, Kohler B, Korystov D, Mikhailovsky A, Bazan GC. J Am Chem Soc. 2005;127:14721–14729. doi: 10.1021/ja052906g. [DOI] [PubMed] [Google Scholar]
- 36.Martell AE, Smith RM. Critical Stability Constants. Vol. 1. New York: Plenum; 1974. pp. 139–199. [Google Scholar]
- 37.Higley MJ, Sabatini BL. Neuron. 2008;59:902–913. doi: 10.1016/j.neuron.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Niggli E. Annu Rev Physiol. 1999;61:311–335. doi: 10.1146/annurev.physiol.61.1.311. [DOI] [PubMed] [Google Scholar]
- 39.Lipp P, Niggli E. J Physiol. 1998;508(Pt 3):801–809. doi: 10.1111/j.1469-7793.1998.801bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindegger N, Niggli E. J Physiol. 2005;565:801–813. doi: 10.1113/jphysiol.2005.084376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogrodnik J, Niggli E. J Physiol (Lond) 2010;588:225–242. doi: 10.1113/jphysiol.2009.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng H, Lederer WJ. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 43.Binkley RW, Flechtner TW. In: Synthetic Organic Photochemistry. Horspool WM, editor. Plenum: New York and London; 1984. pp. 375–423. [Google Scholar]
- 44.McCray JA, Herbette L, Kihara T, Trentham DR. Proc Natl Acad Sci U S A. 1980;77:7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldman YE, Hibberd MG, McCray JA, Trentham DR. Nature. 1982;300:701–705. doi: 10.1038/300701a0. [DOI] [PubMed] [Google Scholar]
- 46.Rapp G, Poole KJV, Maeda Y, Ellis-Davies GCR, Kaplan JH, Mccray J, Goody RS. Berichte Der Bunsen-Gesellschaft-Physical Chemistry Chemical Physics. 1989;93:410–415. [Google Scholar]
- 47.Nerbonne JM, Richard S, Nargeot J, Lester HA. Nature. 1984;310:74–76. doi: 10.1038/310074a0. [DOI] [PubMed] [Google Scholar]
- 48.Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. J Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sjoback R, Nygren J, Kubista M. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy. 1995;51:L7–L21. [Google Scholar]
- 50.Cueto Diaz EJ, Picard S, Chevasson V, Daniel J, Hugues V, Mongin O, Genin E, Blanchard-Desce M. Org Lett. 2015;17:102–105. doi: 10.1021/ol5033046. [DOI] [PubMed] [Google Scholar]
- 51.Rumi M, Barlow S, Wang J, Perry JW, Marder SR. Adv Polym Sci. 2008;213:1–95. [Google Scholar]
- 52.Zheng S, Beverina L, Barlow S, Zojer E, Fu J, Padilha LA, Fink C, Kwon O, Yi Y, Shuai Z, Van Stryland EW, Hagan DJ, Bredas JL, Marder SR. Chem Commun (Camb) 2007:1372–1374. doi: 10.1039/b618265h. [DOI] [PubMed] [Google Scholar]
- 53.Pond SJK, Rumi M, Levin MD, Parker TC, Beljonne D, Day MW, Bredas JL, Marder SR, Perry JW. Journal of Physical Chemistry A. 2002;106:11470–11480. [Google Scholar]
- 54.Makarov NS, Drobizhev M, Rebane A. Optics Express. 2008;16:4029–4047. doi: 10.1364/oe.16.004029. [DOI] [PubMed] [Google Scholar]
- 55.Rumi M, Ehrlich JE, Heikal AA, Perry JW, Barlow S, Hu ZY, McCord-Maughon D, Parker TC, Rockel H, Thayumanavan S, Marder SR, Beljonne D, Bredas JL. J. Am. Chem. Soc. 2000;122:9500–9510. [Google Scholar]
- 56.Powell CE, Morrall JP, Ward SA, Cifuentes MP, Notaras EGA, Samoc M, Humphrey MG. J. Am. Chem. Soc. 2004;126:12234–12235. doi: 10.1021/ja048608l. [DOI] [PubMed] [Google Scholar]
- 57.Ullrich ND, Fanchaouy M, Gusev K, Shirokova N, Niggli E. Am J Physiol Heart Circ Physiol. 2009;297:H1992–H2003. doi: 10.1152/ajpheart.00602.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams SR, Kao JPY, Grynkiewicz G, Minta A, Tsien RY. J Am Chem Soc. 1988;110:3212–3220. [Google Scholar]
- 59.Adams SR, Lev-Ram V, Tsien RY. Chem Biol. 1997;4:867–878. doi: 10.1016/s1074-5521(97)90119-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.