ABSTRACT
Trauma is a leading cause of death in both military and civilian populations worldwide. Although medical advances have improved the overall morbidity and mortality often associated with trauma, additional research and innovative advancements in therapeutic interventions are needed to optimize patient outcomes. Cell-based therapies present a novel opportunity to improve trauma and critical care at both the acute and chronic phases that often follow injury. Although this field is still in its infancy, animal and human studies suggest that stem cells may hold great promise for the treatment of brain and spinal cord injuries, organ injuries, and extremity injuries such as those caused by orthopedic trauma, burns, and critical limb ischemia. However, barriers in the translation of cell therapies that include regulatory obstacles, challenges in manufacturing and clinical trial design, and a lack of funding are critical areas in need of development. In 2015, the Department of Defense Combat Casualty Care Research Program held a joint military–civilian meeting as part of its effort to inform the research community about this field and allow for effective planning and programmatic decisions regarding research and development. The objective of this article is to provide a “state of the science” review regarding cellular therapies in trauma and critical care, and to provide a foundation from which the potential of this emerging field can be harnessed to mitigate outcomes in critically ill trauma patients.
Keywords: Acute kidney injury, acute renal failure, burns, cellular therapies, extremity injury, hemorrhagic shock, neurotrauma, organ injury, orthopedic trauma, spinal cord injury, stem cells, trauma and critical care medicine, traumatic brain injury, wound healing
INTRODUCTION
As the United States emerges from the longest period of combat operations in its history, the military's continuously learning system in trauma—trauma system, trauma research, and trauma training—must take stock of lessons learned (1). This recent period of combat witnessed progress including an understanding of the epidemiology of mortality and morbidity on the battlefield (1). From this informed platform, the requirements-driven research aspect of the learning system delivered knowledge and material solutions to mitigate the effects of hemorrhage and shock—topics that in the year 2000 were poorly defined and generally considered unsolvable. The impact of the military's learning system in trauma in Afghanistan was a reduction in the case fatality rate following wartime injury (2). Although this advance is laudable, it was achieved in a relatively stable theater of war with short medical evacuations and timely access to advanced levels of care. Furthermore, the reduction in case fatality resulted in the survival of more severely injured persons, and thus a challenging “burden of survivorship.”
In 2015, the Department of Defense (DoD) Combat Casualty Care Research Program (CCCRP) is endeavoring to spur innovation in trauma care for both future and potentially more complex operational scenarios, including those in which initial resuscitation and operative intervention are delayed (2, 3). In the area of resuscitation, the CCCRP supports research in the broad categories of procedural adjuncts (i.e., mechanical devices) and medical therapies (i.e., blood and pharmacologics). As the program explores new ways to tackle problems that today seem unsolvable, it is in this latter category of “medical therapies” where the emerging topic of cellular therapies rests. As the following pages outline, cell-based therapy holds promise in the immediate resuscitation phase following severe injury as well as the subacute or later phases of management. This review follows a joint military–civilian meeting convened by the CCCRP as part of its effort to inform the research community and allow more effective planning and programming of research. The participants/presenters of this meeting are acknowledged at the end of the article. As a product of that meeting, the objective of this review is to provide a “state of the science” summary of cellular therapies for trauma and a foundation from which the potential of this emerging field can be harnessed. It is the hope of the authors that this effort will culminate in knowledge and material solutions that will improve survival and lessen the burden of survivorship following trauma, including vexing injury patterns that today seem unsolvable.
THE NEED FOR NOVEL THERAPIES IN TRAUMA AND CRITICAL CARE MEDICINE
In recent years, improved methods to stop bleeding, optimally resuscitate, and correct the coagulopathy of trauma have increased the overall survival rate following severe traumatic injury associated with hemorrhage (4, 5). The US federal government supports research to improve the general health of the nation as well as promote positive outcomes after disease and injury. As a result, overall life expectancy has increased by 11%, from 70.8 to 78.8 years (1970–2012), whereas cancer- and heart disease-related mortality have decreased by 20% (1991–2009) and 31% (2000–2010), respectively. However, during similar periods, injury-related mortality has risen. In the past decade, there has been a 23% increase in trauma-related mortality within the United States (2000–2010) and a 24% increase worldwide (1990–2010). Unfortunately, funding for injury-related research and overall interest in the development of novel therapeutics in this field lags far behind other public health concerns such as cancer, human immunodeficiency virus (HIV) infection, and heart disease (5).
Trauma is the leading cause of death for individuals between the ages of 1 and 44 and the third leading cause of death in the United States overall (6), accounting for approximately 180,000 fatalities each year. Between 2000 and 2010, traumatic injury increased from the leading cause of death among individuals younger than 43 to the leading cause of death for those under age 46. Likewise, traumatic injury increased from the leading cause of life-years lost up to age 65 to age 75. The cause of trauma-related mortality has remained constant over the years, with head injuries accounting for 42% to 52%, hemorrhage causing 30% to 39%, and multiple organ failure (MOF) resulting in 7% to 11% of fatalities.
Deaths from hemorrhage occur rapidly, often within 2 h of admission, whereas successful hemostasis usually occurs within 105 min of hospital admission (4). For patients suffering from severe injury and hemorrhage, mortality rates of greater than 25% are common. Damage control resuscitation and hemorrhage control devices have decreased hemorrhage-related mortality in civilian trauma practice with little effect on improving outcomes following traumatic brain injury (TBI). Furthermore, most of the deaths occurring after 8 h and before 3 days of admission result from head injury, with the median time of TBI-related death occurring at 29 h (7). After 3 days of admission, the remaining 25% of deaths occur at a low but steady rate (see Fig. 1), and result from a complex interplay of infection, inflammation, microvascular compromise, and dysfunctional coagulation, which is known as the endotheliopathy of trauma and is associated with tissue injury, shock, and resuscitation (8). Specifically, these fatalities occur as a result of a combination of sepsis, MOF, acute respiratory distress syndrome (ARDS), acute renal failure (ARF), venous thromboembolic disease, as well as cerebral edema and neuronal death after TBI. In summary, these are all inflammatory conditions causing substantial death after 48 to 72 h in the hospital, and are therefore potential targets for new therapies.
Fig. 1.
Time to death from admission from a retrospective review of 1,029 deaths over 4 years at a single large urban trauma center—the University of Texas Houston, Texas.
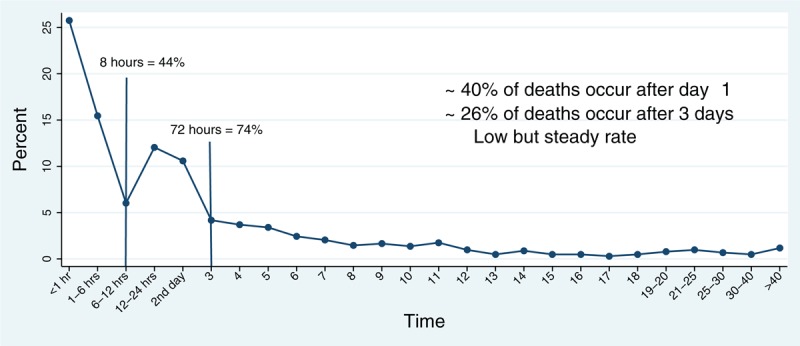
The majority of deaths occur very early, 40% after day 1, but 26% occur after day 3 (4).
To date, the greatest advances in the treatment and care of traumatically injured and bleeding patients have been the development of improved methodologies for hemorrhage control and the effective paradigms for resuscitation and blood product usage (9). Much of the progress and innovation in trauma care and resuscitation of the bleeding patient has been driven by the wars in Iraq and Afghanistan; however, in terms of novel therapeutics, few products, drugs, or biologics have been shown to significantly improve outcomes, with the exception of the antifibrinolytic tranexamic acid (10). This is clearly evident in the case of investigational therapies for TBI, where the majority of clinical trials have failed to demonstrate significant benefits in the designated primary and secondary outcome measures (11). In this instance, the cause of these failures is multifactorial and includes heterogeneity of the disease, complex outcome measures, and a lack of scientific understanding of the disease and its potential therapeutic targets.
Cellular therapies have the unique ability to modulate multiple therapeutic targets such as inflammation, vascular dysfunction, cell death, and tissue loss. These pleiotropic effects can potentially address the complex and heterogeneous patterns of trauma-induced injury. For example, in TBI, cellular therapies administered acutely after injury have been shown to modulate blood brain barrier (BBB) permeability, the production of inflammatory cytokines and chemokines, as well as the activation of critical inflammatory cells such as microglia, neutrophils, and macrophages (12, 13). Aside from the primary physical insult, injury in TBI is also due to a secondary wave of injury characterized by chronic inflammation, progressive neuronal cell loss, and vascular compromise, all of which are endpoints addressable by cell therapies. These multiple levels of dysfunction suggest that TBI patients may benefit tremendously from a multimodal therapeutic approach. Similarly, the treatment of MOF may also benefit from such a multifaceted approach as it too is characterized by a complex heterogeneous pattern of ischemia–reperfusion injury, unregulated inflammatory responses, dysfunctional coagulation, and organ damage. There are multiple cell types with unique and defined properties that have been investigated in traumatic injury and critical care applications. The optimal cell type, safety, efficacy, cell source, delivery route, dose, and timing of administration are still active areas of preclinical investigation and development.
SOURCES AND TYPES OF CELLS, ROUTES OF ADMINISTRATION, DOSE, AND TIMING
Cellular therapies have the potential to address multiple therapeutic targets in injury that are not currently achievable through the optimized resuscitation paradigms and supportive care in standard use. This has garnished increasing excitement over cellular therapies and their therapeutic potential over the past 15 years. There have been a number of goals for stem cell therapies that include: (1) mediating cell replacement such as the replacement of dead cells and tissue, (2) mediating cell protection that prevents further damage, (3) modifying the microenvironment (i.e., inflammatory mediators that exacerbate injury), and (4) stimulation of self-regenerative and reparative processes.
A stem cell, by definition, can be of two kinds—multipotent or pluripotent. A pluripotent stem cell, such as embryonic stem cells (ESCs) (Fig. 2), can continuously proliferate and asymmetrically divide to self-renew and generate daughter cells committed to differentiation. In contrast, a multipotent stem cell demonstrates a limited proliferative capacity and differentiation potential. Cellular therapies can be derived from multiple tissue sources (Fig. 2). In terms of differentiation potential, there are essentially two cell types that have the capacity to differentiate and form all tissues of the body. These pluripotent cells include ESCs derived from fetal tissue and induced pluripotent stem cells (iPSCs), which can be derived from various adult tissue sources, including the skin, blood, or liver (14).
Fig. 2.
Sources of stem cells for infusion or transplantation.
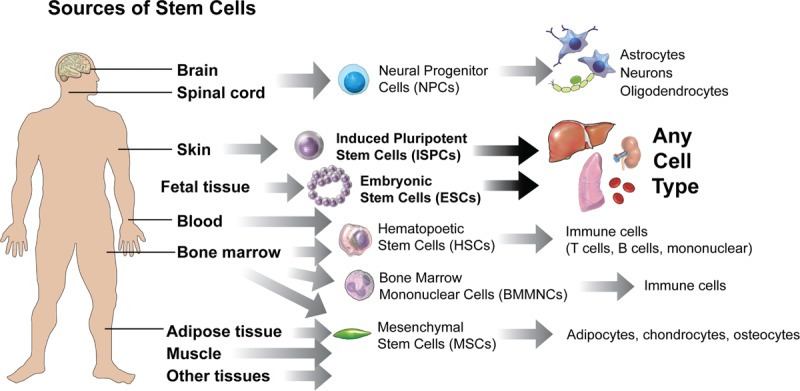
This illustration shows various tissue sources of stem cells, including neural stem cells (NSCs), induced pluripotent stem sells (iPSCs), mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), and direct conversion methods to yield cells for transplantation. NSCs can be isolated from the fetal and adult brain, and spinal cord can be differentiated into progenitor cells, such as oligodendrocyte precursor cells (OPCs) and mature oligodendrocytes, or astrocytes or neurons depending on culture conditions and exposure to growth factors. The same is true for MSCs which can be isolated from a variety of tissues and under the correct culture conditions differentiated to adipocytes, chrondrocytes, and osteocytes. ESCs and iPSCs are pluripotent and can form any cell type in the body if cultured correctly. Bone marrow mononuclear cells (BMMNCs) and hematopoietic stem cells (HSCs) can form cells of blood and immune cell lineages.
There are a number of sources for stem and progenitor cells in the human body (see Fig. 2). In addition, many of these cells have been investigated at the preclinical and clinical levels for their potential to treat a number of trauma- and critical care-related applications (see Table 1). Bone marrow mononuclear cells (BMMNCs) and hematopoietic stem cells (HSCs) are typically derived from bone marrow (BM) or blood, respectively, and these cell types have been used clinically for decades to treat hematologic malignancies and BM failure syndromes (15). Other cell types include neural stem cells (NSCs), endothelial progenitor cells (EPCs), mesenchymal stem cells (MSCs), multipotent adult progenitor cells (MAPCs), iPSCs, and ESC-derived neural precursors, to name a few (16, 17). Other forms of cellular therapies that have been investigated involve fully mature, differentiated adult cells known as somatic cell therapies. However, the majority of cells under investigation clinically are MSCs and MAPCs, which are typically isolated from allogeneic tissue sources.
Table 1.
Stem cell types
| Stem cell type | Source | Progeny | Differentiation potential | Preclinical investigation | Clinical investigation |
| Neural stem cells (40, 41) | Central nervous system | Neurons, astrocytes, and oligodendrocytes | Tripotent | Neurotrauma | Neurotrauma |
| Hematopoietic stem cells (15, 45) | Peripheral blood and the bone marrow | Myeloid and lymphoid blood lineages | Multipotent | Neurotrauma | Neurotrauma |
| Renal and lung | Renal and lung | ||||
| Burns | Burns | ||||
| Embryonic stem cells (52–56) | Inner cell mass of a blastocyst | Any cell type | Pluripotent | Organ injury | Organ injury |
| Mesenchymal stem cells (16, 63) | Bone marrow, cord blood, peripheral blood, fallopian tube, and fetal liver and lung | Osteoblasts, chondrocytes, myocytes, and adipocytes | Multipotent | Neurotrauma | Neurotrauma |
| Induced pluripotent stem cells (69) | Adult cells | Any cell type | Pluripotent | Organ injury | Organ injury |
| Endothelial progenitor cells | Peripheral blood, bone marrow, umbilical cord blood, fetal liver | Endothelial cells | Unipotent | Organ injury | Neurotrauma and organ injury |
MAPCs and MSCs were originally derived from BM and identified as the cell population that has the capacity to adhere and expand on plastic tissue culture dishes (16–19). These cells are currently defined by their expression of distinct cell surface antigens, which do not include HSC markers. MSCs express CD29, CD44, CD73, CD90, and CD105, and lack the expression of CD14, CD34, CD45, and HLA-DR. The major advantage of MSCs/MAPCs is that they can be autologously transplanted and are able to differentiate into cells of adipocyte, chondrocyte, and osteocyte lineage. In addition, under defined culture conditions, MSCs and MAPCs have been shown to differentiate into other cell types such as endothelial cells, neurons, and cardiomyocytes. Importantly, these cells have low immunogenicity, and their reported immunomodulatory properties are of critical value in the therapeutic setting.
MSCs are widespread throughout a variety of tissues, including in Wharton jelly of the umbilical cord, adipose tissue, adult muscle, and the dental pulp of deciduous baby teeth. MSCs and MAPCs require expansion after collection and can be stored in therapeutic aliquots for rapid deployment, which makes them amenable to timely administration after traumatic injury. In terms of optimal doses, timing, and routes of delivery, each cell type has predominantly been investigated in preclinical models. In regards to the route of delivery, direct implantation, intravenous (IV), intra-arterial, and intratracheal delivery have been investigated. Further investigation is warranted in this area to optimally determine the timing, dose, and routes of delivery that maximize the therapeutic effects of these cells while maintaining their safety profile. Logistically, they can be stored as a shelved therapeutic for rapid processing and use. Data suggest that they do not require initial HLA cross matching and can be expanded in large numbers in an automated fashion. Although MSCs have been proven to be safe in multiple clinical trials, further studies are necessary to identify the long-term consequences of treatment. For a bleeding trauma patient, achieving hemostasis is paramount. MSCs have been shown to express tissue factor (TF); however, their role in coagulation and the safety of TF expression by MSCs in hemorrhage and hemostasis has yet to ascertained. After hemostasis is achieved in trauma patients, up to 80% will develop an inflammatory complication that results in organ injury and MOF such as ARF and ARDS.
In terms of timing, there are multiple time points during which cellular therapies could be effective in mitigating outcomes. Early delivery of cells may attenuate the inflammatory cascade that begins immediately after injury and results in progressive tissue damage and organ injury; however, further investigation is required at the preclinical level to determine the optimal time of delivery that promotes and supports repair and recovery. Use of cells at early acute time points will likely require allogeneic sources of cells, whereas autologous cells can be administered at later time points to address chronic disease. In cases where treatments are administered days or weeks after injury, fresh autologous, amplified autologous, or allogeneic cells can be used. Although further investigation at both the preclinical and clinical levels is needed, cellular therapies hold great promise in addressing the morbidity and mortality in trauma associated inflammatory conditions and could lead to therapeutic interventions that significantly improve outcomes in critically injured patients.
CELLULAR THERAPIES IN NEUROTRAUMA
Neurotrauma remains a significant public health concern in both civilian and military populations worldwide (20). TBI is the leading cause of death in all individuals ages 1 to 44 (21). In the United States, TBI affects approximately 1.7 million people each year and is a leading cause of injury-related death and disability (22). There are currently an estimated 5.3 million people living with TBI and 270,000 people living with spinal cord injury (SCI)-related disabilities in the United States alone. Aside from the mortality associated with TBI and SCI, survivors often suffer long-term consequences that include physical, emotional, and cognitive impairments resulting in a profound loss in quality of life and productivity. The primary causes of neurotrauma in the civilian population are motor vehicle accidents, sport-related injuries, falls, and occupational trauma. In the military, neurotrauma can result from similar causes, but combat wounds are predominately penetrating and include the combat-related injuries inflicted by improvised explosive device explosions, and gunshot wounds to the head (23, 24). Penetrating TBI and SCI has received less attention and is often excluded from randomized trials.
Mechanistically, TBI and SCI are similarly characterized by a first wave of damage caused by the primary injury itself, and a secondary wave or cascade that is modulated by ongoing inflammation, neuronal cell death, fluid and electrolyte imbalances, free radical damage, and cerebral edema that leads to further persistent damage (25). Many groups have shown that chronic inflammation and microglial activation continue for extended periods of time in the brain post-TBI (26). The complex mechanisms and unclear therapeutic targets involved in both TBI and SCI have complicated advances in new and effective treatment options, which currently remain limited and generally ineffective.
Although cellular therapies have shown promise and potential in treating TBI and SCI, clinically translatable therapeutics are still early in development. Cell types investigated at the preclinical stages include ESCs, MSCs, MAPCs, neural stem/progenitor cells, Schwann cells, and iPSCs. Cellular therapeutics in neurotrauma have focused on addressing a number of potentially modifiable therapeutic targets, which include neuroprotective approaches aiming to replace lost neurons, reform lost connections, decrease blood–central nervous system barrier permeability and edema, and decrease overall inflammation. Preclinical studies in animal models have provided some insight on the potential advantages and disadvantages of various cellular therapies and their mechanisms of action; however, a major pitfall in the field has been that no single model can fully recapitulate the complex heterogeneity of the disease. This has made translation of preclinical findings (i.e., dose, timing, cell types, and optimal target population) challenging and an area worthy of research and development.
Traumatic brain injury
There are few, if any, effective therapies for the treatment of severe TBI. Treatment options for injured patients are limited to controlling intracranial pressure and optimizing cerebral perfusion (27–29). The main treatment modalities are (1) evacuating extra-axial clots, (2) drainage of cerebrospinal fluid (CSF), (3) decompressive craniectomy, and (4) hyperosmolar infusions. None of these approaches mitigate the primary drivers of the secondary neuroinflammatory response or interrupt the inflammatory cascade. Adult BM stem and progenitor cells have been the main cell types investigated preclinically for the treatment of TBI (30). These cell types include BMMNCs, MSCs, and MAPCSs. All three of these cells have demonstrated significant therapeutic effects on regulating BBB permeability, neuroinflammation, neuroprotection, and neurocognitive outcomes in preclinical rodent models (12, 31–33). In early studies, whole BM infusions administered post-TBI were shown to result in migration of infused cells to the site of injury and were associated with a limited improvement in motor function (34). Cox and colleagues have shown that autologous adult BMMNCs and also multipotent adult MAPCs have potent therapeutic effects on BBB permeability and microglial activation, and can also result in long-term improvements in spatial learning following TBI (13, 32, 35).
Mechanistically, a number of groups have demonstrated the migration of IV-administered cells to injury sites post-TBI and the subsequent survival of some of these cells (i.e., MSCs) with small numbers acquiring markers of neurons and astrocytes (33, 34). However, more recent work strongly suggests that these differentiated cells are not integrated into functional neuronal circuitry, and the beneficial effects of the cells, similar to the observed effects in ARDS studies, are largely mediated by soluble factors secreted by the cells after IV infusion. Biodistribution tracking studies have demonstrated that IV delivery of MSCs and MAPCs results in more than 90% of the cells being lodged in the lungs of injured animals due to a first-pass effect through the pulmonary vasculature (36). Pati and colleagues have shown that IV MSCs, administered 2 and 24 h after TBI in a rodent model of controlled contusion injury, result in significantly decreased BBB permeability, which is associated with decreased inflammatory infiltrates and increased vascular integrity within the injured brain (12, 31). These effects were subsequently found to be in part due to production of the soluble factor Tissue Inhibitor of Matrix Metalloproetinases-3 by the MSCs (31). Prockop and colleagues have also investigated the potential of MSCs and another MSC-derived factor, tumor necrosis factor-stimulated gene 6 (TSG-6), in TBI (37). TSG-6 has been shown to recapitulate many of the protective effects of the MSCs themselves, hence reaffirming the notion that soluble factors or an MSC “secretome” can recapitulate many of the effects of the cells and play a critical role in the mechanism of action of MSCs (38).
NSCs are another cell type that has been investigated preclinically for the treatment of TBI (see Fig. 2). NSCs can be isolated from many parts of the fetal or adult brain, but the most common niche is found within the subventricular zone of the lateral ventricle and the dentate gyrus of the hippocampus (39). NSCs are multipotent and possess the ability to differentiate into cells of neural origin, including neurons, oligodendrocytes, and astroglia (40). Although difficult to isolate and culture, these cells hold significant potential as the progenitor cells of the neural lineage. Administration of these cells has typically been via direct implantation into the injured brain, which is challenging and has implications on safety due to the invasive nature of this delivery route. Classically, transplantation of NSCs into the cortex of injured rats has demonstrated that these cells can differentiate into neurons and produce soluble factors that may also improve cognitive function after TBI (41). Cox and colleagues have demonstrated that direct transplantation of NSCs after cortical impact injury results in 1% to 3% of cells being engrafted within 2 weeks of treatment. NSC engraftment was associated with an improvement in motor function; however, no recovery of cognitive function was identified in these studies (42). A study by Lee et al. investigated the potential anti-inflammatory effect of systemic NSC infusion using a rat model of intracerebral hemorrhage (43). NSCs were IV and administrated at 2 and 24 h after injury. This treatment was compared with NSCs that were directly transplanted at similar time points. Systemically infused NSCs during the acute phase resulted in reduced brain swelling and edema, decreased cerebral inflammation and apoptosis, and improved cognition. Further work is being conducted in TBI using human spinal cord-derived NSCs. Bullock and colleagues at the University of Miami are currently investigating the potential of direct intracerebral implantation of human stem cells of spinal cord origin (NSI-566 from Neuralstem Inc) in penetrating TBI to assess axonal growth rates and neuroprotection (44). The results from these studies are currently pending.
Based on the encouraging rodent data with BMMNCs in TBI, a phase 1 clinical trial investigating the IV administration of autologous BMMNCs for the treatment of severe TBI in children (NCT00254722) was performed to evaluate treatment safety (45) (see Table, Supplemental Digital Content 1). The advantages of HSCs or BMMNCs are that they can be autologously derived and have a record of safety in humans. The drawbacks are that HSCs are rare (1 in 100,000 BM cells) and pose major risks with graft rejection and graft-versus-host disease when no autologous cells are used. In the pediatric TBI study, 10 children (ages 5–14 years) with a Glasgow coma score between 5 and 8 upon hospital admission were treated with 6 × 106 cells/kg body weight BMMNCs (approximately 1 × 106 CD34+, Lin−, CD133+ cells infused in each patient, and approximately 1 × 104 of CD34−, Lin−, CD133+ cells) within 48 h of injury. No patients died during this study, and no adverse events were reported (45). Specifically, there was no effect of treatment on pediatric logistic organ dysfunction scores, which is a measure of multiple organ dysfunction, or on pulmonary, hepatic, renal, or neurological function. Patients significantly improved with regard to neuropsychological and functional scores from the 1- to 6-month assessments. In addition, no volumetric brain differences were observed via magnetic resonance imaging (MRI) between 1 and 6 months, when there should typically be an 8% to 12% reduction in brain volume. Overall, this study indicated that the harvest of and treatment with BMMNCs is feasible, appears to be safe for the early treatment of severe TBI in children, and may improve functional and structural outcomes (45). A recently published retrospective cohort study by Liao et al. compared this same study population with age- and severity-matched controls, finding that IV administration of BMMNCs reduced the intensity of treatment required following TBI (46). Currently, this study is also being followed up on by a phase 2 trial (NCT01851083) examining the effect of IV treatment with autologous BMMNCs in children within 36 h of severe TBI (i.e., GCS 3–8) in which the primary outcome measures are white and gray matter volumetric preservation in treated versus untreated controls. Secondary outcomes of this study will assess volumetric preservation within specific regions of the brain as well as neurocognitive and functional outcomes. In addition, a similar phase 2a study (NCT01575470) has recently been conducted in adults (ages 18–55 years) with severe TBI (GCS 5–8). This study, which ended in March 2015, sought to investigate the safety of dose-escalated (i.e., 6, 9, and 12 × 106 cells/kg bodyweight) BMMNC treatment within 36 h after injury. Compared with a cohort of untreated controls, this study will also evaluate the effect of treatment on functional outcomes, BBB permeability, and volumetric preservation in the brain after severe TBI. To date, there have been more than 33 trials investigating novel therapeutics in TBI, the majority of which have failed to show any effect on primary or secondary outcome measures. The reasons for this lack of success are complex and compounded by the fact that the diseases are heterogeneous with multiple therapeutic targets and because clinical trial design is challenged by identification of the correct modifiable outcome measures.
Spinal cord injury
There are currently few effective therapeutic modalities in clinical use that mitigate SCI. Clinical management of SCI includes preserving blood flow to the cord, timely decompression, prevention of hyperthermia and infection, and the use of pharmacological interventions such as Riluzole. Stem cell therapies offer several promising strategies for the treatment of SCI. Therapeutic targets in SCI include replacement of damaged or lost neurons, mitigation of oxidative damage, remyelination, neuronal connectivity, cavity lesion bridging, and secretion of factors that promote tissue repair and inhibit glial scar formation (47). One of the greatest challenges in spinal cord repair is the physical loss of tissue that results in a large fluid-filled cavity at the site of injury. This cavity enlarges over time, and contains demyelinated axons, inflammatory cells, and extracellular matrix deposition, hence resulting in a glial scar that causes a physical barrier to neural connectivity, regeneration, and repair (48). A number of cell types have been investigated in SCI at the preclinical and clinical levels. These include ESCs, MSCs, NSCs, oligodendrocyte progenitor cells (OPCs), Schwann cells, olfactory ensheathing glia, neurotropin expressing fibroblasts, and activated macrophages (49–51).
Human ESCs are typically obtained from blastocyst stage embryos created during in vitro fertilization or generated by somatic cell nuclear transfer (47). ESCs can be differentiated into a variety of neural precursors (52, 53). In preclinical studies, ESCs differentiated into neural precursors have shown some potential to improve motor function when transplanted into rodent models of SCI (54). SCI can cause significant demyelination, and oligodendrocytes are vulnerable to cell death and apoptosis. ESCs differentiated into OPCs have been transplanted subacutely into injured rat spinal cords and have demonstrated therapeutic potential (55). However, ESCs do have drawbacks. Aside from ethical concerns, ESCs have the potential for karyotypic instability, which raises concerns regarding tumor formation in transplanted patients.
NSCs are another cell type that has been shown to differentiate into oligodendrocytes in vitro(56) and in vivo(47). Transplantation of NSCs is associated with regeneration of nerves and functional recovery in rats with SCI (57). However, most preclinical studies using transplanted NSCs in SCI have shown only modest recovery of the injured spinal cord (49, 58). Clinically, human NSCs have translational pitfalls associated with ethical concerns stemming from their fetal origin, difficulties in expanding large numbers of cells for clinical use, and lack of access and availability to autologous cell sources.
HSCs and MSCs are two cell types that are easier to investigate clinically. Both cell types have been shown to have therapeutic potential in preclinical models of SCI. Transplantation of HSCs within the spine has been demonstrated to improve functional recovery after compression SCI in mice (59). Sources of HSCs include peripheral blood from individuals whose BM cells are mobilized by cytokine (i.e., GM-CSF and glial-cell stimulating factor) administration, and the BMMNC fraction is typically harvested, processed, and administered as an autologous transplant.
Transplantation of MSCs for SCI has been investigated by many groups with some demonstrating significant improvement in motor function (60) and others not (61, 62). These differences can be attributed to a variety of reasons that include variations in experimental set up, differences in the animal model, and cell product variability. Several studies have also shown that MSCs can differentiate into neural lineages in vitro; however, similar to their use in ARDS and acute kidney injury (AKI), the majority of the beneficial effects are due secretion of soluble factors (63). HSCs and MSCs have also shown to have variable efficacy when transplanted intravenously or intrathecally, indicating that route of delivery can significantly impact the biological effects of the cells (64, 65). There are a number of completed and currently ongoing SCI clinical trials involving autologous BMMNCs or MSCs transplantation. In addition, there are reports of small numbers of patients treated with MSC direct transplants showing no adverse effects and improved outcomes that maybe dependent on soluble factor secretion (66–68).
Considering the significant debate that exists with regard to ethical issues surrounding the use of ESCs, iPSCs derived from adult tissues have been considered a viable alternative for use in cellular therapies. However, when compared with ESCs, iPSCs carry a greater likelihood of teratoma formation, therefore necessitating a careful evaluation of safety both in the short term and long term. A 2010 study by Tsuji et al. evaluated the transplantation of iPSC-derived neurospheres for the treatment of SCI in mice and found that cells transplanted 9 days after injury had a survival rate of 18%. In addition, these cells were also able to remyelinate damaged cells (69). Furthermore, some of the transplanted cells also differentiated into neurons, astrocytes, and oligodendrocytes. Transplantation of these iPSCs into the spinal cord of injured mice also improved functional recovery; however, transplantation of some iPSC-derived neurospheres in injured mice resulted in tumor formation and loss of functional recovery. Similar results were observed when human-induced pluripotent stem cells were transplanted into adult NOD-SCID mice after SCI (70), hence highlighting the need for caution and a better understanding of these cells in clinical use for the treatment of SCI.
In 2009, the US Food and Drug Administration (FDA) approved the first human ESC trial following promising preclinical work, indicating that human ESC-derived OPCs may improve outcomes in rats with SCI (55). This phase 1 safety trial, sponsored by Geron Corp., began in 2010 after preclinical safety data were obtained pertaining to abnormal cyst formation in transplanted animals. The GRNOPC1 cell line (human ESC-derived OPC) was transplanted subacutely (i.e., 1–2 weeks after injury) directly into the spinal cord of American Spinal Injury Association (ASIA)-A patients with complete thoracic SCI. Patients received 2 million cells and were immunosuppressed for the first 2 months following transplantation. In 2011, Geron discontinued this trial due to funding challenges. No safety issues were reported in the five patients who received GRNOPC1 transplants; however, complete results have not yet been published (see Table, Supplemental Digital Content 2). Asterias Biotherapeutics is slated to continue this trial in the upcoming year (71).
In a current clinical trial led by the Miami Project to Cure Paralysis at the University of Miami, Guest and colleagues have begun trials with Schwann cell transplantation into patients with ASIA-A, B, and C grade SCIs (NCT02354625 and NCT01739023) (see Table, Supplemental Digital Content 1). The phase 1 trial is open-label, nonblinded, nonrandomized, and nonplacebo controlled with a dose escalation design aimed at investigating treatment safety. The trial also includes robust rehabilitation and training phases both before and after transplantation. In addition, the Schwann cells are derived autologously from a segment of the patient's nerves, which are then cultured ex vivo and transplanted 30 to 42 days postinjury. The hypothesis of this trial is that the Schwann cells will mechanistically support regeneration in peripheral nerves. Currently, four patients have been enrolled with complete thoracic SCI and four more are planned to complete the trial. No complications or safety concerns have been reported to date. An additional trial that is ongoing is an allogeneic phase 2 NSC trial for chronic cervical SCI in progress with StemCells, Inc (NCT02163876).
Preclinical studies have indicated that human NSCs can differentiate and integrate into the spinal cord of both healthy and injured rats (72). Neuralstem Inc, a Maryland-based biotech company focused on regenerative medicine, is currently sponsoring a phase 1, single-site, open-label study (NCT01772810) to evaluate the safety of NSI-566 human spinal cord-derived neural stem cell transplantation for the treatment of chronic T2 to T12 SCI (American Spinal Injury Association Impairment Scale-A complete) at the University of California (UC), San Diego School of Medicine (73). With a targeted enrollment of four patients (ages 18–65 years), patients will receive six injections of NSI-566 either in or around the site of injury, followed by physical therapy and 3 months of immunosuppression to limit graft rejection. Although this trial primarily seeks to evaluate the safety of NSI-566 treatment, secondary outcomes include graft survival at the site of transplantation as evaluated by MRI or autopsy and an evaluation of donor-specific HLA antibodies following immunosuppression. Furthermore, exploratory objectives, including sensory, motor, bowel, bladder function, and electromyogram, will be compared pre- and post-transplantation. Similar studies evaluating NSI-566 in acute SCI are currently awaiting investigational new drug (IND) approval in the pursuit of a phase 1/2 trial in Seoul, South Korea. Notably, preclinical studies are also evaluating the effectiveness of NSI-566 in the treatment of TBI. A similar 15-subject phase 1 trial (NCT01348451) investigating the safety of NSI-566 treatment in patients with amyotrophic lateral sclerosis (ALS) found no adverse effects associated with this treatment (74). In 2013, phase 2 of this trial (NCT01730716) commenced seeking to evaluate the feasibility and safety of dose-escalated treatment with NSI-566 cells, as well as the effect of treatment on the progression of neurological function and graft survival.
Although the use of cellular therapies for the treatment of TBI and SCI holds great promise, this field faces significant challenges in translating these therapies from bench to bedside. Identifying large animal models for the study of cellular therapies in neurotrauma is imperative for the sufficient preclinical evaluation of treatment safety and efficacy. Furthermore, as progress is made toward human clinical trials, the basic optimization of trial logistics (i.e., cell types administered, dosage, timing of treatment, etc.) should remain a major area of focus so as to maximize positive patient outcomes and minimize potential side effects. Improving treatment options and outcomes for these patients will result in significant public health, societal, and economic impacts.
CELLULAR THERAPIES IN HEMORRHAGIC SHOCK AND ORGAN INJURY
Acute lung injury and acute respiratory distress syndrome
In recent years, improved methods to stop bleeding, optimally resuscitate, and correct coagulopathy have increased the overall survival rate of severe traumatic injury due to hemorrhage (1). Aside from mortality, one of the main consequences of severe traumatic injury is MOF. For instance, lung injury induced by hemorrhagic shock (HS) and trauma can result in ARDS and AKI, which eventually can lead to ARF (73). Incidence of MOF in trauma can be as high as 17% to 20% (75, 76). Furthermore, ARDS occurs in 200,000 ventilated patients in the United States annually with a mortality rate as high as 20% to 35% (77). Recent data from the Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial reveal that the rate of ARDS was 14% in trauma patients and MOF was 5% (4). However, the overall complication rate, due mostly to inflammatory conditions (excluding death), was 65%. This is the group of patients that could potentially be addressed by multimodal cellular therapies. Supportive treatment with lung protective ventilation and fluid conservative therapy has been the mainstay of treatment for ARDS (78). There are currently few, if any, effective therapeutic measures aside from supportive therapy that can prevent or mitigate these deleterious consequences of traumatic injury.
BM-derived MSCs have been the primary therapeutic stem cell intervention investigated at the preclinical and clinical levels for ARDS. Other stem cell types, including BMMNCs, EPCs, and ESCs, have been demonstrated to reduce mortality and modulate the inflammatory and remodeling processes in relevant preclinical ARDS models (79). MSCs were originally discovered by Freidenstein and colleagues in 1968, and have been identified in various tissues, including adipose tissue, cord blood, and placental tissue (80). MSCs have been investigated in over 300 trials to date for various applications with no adverse events or Data Safety Monitoring Board (DSMB) halts reported to date; however, many of these studies are early phase trials, and side effects may not become apparent until later stages. Mechanistically, it has been shown that their biological effects are mediated in part or completely by the release of soluble factors they systemically secrete after IV administration (63, 81). Some of these factors have been identified, and the collection of factors has been alluded to as the MSC secretome (63). A number of groups have demonstrated in preclinical models of TBI and HS that MSCs are potent regulators of vascular stability and result in decreased organ injury and inflammation (82). Recent work suggests that stabilization and restoration of the integrity of the injured vasculature are critical to survival and recovery after traumatic hemorrhage (83, 84).
In the 1990s, a new interest in BM-derived MSCs was generated from work conducted by Prockop and colleagues as well as Pittenger and colleagues, which indicated that MSCs possess the uncanny ability to differentiate into various cell types and can home to injured and inflamed organs. In addition, MSCs have potent and promising therapeutic effects in several preclinical models of acute lung injury (ALI) and ARDS in mice, rats, sheep, and swine when delivered intravenously or intratracheally (85). Work by Matthay and colleagues has demonstrated resolution of inflammation and lung injury in mice, rats, and sheep (85). Mechanistically, the potent beneficial effects of MSCs in ALI have been attributed to the secretion of soluble factors such as keratinocyte growth factor and angiopoeitin-1 in the lungs, where 90% of the cells are lodged due to a first-pass effect through the pulmonary vasculature (36). Marked antibacterial effects of MSCs have also been noted in vitro and in vivo(86), which complement their ability to modulate cytokine production and macrophage/monocyte phenotypes. Innovative studies using an ex vivo perfused human lung model have demonstrated resolution of endotoxin- or live bacteria-mediated lung injury upon MSC administration, providing preclinical evidence supporting their functionality in human tissue (87).
Further support for the clinical use of MSCs in trauma care has been demonstrated by preclinical models of HS-induced ALI. Lung contusion (LC) and HS are two of the main inducers of lung injury in trauma patients. In preclinical studies, Pati et al. demonstrated that IV-administered MSCs potently inhibit systemic levels of serum inflammatory cytokines and chemokines using a rat “fixed volume” model of mild-to-moderate HS (82). In the lungs, IV-administered MSCs also inhibit pulmonary endothelial permeability and lung edema with concurrent preservation of the vascular endothelial barrier proteins VE-cadherin, Claudin-1, and Occludin-1, all of which are tight junction markers that regulate vascular leak (82). Leukocyte infiltrates (i.e., CD68 and MPO positive cells) are also decreased in the lungs upon MSC treatment in rodent models of HS-induced lung injury (82). These data suggest that MSCs are potent stabilizers of both the vascular endothelium and inflammation after trauma-induced lung injury. Notably, MSCs are highly modifiable with genes and proteins of interest, and can be used as vehicles to deliver a targeted payload to injured tissues. Further work in this area by Chaudry and colleagues has investigated whether the effects of MSCs on organ injury following trauma can be potentiated by overexpressing or genetically modifying MSCs to express the estrogen receptor, which has been shown to be protective in HS (88). Preconditioning of MSCs to enhance their potency has also been investigated (89).
In light of the numerous positive preclinical studies investigating MSCs in the treatment of ARDS, a National Heart, Lung, and Blood Institute-funded, multicenter trial was initiated by Matthay and colleagues (90) (see Table, Supplemental Digital Content 2). This phase 1/2 trial (NCT01775774) investigated the safety of administrating 1, 5, or 10 × 106 cells/kg MSCs to nine patients with moderate-to-severe ARDS. Although this trial had only a small number of patients, no MSC-related safety issues were identified, and after a DSMB recommendation to continue this trial, a phase 2 randomized, double-blind, multicenter trial in 60 patients with ARDS is currently underway at four university medical centers to test the safety and efficacy of MSC administration (i.e., UCSF, Stanford, Pittsburgh, and Massachusetts General Hospital) (90, 91). Thirty patients have been enrolled in this trial to date. In addition, this trial did not include trauma patients with ARDS, which will become a focus area for future trials. Additional trials by a Canadian group led by Duncan Stewart and colleagues are planned to investigate the therapeutic potential of MSCs in sepsis-induced ARDS. There are currently over 300 clinical trials registered with Clinicaltrials.gov investigating the use of adult MSCs in a number of conditions; however, there are no clinical trials investigating the use of MSCs in HS-induced ARDS despite the significant preclinical data to support their therapeutic efficacy. Together, the preclinical and clinical data generated to date suggest that MSCs may represent a promising treatment for ARDS, which is an important complication of major trauma.
Acute kidney injury and acute renal failure
Current therapies for AKI are primarily supportive and fail to reduce morbidity and mortality (92). There have been few, if any, treatments that have been highly effective in preventing and treating AKI and ARF, both of which are clinical entities that present in critically ill patients after severe hemorrhage and trauma. The incidence of AKI and ARF in trauma patients can be as high as 26%, depending on whether they are critically ill in the intensive care unit (ICU) (93, 94). In the recent PROPPR study in trauma patients, the rate of AKI was 25% (82). In addition, a compelling argument for novel preventative therapies arises from data demonstrating that a significant percentage of patients with AKI go on to develop end-stage renal disease, which is characterized by continued inflammation, fibrotic changes in the kidney, and microvascular compromise even after they recover from AKI (95). Several cellular therapeutic strategies have been investigated in renal failure, including the use of iPSCs or renal resident progenitor cells to replace injured cells in the damaged kidney (96). The most extensively investigated cellular therapy for AKI has involved MSCs derived from various tissues, such as BM and adipose tissue. Numerous rodent studies have consistently documented a protective effect of MSCs on both acute and chronic injury models. In a systematic analysis of 21 of these studies, a consistent reduction in serum creatinine was observed in MSC-treated animals versus controls. Westenfelder and colleagues demonstrated that administration of MSCs to the kidneys of rats with ischemia/reperfusion-induced AKI ameliorated renal function, provided renoprotection, decreased apoptosis, and resulted in anti-inflammatory effects (97). Further work in this area revealed that, after 1 to 3 months, animals treated with MSCs had normal renal function, an absence of interstitial fibrosis, and low expression of profibrotic genes like plasminogen activator inhibitor-1 compared with vehicle and fibroblast-treated control animals (98). Several other studies have investigated the potential utility of MSCs in the context of other models of induced AKI, including glycerol and cis-platinum-induced injury, where they had similar renoprotective effects (99–102). Notably, Camussi and colleagues have demonstrated that soluble factors, specifically microvesicles and exosomes, released by MSCs and other cell types are responsible for these observed renoprotective effects (103).
A phase 1 clinical trial for AKI (NCT00733876) was initiated based on a number of positive preclinical studies. In this trial, Westenfelder and colleagues studied the effect of MSC therapy on adult patients who had undergone on-pump coronary artery bypass grafts and/or cardiac valve surgery (see Table, Supplemental Digital Content 2). In this safety and feasibility trial, adult patients who had undergone on-pump coronary artery bypass graft and/or cardiac valve surgery were infused with allogeneic MSCs via the suprarenal aorta using a dose-escalation protocol. All study subjects presented with the following risk factors for postcardiac surgery-associated AKI: underlying chronic kidney disease of stages 1 to 4, congestive heart failure, diabetes mellitus, hypertension, chronic obstructive pulmonary disease, and age over 65 years (104, 105). Preliminary analysis of the outcomes in this cohort of study subjects showed that the suprarenal, postoperative administration of allogeneic MSCs is feasible and safe, as it did not result in adverse or serious adverse events. In addition, preliminary efficacy data appear promising, indicating that MSC therapy prevented postoperative deterioration in renal function by approximately 20% and also prevented late deterioration in renal function, which closely parallels outcomes obtained in preclinical studies (104). In addition, MSC treatment reduced length of hospital stay and readmission rates by approximately 40% (104). Plans are underway for a double-blind, controlled, multicenter phase 2 efficacy trial. In addition, phase 1/2 clinical trials (NCT00658073) have been conducted in which MSCs were administered to kidney recipients from living, unrelated donors and to patients with chronic allograft nephropathy. Among patients undergoing renal transplant, the use of autologous MSCs resulted in lower incidence of acute rejection, decreased risk of opportunistic infection, and better estimated renal function at 1 year compared with anti-interleukin-2 receptor antibody induction therapy (106). Taken together, the current preclinical and clinical data support a beneficial role of cellular therapies, specifically MSCs, in the treatment of AKI, which is a common complication of major trauma.
Bone marrow dysfunction and wound healing
Following severe injury, patients admitted to the ICU can develop a persistent anemia that is unrelated to ongoing blood loss. Rather, this anemia is due to BM dysfunction and the suppression of hematopoietic progenitor cell (HPC) growth and mobilization into peripheral circulation (107). ICU patients are subjected to severe stresses that include numerous invasive procedures, pain, deregulated circadian rhythms, forced immobility, and mechanical ventilation (108). These patients are also known to have poor wound healing, immune dysfunction, and an increased susceptibility to infection. Livingston and colleagues have shown that BM dysfunction and impaired wound healing develop in a rodent model that combines blunt LC and HS followed by chronic stress (108). In this model, rats demonstrated significant and persistent BM dysfunction as evidenced by decreased cellularity, growth of all HPC lines, and anemia 1 week following LC, HS, and chronic stress. This observation was associated with persistent mobilization of HPCs from the BM out into the peripheral blood and an elevation in plasma granulocyte-colony stimulating factor (G-CSF). Furthermore, the lungs of these animals displayed significantly impaired wound-healing ability. A single IV dose of 1 × 105 rat MSCs restored BM cellularity, HPC growth, and hemoglobin levels. Furthermore, lungs of treated animals significantly improved with regard to wound healing, with plasma G-CSF levels falling to control values. MSC administration was associated with an expansion of the peripheral T regulatory cell population as well as improved BM function and wound healing (109). Further studies examining this novel cell-based therapy for the treatment of both anemia and wounds healing after traumatic injury is needed before clinical application. These studies include optimization of the timing and dose of MSC administration as well as elucidation of the mechanisms by which MSCs interact with the immune system to protect the BM from compromise and facilitate wound healing (110).
EXTREMITY INJURY, ORTHOPEDIC TRAUMA, AND BURNS
Extremity injuries are among the most common injuries to both military and civilian populations (111). Cellular therapies derived from a number of tissue sources have been investigated in regards to their ability to support the healing and regeneration of tissues in animal models (112, 113). However, the safety and efficacy of cellular therapies is still under investigation in humans. In a battlefield setting, extremity trauma is primarily due to penetrating and blast injuries. In contrast, these injuries primarily result from motor vehicle accidents, sport injuries, and chronic disease in civilian populations.
The DoD has recognized the potential therapeutic benefits associated with cellular therapies and the need for regenerative treatments. As a result, the Armed Forces Institute of Regenerative Medicine (AFIRM) was established in March 2008. AFIRM is a multicenter network of civilian and military institutions that focuses on developing new techniques and treatment options for wounded service members. This consortium is managed by the US Army Medical Research and Materiel Command (USAMRMC) and funded by multiple governmental organizations as well as private institutions. The AFIRM funds efforts focused on regenerative medicine that span a large spectrum of research areas, from basic bench research to human clinical trials, with a particular emphasis on extremity injury treatment, cranio-maxillofacial reconstruction, skin injury treatments, vascular composite tissue allo-transplantation, and immunomodulation. AFIRM aims to translate these advances into clinical products that can help mitigate outcomes in trauma and burn patients (see Table, Supplemental Digital Content 2).
Limb ischemia
Jan Nolta and colleagues at the UC Davis Stem Cell Program and Institute for Regenerative Cures have been conducting research in the area of limb ischemia, a frequent complication of extremity injury in trauma (114). It has been reported that hypoxic preconditioning of human MSCs enhances their duration of residence and regenerative properties in a preclinical, acute tissue ischemia model (115). Transplantation of the cells into the tissue in a semisolid or injectable biodegradable matrix also improves their retention. In an effort to optimize potency of the cells, MSCs can be engineered with transgenes to express higher than normal Vascular Endothelial Growth Factor-A (VEGF-A), a key factor involved in angiogenesis that can enhance revascularization and tissue repair. A phase 1 clinical trial is planned to investigate the effect of intramuscular (IM) injection of lentiviral VEGF-A genetically modified MSCs for the treatment of critical limb ischemia. Critical factors involved in the preclinical safety assessment of these modified cells have been karyotype stability, tumorigenic potential, edema formation, long-term engraftment, and dose finding studies. This planned clinical trial, which is a collaboration between John Laird at UC-Davis and Inmaculada Herrera from Hospital Universitario Reina Sofia in Spain, aims to provide an alternative to patients that are either not candidates for traditional revascularization surgery or have received unsuccessful treatment in the past. Ultimately, this study and intervention intend to reduce the number of amputations and improve the quality of life in patients with peripheral arterial disease.
Compartment syndrome
Compartment syndrome (CS) is a devastating complication of extremity trauma, and results from edema-initiated pressure compromising perfusion to extremity muscle compartments. Despite timely surgical intervention, ischemia, infarction, and reperfusion injury occasionally result in catastrophic cell death of skeletal muscle, nerve, and vascular tissues (116). CS magnifies the initial injury, and decreases the chance of a full functional recovery, hence delaying return to normal activity for patients with CS. As a result of the DoD-led initiatives in CS, there are now ongoing animal studies and human clinical trials being planned to investigate BMMNCs in CS.
Gregory and colleagues at Oregon Health Sciences University (OHSU) are currently conducting preclinical trials using autologous BMMNCs in swine. In this study, CS is induced by autologous plasma infusion into anterior tibialis (AT) muscles followed by fasciotomy. On day 7, autologous BM is aspirated and BMMNCs isolated and injected into the AT in a grid pattern. Muscle torque, nerve conduction, and gait are serially measured until the 12-week study endpoint. Study results are still currently pending.
Plans are underway at OHSU to initiate a clinical trial using autologous BMMNCs 1 week after CS injury. This study intends to enroll 20 patients with CS that will receive cell treatments 5 to 10 days postinjury. The patients will undergo BM aspiration, and the BM will be processed and injected directly into the wounded leg. The primary endpoints of this study will be improvement in muscle strength at 3 and 6 months posttreatment as well as the incidence of combined adverse events. Secondary endpoints will include improvement in gait, nerve conduction velocity, and MRI indices of muscle fibrosis and muscle regeneration. Ultimately, the goal of this study is to improve functional recovery and reduce disability in patients with CS.
There are currently eight active clinical trials in the United States, investigating the use of stem cells to heal extremity injuries like CS and critical limb ischemia (see Table, Supplemental Digital Content 2). There is a phase 1 trial (NCT01837264) funded by the DoD and sponsored by Arteriocyte, Inc, that focuses on the prevention of battlefield trauma-induced CS leading to amputation. This trial is ongoing and has a target enrollment of 20 patients with tibial fractures due to motor vehicle or similar orthopedic trauma that develop CS and are treated with a fasciotomy. In this trial, the BM is harvested and separated using the Magellan® (Arteriocyte, Hopkinton, MA). The primary endpoints of this study include adverse events due to the wound and/or therapy, amputation of limb, and/or death, whereas the secondary outcomes will investigate the rate of healing, limb pain, and functional performance as compared with the uninjured limb.
Burn injury
Thermal burns are a significant source of morbidity in times of war, constituting 5% to 20% of all injuries (117). Mortality from burn injury is closely associated with percent total body surface area burned, age, and inhalation injury (118). Although there have been significant improvements in resuscitation and wound healing over the past 50 years that have significantly reduced the rate of burn-related mortality, new methods to heal acute burn wounds more effectively and regenerative approaches to treat burn scars postinjury are urgently needed (119). In addition to wound closure after full-thickness burns, postburn wound issues include altered pigmentation, hypertrophy of the burned area, contractures, pain, pruritus, and defective temperature regulation. Although significant advancements have been achieved, there is currently no reliable full-thickness skin substitute. Currently, available products include dermal substitutes, neonatal-derived products, amniotic membrane products, and cultured epithelial autografts (120).
MSCs have the potential to engraft into wounds and incorporate into mature skin structures that include blood vessels, sebaceous glands, and hair follicles (121, 122). In addition, they can rebuild wound beds and contribute cellular material to help orchestrate tissue repair (123, 124). Limited preclinical and clinical data indicate that these MSC-based treatments appear to be well tolerated, although further study is needed to firmly establish safety, efficacy, and potency. A few cases have been reported of MSCs used to successfully treat burn wounds due to radiation accidents (125); however, these are isolated cases and occurred in studies where there was an absence of controls as well as an undefined burn depth.
Alternate stem cell treatment options are currently being investigated in burns animal models. These include adipose tissue epidermis/debrided skin (126). Many of these therapies are still in the very early stages of development and are being tested in small animal models.
Currently, there are only two active clinical trials in the United States focused directly on burns (see Table, Supplemental Digital Content 2), one of which is a DoD-funded trial entitled “Stem Cell Therapy to Improve Burn Wounds Healing” (NCT02104713). This study aims to test the effect of allogeneic MSCs in patients with second-degree burns to determine treatment safety and effectiveness. Preclinical studies in progress have used MSCs delivered in PEGylated fibrin matrices to treat skin burn injuries in pigs. Another in-progress preclinical study is investigating the use of fractional lasers as a cell delivery method in a swine model. This study has shown laser treatment to increase the skin depth by which the cells are delivered. This treatment is being considered for deep third-degree burns that are characterized by thick scar tissue. The second-burn trial currently in progress is entitled “Use of Autologous Platelet Rich Plasma (PRP) Gel as an Adjunct to the Treatment of Deep 2nd and 3rd Degree Burns” (NCT01843686). This study, which focuses on treating second- and third-degree burns, is currently enrolling participants. The primary outcomes are the safety of platelet rich plasma administration following excision of an acute deep second- and third-degree burn, as well as a decrease in adverse events. In the area of wound healing and burn healing, StrataGraft® (Stratatech, Madison WI), a biologically functional epidermis made of mature cell graft of neonatal immortalized keratinocytes, has been investigated as a potential human skin substitute. This product produced no improvement when compared with control cadaveric skin grafts in a phase 1/2 clinical trial (126).
The morbidity associated with burn and wound healing is profound and devastating for many. A poor outcome often leads to a lifelong physical and emotional disability. The potential impact of stem cell therapy to facilitate wound healing and the regeneration of damaged tissues will have a significant impact on the lives of trauma and burn victims.
Orthopedic trauma
Orthopedic trauma is another area that may benefit from cellular therapies. The investigation of stem cells and cellular therapies in orthopedic trauma shares many of the same sources as those described in the previous sections: BM, adipose tissue, placenta, blood vessels, muscle, and dental pulp (127–129). Currently, there is no consensus in the field regarding which cell type and source are optimal for use in osteogenic and reparative interventions. Most of the preclinical work in this area involves the repair of bone defects and fractures, often using a combination of biomaterials (i.e., scaffolds), growth factors, antibiotics, and stem cells together. Novel treatments are emerging to also heal muscle, tendons, ligaments, cartilage, and nerves.
A recently completed study (NCT02177565) led by James Richardson of the Robert Jones and Agnes Hunt Orthopedic Hospital in the United Kingdom investigated the effect of treatment with MSCs on the healing of nonunion fractures. Although results have not yet been made available, the primary outcomes investigated in this study included computed tomography scans and radiographs for up to 12 months to investigate callus formation at the fracture site posttreatment with MSCs or the carrier control. In addition, a prospective, randomized, blinded, sham-controlled phase 2 study (NCT02448849) sponsored by the Royan Institute in Iran is currently seeking to recruit 40 participants to investigate the effect of allogeneic adipose-derived MSC transplantation on tibial fracture healing.
Studies investigating the use of cellular therapies for osteoregeneration outside of the context of orthopedic injury, such as for noninjury-induced musculoskeletal deformities, are ongoing and may provide the foundation for therapeutics in this field as well. Furthermore, because orthopedic injuries can be complex and involve many aspects of the musculoskeletal system, cellular therapies with multi-tissue healing capabilities hold the most promise for use in the treatment of these injuries. Indeed, the future of regenerative medicine in orthopedic trauma focuses on traumatic injuries that affect more than a single system. New literature focuses on polytrauma and composite injury models to investigate the effects of multiple injuries on healing (130). These platforms are appropriate for testing the application of cellular therapies in in vivo animal models of trauma, and bring us a step closer to acute treatments of orthopedic injuries in human trials.
BARRIERS IN TRANSLATION FOR CELLULAR THERAPIES IN TRAUMA AND CRITICAL CARE MEDICINE
Regulatory concerns for cellular therapies in the United States
Investigational cell therapy products, which include cells derived from stem cells or other progenitors, hold great promise for addressing unmet medical needs. However, significant challenges related to safety, efficacy, characterization, and the design of clinical trials impede progress in this field. The research, development, and implementation considerations required for cellular therapies in any disease are extensive and require consideration of multiple areas: (1) the stem cell products themselves (i.e., allogeneic vs. autologous, cell types, tissue sources, stored vs. expanded cells, effect of passage number and culture conditions on expanded cells, pluripotent vs. multipotent cells); (2) collection, storage, handling, labeling, delivery, quality assurance/quality control considerations; (3) infectious risks and biovigilance; (4) challenges in testing safety and efficacy of the cells in the appropriate models, biopharmacokinetics and potency measures, cell clearance versus chimerism; (5) interaction with other complex systems (i.e., hematopoiesis, immune function, coagulation, and the endothelium); (6) integration into transfusion protocols and clinical practice; and (7) training involved in handling of cells for collection, distribution, and administration. In the past 15 years, significant advances have been made in all of these areas that have permitted rapid translational progress and the clinical investigation of a number of cellular therapies in a wide variety of human diseases.
The US FDA has adapted to the changing landscape of this evolving field by applying flexible regulatory standards that balance benefits and risks to those who take part in clinical trials (131). Aside from HSC transplantation, there is currently no cellular therapy that is FDA-approved for commercial production and clinical use in trauma or critical care medicine in the United States; however, there are some trials that are currently underway. The FDA evaluates potential risk based on results derived from an analytical assessment of product characteristics as well as preclinical proof-of-concept and safety testing, which, collectively, are considered within the context of a proposed clinical study (132). It is clear that a number of factors come into play in defining the regulatory roadmap and path forward to developing and evaluating these therapies for clinical use. The type of cell and clinical application will often define the rigor by which a particular therapy is scrutinized. Considerable progress has indeed been made through active participation of small biotech companies, academic physicians, scientists, and funding agencies that have pursued the development and investigation of cell therapies for critically ill patients where there are few, if any, effective therapeutic options.
The Center for Biologics Evaluation and Research (CBER) regulates cellular therapies and cell therapy products. Specifically, the Office of Cellular, Tissue, and Gene Therapies (OCTGT) conducts regulatory reviews, regulatory policy and guidance development, international activities and standards, and outreach to the scientific and medical community. OCTGT regulates stem cells and stem cell-derived products, including BMMNCs, MSCs, HSCs, iPSCs, ESCs, and somatic cell therapies. INDs are submitted to OCTGT for review and subject to standard considerations of safety, efficacy, purity, and potency. Oversight of the products and processes are included via review of quality control, product intermediates, and reproducibility. Draft guidance documents have been generated by CBER to guide IND preparation for cell therapies and take into consideration the following: (1) chemistry, manufacturing, and controls (CMC); (2) pharmacology and toxicology; and (3) the clinical protocols. The CMC review is critical and aims to deliver a safe cellular product to the patients in the study. Meticulous attention is given to the source of the cells and to preventing the introduction of microorganisms during the manufacturing process (133). Because cellular therapies are highly diverse compared with, for example, small molecule drugs, CBER recognizes they do not lend themselves to a standard review process, and therefore, flexibility is built into its processes to facilitate the fulfillment of their goal of ultimately generating regulatory roadmaps for cellular therapeutics in the United States (133). Accelerating and translating emerging stem cell therapies to patients, particularly critically ill patients, is a high priority in the United States and elsewhere in the world. The FDA is increasingly invoking new regulatory mechanisms for the accelerated review of INDs, and it is likely that these mechanisms will be applied specifically to new stem cell biologics. With a fixed budget and the number of new applications submitted to the FDA increasing, the FDA has stepped up to the challenge to meet the needs of this rapidly growing new frontier.
Aside from the FDA, the American Association of Blood Banks (AABB) has taken a new interest in developing a role for the standardization of cell therapy production and processing. The AABB has been a leader in the development of voluntary standards in blood bank blood component collection, processing, and transfusion since 1957. Over the past two decades, AABB's standard-setting program has moved beyond the blood bank to encompass new disciplines and activities, including cellular therapies. Blood banks have also been involved in cellular therapies for years, with extensive experience in cord blood processing and BM transplants, and are now venturing into stem cell expansion, production, and processing. Each set of standards is developed by a volunteer committee of experts, known as a Standards Program Unit (SPU). Each SPU is composed of AABB members that serve as technical experts, liaisons from other AABB committees, clinicians, and representatives from other organizations. The Cellular Therapy SPU includes individuals with expertise in HSCs, cord blood, pancreatic islets, MSCs, etc. AABB Cellular Therapy Standards cover all processes involved with the procurement, processing, and administration of cellular therapies (134).
The AABB Standards requirements are based on good medical practice, current scientific data, principles of good manufacturing practices (GMPs) and quality assurance, and applicable regulations. The US Cellular Therapy Standards are revised on a 24-month cycle with revisions based on changes to industry practice, technical/scientific advances, regulatory changes, and feedback from members and assessors compiled during a 60-day public comment period. With the emergence of novel cellular and regenerative medicine therapies, AABB, along with other standard setting organizations and the FDA, has been an active participant in efforts to harmonize standards, and will likely play a large role as the field develops (134).
International regulatory standards and trials
It is of interest to note that a number of trials using cellular therapies have been conducted in the international arena. According to a 2014 study by Li et al., a search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Search Portal indicated that 4,749 stem cell-related clinical trials had been registered worldwide as of January 1, 2013 (135). The vast majority of these trials were in early stages, highlighting the infancy of this field in its progress toward effective, well-characterized therapeutics for human applications. As the number of stem cell trials is steadily rising, internationally founded collaborative efforts are needed to strengthen scientific outcomes and standardize regulatory processes. Although some of these unified regulatory efforts exist, such as those between the FDA and European Medicines Agency (EMA), wider scale global collaborations are needed and the roadmap for collaborations between the FDA and EMA in regards to cellular therapies, which has yet to be established (136).
In the EU, cell-based therapies are regulated by the EMA under Advanced Therapy Medicinal Product Regulations, which regulate such products as pharmaceuticals while ensuring their free movement within the EU market to promote competition and ensure patient safety is being met. Furthermore, clinical trials are regulated by individual EU member states. Other countries have less stringent regulations meant to allow for the fast tracking of potentially beneficial therapeutics to market. In 2013, Japan approved a revision to its Pharmaceutical Affairs Law, which simplified the processes in place for the approval of regenerative therapies. The revised processes will expedite the approval of therapeutics that have been shown to be safe and effective in pilot studies with small sample sizes (137). Furthermore, regulatory authorities in countries such as China, Russia, and India have been widely criticized in recent years for their lax guidelines that lack significant legal implications (138).
Although the majority of stem cell-related clinical trials are executed by the United States and EU, respectively, other countries, including China, South Korea, India, Japan, and Australia, are increasingly executing a greater number of cell therapy trials. Notably, as of 2008, Asian countries have more novel clinical trials in this area than the United States and EU (135). In India, the Neurogen Brain and Spine Institute is conducting a phase 1 trial (NCT02028104) to assess the safety and efficacy of autologous BMMNCs on pediatric and adult patients (6 months to 65 years) with TBI, focusing on measures of disability after injury as their primary outcome. This institute completed a similar study examining the intrathecal administration of these cells in patients with TBI (NCT02027246) for which preliminary results from the pilot study of 14 patients indicated that treatment improved functional outcomes up to 6 months posttreatment (139). In addition, Kasiak Research Pvt. Ltd of Maharashtra, India, is currently recruiting participants for a phase 1/2 clinical trial to examine the safety and efficacy of IV and IM injection of adipose-derived stromal vascular fraction and MSCs for the treatment of critical limb ischemia in patients 18 to 65 years of age.
A study by K-Stemcell Co Ltd of Korea is currently recruiting participants for a phase 1/2 trial (NCT01769872) evaluating the safety and efficacy of autologous adipose tissue-derived MSC transplantation in adults (ages 19–70) with SCI. This study will evaluate ASIA-A scale scores at 3 and 6 months posttreatment, as well as MRI changes and other measures of functional outcome at these time points. Furthermore, a phase 1 study (NCT01325103) of 14 patients with chronic traumatic SCIs out of Brazil evaluated the effect of intralesion injection of MSCs. Another phase 1/2 study (NCT01903044) from Pontifícia Universidade Católica do Paraná of Brazil will be investigating the effect of IM injection of autologous BM-derived stem cells on lower limb ischemia.
In regards to organ injury, there are several international studies investigating the use of stem cells for the treatment of lung injury. Shaoxing Second Hospital of China is currently recruiting patients for a phase 1 trial (NCT01902082), investigating the safety and efficacy of one IV dose of 1 × 106 allogeneic adipose-derived MSCs per kg body weight for the treatment of ARDS in adults (ages 18–90 years). Asan Medical Center in Seoul, South Korea, is conducting a phase 2 study (NCT02112500), also investigating stem cell treatments for ARDS. However, this study will be examining IV infusion of autologous BM-derived MSCs rather than adipose-derived MSCs. Overall, the international scientific community is making great strides in the field of cell therapies; however, significant coordination and regulation of efforts is needed to promote efficient progress in the field that prioritizes patient safety.
Cell processing and production
One of the greatest challenges in the area of cellular therapies and the execution of clinical trials is and will be where to source clinical grade cells in sufficient quantities for trials and future licensure. A number of steps are necessary when an organization, industry, blood banks, or academic center is considering the development of a cell therapy program. Most attention is focused on the development of a specialized facility dedicated to the manufacture of cellular therapies. However, far more is needed for a functional cell therapy program to become established. A team of people working in several laboratories and centers are required to effectively manufacture and deliver the cellular therapies. Mechanisms must be in place to recruit, screen, and test donors willing to donate the cells that serve as the starting material for the manufacturing process. Once individuals meeting donation criteria are identified, a process must be in place to sterilely collect and process the starting material. The product must be tested both during and at the end of the manufacturing process to ensure that it meets established criteria required by the FDA. A laboratory is needed to perform these in-process and lot release assays, which can potentially be outsourced to established labs. This laboratory is needed to perform the assays necessary to determine if the people who donate the starting cellular material meet criteria established by regulatory groups and the manufacturing protocols for donating. After the manufacturing process is complete, the final product must be stored, possibly shipped, and finally issued and administered to the recipient.
Ideally, coupled into this effort would be the establishment of a new product development group that is specialized in working with research laboratory and clinical investigators to bring new cellular therapies from the lab bench to a clinic. This process involves scaling up manufacturing, replacing research grade reagents with GMPs grade reagents when possible, and validating the manufacturing process. For the cell collection group, the cell processing laboratory, the product and donor testing laboratories, the product development laboratory, and the product storage and issue laboratory, written and validated standard operating procedures must be in place and staff must be trained on how to perform these procedures. The medical personnel who administer the cellular therapies must also be trained and their responsibilities explicitly described in written procedures. To ensure that high-quality cellular therapies are consistently produced, staff dedicated to quality control and regulatory affairs are essential. Establishing the facilities required for cell therapy centers and assembling the large and diverse group of people needed to operate such a facility requires considerable investment.
Most new cellular therapies are developed and taken through phase 1 and phase 2 clinical trials at academic medical centers (140). The resources needed to initiate and complete phase 1 and phase 2 cellular therapy clinical trials are in place at many academic medical centers. Although phase 1 and phase 2 trials have been run in multiple areas, the funding for phase 3 clinical trials has been and will be a challenge in the future, as well as a limiting barrier for the commercialization of any of the cell therapy products currently under investigation. Large pharmaceutical companies that have the capacity to support phase 3 trials have conservatively embarked into cellular therapies, although this may be changing as more recently Novartis has engaged in the development of a program to produce anti-CD19 Chimeric Antigen Receptor T cells for the treatment of B cell leukemia and lymphoma. This has led to an increase in the manufacturing of these and other genetically engineered T cells as cancer immunotherapies (141).
Currently, most academic-based cellular therapy centers are focused on producing immunotherapies for cancer and hematologic malignancies as well as genetically engineered HSCs to treat monogene disorders. Many academic cellular therapy facilities are part of hematology or oncology departments or cancer centers, and therefore preparing cellular therapies for trauma and critical care medicine is outside their area of expertise; however, modifications to suit the needs of a trauma or critical care trial with the right support and infrastructure are possible. The application of cell therapies for trauma and critical care medicine relies on readily accessible banked products (i.e., third party, stabile/preserved, and easily prepared for infusion). MSCs are one candidate for such application because large-scale, cGMP manufacturing has already been established. Although several trials have been completed and others are ongoing, there remains a need for optimization of manufacturing, including both cell culture and bio/cryopreservation, which may be application-dependent.
Funding
To date, obtaining funding for research and clinical trials investigating stem cell therapies has been challenging. The majority of clinical trials in cellular therapies have either been funded by entities such as small biotech companies, the DoD, or the NIH. The NHBLI developed the Production Assistance for Cell Therapy (PACT) program to advance the development and production of clinical grade cellular therapies to treat ALI, left ventricular failure, and hematologic diseases (142); however, this funding has ceased, and the direction PACT funding is taking now is to support research-grade cell production facilities that will support preclinical research.
Lack of funding is a major barrier in translation for trauma care as well as for cellular therapies in general. The majority of clinical trial runs in trauma in civilian populations have been conducted at Level 1 trauma centers, and few large multicenter trials have been conducted to date. The DoD has strongly supported efforts in the area of trauma research and also regenerative medicine through entities such as USAMRMC, Congressionally Directed Medical Research Program, and AFIRM. However, there is currently no true “home” or institute to support trauma research or highly translational cellular therapy efforts, hence compounding the truly challenging prospects for advancement in both fields. Strong support from federal and state governments, private foundations, the general public, and industry are needed to make significant advances in this field. The interest and capability from all of these involved parties is growing, but will require accelerated action from all sides. Another problem is the conceptual barrier that trauma is not a disease in itself and, although it is one of the leading causes of death worldwide, has yet to receive the interest and support that other diseases such as cancer, HIV, and cardiovascular disease have received (143, 144). This would need to change for major advances to be made in a field with strong potential to offer novel therapies that can significantly alter outcomes for gravely ill patients.
The challenges of conducting randomized controlled trials in trauma and critical care
A major challenge and translational barrier for novel therapies in trauma and critical care medicine is the difficulty posed in the planning, design, and execution of successful randomized controlled clinical trials. Challenges that arise as the field progresses toward clinical trials for the investigation of cellular therapies in trauma and critical care will likely include selection of the appropriate target patient population, relevant and modifiable outcome measures that are accepted by the FDA, therapeutic targets that occur at sufficient frequency, timing of delivery of treatment, and adequate dosing. Preclinical data on timing, dose and route of delivery as a well as characterization of the optimal cell product, its mechanisms and potency are critical to the success of cell therapies in trauma and critical care. All of these components will be required to optimally design and successfully execute trials for cell therapies in trauma.
In general, prospective randomized trials in critically ill patients require consent. Critically ill patients are frequently unable to give consent, and therefore, these trials may be governed by FDA code of federal regulations Title 21 Part 50, rules for exception from informed consent. An interesting area of development and a true barrier in the translation of stem cell therapies from bench to bedside in the acute care of trauma patients is the issue of patient consent. In recent years, it has become clear that the “Golden Hour” of prehospital care is a critical period to medically intervene (3). Intervention with novel methods for hemorrhage control, optimized blood product administration, and supportive measures have been shown to dramatically improve outcomes in critically injured patients and trials during this period and during the early stages of arrival in the hospital require exception from informed consent (EFIC). The issue of EFIC will likely arise for cellular therapies, particularly in trials where acute administration of cells is desired for the injured patient incapable of providing individual consent. The 1996 Final Rule 21 CFR 50.24 and 45 CFR 46.101 of the FDA and Department of Health and Human Services was passed and specifies that, under strict criteria, research can be conducted without obtaining individual informed consent of the human subject participant. This rule allows for out-of-hospital and early in-hospital randomized controlled trials for research that carries the potential to improve clinical outcomes for patient populations with a poor prognosis. The idea being that early, acute care may stem some of the deleterious processes that occur well before the patient receives targeted care in the emergency department or operating room. Exception from informed consent studies have historically been required to have 30-day mortality as a primary outcome measure. This is difficult to achieve, and several studies have been stopped for futility (i.e., the Hypertonic Saline Trials) (145–147). Alternatively, in nonemergent settings, family members or legal representatives must give consent in situations where research is often not a priority, hindering study enrollment.
Target patient selection in trauma and critical care trials is paramount for their success. In trauma-relevant trials, it is difficult to identify the population at risk that has the optimal opportunity to benefit from the planned study intervention. Patients may either be too ill or not ill enough to benefit. Consideration of these issues is required in trial design. Furthermore, another issue is that careful study of a population may improve the outcome in question in both the intervention group and the control group. These studies tend to be underpowered as the difference between the groups narrows. ICU patients are complex and numerous factors alter their outcomes. These factors may overcome the benefits of a proposed study intervention, resulting in a negative study. Finally, adverse events occur commonly within a critically ill population, and it may therefore be difficult to attribute an adverse event to a study intervention as opposed to the underlying disease. Some of these issues have been successfully addressed in current trauma trials investigating novel treatment paradigms in trauma patients; however careful thought, a better understanding of mechanisms of action and the disease condition itself are required to ensure future trial success.
IN SUMMARY
Cellular therapies show considerable promise in the acute care of trauma patients, a field where there are few, if any, effective therapeutic options that mitigate the immediate and long-term consequences of traumatic injury.
In 2015, the military and civilian trauma communities must lean forward to innovate new ways to improve survival and recovery from severe injury. As part of a joint military–civilian effort, this state-of-the-science review demonstrates the significant potential of cell-based therapies to do just that—to serve as a resuscitative adjunct with potential applicability in the acute, subacute, and longer-term phases of trauma care. Unlike any other therapy studied today, cell-based treatments have immunomodulatory and restorative capacity and have already been shown to be clinically feasible and, in some settings, effective. Given the breadth and comprehensive nature of the topic, building on this promising momentum will require a dedicated research approach that includes strong and ideally coordinated programs in each phase of investigation—basic, translational, and clinical. In addition to advancing these lines of research with programmed and managed funding, the military medical research and development enterprise stands to serve in a partnership role as it pertains to regulatory, cell procurement and processing and large-scale production efforts, be they private, federal, or some combination of the two. Finally, the military's experience with clinical management and research of HS and brain injury position it well to guide civilian investigators and regulatory agencies in identification of relevant and impactful endpoints to be used in clinical study of trauma. To facilitate these efforts, it is the hope of the authors that this structured and contemporary review will serve as a call to action, one that culminates in knowledge and material solutions to improve mortality and lessen the burden of survivorship following trauma, including vexing injury patterns that today seem unsolvable.
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all the presenters and the working group who helped with this manuscript: John B. Holcomb, MD, FACS, Texas Trauma Institute University of Texas Health Science Center, Houston, TX; Michael A. Matthay, MD, University of California, San Francisco, CA; Darwin J. Prockop, MD, PhD, FACC, Institute for Regenerative Medicine, Texas A&M University; Andriy I. Batchinsky, MD, U.S. Army Institute of Surgical Research, Fort Sam, Houston, TX; Anthony Atala, MD, Wake Forest School of Medicine, Winston-Salem, NC; M. Ross Bullock, MD, PhD, University of Miami/Jackson Memorial Hospital, Miami, FL; Charles S. Cox, Jr., MD, University of Texas-Houston Medical School, Houston, TX; James D. Guest MD, PhD, FACS, FRCS(C), University of Miami, Miami, FL; Kenton W. Gregory, MD, Oregon Health and Science University, Portland, OR; Jan A. Nolta, PhD, University of California Davis, Sacramento, CA; Carl I. Schulman, MD, PhD, MSPH, FACS, University of Miami, Miami, FL; Shibani Pati, MD, PhD, Blood Systems Research Institute, San Francisco, CA; Irshad H. Chaudry, PhD, University of Alabama at Birmingham, Birmingham, AL; Pranela Rameshwar, PhD, Rutgers New Jersey Medical School, NJ; David H. Livingston, MD, Rutgers New Jersey Medical School, NJ; LTC Andrew P. Cap, MD, PhD, FACP, U.S. Army Institute of Surgical Research, Fort Sam, Houston, TX; Marc S. Penn, MD, PhD, FACC, Northeast Ohio Medical University, Rootstown, OH; Celia M. Witten, PhD, MD, United States Food and Drug Administration, Silver Spring, MD; David Stroncek, MD, National Institutes of Health, Bethesda, MD; David H. McKenna, Jr., MD, University of Minnesota, Saint Paul, MN; LTC Kevin K. Chung, MD, FCCM, FACP, U.S. Army Institute of Surgical Research, Fort Sam, Houston, TX; Naynesh Kamani, MD, American Association of Blood Banks, Washington DC; Martin Schreiber, MD, FACS, Oregon Health and Science University, Portland, OR; Charles Wade, PhD, University of Texas Health Science Center, Houston, TX; Frank Lebeda, PhD, US Army Medical Research and Materiel Command; Lisbeth Welniak, PhD, National Heart, Lung, and Blood Institute. The authors would like to extend their gratitude to the meeting participants as well: Robert Christy, PhD, US Army Institute of Surgical Research; Kenneth Curley, MD, US Army Medical Research and Materiel Command; Jurandir Dalle Lucca, MD, PhD, US Army Institute of Surgical Research; Thomas Davis, PhD, Naval medical Research Center; Simone Glynn, MDCM, MSc, MPH, National Institutes of Health/National Heart, Lung, and Blood Institute; Cynthia Gregory, PhD, Oregon Health & Science University; Crystal Hill-Pryor, PhD, MPH, US Army Medical Research and Materiel Command; W. Keith Hoots, MD, National Heart, Lung, and Blood Institute; Lt. Col. Debra Malone, MD, Walter Reed National Military Medical Center; Traci Mondoro, PhD, National Institutes of Health/National Heart, Lung, and Blood Institute; Brian J. Pfister, PhD, US Army Medical Research and Materiel Command; Anthony E. Pusateri, PhD, US Army Medical Research and Materiel Command; Deb Shear, Walter Reed National Military Medical Center; Frank C. Tortella, ST, PhD, Walter Reed National Military Medical Center; Tom Walters, PhD, US Army Institute of Surgical Research; Joseph C. Wenke, PhD, US Army Institute of Surgical Research. Special thanks to Scott Holmes for his help with the illustrations.
Footnotes
This manuscript is a result of the Department of Defense Meeting in Cellular Therapies in Trauma and Critical Care Medicine that took place February 5th in Fort Detrick, MD. At this meeting experts in the field of cellular therapies in trauma and critical care medicine were invited to present the state of the science in their respective areas of expertise. All speakers were asked to provide slides of their presentations, written abstracts, key pertinent references, and many were asked to review the article for accuracy and clarity. Only peer-reviewed publications were referenced in this article to support all facts, studies, and findings described in the article. The article hence provides a broad spanning overview of the state of the science in the field.
The authors report no conflicts of interest.
REFERENCES
- 1.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg 2012; 73 (6 suppl 5):S431–S437. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen TE, Gross KR, Baer DG. Where do we go from here? Preface. US Military Health System Research Symposium, August 2013. J Trauma Acute Care Surg 75(2 suppl 2):S105–S106, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen TE, Baer DG, Doll BA, Caravalho J., Jr In the ‘golden hour’ - combat casualty care research drives innovation to improve survivability and reimagine future combat care. Army AL & T Magazine Jan-Mar:80–85, 2015. [Google Scholar]
- 4.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015; 313 5:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holcomb JB, Hoyt DB. Comprehensive injury research. JAMA 2015; 313 14:1463–1464. [DOI] [PubMed] [Google Scholar]
- 6.Langan NR, Eckert M, Martin MJ. Changing patterns of in-hospital deaths following implementation of damage control resuscitation practices in US forward military treatment facilities. JAMA Surg 2014; 149 9:904–912. [DOI] [PubMed] [Google Scholar]
- 7.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann Surg 2015; 261 3:586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holcomb JB, Pati S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: the surgeon's perspective. Hematology Am Soc Hematol Educ Program 2013; 2013:656–659. [DOI] [PubMed] [Google Scholar]
- 9.Santry HP, Alam HB. Fluid resuscitation: past, present, and the future. Shock 2010; 33 3:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, Cook L, Kawahara T, Perel P, Prieto-Merino D, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 2013; 17 10:1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelnick LR, Morrison LJ, Devlin SM, Bulger EM, Brasel KJ, Sheehan K, Minei JP, Kerby JD, Tisherman SA, Rizoli S, et al. Addressing the challenges of obtaining functional outcomes in traumatic brain injury research: missing data patterns, timing of follow-up, and three prognostic models. J Neurotrauma 2014; 31 11:1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pati S, Khakoo AY, Zhao J, Jimenez F, Gerber MH, Harting M, Redell JB, Grill R, Matsuo Y, Guha S, et al. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/beta-catenin signaling. Stem Cells Dev 2011; 20 1:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker PA, Bedi SS, Shah SK, Jimenez F, Xue H, Hamilton JA, Smith P, Thomas CP, Mays RW, Pati S, et al. Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: modulation of the resident microglia population. J Neuroinflammation 2012; 9:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126 4:663–676. [DOI] [PubMed] [Google Scholar]
- 15.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 2006; 6 2:93–106. [DOI] [PubMed] [Google Scholar]
- 16.Picinich SC, Mishra PJ, Glod J, Banerjee D. The therapeutic potential of mesenchymal stem cells. Cell- & tissue-based therapy. Expert Opin Biol Ther 2007; 7 7:965–973. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418 6893:41–49. [DOI] [PubMed] [Google Scholar]
- 18.Kusadasi N, Groeneveld AB. A perspective on mesenchymal stromal cell transplantation in the treatment of sepsis. Shock 2013; 40 5:352–357. [DOI] [PubMed] [Google Scholar]
- 19.Weil BR, Herrmann JL, Abarbanell AM, Manukyan MC, Poynter JA, Meldrum DR. Intravenous infusion of mesenchymal stem cells is associated with improved myocardial function during endotoxemia. Shock 2011; 36 3:235–241. [DOI] [PubMed] [Google Scholar]
- 20.Duckworth JL, Grimes J, Ling GS. Pathophysiology of battlefield associated traumatic brain injury. Pathophysiology 2013; 20 1:23–30. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Traumatic Brain Injury in the United States: Fact Sheet. [Internet.] Available at: http://www.cdc.gov/traumaticbraininjury/get_the_facts.html, accessed August 15, 2015. [Google Scholar]
- 22.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 23.Santiago LA, Oh BC, Dash PK, Holcomb JB, Wade CE. A clinical comparison of penetrating and blunt traumatic brain injuries. Brain Inj 2012; 26 2:107–125. [DOI] [PubMed] [Google Scholar]
- 24.Masel BE, Bell RS, Brossart S, Grill RJ, Hayes RL, Levin HS, Rasband MN, Ritzel DV, Wade CE, DeWitt DS. Galveston Brain Injury Conference 2010: clinical and experimental aspects of blast injury. J Neurotrauma 2012; 29 12:2143–2171. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun 2012; 26 8:1191–1201. [DOI] [PubMed] [Google Scholar]
- 26.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013; 136 (pt 1):28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alali AS, Fowler RA, Mainprize TG, Scales DC, Kiss A, de Mestral C, Ray JG, Nathens AB. Intracranial pressure monitoring in severe traumatic brain injury: results from the American College of Surgeons Trauma Quality Improvement Program. J Neurotrauma 2013; 30 20:1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, Kossmann T, Ponsford J, Seppelt I, Reilly P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 2011; 364 16:1493–1502. [DOI] [PubMed] [Google Scholar]
- 29.Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, et al. Guidelines for the management of severe traumatic brain injury. XI. Anesthetics, analgesics, and sedatives. J Neurotrauma 2007; 24 suppl 1:S71–S76. [DOI] [PubMed] [Google Scholar]
- 30.Aertker BM, Bedi S, Cox CS., Jr Strategies for CNS repair following TBI. Exp Neurol 2015; Jan 28. pii: S0014-4886(15)00021-7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31.Menge T, Zhao Y, Zhao J, Wataha K, Gerber M, Zhang J, Letourneau P, Redell J, Shen L, Wang J, et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci Transl Med 2012; 4 161:161ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedi SS, Walker PA, Shah SK, Jimenez F, Thomas CP, Smith P, Hetz RA, Xue H, Pati S, Dash PK, et al. Autologous bone marrow mononuclear cells therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. J Trauma Acute Care Surg 2013; 75 3:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery 2003; 53 3:697–702. [DOI] [PubMed] [Google Scholar]
- 34.Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery 2001; 49 5:1196–1203. [PubMed] [Google Scholar]
- 35.Walker PA, Shah SK, Jimenez F, Gerber MH, Xue H, Cutrone R, Hamilton JA, Mays RW, Deans R, Pati S, et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp Neurol 2010; 225 2:341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS., Jr Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 2009; 18 5:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe J, Shetty AK, Hattiangady B, Kim DK, Foraker JE, Nishida H, Prockop DJ. Administration of TSG-6 improves memory after traumatic brain injury in mice. Neurobiol Dis 2013; 59:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crisostomo PR, Wang M, Markel TA, Lahm T, Abarbanell AM, Herrmann JL, Meldrum DR. Stem cell mechanisms and paracrine effects: potential in cardiac surgery. Shock 2007; 28 4:375–383. [DOI] [PubMed] [Google Scholar]
- 39.Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Generalized potential of adult neural stem cells. Science 2000; 288 5471:1660–1663. [DOI] [PubMed] [Google Scholar]
- 40.Sohur US, Emsley JG, Mitchell BD, Macklis JD. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos Trans R Soc Lond B Biol Sci 2006; 361 1473:1477–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao J, Prough DS, McAdoo DJ, Grady JJ, Parsley MO, Ma L, Tarensenko YI, Wu P. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp Neurol 2006; 201 2:281–292. [DOI] [PubMed] [Google Scholar]
- 42.Harting MT, Jimenez F, Adams SD, Mercer DW, Cox CS., Jr Acute, regional inflammatory response after traumatic brain injury: implications for cellular therapy. Surgery 2008; 144 5:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 2008; 131 (pt 3):616–629. [DOI] [PubMed] [Google Scholar]
- 44.Tajiri N, Quach DM, Kaneko Y, Wu S, Lee D, Lam T, Hayama KL, Hazel TG, Johe K, Wu MC, et al. Behavioral and histopathological assessment of adult ischemic rat brains after intracerebral transplantation of NSI-566RSC cell lines. PLoS One 2014; 9 3:e91408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox CS, Jr, Baumgartner JE, Harting MT, Worth LL, Walker PA, Shah SK, Ewing-Cobbs L, Hasan KM, Day MC, Lee D, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery 2011; 68 3:588–600. [DOI] [PubMed] [Google Scholar]
- 46.Liao GP, Harting MT, Hetz RA, Walker PA, Shah SK, Corkins CJ, Hughes TG, Jimenez F, Kosmach SC, Day MC, et al. Autologous bone marrow mononuclear cells reduce therapeutic intensity for severe traumatic brain injury in children. Pediatr Crit Care Med 2015; 16 3:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest 2012; 122 11:3824–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 1991; 75 1:15–26. [DOI] [PubMed] [Google Scholar]
- 49.Enzmann GU, Benton RL, Talbott JF, Cao Q, Whittemore SR. Functional considerations of stem cell transplantation therapy for spinal cord repair. J Neurotrauma 2006; 23 (3–4):479–495. [DOI] [PubMed] [Google Scholar]
- 50.Fehlings MG, Vawda R. Cellular treatments for spinal cord injury: the time is right for clinical trials. Neurotherapeutics 2011; 8 4:704–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sahni V, Kessler JA. Stem cell therapies for spinal cord injury. Nat Rev Neurol 2010; 6 7:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol 2001; 19 12:1134–1140. [DOI] [PubMed] [Google Scholar]
- 53.Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 2001; 30 1:65–78. [DOI] [PubMed] [Google Scholar]
- 54.McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 1999; 5 12:1410–1412. [DOI] [PubMed] [Google Scholar]
- 55.Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 2010; 28 1:152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kulbatski I, Mothe AJ, Keating A, Hakamata Y, Kobayashi E, Tator CH. Oligodendrocytes and radial glia derived from adult rat spinal cord progenitors: morphological and immunocytochemical characterization. J Histochem Cytochem 2007; 55 3:209–222. [DOI] [PubMed] [Google Scholar]
- 57.Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci 2005; 8 3:346–353. [DOI] [PubMed] [Google Scholar]
- 58.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma 2011; 28 8:1611–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koda M, Okada S, Nakayama T, Koshizuka S, Kamada T, Nishio Y, Someya Y, Yoshinaga K, Okawa A, Moriya H, et al. Hematopoietic stem cell and marrow stromal cell for spinal cord injury in mice. Neuroreport 2005; 16 16:1763–1767. [DOI] [PubMed] [Google Scholar]
- 60.Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport 2000; 11 13:3001–3005. [DOI] [PubMed] [Google Scholar]
- 61.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant 2007; 40 7:609–619. [DOI] [PubMed] [Google Scholar]
- 62.Wong RS. Mesenchymal stem cells: angels or demons? J Biomed Biotechnol 2011; 2011:459510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee RH, Oh JY, Choi H, Bazhanov N. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. J Cell Biochem 2011; 112 11:3073–3078. [DOI] [PubMed] [Google Scholar]
- 64.Bakshi A, Hunter C, Swanger S, Lepore A, Fischer I. Minimally invasive delivery of stem cells for spinal cord injury: advantages of the lumbar puncture technique. J Neurosurg Spine 2004; 1 3:330–337. [DOI] [PubMed] [Google Scholar]
- 65.Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine (Phila Pa 1976) 2009; 34 4:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Attar A, Ayten M, Ozdemir M, Ozgencil E, Bozkurt M, Kaptanoglu E, Beksac M, Kanpolat Y. An attempt to treat patients who have injured spinal cords with intralesional implantation of concentrated autologous bone marrow cells. Cytotherapy 2011; 13 1:54–60. [DOI] [PubMed] [Google Scholar]
- 67.Kumar AA, Kumar SR, Narayanan R, Arul K, Baskaran M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: a phase I/II clinical safety and primary efficacy data. Exp Clin Transplant 2009; 7 4:241–248. [PubMed] [Google Scholar]
- 68.Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells 2010; 28 8:1446–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A 2010; 107 28:12704–12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A 2011; 108 40:16825–16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brower V. BioTime acquires Geron's stem cell program. Nat Biotechnol 2013; 31 2:94. [DOI] [PubMed] [Google Scholar]
- 72.Xu XM, Onifer SM. Transplantation-mediated strategies to promote axonal regeneration following spinal cord injury. Respir Physiol Neurobiol 2009; 169 2:171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heegard KD, Stewart IJ, Cap AP, Sosnov JA, Kwan HK, Glass KR, Morrow BD, Latack W, Henderson AT, Saenz KK, et al. Early acute kidney injury in military casualties. J Trauma Acute Care Surg 2015; 78 5:988–993. [DOI] [PubMed] [Google Scholar]
- 74.Feldman EL, Boulis NM, Hur J, Johe K, Rutkove SB, Federici T, Polak M, Bordeau J, Sakowski SA, Glass JD. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol 2014; 75 3:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012; 122 8:2731–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342 18:1334–1349. [DOI] [PubMed] [Google Scholar]
- 77.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353 16:1685–1693. [DOI] [PubMed] [Google Scholar]
- 78.Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. JAMA 283(15):1995–2002, 2000. [DOI] [PubMed] [Google Scholar]
- 79.Maron-Gutierrez T, Laffey JG, Pelosi P, Rocco PR. Cell-based therapies for the acute respiratory distress syndrome. Curr Opin Crit Care 2014; 20 1:122–131. [DOI] [PubMed] [Google Scholar]
- 80.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968; 6 2:230–247. [PubMed] [Google Scholar]
- 81.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells 2011; 29 6:913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pati S, Gerber MH, Menge TD, Wataha KA, Zhao Y, Baumgartner JA, Zhao J, Letourneau PA, Huby MP, Baer LA, et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One 2011; 6 9:e25171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rahbar E, Cardenas JC, Baimukanova G, Usadi B, Bruhn R, Pati S, Ostrowski SR, Johansson PI, Holcomb JB, Wade CE. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med 2015; 13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Potter DR, Baimukanova G, Keating SM, Deng X, Chu JA, Gibb SL, Peng Z, Muench MO, Fomin ME, Spinella PC, et al. Fresh frozen plasma and spray-dried plasma mitigate pulmonary vascular permeability and inflammation in hemorrhagic shock. J Trauma Acute Care Surg 2015; 78 (6 suppl 1):S7–S17. [DOI] [PubMed] [Google Scholar]
- 85.Gotts JE, Matthay MA. Mesenchymal stem cells and acute lung injury. Crit Care Clin 2011; 27 3:719–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012; 67 6:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A 2009; 106 38:16357–16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu HP, Shimizu T, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. J Leukoc Biol 2006; 79 5:963–970. [DOI] [PubMed] [Google Scholar]
- 89.Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock 2010; 33 1:24–30. [DOI] [PubMed] [Google Scholar]
- 90.Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 2015; 3 1:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu KD, Wilson JG, Zhuo H, Caballero L, McMillan ML, Fang X, Cosgrove K, Calfee CS, Lee JW, Kangelaris KN, et al. Design and implementation of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate-severe acute respiratory distress syndrome. Ann Intensive Care 2014; 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8 4:R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Case J, Khan S, Khalid R, Khan A. Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract 2013; 2013:479730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Podoll AS, Kozar R, Holcomb JB. KW: Finkel: incidence and outcome of early acute kidney injury in critically-ill trauma patients. PLoS One 2013; 8 10:e77376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldberg R, Dennen P. Long-term outcomes of acute kidney injury. Adv Chronic Kidney Dis 2008; 15 3:297–307. [DOI] [PubMed] [Google Scholar]
- 96.Imberti B, Tomasoni S, Ciampi O, Pezzotta A, Derosas M, Xinaris C, Rizzo P, Papadimou E, Novelli R, Benigni A, et al. Renal progenitors derived from human iPSCs engraft and restore function in a mouse model of acute kidney injury. Sci Rep 2015; 5:8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 2005; 289 1:F31–F42. [DOI] [PubMed] [Google Scholar]
- 98.Togel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev 2009; 18 3:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med 2004; 14 6:1035–1041. [PubMed] [Google Scholar]
- 100.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 2003; 112 1:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 2003; 14 5:1188–1199. [DOI] [PubMed] [Google Scholar]
- 102.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 2004; 15 7:1794–1804. [DOI] [PubMed] [Google Scholar]
- 103.Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant 2012; 27 8:3037–3042. [DOI] [PubMed] [Google Scholar]
- 104.Westenfelder C, Togel FE. Protective actions of administered mesenchymal stem cells in acute kidney injury: relevance to clinical trials. Kidney Int Suppl 2011; 1 3:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosner MH, Portilla D, Okusa MD. Cardiac surgery as a cause of acute kidney injury: pathogenesis and potential therapies. J Intensive Care Med 2008; 23 1:3–18. [DOI] [PubMed] [Google Scholar]
- 106.Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA 2012; 307 11:1169–1177. [DOI] [PubMed] [Google Scholar]
- 107.Sifri ZC, Kaiser VL, Ananthakrishnan P, Wang L, Mohr AM, Hauser CJ, Rameshwar P, Deitch EA, Livingston DH. Bone marrow failure in male rats following trauma/hemorrhagic shock (T/HS) is mediated by mesenteric lymph and modulated by castration. Shock 2006; 25 1:12–16. [DOI] [PubMed] [Google Scholar]
- 108.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, Rameshwar P. Bone marrow failure following severe injury in humans. Ann Surg 2003; 238 5:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hannoush EJ, Elhassan I, Sifri ZC, Mohr AA, Alzate WD, Livingston DH. Role of bone marrow and mesenchymal stem cells in healing after traumatic injury. Surgery 2013; 153 1:44–51. [DOI] [PubMed] [Google Scholar]
- 110.Gore AV, Bible LE, Livingston DH, Mohr AM, Sifri ZC. Can mesenchymal stem cells reverse chronic stress-induced impairment of lung healing following traumatic injury? J Trauma Acute Care Surg 2015; 78 4:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stannard A, Scott DJ, Ivatury RA, Miller DL, Ames-Chase AC, Feider LL, Porras CA, Gifford SM, Rasmussen TE. A collaborative research system for functional outcomes following wartime extremity vascular injury. J Trauma Acute Care Surg 2012; 73 (2 suppl 1):S7–S12. [DOI] [PubMed] [Google Scholar]
- 112.Crowley C, Wong JM, Fisher DM, Khan WS. A systematic review on preclinical and clinical studies on the use of scaffolds for bone repair in skeletal defects. Curr Stem Cell Res Ther 2013; 8 3:243–252. [DOI] [PubMed] [Google Scholar]
- 113.Granero-Molto F, Weis JA, Longobardi L, Spagnoli A. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther 2008; 8 3:255–268. [DOI] [PubMed] [Google Scholar]
- 114.Percival TJ, Rasmussen TE. Reperfusion strategies in the management of extremity vascular injury with ischaemia. Br J Surg 2012; 99 suppl 1:66–74. [DOI] [PubMed] [Google Scholar]
- 115.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008; 26 8:2173–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harvey EJ, Sanders DW, Shuler MS, Lawendy AR, Cole AL, Alqahtani SM, Schmidt AH. What's new in acute compartment syndrome? J Orthop Trauma 2012; 26 12:699–702. [DOI] [PubMed] [Google Scholar]
- 117.Cancio LC, Horvath EE, Barillo DJ, Kopchinski BJ, Charter KR, Montalvo AE, Buescher TM, Brengman ML, Brandt MM, Holcomb JB. Burn support for Operation Iraqi Freedom and related operations, 2003 to 2004. J Burn Care Rehabil 2005; 26 2:151–161. [DOI] [PubMed] [Google Scholar]
- 118.Osler T, Glance LG, Hosmer DW. Simplified estimates of the probability of death after burn injuries: extending and updating the baux score. J Trauma 2010; 68 3:690–697. [DOI] [PubMed] [Google Scholar]
- 119.Thomas SJ, Kramer GC, Herndon DN. Burns: military options and tactical solutions. J Trauma 2003; 54 (5 suppl):S207–S218. [DOI] [PubMed] [Google Scholar]
- 120.Philandrianos C, Andrac-Meyer L, Mordon S, Feuerstein JM, Sabatier F, Veran J, Magalon G, Casanova D. Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns 2012; 38 6:820–829. [DOI] [PubMed] [Google Scholar]
- 121.Lewis CJ. Stem cell application in acute burn care and reconstruction. J Wound Care 22(1): 7–8, 10, 12-6, 2013. [DOI] [PubMed] [Google Scholar]
- 122.Badiavas AR, Badiavas EV. Potential benefits of allogeneic bone marrow mesenchymal stem cells for wound healing. Expert Opin Biol Ther 2011; 11 11:1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Badiavas EV, Ford D, Liu P, Kouttab N, Morgan J, Richards A, Maizel A. Long-term bone marrow culture and its clinical potential in chronic wound healing. Wound Repair Regen 2007; 15 6:856–865. [DOI] [PubMed] [Google Scholar]
- 124.Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res 2009; 12 5:359–366. [DOI] [PubMed] [Google Scholar]
- 125.Bey E, Prat M, Duhamel P, Benderitter M, Brachet M, Trompier F, Battaglini P, Ernou I, Boutin L, Gourven M, et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen 2010; 18 1:50–58. [DOI] [PubMed] [Google Scholar]
- 126.Schurr MJ, Foster KN, Centanni JM, Comer AR, Wicks A, Gibson AL, Thomas-Virnig CL, Schlosser SJ, Faucher LD, Lokuta MA, et al. Phase I/II clinical evaluation of StrataGraft: a consistent, pathogen-free human skin substitute. J Trauma 2009; 66 3:866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Asatrian G, Pham D, Hardy WR, James AW, Peault B. Stem cell technology for bone regeneration: current status and potential applications. Stem Cells Cloning 2015; 8:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fan ZX, Lu Y, Deng L, Li XQ, Zhi W, Li-Ling J, Yang ZM, Xie HQ. Placenta- versus bone-marrow-derived mesenchymal cells for the repair of segmental bone defects in a rabbit model. FEBS J 2012; 279 13:2455–2465. [DOI] [PubMed] [Google Scholar]
- 129.Usas A, Maciulaitis J, Maciulaitis R, Jakuboniene N, Milasius A, Huard J. Skeletal muscle-derived stem cells: implications for cell-mediated therapies. Medicina (Kaunas) 2011; 47 9:469–479. [PubMed] [Google Scholar]
- 130.Uhrig BA, Boerckel JD, Willett NJ, Li MT, Huebsch N, Guldberg RE. Recovery from hind limb ischemia enhances rhBMP-2-mediated segmental bone defect repair in a rat composite injury model. Bone 2013; 55 2:410–417. [DOI] [PubMed] [Google Scholar]
- 131.Knoepfler PS. From bench to FDA to bedside: US regulatory trends for new stem cell therapies. Adv Drug Deliv Rev 2015; 82-83:192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fink DW., Jr FDA regulation of stem cell-based products. Science 2009; 324 5935:1662–1663. [DOI] [PubMed] [Google Scholar]
- 133.Au P, Hursh DA, Lim A, Moos MC, Jr, Oh SS, Schneider BS, Witten CM. FDA oversight of cell therapy clinical trials. Sci Transl Med 2012; 4 149:149fs31. [DOI] [PubMed] [Google Scholar]
- 134.American Association of Blood Banks. Standards for Cellular Therapy Services. 6th edition. AABB Press, 2013. [Google Scholar]
- 135.Li MD, Atkins H, Bubela T. The global landscape of stem cell clinical trials. Regen Med 2014; 9 1:27–39. [DOI] [PubMed] [Google Scholar]
- 136.Arcidiacono JA, Blair JW, Benton KA. US Food and Drug Administration international collaborations for cellular therapy product regulation. Stem Cell Res Ther 2012; 3 5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cyranoski D. Japan to offer fast-track approval path for stem cell therapies. Nat Med 2013; 19 5:510. [DOI] [PubMed] [Google Scholar]
- 138.Rosemann A. Medical innovation and national experimental pluralism: insights from clinical stem cell research and applications in China. BioSocieties 8:58–74, 2013. [Google Scholar]
- 139.Sharma A, Sane H, Kulkarni P, Yadav J, Gokulchandran N, Biju H, Badhe P. Cell therapy attempted as a novel approach for chronic traumatic brain injury—a pilot study. Springerplus 2015; 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sabatino M, Ren J, David-Ocampo V, England L, McGann M, Tran M, Kuznetsov SA, Khuu H, Balakumaran A, Klein HG, et al. The establishment of a bank of stored clinical bone marrow stromal cell products. J Transl Med 2012; 10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368 16:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McCullough J, Wagner J, Gee A, Rooney C, Whiteside T, Donnenberg A, Lindblad R. Production assistance for cellular therapies. Transfusion 2004; 44 12:1793–1795. [DOI] [PubMed] [Google Scholar]
- 143.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Ann Surg 2014; 260 1:13–21. [DOI] [PubMed] [Google Scholar]
- 144.Trunkey DD. Trauma. Accidental and intentional injuries account for more years of life lost in the U. S. than cancer and heart disease. Among the prescribed remedies are improved preventive efforts, speedier surgery and further research. Sci Am 1983; 249 2:28–35. [PubMed] [Google Scholar]
- 145.Bulger EM, May S, Brasel KJ, Schreiber M, Kerby JD, Tisherman SA, Newgard C, Slutsky A, Coimbra R, Emerson S, et al. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA 2010; 304 13:1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bulger EM, May S, Kerby JD, Emerson S, Stiell IG, Schreiber MA, Brasel KJ, Tisherman SA, Coimbra R, Rizoli S, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg 2011; 253 3:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Delano MJ, Rizoli SB, Rhind SG, Cuschieri J, Junger W, Baker AJ, Dubick MA, Hoyt DB, Bulger EM. Prehospital resuscitation of traumatic hemorrhagic shock with hypertonic solutions worsens hypocoagulation and hyperfibrinolysis. Shock 2015; 44 1:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


