Abstract
Purpose
Potentials of 68Ga-NEB as a PET tracer in the evaluation of a variety of lymphatic drainage disorders were analyzed.
Methods
68Ga-NEB was injected subcutaneously, and the PET/CT images were acquired in 13 patients with different suspected lymphatic drainage abnormality. The 68Ga-NEB PET/CT findings were compared with 99mTc-SC lymphoscintigraphy.
Results
68Ga-NEB activity could be clearly observed in the lymphatic route on the PET/CT images from all the patients. In 5 (38.5%) of 13 patients tested, 68Ga-NEB PET/CT provided more information than the 99mTc-SC lymphoscintigraphy.
Conclusions
68Ga-NEB PET/CT can be used as an alternative of 99mTc-SC lymphoscintigraphy in the evaluation of lymphatic disorders, which enables fast results and might be more accurate than the conventional 99mTc-SC lymphoscintigraphy.
Key Words: 68Ga; chyloperitoneum; chylothorax; Evans blue; lymphangioleiomyomatosis; lymphedema, chylothorax, chyloperitoneum; lymphoscintigraphy; PET/CT
Disorders of the lymphatic system are diverse and include lymphedema, chyloperitoneum, chyluria, and chylothorax. Lymphoscintigraphy using 99mTc-SC is inexpensive with a safety profile and has long been used in the evaluation of pathologies involving lymphatic drainage.1–7 However, 99mTc-SC scintigraphy also has several disadvantages, including minimal absorption from the injection site and slow transport from the injection site after subcutaneous administration.8 Evans blue dye is used for the determination of plasma volume and map for the drainage basins of sentinel node localization.9 It has fast rate of migration through the lymphatic channels when subcutaneously administered.9 It is reported before that when scintigraphy and Evans blue dye are used in tandem, false-negative rate for sentinel node localization is decreased compared with using either agent alone.10
Previous studies have reported the99mTc-labeled Evans blue (99mTc-EB) for lymphatic mapping in animal models.11–14 It demonstrated that 99mTc-EB had the same pharmacodynamic properties as Evans blue when incorporated into the cells of the lymph node or became bound to the parenchyma in the lymphatic system. However, those studies also found its limitation in visualizing deep lymph nodes.13 A recent report showed that 18F-AlF-NEB can distinguish lymph nodes by the apparent blue color and high=intensity PET signal in both inflammation and orthotropic breast cancer animal models.15 More recently, NOTA-conjugated truncated Evans blue was labeled with 68Ga-NEB and tested for its safety and diagnosis of hepatic hemangioma using PET/CT.16 In this current investigation, which was approved by the institutional review board of our hospital, we tried to assess the 68Ga-NEB PET/CT in the evaluation of lymphatic disorders.
METHODS AND PATIENTS
Patients and Methods
A total of 13 patients (5 men, 8 women; aged 17–66 years [41 ± 16 years]) were recruited in the analysis. These included 3 patients with lymphedema without known causes. Another 3 patients had postsurgical limb swelling (1 after mastectomy for breast cancer and 2 after oophorectomy for ovary cancers). Seven patients had other types of chyle leak including chylothorax, chyloperitoneum, and chyluria determined by the referring physicians (Table 1). The chylothorax, chyloperitoneum, and chyluria were confirmed by laboratory examination. All of them underwent both 68Ga-NEB PET/CT and 99mTc-SC scintigraphy within 2 days of each other for suspected lymphatic system disorders. The exclusion criteria included the following: liver and kidney function impairment, low white blood cell count, or currently pregnant or breast-feeding. All patients signed a written informed consent form and were informed of the potential benefits and risks of participating in the investigation.
TABLE 1.
Patient Characteristics
99mTc-SC Scintigraphy
Within 2 days of 68Ga-NEB PET/CT, 99mTc-SC lymphoscintigraphy was performed. For most patients, the tracer was injected into first and second interdigital spaces of both feet (0.5 mL, 37 MBq). For the patient with upper limb swelling (patient 1), the tracer was administered subcutaneously between the thumb and index finger of each hand. Images were acquired with a double-head gamma camera with a low-energy high-resolution parallel whole collimator in whole-body scanning mode at a speed of 10 cm/min. Spot and whole-body images are obtained for up to 24 hours if necessary. The images were read jointly by 2 experienced nuclear medicine physicians.
68Ga-NEB PET/CT
All patients underwent 68Ga-NEB PET/CT. Preparation of NEB and 68Ga labeling were performed as described in previous publications.17 A Biograph 64 True Point TrueV PET/CT system (Siemens Medical Solutions, Erlangen, Germany) was used. For the patient with upper extremity swelling, the tracer (0.5 mL, 37 MBq/hand) was injected into the subcutaneous tissue between the thumb and index finger of each hand. For other patients, 68Ga-NEB was injected subcutaneously into the bilateral first web spaces of the feet (0.5 mL, 37 MBq/foot), followed by massage of the injection sites. The patients were requested to walk after tracer injection. For those patients with suspected chylothorax, chyloperitoneum, or chyluria, the images were acquired 5 to 20 minutes after tracer injection. For those with lymphedema, longer interval up to 1 hour between the tracer injection and image acquisition was applied. Whole-body images were acquired using a low-dose CT scan (120– kV, 35 mA, 3-mm layer, 512 × 512 matrix, 70 cm FOV). PET acquisition was performed (8–11 bed positions, 2 min/bed).
RESULTS
68Ga-NEB Distribution
68Ga-NEB activity can clearly visualize lymphatic vessels and lymph nodes by PET/CT in all patients. Distribution of 68Ga NEB was also found in the heart and major vessels. Renal and hepatic activity gradually increased radioactivity over time. The spleen can also have mild activity.
Time Differences Between 99mTc-SC Lymphoscintigraphy and 68Ga-NEB PET/CT in Acquiring Sufficient Information for Diagnosis
68Ga NEB PET/CT needs significantly less time waiting after tracer injection to perform than the 99mTc-SC lymphoscintigraphy. In our patient population, the 68Ga NEB PET/CT images were acquired between 10 and 90 minutes after tracer administration. In comparison, the images of 99mTc-SC lymphoscintigraphy had to be acquired much later. The time of completing image acquisition after tracer injection is 0.9 ± 0.2 hour (mean ± SD) for 68Ga NEB PET/CT scan, which is significantly shorter (P < 0.01) than for the 99mTc-SC lymphoscintigraphy (7.8 ± 6.9 hours).
Image Findings Between 99mTc-SC Lymphoscintigraphy and 68Ga NEB PET/CT
Among all 13 patients, the results of 99mTc-SC lymphoscintigraphy and 68Ga NEB PET/CT were consistent in 8 of them (Figs. 1, 2), although the results of 68Ga NEB PET/CT were obtained much faster. 68Ga NEB PET/CT provided more information in 5 patients (38.5%). In 1 patient with chyloperitoneum, 99mTc-SC lymphoscintigraphy was unable to localize the site of the chyle leak, whereas 68Ga NEB PET/CT successfully identified leak (Fig. 3). In 2 patients with chylothorax, 99mTc-SC lymphoscintigraphy was unable to identify the sites of the leak. In contrast, the sites of the leakage were found after 68Ga-NEB PET/CT (Fig. 4). In 1 patient who had postsurgical limp swelling, the site of the chyle leak was identified only by 68Ga-NEB PET/CT but not by 99mTc-SC lymphoscintigraphy (Fig. 5). In a young female patient who had cystic lesions and pleural effusion, the site of chest abnormality was visualized on 68Ga-NEB PET/CT but not on 99mTc-SC lymphoscintigraphy (Fig. 6). The abdominal and pelvic lesions of the same patient were also better visualized by 68Ga-NEB study than the 99mTc-SC images (Figs. 6, 7). A diagnosis of lymphangioleiomyomatosis, a rare slowly progressive, low-grade, metastasizing neoplasm of women that spreads primarily through the lymphatic channels,18,19 was subsequently made in this patient.
FIGURE 1.
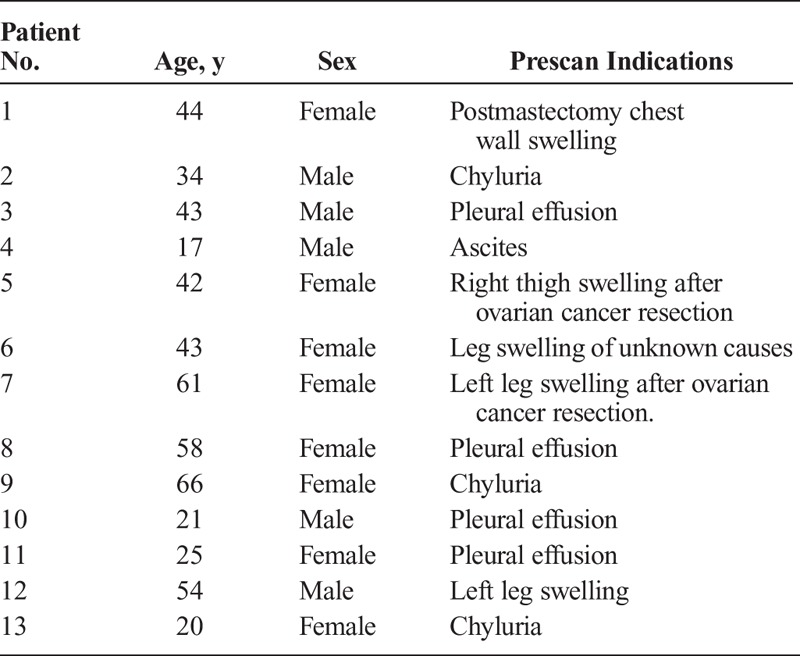
A 43-year-old woman (patient 6) with symptoms of swollen lower limbs for more than 4 years. Both anterior 99mTc-SC scintigraphy (A) and 68Ga-NEB PET/CT MIP image (B) acquired 60 minutes after tracer injection showed dermal backflow on bilateral ankles and feet (small arrows) and lateral left calf (large arrows). On both transaxial (C and D) and coronal (E and F) images of 68Ga-NEB PET/CT, the abnormal radioactivity accumulation corresponded to tubular low density (large arrows), which was consistent with a diagnosis of lymphatic cyst. Dermal backflow in the feet/ankle was also clearly visualized on the tomographic image (E: small arrow).
FIGURE 2.
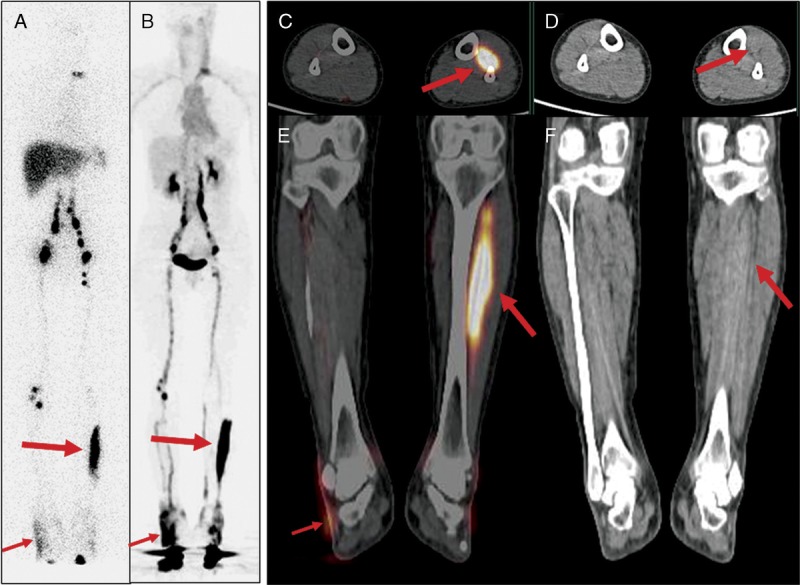
A 42-year-old woman (patient 5) developed swelling in her right upper thigh and right lower abdomen and pelvis after total abdominal hysterectomy with bilateral salpingo-oophorectomy due to ovary cancer. On both anterior (A) and posterior (B) images of 99mTc-SC scintigraphy acquired 5 hours after injection, there was significant dermal backflow (thin red arrows) in the right lower abdomen/right pelvis and right thigh, consistent with lymphedema and the clinical symptoms. Inguinal nodes (thick arrow) and right iliac nodes (curved arrow) were also noted. Similar findings could be found on the MIP image (C) of 68Ga-NEB PET, which was acquired 1 hour after tracer injection. All findings (dermal backflow: thin red arrow; inguinal nodes: thick arrows; and iliac nodes: curved arrow) were better appreciated on transaxial fusion images (D) of 68Ga-NEB PET/CT.
FIGURE 3.
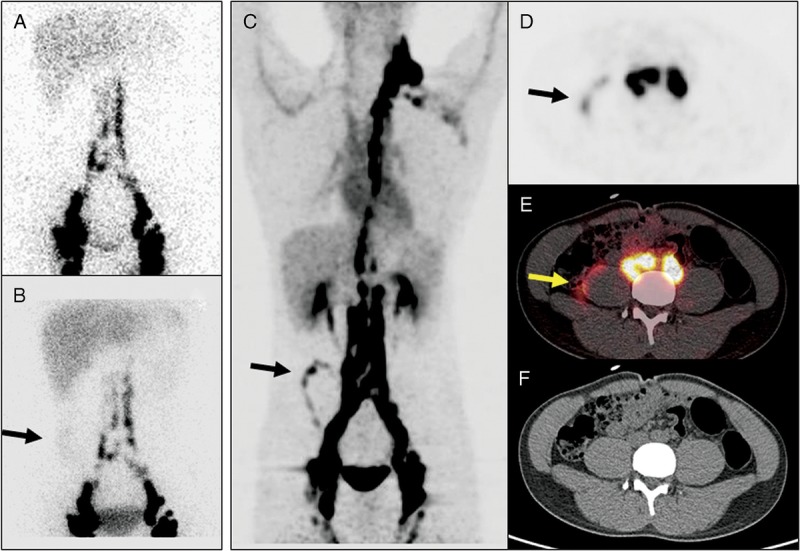
A 17-year-old man (patient 4) with abdominal discomfort for more than 6 months. Chylous ascites was suspected, and nuclear medicine was consulted. 99mTc-SC anterior image of the abdomen acquired 60 minutes after injection (A) was unremarkable. The image acquired 6 hours after injection (B), however, revealed diffuse, mild radioactivity on the right side of the lower abdomen (arrow). On 68Ga-NEB MIP image (C) acquired 5 minutes after tracer injection, clear linear activity (arrow) could be seen in the right abdomen. On transaxial PET (D), fusion (E), and CT (F) images, this activity (arrows) was located in the immediate anterolateral border of the right psoas muscle, indicating the site of the chyle leak. Intense activity in the inguinal, iliac, and paraspinal lymph nodes and thoracic duct was also better appreciated on 68Ga-NEB PET/CT images.
FIGURE 4.
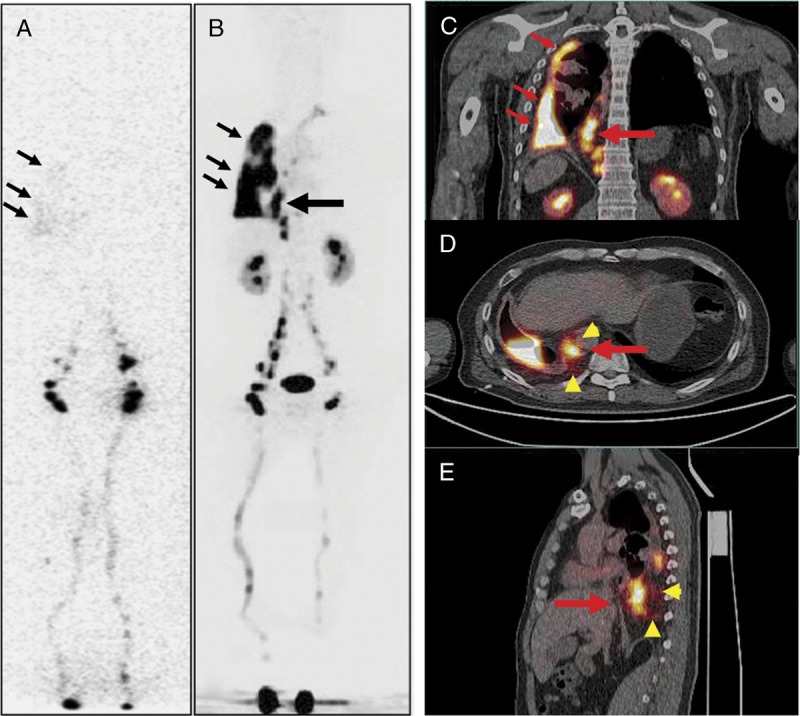
A 43-year-old man with the right side of persistent pleural effusion (patient 3). The patient had a remote history of motor vehicle accident. Laboratory examination of the fluid after thoracocentesis demonstrated chylothorax. 99mTc-SC scintigraphy (A) revealed diffuse, mild activity in the right chest, consistent with the clinical findings of right chylothorax. However, the potential site of the chyle leak could not be identified. In comparison, 68Ga-NEB PET/CT (B, MIP; C: coronal fusion; D: axial fusion; E: sagittal fusion) not only showed activity in the right chest pleural effusion (small arrows), but also clearly revealed an additional vertically linear intense activity (large arrow) centered in the dilated thoracic duct and cisterna chyli with mild activity surrounding (arrowheads), consistent with the site of the leak.
FIGURE 5.
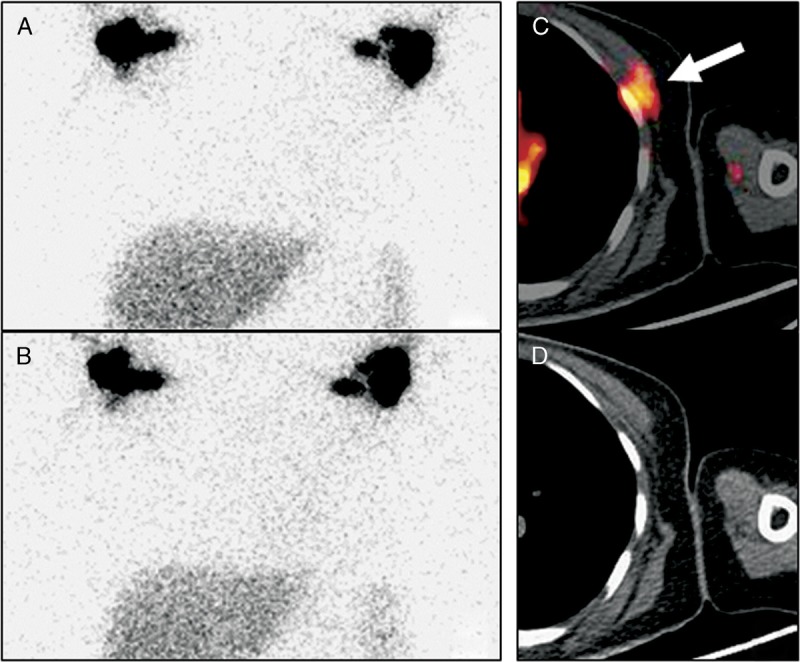
Anterior 99mTc-SC images were acquired at 2 hours (A) and 6 hours (B) after subcutaneous tracer injection between the thumb and index finger of the hands in a 44-year-old woman (patient 1) who had left chest swelling and was status post left mastectomy for breast cancer. The images revealed axillary lymph nodes bilaterally and minimally more tracer activity in the left chest without evidence of the site of the leak. In comparison, the transaxial images (C: fusion; D: CT) of the 68Ga-NEB PET/CT acquired at 30 minutes after injection demonstrated a focal activity (arrow) underneath the left pectoralis major muscle, at the level of the left anterior fourth rib, indicating the site of the chyle leak.
FIGURE 6.
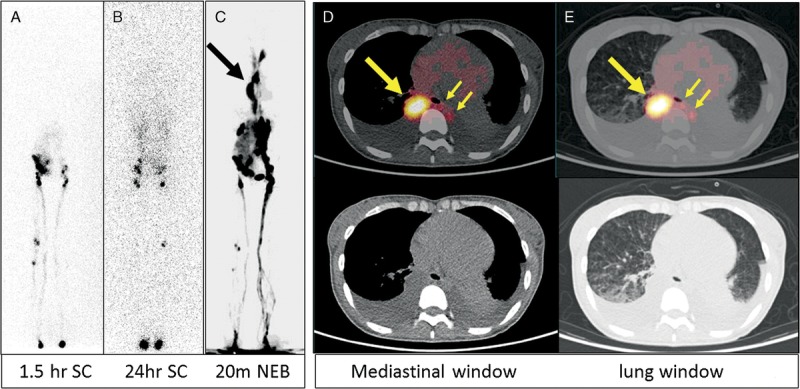
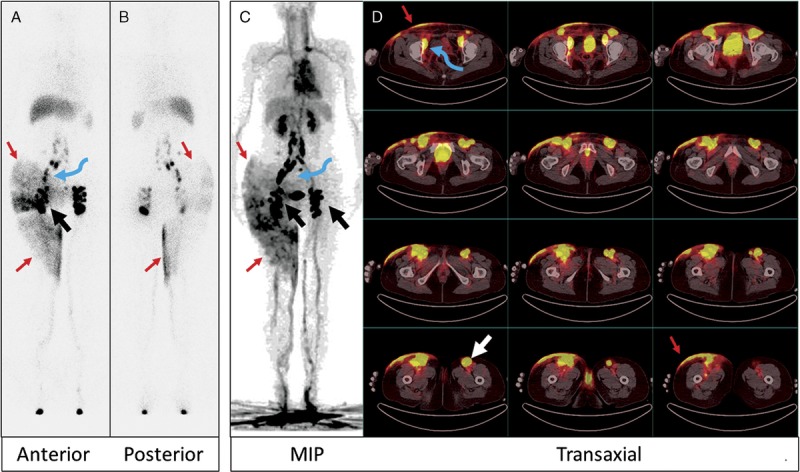
A 25-year-old woman presented with shortness of breath for 3 months (patient 11). A diagnostic CT (images not shown) revealed many cystic structures in the chest, abdomen, and pelvis. In addition, bilateral pleural effusion was noted, which was subsequently shown as chylothorax. For this reason, lymphoscintigraphy was ordered. The 99mTc-SC images at 1.5 hours (A) and 24 hours (B) after injection in the feet both showed that the tracer reached abdomen/pelvis without much chest activity. However, on 68Ga-NEB MIP PET image (C) acquired 20 minutes after tracer injection, there was clear, intense vertical activity (large arrow) in the thorax. On transaxial images (D: mediastinal window; E: lung window), the vertical activity was in an enlarged thoracic duct (large arrows). The chyle leak (small arrows) from the thoracic duct to the left chest was also noted. There were also many small cysts in both lungs (E).
FIGURE 7.
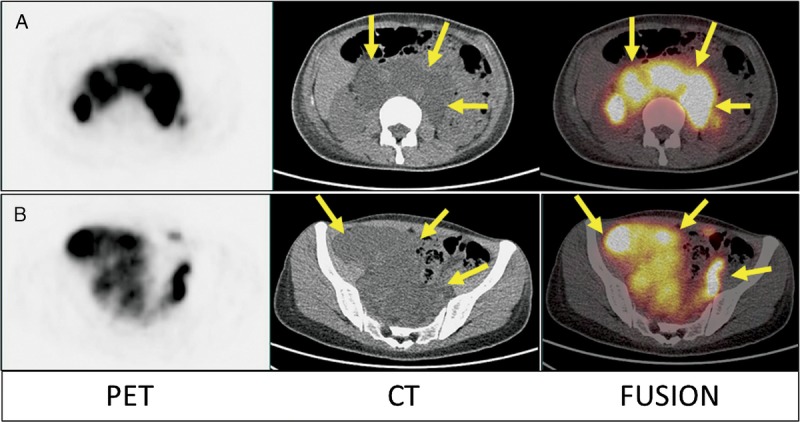
Furthermore, the transaxial images of the abdomen (A) and pelvis (B) of 68Ga-NEB PET/CT from the same patient demonstrated that all of the hypodense cystic structures were filled with radioactive lymph fluid (small arrows), which was not impressively seen on 99mTc-SC scintigraphy. Eventually, the patient was proven to have lymphangioleiomyomatosis.
DISCUSSION
Evans blue dye is usually used in defining the drainage basins and mapping for the localization of tumor sentinel node before surgery. When Evans blue diffuses into the cellular matrix, it binds to endogenous proteins forming an Evans blue–protein complex, which can specifically penetrate the single layers of gracile lymphatic endothelial cells, but does not migrate into the venous capillary network as the size of the complex is too large to enter arteriovenous capillaries.20–23 Our results show that 68Ga-NEB can be clearly observed in the lymphatic vessels and lymph nodes on the PET/CT images of the patients, which shows similar migration properties as Evans blue and 99mTc-labeled Evans.11–13 Evans blue is known to be metabolized in the liver by cytochrome C (P450) reductase and then excreted through the renal route as colorless, radioactive metabolites.24 We also observed slight distribution of radioactivity in the liver and prominent visualization of the kidneys over time on 68Ga-NEB PET/CT scans. The cardiac blood pool and spleen also had radioactivity distribution. In our patient population, when comparing with 99mTc-SC lymphoscintigraphy, 68Ga NEB PET/CT can be completed much faster. This is because99mTc-SC lymphoscintigraphy requires long waiting time before scan can be completed because of the problem that the radiolabeled SCs are relatively large particle sizes that do not move effectively. In addition, 68Ga-NEB PET/CT appears more revealing than 99mTc-SC lymphoscintigraphy because in 30.7% (4/13) patients 68Ga NEB PET/CT presented more clinically important information than did 99mTc-SC lymphoscintigraphy. This is conceivable because 68Ga NEB PET/CT images are 3-dimensional and have CT to correlation, whereas traditional 99mTc-SC lymphoscintigraphy acquires only static images without CT correlation, which has intrinsic disadvantage compared with PET/CT.
We feel that much faster moving of 68Ga-NEB along the lymphatic route itself at least also plays a role in increasing the accuracy of the study. When 99mTc-SC slowly leaked over several hours, the leaked radioactivity could spread to a larger region, which might obscure the true site of the leak (Figs. 3B, 8A). In contrast, the high concentration of 68Ga-NEB activity at the leak site can be determined prior to the activity diffused into adjacent region (Figs. 3C–E, 8B, C). This difference between speeds of moving along the lymphatic route between these 2 tracers might also be a contributor of higher accuracy of 68Ga-NEB PET/CT.
FIGURE 8.
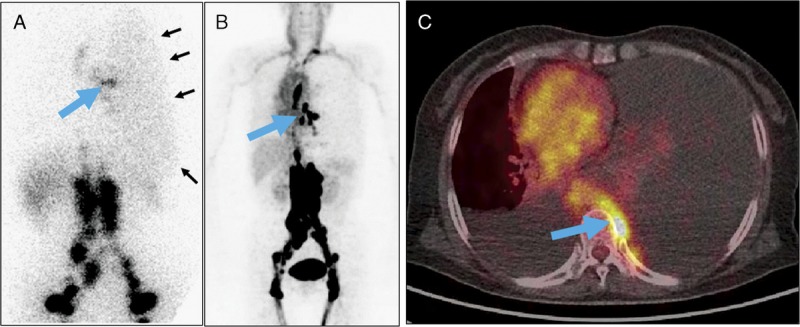
A 58-year-old woman (patient 8) presented with chest tightness for 8 months. Routine examination revealed pleural effusion in both sides of the thorax, more severe on the left side. Laboratory examination of thoracocentesis drainage confirmed chylothorax. 99mTc-SC scintigraphy (A) at 1 hour after injection showed relatively focal radioactivity (large arrow) in the left middle chest. In addition, mild, diffuse radioactivity (small arrows) in the entire left thorax was noted. The abnormal radioactivity distribution in the left mediastinal area is much more obvious on 68Ga-MIP image (B) of NEB PET/CT acquired 15 minutes after tracer injection. Interestingly, the activity in the left pleural effusion was not as obvious on PET images as the 99mTc-SC image (compared with A). The PET/CT fusion image demonstrated that this activity (large arrow) was on the left of T7 vertebral body (C).
Many publications in recent years have shown that adding SPECT/CT images to routine planar 99mTc-SC lymphoscintigraphy can significantly add diagnostic value of the study.25–30 However, 68Ga NEB PET/CT still has advantage over 99mTc-SC lymphoscintigraphy with SPECT/CT image acquisition. For example, acquisition of images covering from feet to the shoulder is generally necessary to evaluate different lymphatic drainage disorders. However, it takes only approximately 20 minutes for modern PET/CT scanner to acquire images from feet to the neck. In contrast, it needs several hours to acquire multiple bed position SPECT/CT images. Long time under scanner is causing not only inconvenience to the patients and nuclear medicine technologists, but also a source of mismatch between SPECT and CT images because patient movement becomes difficult to avoid during very long scanning time. In addition, SPECT/CT images per se will be unable to solve the necessary long-waiting period between the 99mTc-SC injection and final image acquisition.
CONCLUSION
68Ga-NEB PET/CT is a promising technique in the evaluation of lymphatic drainage abnormality, which can reach the diagnosis much earlier than the conventional 99mTc-SC lymphoscintigraphy and is also possibly more accurate. However, more comparison studies that include SPECT/CT images in 99mTc-SC lymphoscintigraphy are necessary to determine whether 68Ga-NEB PET/CT is more accurate than 99mTc-SC lymphoscintigraphy with SPECT/CT.
Footnotes
Conflicts of interest and sources of funding: none declared.
REFERENCES
- 1. Witte MH, Williams WH. Chylothorax and chyloperitoneum. N Engl J Med. 2006; 354: 879; author reply 879. [DOI] [PubMed] [Google Scholar]
- 2. Kazemzadeh GH, Sadeghi R, Ebrahimi E, et al. A successful experience in managing a chylous reflux: importance of lymphoscintigraphy. Clin Nucl Med. 2014; 39: 485– 487. [DOI] [PubMed] [Google Scholar]
- 3. Kim DW, Kim MH, Kim CG. Lymphoscintigraphy revealed chyloperitoneum after gastrectomy for gastric cancer. Clin Nucl Med. 2015; 40: 41– 44. [DOI] [PubMed] [Google Scholar]
- 4. Pui MH, Yueh TC. Lymphoscintigraphy in chyluria, chyloperitoneum and chylothorax. J Nucl Med. 1998; 39: 1292– 1296. [PubMed] [Google Scholar]
- 5. Pena Quian Y, Hernandez Ramirez P, Batista Cuellar JF, et al. Lymphoscintigraphy for the assessment of autologous stem cell implantation in chronic lymphedema. Clin Nucl Med. 2015; 40: 217– 219. [DOI] [PubMed] [Google Scholar]
- 6. Bender B, Murthy V, Chamberlain RS. The changing management of chylothorax in the modern era. Eur J Cardiothorac Surg. 2016; 49: 18– 24. [DOI] [PubMed] [Google Scholar]
- 7. Oh JK, Yoon HE, Chung YA. Lymphoscintigraphic demonstration of chyle leak after kidney transplantation and gamma camera detection of radioactivity in chylous aspirate. Clin Nucl Med. 2014; 39: 760– 761. [DOI] [PubMed] [Google Scholar]
- 8. Hung JC, Wiseman GA, Wahner HW, et al. Filtered technetium-99m-sulfur colloid evaluated for lymphoscintigraphy. J Nucl Med. 1995; 36: 1895– 1901. [PubMed] [Google Scholar]
- 9. Margouleff D. Blood volume determination, a nuclear medicine test in evolution. Clin Nucl Med. 2013; 38: 534– 537. [DOI] [PubMed] [Google Scholar]
- 10. Wong SL, Edwards MJ, Chao C, et al. Sentinel lymph node biopsy for breast cancer: impact of the number of sentinel nodes removed on the false-negative rate. J Am Coll Surg. 2001; 192: 684– 689; discussion 689–691. [DOI] [PubMed] [Google Scholar]
- 11. Green M, Farshid G, Kollias J, et al. The tissue distribution of Evans blue dye in a sheep model of sentinel node biopsy. Nucl Med Commun. 2006; 27: 695– 700. [DOI] [PubMed] [Google Scholar]
- 12. Sutton R, Tsopelas C, Kollias J, et al. Sentinel node biopsy and lymphoscintigraphy with a technetium 99m labeled blue dye in a rabbit model. Surgery. 2002; 131: 44– 49. [DOI] [PubMed] [Google Scholar]
- 13. Tsopelas C, Bellon M, Bevington E, et al. Lymphatic mapping with 99mTc-Evans blue dye in sheep. Ann Nucl Med. 2008; 22: 777– 785. [DOI] [PubMed] [Google Scholar]
- 14. Tsopelas C, Bevington E, Kollias J, et al. 99mTc-Evans blue dye for mapping contiguous lymph node sequences and discriminating the sentinel lymph node in an ovine model. Ann Surg Oncol. 2006; 13: 692– 700. [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, Lang L, Huang P, et al. In vivo albumin labeling and lymphatic imaging. Proc Natl Acad Sci U S A. 2015; 112: 208– 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Lang L, Zhu Z, et al. Clinical translation of an albumin-binding PET radiotracer 68Ga-NEB. J Nucl Med. 2015; 56: 1609– 1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Niu G, Lang L, Kiesewetter DO, et al. In vivo labeling of serum albumin for PET. J Nucl Med. 2014; 55: 1150– 1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gupta R, Kitaichi M, Inoue Y, et al. Lymphatic manifestations of lymphangioleiomyomatosis. Lymphology. 2014; 47: 106– 117. [PubMed] [Google Scholar]
- 19. Steagall WK, Taveira-DaSilva AM, Moss J. Clinical and molecular insights into lymphangioleiomyomatosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005; 22(suppl 1): S49– S66. [PubMed] [Google Scholar]
- 20. Moghimi SM, Bonnemain B. Subcutaneous and intravenous delivery of diagnostic agents to the lymphatic system: applications in lymphoscintigraphy and indirect lymphography. Adv Drug Deliv Rev. 1999; 37: 295– 312. [DOI] [PubMed] [Google Scholar]
- 21. Pfister G, Saesseli B, Hoffmann U, et al. Diameters of lymphatic capillaries in patients with different forms of primary lymphedema. Lymphology. 1990; 23: 140– 144. [PubMed] [Google Scholar]
- 22. Warren AG, Brorson H, Borud LJ, et al. Lymphedema: a comprehensive review. Ann Plast Surg. 2007; 59: 464– 472. [DOI] [PubMed] [Google Scholar]
- 23. Tsopelas C, Sutton R. Why certain dyes are useful for localizing the sentinel lymph node. J Nucl Med. 2002; 43: 1377– 1382. [PubMed] [Google Scholar]
- 24. Muskhelishvili L, Thompson PA, Kusewitt DF, et al. In situ hybridization and immunohistochemical analysis of cytochrome P450 1B1 expression in human normal tissues. J Histochem Cytochem. 2001; 49: 229– 236. [DOI] [PubMed] [Google Scholar]
- 25. Atkinson C, Banks K. Imaging idiopathic chylopericardium with 99mTc-SC lymphoscintigraphy and SPECT/CT. Clin Nucl Med. 2015; 40: e508– e510. [DOI] [PubMed] [Google Scholar]
- 26. Baulieu F, Bourgeois P, Maruani A, et al. Contributions of SPECT/CT imaging to the lymphoscintigraphic investigations of the lower limb lymphedema. Lymphology. 2013; 46: 106– 119. [PubMed] [Google Scholar]
- 27. Han DY, Cheng MF, Yen RF, et al. Postoperative lymphocele demonstrated by lymphoscintigraphy SPECT/CT. Clin Nucl Med. 2012; 37: 374– 376. [DOI] [PubMed] [Google Scholar]
- 28. Yang J, Codreanu I, Zhuang H. Minimal lymphatic leakage in an infant with chylothorax detected by lymphoscintigraphy SPECT/CT. Pediatrics. 2014; 134: e606– e610. [DOI] [PubMed] [Google Scholar]
- 29. Prevot N, Tiffet O, Avet J, Jr, et al. Lymphoscintigraphy and SPECT/CT using 99mTc filtered sulphur colloid in chylothorax. Eur J Nucl Med Mol Imaging. 2011; 38: 1746. [DOI] [PubMed] [Google Scholar]
- 30. Weiss M, Schwarz F, Wallmichrath J, et al. Chylothorax and chylous ascites. Clinical utility of planar scintigraphy and tomographic imaging with SPECT/CT. Nuklearmedizin. 2015; 54: 231– 240. [DOI] [PubMed] [Google Scholar]


