Abstract
Little is known about how DNA damage and metabolism are interconnected. In this issue of Cancer Cell, Jeong and colleagues report that an important component of the DNA damage response is the SIRT4-mediated blockade of glutamine catabolism. Failure to shut down glutamine consumption results in unscheduled proliferation, genomic instability, and cancer.
DNA damage is a natural process that occurs in all cells under normal physiological conditions. Multiple cellular mechanisms sense the presence of DNA damage and trigger adaptive responses (collectively known as DNA damage response or DDR), which culminate in a proliferative arrest that allows for DNA repair. Defects in the DDR can lead to unrepaired DNA damage, including oncogenic DNA alterations, and eventually to cancer. In recent years, it has become evident that cell proliferation and metabolism are intimately connected (DeBerardinis et al., 2008), therefore implying that DDR-induced proliferative arrest must be accompanied by metabolic changes. However, although much is known about the molecular biology of the DDR, the metabolic consequences of DNA damage have remained largely unexplored until very recently. The DDR has been shown to increase the cellular antioxidant defenses through the production of NADPH by the pentose phosphate pathway (Bensaad et al., 2006; Cosentino et al., 2011). In other settings, the DDR downregulates glucose uptake and glycolysis (Zhou et al., 2002). These precedents support the concept that metabolic changes constitute an intrinsic aspect of the DDR.
A new study by Jeong et al. (2013) in this issue of Cancer Cell uncovers a new connection between the DDR and metabolism. By performing metabolic analyses of cells in the presence or absence of DNA damage, the authors confirmed previous reports indicating that DNA damage increases the flux through the pentose phosphate pathway (Bensaad et al., 2006; Cosentino et al., 2011). In addition, they found an unexpected decrease in glutamine uptake and in intermediates of the tricarboxylic acid (TCA) cycle. These initial observations set the focus on the connection between DNA damage, glutamine, and the TCA cycle.
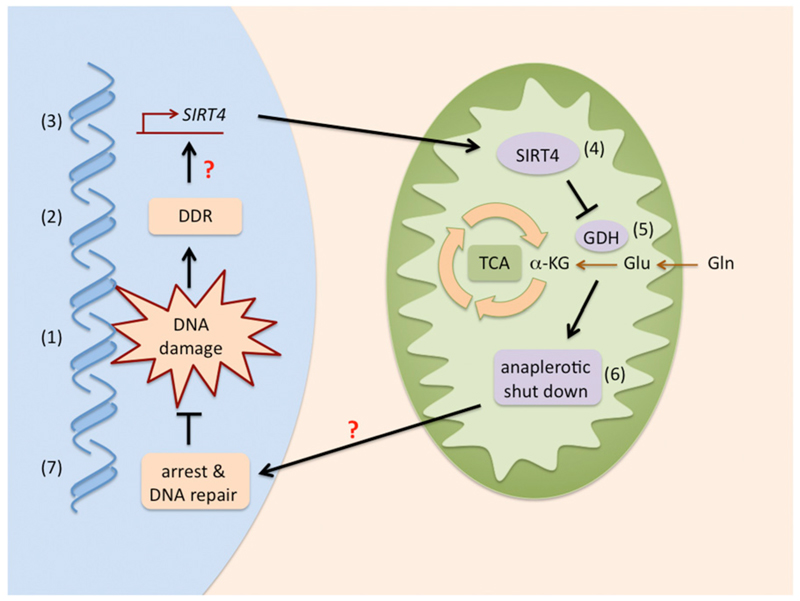
Quiescent cells use the TCA cycle to produce energy from glucose. In contrast, proliferating cells mostly use it for a completely different purpose: as a carbon source for lipogenesis through the mitochondrial efflux of citric acid. This efflux must be compensated by an influx of TCA cycle intermediates, a process known as anaplerosis. Of relevance, glutamine is the main source for TCA anaplerosis in proliferating cells (DeBerardinis et al., 2008). In a first reaction, glutamine is converted into glutamate by glutaminase (GLS) and then into α-ketoglutarate (αKG) by either glutamate dehydrogenase (GDH) or, less prominently, by transamination-coupled reactions. Jeong et al. (2013) characterize how several types of DNA damage block glutamine anaplerosis in proliferating cells. They had previously shown that SIRT4 ADP-ribosylates and inhibits GDH (Haigis et al., 2006), and based on this, they reasoned that SIRT4 might be involved in the inhibition of glutamine uptake and anaplerosis triggered by DNA damage. SIRT4 is a member of the sirtuin family (SIRT1–7) of protein deacetylases and ADP-ribosylases involved in multiple cellular processes, including the maintenance of genomic stability and regulation of metabolism (Sebastián et al., 2012). Interestingly, SIRT4 mRNA levels were highly induced upon different types of DNA damage, even higher than other sirtuin members previously related to the DDR, such as SIRT1 or SIRT3. Importantly, the authors demonstrate that SIRT4-mediated inhibition of glutamine anaplerosis is necessary for efficient cell cycle arrest upon DNA damage (Figure 1). In the absence of SIRT4, failure to arrest the cell cycle in response to DNA damage results in delayed DNA repair and increased chromosomal aneuploidies. Even more, SIRT4-deficient primary fibroblasts already show aberrant levels of polyploidy, suggesting that SIRT4 is important not only in response to exogenously inflicted DNA damage, but also to protect cells from spontaneous damage.
Figure 1. SIRT4: The Glutamine Gatekeeper.
DNA damage (1) elicits the DDR (2) that, through undefined mechanisms (indicated with a question mark), results in enhanced SIRT4 transcription (3) and higher SIRT4 activity in the mitochondria (4). In turn, SIRT4 inhibits glutamine conversion into αKG by inhibiting glutamate dehydrogenase (GDH) (5). Decreased αKG shuts down the anaplerotic replenishment of the tricarboxylic acid cycle (TCA) (6), which, through mechanisms that remain to be clarified (question mark), result in arrest and DNA repair (7).
The above findings suggest that the SIRT4-mediated blockade of glutamine anaplerosis could be a tumor suppressor mechanism. Indeed, Jeong et al. (2013) present multiple lines of evidence. First, they show that SIRT4-deficient fibroblasts grow faster than their wild-type counterparts. Also, neoplastic SIRT4-deficient fibroblasts are less dependent on glucose and form larger allograft tumors than SIRT4-proficient cells. These pro-tumorigenic phenotypes were reversed when cells were treated with GLS1 or GDH inhibitors or upon ectopic expression of catalytically active, but not catalytically dead, SIRT4. Moreover, several human malignancies present reduced SIRT4 mRNA levels, and this is associated with a poorer outcome in the case of lung adenocarcinomas.
The authors recapitulate their main findings in genetically modified mice lacking SIRT4 (Jeong et al., 2013). Importantly, two independently generated strains of SIRT4-deficient mice present a significant incidence of spontaneous lung tumors compared to their wild-type littermates. In support of a direct inhibitory effect of SIRT4 on GDH (Haigis et al., 2006), lung extracts from SIRT4-deficient mice presented higher constitutive levels of GDH activity. Moreover, ionizing irradiation decreased GDH activity in wild-type but not SIRT4-deficient lungs. Together, these observations compellingly demonstrate that SIRT4 is a tumor suppressor contributing to the DDR by shutting down glutamine metabolism (Figure 1).
The new findings by Jeong et al. (2013) strongly reinforce previous evidences pointing to glutamine-dependent anaplerosis as an attractive Achilles’ heel of cancer cells. For example, GLS1 inhibition impairs neoplastic transformation (Wang et al., 2010). Also, estrogen receptor-negative breast cancers present a particular type of glutamine-dependent anaplerosis characterized by elevated levels of the gene encoding phosphoglycerate dehydrogenase (PHGDH) (Possemato et al., 2011). This enzyme diverts phosphoglycerate (a glycolytic intermediate) into the so-called serine pathway. The relevance of this pathway for cancer does not reside in the synthesis of serine but on the fact that its transamination step is coupled to the conversion of glutamate into αKG, thereby directly contributing to TCA anaplerosis independently of GDH (Possemato et al., 2011). Importantly, inhibition of PHGDH in breast cancer cell lines induces a metabolic collapse in TCA cycle intermediates that is highly reminiscent to the one observed upon DDR-induced SIRT4 upregulation and the ensuing GDH inhibition (Jeong et al., 2013; Possemato et al., 2011).
A few aspects in the DDR-mediated block in glutamine anaplerosis still remain to be elucidated. Not much is known about the mechanisms that upregulate SIRT4 mRNA by DNA damage, apart from the lack of involvement of p53 (Jeong et al., 2013). Finding the exact components of the DDR pathway responsible for SIRT4 induction could point to new strategies to shut down glutamine-dependent anaplerosis. It is also unclear yet how the glutamine-dependent anaplerotic blockade contributes to the implementation of DDR-induced cell cycle arrest.
Intriguingly, with the current report, essentially all members of the sirtuin family have been involved in cancer protection and metabolism (Sebastián et al., 2012). The fact that sirtuin activity depends on the intracellular levels of the co-substrate NAD+ opens the possibility that enhancing NAD+ biosynthetic pathways could result in a general activation of sirtuins and a concurrent enhancement of tumor suppression. As a proof of principle, treating mice with an NAD+ precursor, nicotinamide riboside, activates at least SIRT1 and SIRT3, improves energy expenditure and fatty acid oxidation, and protects mice from diet-induced obesity and metabolic syndrome (Cantó et al., 2012). How these metabolic effects will apply to a cancer scenario is not clear yet, but the increasing body of evidence suggests that global sirtuin activation will protect against cancer.
In summary, the current paper together with previous evidences reinforces two parallel strategies for treating cancer: (1) SIRT4 activation either through specific approaches or through a general activation of sirtuins, and (2) inhibition of glutamine-dependent anaplerosis through inhibition of GLS, GDH, or even PHGDH.
References
- Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino C, Grieco D, Costanzo V. EMBO J. 2011;30:546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, et al. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Jeong SM, Xiao C, Finley LWS, Lahusen T, Souza AL, Pierce K, Li Y-H, Wang X, Laurent G, German NJ, et al. Cancer Cell. 2013;23(this issue):450–463. doi: 10.1016/j.ccr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, et al. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastián C, Satterstrom FK, Haigis MC, Mostoslavsky R. J Biol Chem. 2012;287:42444–42452. doi: 10.1074/jbc.R112.402768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV, et al. Cancer Cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Vander Heiden MG, Rudin CM. Cancer Res. 2002;62:3515–3520. [PubMed] [Google Scholar]