Abstract
In phylogenetic analyses of the genus Streptomyces using 16S rRNA gene sequences, Streptomyces albus subsp. albus NRRL B-1811T forms a cluster with five other species having identical or nearly identical 16S rRNA gene sequences. Moreover, the morphological and physiological characteristics of these other species, including Streptomyces almquistii NRRL B-1685T, Streptomyces flocculus NRRL B-2465T, Streptomyces gibsonii NRRL B-1335T and Streptomyces rangoonensis NRRL B-12378T are quite similar. This cluster is of particular taxonomic interest because Streptomyces albus is the type species of the genus Streptomyces. The related strains were subjected to multilocus sequence analysis (MLSA) utilizing partial sequences of the housekeeping genes atpD, gyrB, recA, rpoB and trpB and confirmation of previously reported phenotypic characteristics. The five strains formed a coherent cluster supported by a 100 % bootstrap value in phylogenetic trees generated from sequence alignments prepared by concatenating the sequences of the housekeeping genes, and identical tree topology was observed using various different tree-making algorithms. Moreover, all but one strain, S. flocculus NRRL B-2465T, exhibited identical sequences for all of the five housekeeping gene loci sequenced, but NRRL B-2465T still exhibited an MLSA evolutionary distance of 0.005 from the other strains, a value that is lower than the 0.007 MLSA evolutionary distance threshold proposed for species-level relatedness. These data support a proposal to reclassify S. almquistii, S. flocculus, S. gibsonii and S. rangoonensis as later heterotypic synonyms of S. albus with NRRL B-1811T as the type strain. The MLSA sequence database also demonstrated utility for quickly and conclusively confirming that numerous strains within the ARS Culture Collection had been previously misidentified as subspecies of S. albus and that Streptomyces albus subsp. pathocidicus should be redescribed as a novel species, Streptomyces pathocidini sp. nov., with the type strain NRRL B-24287T.
The characterization and systematics of the species of the genus Streptomyces has evolved from a largely morphology-based classification system, with attempts to fine-tune these methods through cooperative projects such as the International Streptomyces Project. This project, however, did not attempt to identify species that were likely to be synonymous. The application of numerical taxonomy to classification of members of the genus Streptomyces (Williams et al., 1983) attempted to bring some order to the confused state of taxonomy of the genus through the evaluation of significantly more phenotypic traits than the classical morphology-based classification, and although it succeeded in grouping phenotypically related strains for further investigation, this approach still was not a useful solution to the problem. The introduction of classification schemes based on molecular biological tools provided meaningful insights into species relationships in the genus Streptomyces. Whole-genomic DNA relatedness determinations among species of the genus Streptomyces (Labeda & Lyons, 1991a, b; Labeda, 1992, 1993, 1998) provided evidence that the numerical taxonomic schemes were not always valid, but the onerous nature of these determinations precluded their widespread usage for routine identification of novel species. A comprehensive phylogenetic analysis of all species within the family Streptomycetaceae with validly published names based on the sequence of the 16S rRNA gene has been published (Labeda et al., 2012) and although it was not possible to assess the evolutionary relationships among all species within the genus due to the highly conserved nature of the gene sequenced, the nearest neighbouring taxa to an individual species could be easily determined. The use of multi-locus sequence analysis (MLSA) in systematics of the genus Streptomyces has been pioneered by the studies at the Institute of Microbiology, Chinese Academy of Sciences by Dr Y. Huang and her colleagues (Guo et al., 2008; Rong et al., 2009; Rong & Huang, 2010, 2012) and has also been applied to the study of phytopathogenic species of the genus Streptomyces by Labeda (2011) as well as homologous recombination in species of the genus Streptomyces by Doroghazi & Buckley (2010). This technique was found to be extremely valuable in determining species-level relationships because of the increased phylogenetic signal available in even partial sequences of single-copy housekeeping-protein-coding genes.
It has been observed in the phylogenetic study of the family Streptomycetaceae based on sequences of the 16S rRNA gene (Labeda et al., 2012) that several species, including Streptomyces almquistii, Streptomyces flocculus, Streptomyces gibsonii and Streptomyces rangoonensis, are very closely related phylogenetically to Streptomyces albus subsp. albus, the type species of the genus. The 16S rRNA gene sequence similarity between S. albus subsp. albus NRRL B-1811T and S. almquistii NBRC 13015T, S. gibsonii LMG 19912T and S. rangoonensis NBRC 13078T is 99.93 % and the similarity between S. flocculus NRRL B-2465T and the rest of the clade members is 99.73 % based on searches in EzTaxon-e (Kim et al., 2012). Kämpfer (2012) made a similar observation in the description of these species in his recent chapter in Bergey’s Manual of Systematic Bacteriology. In the present investigation, an MLSA study was carried out utilizing the type strains of the species within the S. albus subsp. albus 16S rRNA gene clade [i.e. Clade 126 of Labeda et al. (2012)] along with the type strain of Streptomyces albus subsp. pathocidicus NRRL B-24287T and other strains designated as subspecies of S. albus within the ARS Culture Collection in order to clarify their taxonomic status.
Methods
The strains used in the study were obtained from the ARS Culture Collection, Peoria, IL, and are listed in Table 1. Strains were cultivated on yeast extract–malt extract agar (YM) ISP-2 medium (Shirling & Gottlieb, 1966) at 28 °C.
Table 1. Strains used in the present study and their provenance.
| Species | Strain | Provenance |
| Streptomyces albus subsp. albus | NRRL B-1811T | ATCC 3004 ← Waksman 3004 |
| S. albus subsp. albus | NRRL B-2208T | Waksman 3004 |
| ‘Streptomyces albus subsp. cobaltofaciens’ | NRRL B-3902 | Lederle Laboratories AC-630 |
| ‘Streptomyces albus subsp. cretosus’ | NRRL B-1812 | ATCC 3005 ← CBS 137.21 |
| ‘Streptomyces albus subsp. ochroleucus’ | NRRL B-1813 | ATCC 3006 |
| Streptomyces albus subsp. pathocidicus | NRRL B-24287T | DSM 40799 ← Lederle Laboratories BK-513 |
| Streptomyces almquistii | NRRL B-1685T | Waksman Collection ←ATCC 618 |
| Streptomyces flocculus | NRRL B-2465T | Waksman 3863 ← ETH 24454← Nicot 373 |
| Streptomyces gibsonii | NRRL B-1335T | ATCC 6852 ←NCTC 4575 |
| Streptomyces rangoonensis | NRRL B-12378T | ISP 5452←ATCC 6860 |
| Streptomyces willmorei | NRRL B-1332T | ATCC 6867← NCTC 1856 ← J. Willmore |
Genomic DNA was isolated from all strains using UltraClean Microbial DNA isolation kits (MoBio Laboratories) following the instructions of the manufacturer. The 16S rRNA gene of S. albus subsp. albus NRRL B-1811T was amplified and sequenced as described by Labeda et al. (2012) and partial sequences of the housekeeping genes atpD (ATP synthase F1, beta subunit), gyrB (DNA gyrase B subunit) and rpoB (RNA polymerase beta subunit) were amplified and sequenced using the primers and protocols described previously by Guo et al. (2008) and Rong et al. (2009). Modified primers shown in Table S1, available in the online Supplementary Material, were designed for the amplification and sequencing of the housekeeping genes recA (recombinase A) and trpB (tryptophan synthetase, beta subunit) because the previously described primers did not work adequately for the species of the genus Streptomyces being studied. PCR conditions were those described by Guo et al. (2008) and Rong et al. (2009). Amplified products were purified using ExoSAP-IT (Affymetrix) and sequenced using BigDye 3.1 on an ABI model 3730 sequencer in the National Center for Agricultural Utilization Research (NCAUR) core sequencing facility.
Sequence data for the five housekeeping loci for each strain were deposited in GenBank (Table 2) and the multilocus sequences for these and those in the literature were also organized using version 1.5.1 of the Bacterial Isolate Genomic Sequence Database (BIGSdb) software package (Jolley & Maiden, 2010) that is publicly available on the ARS Microbial Genomic Sequence Database server (http://ars.usda.gov/amgsdb). The sequences for all loci for each strain were concatenated head to tail in-frame and exported in fasta format, providing a dataset of 153 strains and 2488 positions. Sequences were aligned with muscle (Edgar, 2004) in mega 5.1 (Tamura et al., 2011) and phylogenetic relationships were reconstructed using the maximum-likelihood algorithm based on the general time reversible model (Nei & Kumar, 2000), that had been determined to be the optimal model for these data using jmodeltest2 (Darriba et al., 2012; Guindon & Gascuel, 2003). Trees were also inferred using the evolutionary distance method (Tamura & Nei, 1993) with the neighbour-joining algorithm of Saitou & Nei (1987) and maximum-parsimony models in mega 5.1. Bootstrap support for all analyses was determined from 1000 resampled datasets (Felsenstein, 1985). Nocardiopsis dassonvillei NRRL B-16336 ( = CGMCC 4.1617), formerly classified as Streptomyces flavidofuscus (Tamura et al., 2008), was used as the outgroup for all phylogenetic analyses. The full phylogenetic tree resulting from these analyses can be seen in Fig. S1. MLSA evolutionary distances were determined using mega 5.1 to calculate the Kimura two-parameter distances (Kimura 1980) as shown in Table 2. Strain pairs having less than 0.007 MLSA distance were considered conspecific based on the guideline empirically determined by Rong & Huang (2012) to be the distance that equates to 70 % DNA–DNA homology.
Table 2. Gene sequences of species of the genus Streptomyces deposited for the present study.
| Species | Strain | atpD | gyrB | recA | rpoB | trpB |
| S. albus subsp. albus | NRRL B-1811T | JX486035 | JX486040 | JX486045 | JX486050 | JX486055 |
| S. albus subsp. albus | NRRL B-2208T | KF528055 | KF528056 | KF528057 | KF528058 | KF528059 |
| ‘S. albus subsp. cobaltofaciens’ | NRRL B-3902 | KC965070 | KC965078 | KC965086 | KC965094 | KC965102 |
| ‘S. albus subsp. cretosus’ | NRRL B-1812 | KC965066 | KC965074 | KC965082 | KC965090 | KC965098 |
| ‘S. albus subsp. ochroleucus’ | NRRL B-1813 | KC965067 | KC965075 | KC965083 | KC965091 | KC965099 |
| S. albus subsp. pathocidicus | NRRL B-24287T | KC965072 | KC965080 | KC965086 | KC965096 | KC965104 |
| S. almquistii | NRRL B-1685T | JX486034 | JX486039 | JX486044 | JX486049 | JX486054 |
| S. flocculus | NRRL B-2465T | JX486036 | JX486041 | JX486046 | JX486051 | JX486056 |
| S. gibsonii | NRRL B-1335T | JX486033 | JX486038 | JX486043 | JX486048 | JX486053 |
| S. rangoonensis | NRRL B-12378T | JX486032 | JX486037 | JX486042 | JX486047 | JX486052 |
| S. willmorei | NRRL B-1332T | KC965065 | KC965073 | KC965081 | KC965089 | KC965097 |
Morphological and physiological properties of the strains in the S. albus 16S rRNA phylogenetic clade were confirmed using the methods of the International Streptomyces Project (Shirling & Gottlieb, 1966). In addition, tolerance to NaCl was evaluated using YM agar to which 2 %, 2.5 %, 3 %, 5 % or 7 % (w/v) NaCl was added prior to sterilization. Additional physiological properties were evaluated using ApiCoryne and ApiZym test strips (bioMérieux) following the manufacturer’s instructions.
Results and Discussion
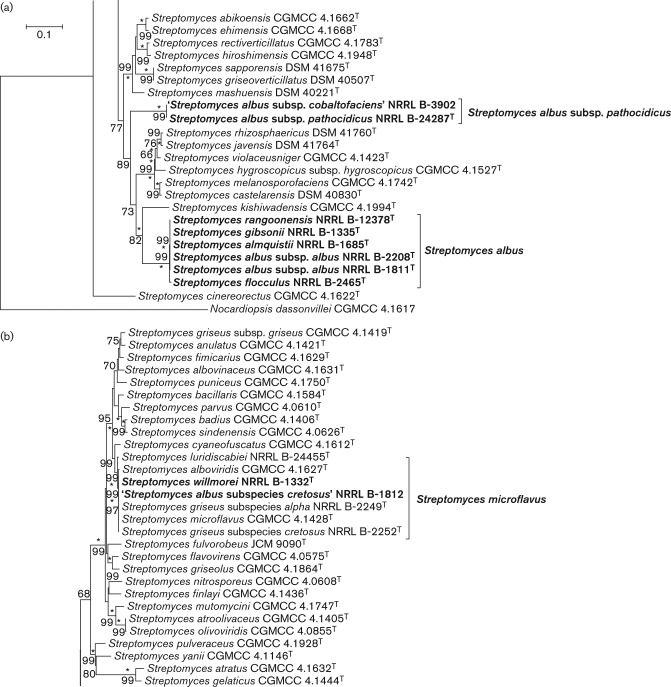
The close phylogenetic relationship of the species S. albus subsp. albus, S. almquistii, S. flocculus, S. gibsonii and S. rangoonensis described in the reports of Labeda et al. (2012) and Kämpfer (2012) was confirmed by the results of the phylogenetic analyses based on the 2488 bp alignment of the concatenated partial sequences of the housekeeping genes atpD, gyrB, recA, rpoB and trpB as can be seen in Fig. 1a. The second isolate of the type strain of Streptomyces albus held in the ARS Culture Collection, NRRL B-2208T, was included in the study to confirm identity with NRRL B-1811T and it was observed to contain identical alleles for all five housekeeping loci. The other species formed a very tight cluster which is extremely well supported by high bootstrap values and similar tree topology was observed in phylogenetic trees reconstructed using the neighbour joining and maximum-parsimony algorithms (not shown). S. flocculus NRRL B-2465T is slightly distant from the other species in this cluster, whose sequences for all of the housekeeping genes are 100 % similar, but the differences amount to only 13 bp over the total alignment of 2488 bp (4 bp in atpD, 2 bp in gyrB, 2 bp in recA, 2 bp in rpoB and 3 bp in trpB). These differences are reflected as an MLSA distance of 0.005 from the other strains in this clade, as can be seen in Table 3, and this value is less than the species level threshold of 0.007 proposed by Rong & Huang (2012). All of these strains were observed to form white spores in spiral chains, did not produce melanin pigments from tyrosine and had physiological properties that were also very similar, as shown in Table S2, supporting the conclusion based on MLSA distances that S. albus, S. almquistii, S. flocculus, S. gibsonii and S. rangoonensis should be considered as a single species with the name Streptomyces albus having priority. The other species names should be classified as later heterotypic synonyms.
Fig. 1.
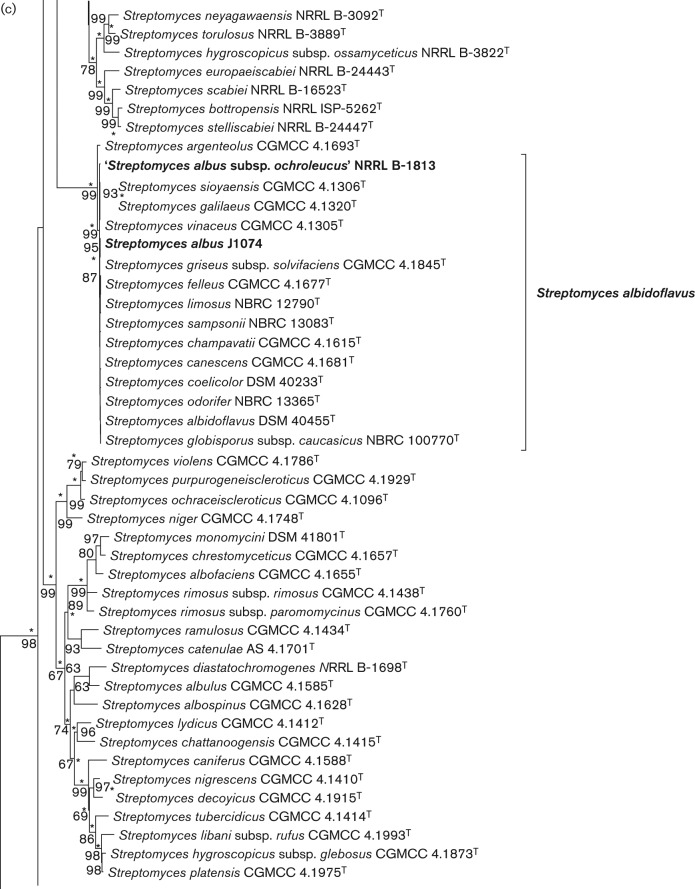
Subsections of the phylogenetic tree inferred in mega 5.1 (Tamura et al., 2011) using the maximum-likelihood method based on the general time reversible model (Nei & Kumar, 2000). There were 2488 positions in the final dataset. Trees were also inferred using the evolutionary distance method (Tamura & Nei, 1993) with the neighbour-joining algorithm of Saitou & Nei (1987), and the neighbour-joining and maximum-parsimony models in mega 5.1 and branches conserved in all methods are marked with asterisks. Percentages at the nodes represent levels of bootstrap support from 1000 resampled datasets (Felsenstein, 1985) with values less than 60 % not shown. The strains within the Streptomyces albus subsp. albus clade and all other valid and invalid subspecies of Streptomyces albus are indicated with bold node labels. Bar, 0.1 substitutions per site. (a) Subtree showing the phylogenetic positions of species in the S. albus subsp. albus clade and Streptomyces albus subsp. pathocidicus. (b) Subtree showing the phylogenetic position of ‘Streptomyces albus subsp. cretosus’ NRRL B-1812 and Streptomyces willmorei NRRL B-1332 confirming their identity as strains of Streptomyces microflavus. (c) Subtree showing the phylogenetic position of ‘Streptomyces albus subsp. ochroleucus’ NRRL B-1813 and Streptomyces albidoflavus J1074 and demonstrating their identity as strains of Streptomyces albidoflavus.
Table 3. MLSA distance values for selected strains in this study.
Strains: 1, Streptomyces microflavus CGMCC 4.1428T; 2, ‘Streptomyces albus subsp. cretosus’ NRRL B-1812; 3, Streptomyces willmorei NRRL B-1332T; 4, Streptomyces albidoflavus DSM 40455T; 5, ‘Streptomyces albus subsp. ochroleucus’ NRRL B-1813; 6, Streptomyces albus J1074; 7, Streptomyces albus subsp. pathocidicus NRRL B-24287T; 8, ‘Streptomyces albus subsp. cobaltofaciens’ NRRL B-3902; 9, Streptomyces rangoonensis NRRL B-12378T; 10, Streptomyces gibsonii NRRL B-1335T; 11, Streptomyces almquistii NRRL B-1685T; 12, Streptomyces albus subsp. albus NRRL B-1811T; 13, Streptomyces albus subsp. albus NRRL B-2208T; 14, Streptomyces flocculus NRRL B-2465T. Bold type indicates distances below the threshold for classification of strains as representatives of distinct species.
| Strain | MLSA distance (Kimura two-parameter) | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| 1 | |||||||||||||
| 2 | 000.0 | ||||||||||||
| 3 | 000.0 | 000.0 | |||||||||||
| 4 | 0.115 | 0.114 | 0.114 | ||||||||||
| 5 | 0.113 | 0.112 | 0.112 | 0.003 | |||||||||
| 6 | 0.115 | 0.115 | 0.115 | 0.001 | 0.002 | ||||||||
| 7 | 0.137 | 0.137 | 0.137 | 0.135 | 0.135 | 0.136 | |||||||
| 8 | 0.137 | 0.137 | 0.137 | 0.135 | 0.135 | 0.136 | 0.000 | ||||||
| 9 | 0.128 | 0.128 | 0.128 | 0.144 | 0.143 | 0.145 | 0.132 | 0.132 | |||||
| 10 | 0.128 | 0.128 | 0.128 | 0.144 | 0.143 | 0.145 | 0.132 | 0.132 | 0.000 | ||||
| 11 | 0.128 | 0.128 | 0.128 | 0.144 | 0.143 | 0.145 | 0.132 | 0.132 | 0.000 | 0.000 | |||
| 12 | 0.128 | 0.128 | 0.128 | 0.144 | 0.143 | 0.145 | 0.132 | 0.132 | 0.000 | 0.000 | 0.000 | ||
| 13 | 0.128 | 0.128 | 0.128 | 0.144 | 0.143 | 0.145 | 0.132 | 0.132 | 0.000 | 0.000 | 0.000 | 0.000 | |
| 14 | 0.133 | 0.132 | 0.132 | 0.145 | 0.144 | 0.145 | 0.135 | 0.135 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 |
The phylogenetically distant position of S. albus subsp. pathocidicus NRRL B-24287T relative to the S. albus subsp. albus clade (see Fig. 1a.) demonstrates that it cannot represent a subspecies of S. albus. Moreover, this observation is further supported by the fact that the 16S rRNA sequence similarity of this strain with S. albus subsp. albus NRRL B-1811T is 94.3 % (51 mismatches over 1459 bp) and the MLSA distance from the S. albus MLSA clade (Table 3) is 0.132, well above the species-definitive MLSA distance of 0.007. During the course of the present investigation it was also observed that the sequences for all five of the housekeeping genes of strain NRRL B-3902, deposited in the ARS Culture Collection in 1970 as the invalid subspecies ‘S. albus subsp. cobaltofaciens’, were identical to those of NRRL B-24287T (MLSA distance = 0.000), thus indicating that they represent the same species. It is proposed that the taxon represented by these two strains be classified as the novel species Streptomyces pathocidini sp. nov. with NRRL B-24287T as the type strain.
The growing multigene database for members of the genus Streptomyces proved to be a very useful tool for rapid and accurate identification of strains of questionable taxonomic status in the ARS Culture Collection. Two strains whose accession records indicated that they represented subspecies of S. albus for which details are unpublished were included in the present study and were conclusively identified (MLSA distance = 0.000) as representatives of other species, as can be seen in Figs 1b and 1c. ‘S. albus subsp. cretosus’ NRRL B-1812 was identified as a strain of Streptomyces microflavus, while ‘S. albus subsp. ochroleucus’ NRRL B-1813 was found to be a strain of Streptomyces albidoflavus. The five housekeeping gene sequences for Streptomyces willmorei NRRL B-1332T were also determined in the present study and the MLSA distance of 0.000 from S. microflavus CGMCC 4.1428T that was observed (Table 3) confirmed the proposal by Lanoot et al. (2005) that this species should be considered as a later synonym of S. microflavus.
S. albus J1074, whose genome had been sequenced by the Broad Institute (GenBank accession number NZ_ABYC00000000), was included in this study and was found to be misidentified. The sequences for the five housekeeping gene loci were extracted from the draft genome sequence using the capabilities of the BIGSdb package for inclusion in the phylogenetic analyses. The phylogenetic position of this strain (Fig. 1c) is within Streptomyces albidoflavus and the MLSA distance of 0.001 to the type strain, S. albidoflavus DSM 40455T, confirms its identity as a representative of this species.
The results reported in this study conclusively support a proposal to emend the description of S. albus to include S. almquistii, S. gibsonii, S. flocculus and S. rangoonensis as later heterotypic synonyms. The data also support the redescription of Streptomyces albus subsp. pathocidicus as a novel species for which the name Streptomyces pathocidini is proposed. The formal descriptions of Streptomyces albus and Streptomyces pathocidini sp. nov. follow:
Emended description of Streptomyces albus (Rossi Doria 1891) Waksman and Henrici 1943, 339AL
Later heterotypic synonyms: Streptomyces almquistii (Duché 1934) Pridham et al. 1958AL, Streptomyces flocculus (Duché 1934) Waksman and Henrici 1948AL, Streptomyces gibsonii (Erikson 1935) Waksman and Henrici 1948AL and Streptomyces rangoonensis corrig. (Erikson 1935) Pridham et al. 1958AL.
Morphology is as described by Kämpfer (2012). Growth on d-fructose, d-galactose, d-glucose, i-inositol, d-mannitol, salicin and d-xylose as sole carbon source; variable growth on raffinose; weak to no growth on l-arabinose, rhamnose and sucrose. Good growth in the presence of up to 5 % NaCl and variable growth in the presence of 7 % NaCl. Utilizing apiCoryne and apiZym test strips, the following positive test reactions are observed: pyrolidonyl arylamidase, alkaline phosphatase, N-acetyl-glucosaminidase, esculin hydrolysis, gelatin hydrolysis, leucine arylamidase; the following negative test reactions are observed: nitrate reduction, β-glucuronidase, β-glucosidase, esterase (C4), esterase (C6), lipase (C14), cystine arylamidase, trypsin, α-chymotrypsin, naphthol-AS-BI-phosphohydrolase, α-fucosidase; the following variable test reactions are observed: pyrazymidase (3 of 5 positive), β-galactosidase (4 of 5 positive), urease (4 of 5 positive), valine arylamidase (2 of 5 weakly positive), acid phosphatase (3 of 5 positive), α-mannosidase (1 of 5 positive). Fermentation of glucose, ribose and xylose was weak for all strains using the apiCoryne test strips. The test results for α-glucosidase varied between the apiCoryne test strips where results were variable (3 of 5 positive) and apiZym test strips where all were negative.
Type strain is NRRL B-1811T ( = ATCC 25426T = ATCC 3004T = CBS 410.63T = CBS 924.69T = BCRC 10802T = CCUG 33990T = CECT 3077T = CGMCC 4.1640T = CIP 104432T = DSM 40313T = HUT 6613T = IFM 1119T = IMET 40241T = IMRU 3004T = JCM 4450T = JCM 4177T = KCTC 1082T = NBRC 13014T = NBRC 3710T = NCIMB 9558T = NRRL B-2208T = RIA 1206T = VKM Ac-35T). The GenBank/DDBJ/EMBL accession number for the 16S rRNA gene for the type strain is JX486031.
Description of Streptomyces pathocidini sp. nov.
Streptomyces pathocidini. (pa.tho.ci.di′ni. N.L. neut n. pathocidini referring to the antibiotic pathocidin).
Basonym Streptomyces albus subsp. pathocidicus.
Phenotypic description is that of Nagatsu et al. (1962).
Type strain is NRRL B-24287T ( = ATCC 14510T = B-28T = BCRC 12331T = CCRC 12331T = CGMCC 4.1633T = CIP 104431T = DSM 40799T = JCM 4166T = KCTC 9671T = NBRC 13812T = VKM Ac-598T). The GenBank/DDBJ/EMBL accession number for the 16S rRNA gene of the type strain is AB184501.
Acknowledgements
The able technical assistance of E. Basehoar in determining the housekeeping gene sequences is gratefully acknowledged. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture (USDA). USDA is an equal opportunity provider and employer.
Abbreviations:
- MLSA
multi-locus sequence analysis
References
- Darriba D., Taboada G. L., Doallo R., Posada D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9, 772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroghazi J. R., Buckley D. H. (2010). Widespread homologous recombination within and between Streptomyces species. ISME J 4, 1136–1143. 10.1038/ismej.2010.45 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2004). muscle: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791. 10.2307/2408678 [DOI] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52, 696–704. 10.1080/10635150390235520 [DOI] [PubMed] [Google Scholar]
- Guo Y., Zheng W., Rong X., Huang Y. (2008). A multilocus phylogeny of the Streptomyces griseus 16S rRNA gene clade: use of multilocus sequence analysis for streptomycete systematics. Int J Syst Evol Microbiol 58, 149–159. 10.1099/ijs.0.65224-0 [DOI] [PubMed] [Google Scholar]
- Jolley K. A., Maiden M. C. (2010). BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11, 595. 10.1186/1471-2105-11-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämpfer P. (2012). Genus Streptomyces Waksman and Henrici 1943, 339AL emend. Witt and Stackebrandt 1990, 370, emend. Wellington, Stackebrandt, Sanders, Wolstrup and Jorgensen, 1992, 159. In Bergey’s Manual of Systematic Bacteriology, 2nd edn, vol. 5, pp. 1455–1767. Edited by Goodfellow M., Kämpfer P., Busse H.-J., Trujillo M., Suzuki K. E., Ludwig W., Whitman W. B. New York: Springer. [Google Scholar]
- Kim O.-S., Cho Y.-J., Lee K., Yoon S.-H., Kim M., Na H., Park S.-C., Jeon Y. S., Lee J.-H. & other authors (2012). Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62, 716–721. 10.1099/ijs.0.038075-0 [DOI] [PubMed] [Google Scholar]
- Kimura M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16, 111–120. 10.1007/BF01731581 [DOI] [PubMed] [Google Scholar]
- Labeda D. P. (1992). DNA-DNA hybridization in the systematics of Streptomyces. Gene 115, 249–253. 10.1016/0378-1119(92)90566-8 [DOI] [PubMed] [Google Scholar]
- Labeda D. P. (1993). DNA relatedness among strains of the Streptomyces lavendulae phenotypic cluster group. Int J Syst Evol Microbiol 43, 822–825. 10.1099/00207713-43-4-822 [DOI] [Google Scholar]
- Labeda D. P. (1998). DNA relatedness among the Streptomyces fulvissimus and Streptomyces griseoviridis phenotypic cluster groups. Int J Syst Evol Microbiol 48, 829–832. 10.1099/00207713-48-3-829 [DOI] [Google Scholar]
- Labeda D. P. (2011). Multilocus sequence analysis of phytopathogenic species of the genus Streptomyces. Int J Syst Evol Microbiol 61, 2525–2531. 10.1099/ijs.0.028514-0 [DOI] [PubMed] [Google Scholar]
- Labeda D. P., Lyons A. J. (1991a). Deoxyribonucleic acid relatedness among species of the “Streptomyces cyaneus” cluster. Syst Appl Microbiol 14, 158–164. 10.1016/S0723-2020(11)80295-X [DOI] [Google Scholar]
- Labeda D. P., Lyons A. J. (1991b). The Streptomyces violaceusniger cluster is heterogeneous in DNA relatedness among strains: Emendation of the descriptions of S. violaceusniger and Streptomyces hygroscopicus. Int J Syst Bacteriol 41, 398–401. 10.1099/00207713-41-3-398 [DOI] [Google Scholar]
- Labeda D. P., Goodfellow M., Brown R., Ward A. C., Lanoot B., Vanncanneyt M., Swings J., Kim S. B., Liu Z. & other authors (2012). Phylogenetic study of the species within the family Streptomycetaceae. Antonie van Leeuwenhoek 101, 73–104. 10.1007/s10482-011-9656-0 [DOI] [PubMed] [Google Scholar]
- Lanoot B., Vancanneyt M., Van Schoor A., Liu Z., Swings J. (2005). Reclassification of Streptomyces nigrifaciens as a later synonym of Streptomyces flavovirens; Streptomyces citreofluorescens, Streptomyces chrysomallus subsp. chrysomallus and Streptomyces fluorescens as later synonyms of Streptomyces anulatus; Streptomyces chibaensis as a later synonym of Streptomyces corchorusii; Streptomyces flaviscleroticus as a later synonym of Streptomyces minutiscleroticus; and Streptomyces lipmanii, Streptomyces griseus subsp. alpha, Streptomyces griseus subsp. cretosus and Streptomyces willmorei as later synonyms of Streptomyces microflavus. Int J Syst Evol Microbiol 55, 729–731. 10.1099/ijs.0.63391-0 [DOI] [PubMed] [Google Scholar]
- Nagatsu J., Anzai K., Suzuki S. (1962). Pathocidin, a new antifungal antibiotic; II. Taxonomic studies on the pathocidin-producing organism Streptomyces albus var. pathocidicus . J Antibiot (Tokyo) 15, 103–106. [Google Scholar]
- Nei M., Kumar S. (2000). Molecular Evolution and Phylogenetics. New York: Oxford University Press. [Google Scholar]
- Rong X., Huang Y. (2010). Taxonomic evaluation of the Streptomyces griseus clade using multilocus sequence analysis and DNA–DNA hybridization, with proposal to combine 29 species and three subspecies as 11 genomic species. Int J Syst Evol Microbiol 60, 696–703. 10.1099/ijs.0.012419-0 [DOI] [PubMed] [Google Scholar]
- Rong X., Huang Y. (2012). Taxonomic evaluation of the Streptomyces hygroscopicus clade using multilocus sequence analysis and DNA-DNA hybridization, validating the MLSA scheme for systematics of the whole genus. Syst Appl Microbiol 35, 7–18. 10.1016/j.syapm.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Rong X., Guo Y., Huang Y. (2009). Proposal to reclassify the Streptomyces albidoflavus clade on the basis of multilocus sequence analysis and DNA-DNA hybridization, and taxonomic elucidation of Streptomyces griseus subsp. solvifaciens. Syst Appl Microbiol 32, 314–322. 10.1016/j.syapm.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Shirling E. B., Gottlieb D. (1966). Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16, 313–340. 10.1099/00207713-16-3-313 [DOI] [Google Scholar]
- Tamura K., Nei M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10, 512–526. [DOI] [PubMed] [Google Scholar]
- Tamura T., Ishida Y., Otoguro M., Hatano K., Suzuki K. (2008). Reclassification of Streptomyces flavidofuscus as a synonym of Nocardiopsis dassonvillei subsp. dassonvillei. Int J Syst Evol Microbiol 58, 2321–2323. 10.1099/ijs.0.65559-0 [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28, 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. T., Goodfellow M., Alderson G., Wellington E. M. H., Sneath P. H. A., Sackin M. J. (1983). Numerical classification of Streptomyces and related genera. J Gen Microbiol 129, 1743–1813. [DOI] [PubMed] [Google Scholar]