Abstract
Background:
Increasingly, data are implicating muscarinic receptors in the aetiology and treatment of mood disorders. This led us to measure levels of different muscarinic receptor-related parameters in the cortex from people with mood disorders and the CNS of rats treated with mood stabilisers and antidepressant drugs.
Methods:
We measured [3H]AF-DX 384 binding in BA 46 and BA 24 from subjects with bipolar disorders (n = 14), major depressive disorders (n = 19), as well as age- and sex-matched controls (n = 19) and the CNS of rats treated with fluoxetine or imipramine. In addition, we used Western blots to measure levels of CHRM2 protein and oxotremorine-M stimulated [35S]GTPγS binding as a measure of CHRM 2 / 4 signaling.
Results:
Compared with controls, [3H]AF-DX 384 binding was lower in BA 24 and BA 46 in bipolar disorders and major depressive disorders, while CHRM2 protein and oxotremorine-M stimulated [35S]GTPγS binding was only lower in BA 24. Compared with vehicle, treatment with mood stabilisers, antidepressant drugs for 10 days, or imipramine for 28 days resulted in higher levels of in [3H]AF-DX 384 binding select regions of rat CNS.
Conclusions:
Our data suggest that levels of CHRM2 are lower in BA 24 from subjects with mood disorders, and it is possible that signalling by that receptor is also less in this cortical region. Our data also suggest increasing levels of CHRM2 may be involved in the mechanisms of action of mood stabilisers and tricyclic antidepressants.
Keywords: Cortex, bipolar disorders, major depressive disorders, muscarinic receptors, lithium
Introduction
Manipulating the cholinergic system has been shown to affect mood (Rowntree et al., 1950; Janowsky et al., 1974; Furey and Drevets, 2006), suggesting depression and mania could result from changes in cholinergic neurotransmission (Janowsky et al., 1974). The finding that there was an altered risk for mood disorders associated with variance in the sequence of the muscarinic M2 receptor (CHRM2) gene (Comings et al., 2002; Wang et al., 2004; Luo et al., 2005), one of 5 CHRMs in the human CNS (Wess, 2003), focused attention on the role of that receptor in the aetiology of mood disorders. More recently, a positron emission tomography study reported lower levels of CHRM2 (measured as the binding of 3-(3-(3-fluoroproply) thio)-1,2,5-thiadiazol-4-yl] -1,2,5,6-tetrahydro-1-methylpyridine labelled with F-18, a positron emitting radionuclide, ([18F]FP-TZTP)) in Brodmann’s area [BA] 24 in bipolar disorders (BPD) (Cannon et al., 2006). Interestingly, the lower levels of [18F]FP-TZTP binding in BPD was most apparent in subjects who were homozygous T genotype at rs324650 (Cannon et al., 2011), an intronic CHRM2 single nucleotide polymorphism, that had previously been associated with an altered risk for mood disorders (Wang et al., 2004; Luo et al., 2005).
We have reported lower levels of N- [[2,3-Dipropylamino-3H]-methyl]-1-piperidinyl]ethyl]-5,6-dihydro-6-oxo-11H-pyrido[2,3-b][1,4]benzodiazepine-11-carboxamide ([3H]AF-DX 384) binding to BA 46 from subjects with BPD and major depressive disorder (MDD) (Gibbons et al., 2009). This is relevant, because studies using human cloned receptors show [3H]AF-DX 384 binds selectively to CHRM2 and CHRM4 (Miller et al., 1991). Hence, our data seem to partly agree with the neuroimaging study showing lower cortical CHRM2 in BPD. Our data differ from that of another study that reported [3H]AF-DX 384 binding was not altered in BA 24 from people with BPD (Zavitsanou et al., 2005); these apparently contradictory data could be explained by regionally selective changes in cortical [3H]AF-DX 384 in mood disorders. To challenge this hypothesis, we decided to measure levels of [3H]AF-DX 384 binding in BA 24, as well as BA 46, using a larger cohort of subjects with MDD, BPD, and age-/sex-matched controls than was previously available. We also determined levels of CHRM2 protein and oxotremorine-M-stimulated GTPγS binding, a surrogate measure of CHRM2 / 4 signalling in postmortem tissue (Scarr et al., 2006), in the same cortical samples. Finally, we determined if changes in [3H]AF-DX 384 binding could be part of the mechanism of action of drugs used to treat mood disorders by measuring the binding of that radioligand in rat cortex after treatment with mood stabilisers (lithium and valproate) or antidepressant drugs (imipramine and fluoxetine).
Methods and Materials
Human CNS
Human tissue was sourced through the Victorian Brain Bank Network at the Florey Institute of Neuroscience and Mental Health. The tissue was collected at the Victorian Institute of Forensic Medicine after approval from that Institution’s Ethics Committee.
BA 24 (defined as the ventral anterior cingulate gyrus around the genu of the corpus callosum) and BA 46 (defined as the lateral surface of the frontal lobe, including approximately the middle one-third of the middle frontal gyrus and the most rostral portion of the inferior frontal gyrus) were taken from the left hemisphere of 15 subjects with MDD, 14 subjects with BPD, and 19 subjects with no history of psychiatric illness (controls) (Table 1; supplementary Table 1). Psychiatric diagnoses were made by consensus according to DSMIV criteria (American Psychiatric, 1994) using the Diagnostic Instrument for Brain Studies (Hill et al., 1996; Roberts et al., 1998). The designation of nonpsychiatric controls could be reached only where no indication of any psychiatric history was found after an extensive case history review and interviews with relatives. As part of the case history review, duration of illness (DI) was calculated as the time from first contact with a clinical service to death. Postmortem interval (PMI) was calculated as the time between death and autopsy or, where death was not witnessed but the subject was seen alive within 5 hours of being found dead, the midpoint between the subject being found and being last seen alive. Importantly, all cadavers were refrigerated within 6 hours and the brains frozen to -80oC within 30 minutes of autopsy to reduce the impact of autolysis (Stan et al., 2006). Tissue quality was assessed by measuring CNS pH (Kingsbury et al., 1995), which is now regarded as the best measure of tissue preservation postmortem (Stan et al., 2006).
Table 1.
Demographic and CNS Collection Data for the Cases Used in This Study
| Diagnosis | Age (y) |
Sex M/F |
PMI (h) |
pH | DI (y) |
Sui (y/n) |
|---|---|---|---|---|---|---|
| BPD | 57±4.0 | 8 / 6 | 37±4.3 | 6.29±0.05 | 17±3.4 | 5 / 9 |
| MDD | 56±4.5 | 8 / 7 | 44±4.1 | 6.52±0.05 | 15±2.7 | 12 / 3 |
| Controls | 48±3.8 | 13 / 6 | 36±3.4 | 6.28±0.05 | 0 / 19 | |
| Statistics | ||||||
| F | 1.57 | 1.27 | 6.60 | |||
| d.f. | 2,45 | 2,45 | 2,45 | |||
| P | .2 | .64 | .28 | .003 | .67 | <.0001 |
| Bipolar vs controls | n.s. | .005 | ||||
| MDD vs controls | P < .01 | <.0001 | ||||
| BPD vs MDD | P <0.05 | .02 | ||||
Abbreviations: BPD, bipolar disorder; DI, duration of illness; F, female; M, male; MDD, major depressive disorder; n, no; PMI, postmortem interval; Sui, suicide; y, yes.
Neuropsychopharmacological Studies
Animal experimentation was performed with the approval of the Florey Institute for Neuroscience and Mental Health’s animal ethics committee.
Six-week-old, male Sprague Dawley rats were treated with fluoxetine, imipramine, lithium carbonate, and sodium valproate according to accepted protocols, which have been shown to result in clinically relevant levels of drug in rat blood (Dean et al., 1997, 2006). Thus, groups of 20 rats were given diet supplemented with 1.8g lithium carbonate (Sigma Aldrich, Sydney, Australia)/kg diet, 12g sodium valproate (Sigma Aldrich, Sydney, Australia)/kg diet, or control chow for 4 weeks. In addition, groups of 20 rats were administered 10mg/kg/d fluoxetine (Eli Lilly West Ryde, Australia), 20mg/kg/d imipramine (Sigma Aldrich, Sydney, Australia), or 0.9% saline via i.p. injection for either 10 (acute) or 28 (chronic) days. These 2 treatment periods are frequently studied in rats, as they result in differing CNS molecular (Dean et al., 1997) and behavioral (Naudon et al., 2007) effects. At the completion of the treatment period, rats were sacrificed by decapitation, and the brain tissue was removed, rapidly frozen in isopentane on dry ice, and stored at -80oC until required.
In Situ Radioligand Binding
[3H]AF-DX 384 binding was performed as previously described (Crook et al., 1999). Thus, 20-µm frozen sections were cut from BA 24 and BA 46 and from rats at ~2mm rostral to bregma and thaw-mounted on to gelatinized slides. All sections were incubated at room temperature in assay buffer (10mM KH2PO4, 10mM Na2HPO4 [pH 7.4]) for 30 minutes, then rinsed in water and dried. Tissue sections were then incubated with [3H]AF-DX 384 (7nM; PerkinElmer, Waltham, MA) in the presence (2 sections; nonspecific binding [NSB]) or absence (3 sections; total binding [TOT]) of 1 µM tropicamide (Tocris Bioscience, Bristol, UK) in assay buffer for 1 hour at room temperature. Importantly, the level of [3H]AF-DX 384 is greater than 3x the Kd of binding of the radioligand to human CNS homogenate (Crook et al., 1999), and under such conditions the specific binding of [3H]AF-DX 384 is a good measure of the total number of binding sites in a tissue section (McKinney, 2006). Sections were then washed twice for 2 minutes in ice-cold assay buffer, rinsed in ice-cold distilled water, and dried in a stream of cool air before being fixed overnight in paraformaldehyde vapor; this gives a level of fixation that stabilizes the tissue section without affecting radioligand binding properties (Liberatore et al., 1999).
Oxotremorine-M-Stimulated, [35S]GTPγS Binding Assay
[35S]GTPγS binding was essentially performed as described previously (Scarr et al., 2006), and hence, 20-µm frozen sections were cut from BA 24 and BA 46 and thaw-mounted on to gelatinized slides. Sections (n = 5) from each subject were incubated in assay buffer (20mM HEPES containing 100mM NaCl and 5mM MgCl2, pH 7.4 and 0.2mM guanosine diphosphate) for 30 minutes at room temperature before being rinsed in distilled water and dried in a stream of cool air. Sections were then incubated with 0.05nM [35S]GTPγS (Perkin Elmer, Waltham, MA) in assay buffer with (stimulated-binding; 3 sections/subject) or without (basal binding; 2 sections/subject) 100 μM oxotremorine-M for 60 minutes at room temperature. Slides were then washed twice for 15 minutes in ice-cold assay buffer without GDP, rinsed in dH2O, dried in a stream of cool air, and partially fixed overnight in paraformaldehyde vapor.
Autoradiography
For both [3H]AF-DX 384 and [35S]GTPγS, the partially fixed frozen sections were apposed to BAS-TR2025 plates (Fujifilm, Tokyo, Japan) for 7 days or BAS-SR2025 plates (Fujifilm, Tokyo, Japan) overnight, respectively. Each plate was also exposed to autoradiographic microscales (Amersham Biosciences, Little Chalfort, UK). The plates were imaged using a BAS5000 high-resolution phosphoimager (Fujifilm, Tokyo, Japan) and analyzed using AIS imaging software (Imaging Research, St. Catharines, ON, Canada). As [3H]AF-DX 384 and [35S]GTPγS binding was homogenous throughout the grey matter of the human and rat cortex, the intensity of binding was measured as an integrated measurement across the entire grey matter. Calibrating these measurements against the microscales allowed the measurement of TOT and NSB for [3H]AF-DX 384 and stimulated binding and basal binding for [35S]GTPγS. Specific [3H]AF-DX 384 binding was measured as TOT-NSB, while oxotremorine-M-stimulated [35S]GTPγS binding was measured as stimulated – basal [35S]GTPγS binding. All data was then converted to fmol bound radiolabel/mg estimated tissue equivalence (ETE).
CHRM2 Protein
To validate the specificity of the CHRM2 antibody, Western blots were initially performed using homogenates from BA 46 and the frontal cortex of muscarinic M2 receptor knockout (CHRM2-/-) and wild-type (w/t) mice (Gomeza et al., 1999) (gift from Lilly Research Laboratories). In completing these and the experiments comparing CHRM2 protein levels across psychiatric disorders, our experience agrees with the proposition that it is extremely difficult to find CNS reference proteins that, by definition, do not change levels between CNS regions, with disorders of the human CNS, or after drug treatments (Eaton et al., 2013; Rena Li and Shen, 2013). Hence, we followed the suggestion that protein load should be controlled for by carefully measuring protein levels in each cortical homogenate and carefully controlling the volume of each sample loaded on each gel, rather than using a single reference as a loading control (Eaton et al., 2013). Thus, to measure CHRM2 levels, samples of BA 24, BA 46, or mouse frontal cortex were homogenized with 5 strokes of a Teflon-glass homogenizer into 10mM Tris-HCl containing 1% SDS and 0.1mM Na3VO4 (pH 7.4) on ice, with the Na3VO4 being added on the day of use. The protein concentration in each homogenate was then measured using the BioRad protein assay with internally produced quality controls in every assay to allow the identification of unacceptable assay to assay variation.
An aliquot of each cortical homogenate containing exactly 30 μg protein was then electrophoresed, in duplicate, on a 7.5% polyacrylamide gel and transferred onto nitrocellulose membranes. Each membrane was then stained with Ponceau S and examined for any variation in overall levels of protein transfer (Klein et al., 1995). To visualize CHRM2, the nitrocellulose membranes were blocked with 5% nonfat milk powder (NFMP) in Tris buffered saline and 0.1% Tween-20 (TTBS) for 1 hour before being incubated overnight with 1:1000 rabbit anti-CHRM2 antibody in 5% NFMP (AB-5166 Merck Millipore, Kilsyth, Australia) at 4°C, washed thrice for 10 minutes in TTBS, and then incubated with 1:2000 HRP-conjugated, goat anti-rabbit secondary antibody (DAKO, Glostrup, Denmark) in 5% NFMP for 1 hour at room temperature. The membranes were washed thrice in TTBS, incubated for 5 minutes in SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific Pierce, Rockford, IL), and the intensity of the immunogenic bands was measured using the Kodak CF 440 image station. To further limit any variation, the exposure of each gel was standardized to an Internal Control (IC), which was run on each gel. The IC is a homogenate prepared from tissue from a donor who is not part of the study cohort and is first run, under the optimized conditions, in every lane of 2 gels to allow the intensity of immunogenic labelling of the protein of interest to be measured 24 times. These readings must not vary by more than 10% and are used to establish target range for the protein of interest (mean ± 2SD of all repeated measures). The IC was then run on every subsequent gel, along with the unknowns, and chemiluminesence of the IC used to guide exposure time so that the intensity of the IC fell within the established range. The intensity of the immunogenic band representing the protein of interest was then expressed as a ratio of the IC.
Statistics
D’Agostino & Pearson omnibus normality test was used to determine whether data sets followed a Gaussian distribution. Demographic and CNS collection data as well as experimental data from the analyses of human and rat CNS tissue was compared using 1-way ANOVA followed by Tukey’s posttest. Chi-square tests were used to determine if the frequency of categorical data (sex and suicide) varied between diagnoses. Data from suicide completers and death by other causes (see below) were compared using Students t test. Effect sizes were calculated as Cohen’s d (Watson M.W. (2003) Effect size calculator: Computer software; College PA: Ed. Psych. Associates). Analyses were conducted using Prism 6.01 (Graphpad Software, La Jolla, CA) or Minitab 16 (Minitab, State College, PA).
Results
Demographic and CNS Collection Data
There were no significant differences in age, duration of illness, and PMI or the gender balance between diagnostic cohorts (Table 1; supplementary Table 1). There was significant variance in CNS pH, because pH was higher in MDD. There was significant difference in frequency of suicide completion across diagnoses even when rates of suicide were compared in BPD and MDD.
Studies Using Human Cortex
[3H]AF-DX 384 Radioligand Binding
As with our previous study (Gibbons et al., 2009), [3H]AF-DX 384 binding did not show any significant variation across the human cortex (Figure 1A); we therefore took an integrated measure of [3H]AF-DX 384 binding across the cortex.
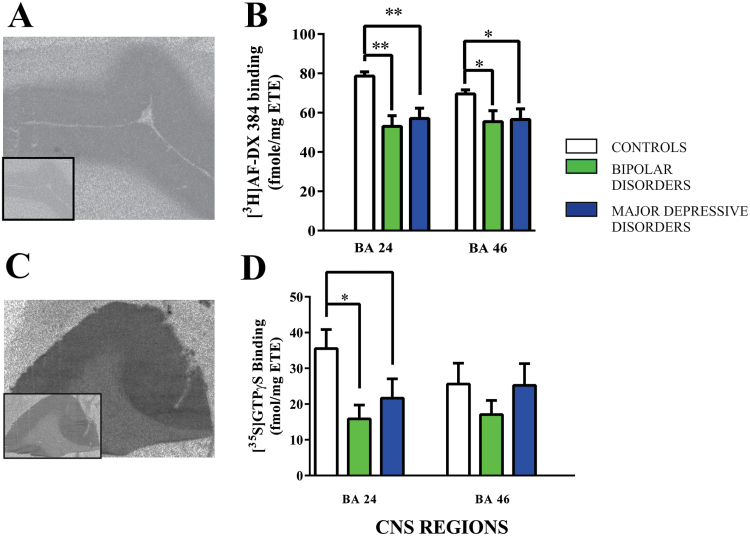
Figure 1.
(A) A representative autoradiograph showing the total binding of [3H]AF-DX 384 to human cortex with non-specific binding shown in the boxed insert.
(B) The specific binding (mean ± SEM) of [3H]AF-DX 384 to Brodmann’s areas (BA) 24 and 46 from subjects with bipolar disorder, major depressive disorder and age and sex matched controls.
(C) A representative autoradiograph showing activated [35S]GTPγS binding in the presence of oxotremorine-M with basal levels of binding shown in the boxed insert.
(D) Oxotremorine-M stimulated [35S]GTPγS binding (mean ± SEM) to Brodmann’s areas (BA) 24 and 46 from subjects with bipolar disorder, major depressive disorder and age and sex matched controls.
*p < 0.05, **p < 0.01.
Compared with controls, levels of [3H]AF-DX 384 binding were lower in BA 24 and BA 46 from subjects with BPD (BA 24: P < .01; Cohen’s d = - 1.70; BA46: P < .05; Cohen’s d = - 0.99) and MDD (BA 24: P < .01; Cohen’s d = - 1.41; BA 46: P < .05; Cohen’s d = - 0.91)(Figure 1B).
Oxotremorine-M Stimulated, [35S]GTPγS Binding
[35S]GTPγS binding in both the basal and activated state was uniformly distributed across the human cortex (Figure 1C) and therefore an integrated measure across the whole cortex was taken.
Oxotremorine-M stimulated, [35S]GTPγS binding showed significant variance with diagnoses in BA 24 (F = 4.14; df = 2,45; P = .02), but not BA 46 (F = 0.71; df = 2,45; P = .50) (Figure 1D). The variation in BA 24 was due to lower oxotremorine-M stimulated, [35S]GTPγS binding in BPD (P = .02; Cohen’s d = - 1.09) and MDD (P = .05; Cohen’s d = - 0.94) compared to controls (Figure 1D).
Western Blotting
To validate the specificity of the anti-CHRM2 antibody, we showed it recognized a 66-kDA immunogenic band in the cortex of wild type but not Chrm2-/- mice (Figure 2A). These data showed the anti-CHRM2 antibody targets an epitope on the mammalian Chrm2. In every sample of human cortex, we visualized the CHRM2 at 65 kDA as predicted from human fibroblasts (Buchli et al., 1999). The antibody cross reacted with an immunogenic band at 71kDa of unknown identity in all human cortical samples.
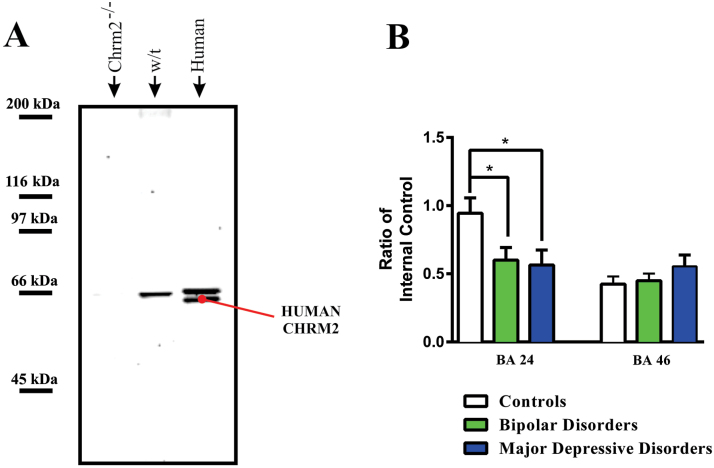
Figure 2.
(A) A Western blot showing immunogenic bands visualised in cortex from muscarinic M2 receptor knock out mouse (Chrm2-/-), wild type mouse (w/t) and human cortex (human) with the anti-muscarinic M2 receptor antibody used to quantify muscarinic M2 receptor protein in human cortex.
(B) Levels (mean ± SEM) of muscarinic M2 receptors in Brodmann’s areas (BA) 24 and 46 from subjects with bipolar disorder, major depressive disorder and age and sex matched controls.
*p < 0.05
There was significant variation in the CHRM2 protein (F = 3.88; df = 2,44; P = .03) with diagnoses in BA 24 (Figure 2B); the variance in CHRM2 protein approached, but did not reach, significance (F = 1.11, df = 2,44, P = .39; F = 2.92, df = 2,43, P = .07) in BA 46. Post-hoc analyses showed that, compared to controls, the variance in BA 24 was due to lower levels of CHRM2 in BPD (P = .04; Cohen’s d = - 0.77) and MDD (P = .04; Cohen’s d = - 0.82).
Potential Confounding Factors
Our experimental data did not vary with gender (males vs females P = .11 to 0.84) and there were no strong relationships between our experimental data and age (r2 from 3.3 x 10–4 to 0.13; p from 0.13 to 0.95), DI (r2 from 0.03 to 0.27; p from 0.07 to 0.85), PMI (r2 from 1.8 x 10–5 to 0.20, p from 0.11 to 0.99), final recorded dose of mood stabiliser (r2 from 0.008 to 0.02; p from 0.59 to 0.76), final recorded dose of antidepressant (r2 from 0.21 to 0.29; p from 0.27 to 0.36) or final recorded antipsychotic drug dose (r2 from 0.03 to 0.27; p from 0.68 to 0.79) (supplementary Table 1). Importantly, there was no strong correlation between CNS pH and our experimental data with all but one regression line not deviating significantly from the horizontal (supplementary Table 2). The regression line describing the relationship between CNS pH and [35S]GTPγS binding in BA24 from the controls did deviate significantly from the horizontal but the relationship was too weak to impact on the outcome of comparing experimental data across diagnoses (Cook and Weisberg, 1999). Finally, there were no strong correlations between levels of [3H]AF-DX 384 binding (BA 24: r2 = 0.01; BA 46 r2 < 0.001), [35S]GTPγS binding (BA 24: r2 = 0.15; BA 46 r2 = 0.007) or CHRM protein (BA 24: r2 = 0.11; BA 46 r2 = 0.005) and freezer storage time.
The incidence of suicide varied between groups (Table 1) and, as a nonnumeric variable, could not be interrogated with linear regression, its impact was therefore assessed by it being analysed as a primary variable. When these analyses were completed using non-psychiatric subjects and psychiatric nonsuicides, [3H]AF-DX 384 binding was shown to be lower in BA 24 (P < .01, Cohen’s d = - 0.84) and BA 46 (P = .01, Cohen’s d = - 0.76) from suicide-completers, levels of CHRM2 protein (P < .01, Cohen’s d = - 1.49) were lower in BA 24, but not BA 46 and oxotremorine-M stimulated, [35S]GTPγS binding did not vary with suicide completion (Table 2). When the same data was analysed without data from the controls [3H]AF-DX 384 binding, CHRM2 protein levels and oxotremorine-M stimulated [35S]GTPγS binding did not vary between suicide completers and those who died from other causes.
Table 2.
A Comparison of Levels (mean ± SEM) of [3H]AF-DX 384 Binding, CHRM2 Protein, and Oxotremorine-M-Stimulated [35S]GTPγS Binding in BA 24 and BA 46 from Suicide Completers and Those Who Died from Other Causes
| Comparison between all cases | ||||
|---|---|---|---|---|
| Assay | Region | Suicide Completion | Nonsuicide | P value |
| [3H]AF-DX 384 bindinga | BA 24 | 53.5±5.80 | 70.4±2.80 | P<.01 |
| BA 46 | 52.5±5.50 | 66.3±2.30 | P=.01 | |
| CHRM2 proteinb | BA 24 | 0.52±0.08 | 0.83±0.09 | P=.02 |
| BA 46 | 0.51±0.08 | 0.45±0.04 | P=.49 | |
| [35S]GTPγS bindinga | BA 24 | 18.5±4.60 | 29.3±4.00 | P=.10 |
| BA 46 | 19.8±4.80 | 24.8±4.20 | P=.46 | |
| Comparison between mood disorder cases | ||||
| [3H]AF-DX 384 bindinga | BA 24 | 53.5±5.80 | 57.4±4.00 | P=.61 |
| BA 46 | 52.5±5.50 | 61.1±4.70 | P=.27 | |
| CHRM2 proteinb | BA 24 | 0.52±0.08 | 0.67±0.13 | P=.31 |
| BA 46 | 0.51±0.08 | 0.50±0.06 | P=.91 | |
| [35S]GTPγS bindinga | BA 24 | 18.5±4.60 | 19.3±5.10 | P=.91 |
| BA 46 | 19.8±4.80 | 23.3±5.90 | P=.64 | |
Abbreviations: BA, Brodmann’s area; CHRM2, muscarinic M2 receptor.
afmol / mg estimated tissue equivalents.
bratio of internal control.
[3H]AF-DX 384 Binding in Rats
The binding of [3H]AF-DX 384 to the rat cortex was more complex than observed using human tissue (Figure 3A). Two layers of radioligand binding were observed in the cingulate and frontal cortices with three layers of binding visible in the parietal cortex. The outer layer of binding in the cingulate and frontal cortices included cortical laminae I, II, III and part of lamina IV whilst the inner layer included the remainder of lamina IV and laminae V, Via, and VIb. In the parietal cortex the outer binding layer included cortical laminae I, II, III, and part of lamina IV, the middle layer included the remainder of lamina IV and the inner layer contained laminae V, Via, and VIb. There was what appeared to be a patch matrix patterning of [3H]AF-DX 384 binding in the striatum. [3H]AF-DX 384 binding in the patches was close to background, while binding across the matrix appeared uniform and so an integrated measure of all binding that was greater than background across the striatum was measured. Binding in the nucleus accumbens and olfactory tubule was more homogenous.
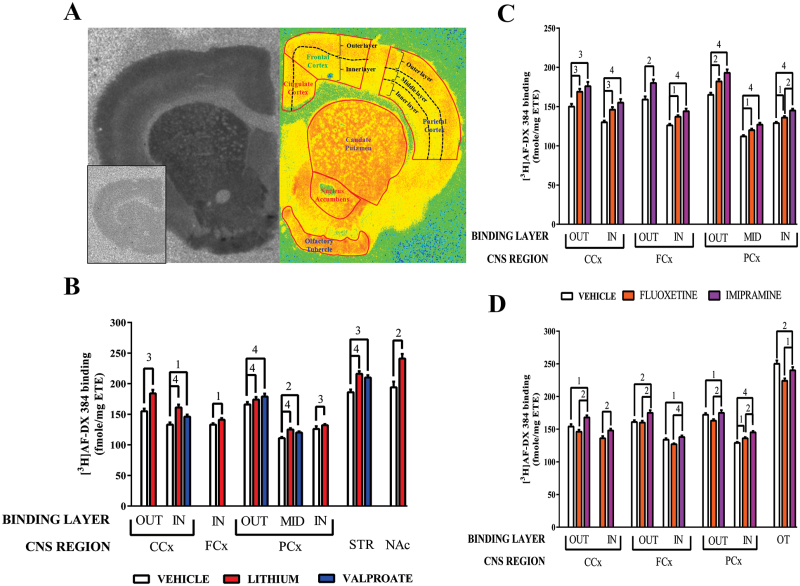
Figure 3.
(A) A typical autoradiograph of the total binding of [3H]AF-DX 384 to rat CNS (left hemisphere) with non-specific binding shown as a boxed insert. The right hemisphere is a pseudo-colour section showing the delineation of CNS regions based on the basis of cytoarchitecture and layers of radioligand binding in the rat cortex.
(B) The specific binding (mean ± SEM) of [3H]AF-DX 384 to rat CNS following 28 days treatment with vehicle, lithium (1.8g lithium carbonate / day) or valproate (12g sodium valproate / day).
(C) The specific binding (mean ± SEM) of [3H]AF-DX 384 to rat CNS following 10 days treatment with vehicle, fluoxetine (20mg / kg / day) or imipramine (10mg / kg / day).
(D) The specific binding (mean ± SEM) of [3H]AF-DX 384 to rat CNS following 28 days treatment with vehicle, fluoxetine (20mg / kg / day) or imipramine (10mg / kg / day).
1p < 0.05, 2p < 0.01, 3p < 0.001, 4p < 0.0001 In = inner layer, Out = outer layer
Neuropsychopharmacological Studies
All drug treatments had complex effects on [3H]AF-DX 384 binding in rat CNS which raises the possibility that both mood stabilisers and antidepressant drugs may act, in part, by altering levels of muscarinic receptors in the CNS.
Mood Stabilisers
The most profound effect of drug treatment was an increase in [3H]AF-DX 384 binding after treatment with lithium (supplementary Table 3). Compared to vehicle, treatment with lithium was associated with higher levels of [3H]AF-DX 384 in all cortical regions, except the outer binding layer of the frontal cortex, and with higher levels of binding in the striatum and nucleus accumbens (Figure 3B). Compared to vehicle, there were higher levels of [3H]AF-DX 384 binding in inner layer of the cingulate cortex, the outer and middle layer of the parietal cortex and the striatum from rats treated with valproate.
Antidepressant Drug Treatment
The effects of antidepressant drug treatments on [3H]AF-DX 384 binding were more complex, showing differing changes with drug and treatment duration.
Compared to vehicle, rats treated with imipramine for 10 days had higher levels of [3H]AF-DX 384 binding in the outer and inner binding layer of the cingulate and frontal cortices as well as in the outer middle and inner layer of the parietal cortex (Figure 3C; Supplementary Table 4). Higher levels of [3H]AF-DX 384 binding was also present in all these regions and layers except for the outer layer of the frontal cortex after treatment with fluoxetine. In the inner layer of the parietal cortex levels of [3H]AF-DX 384 binding were higher in rats treated with imipramine compared with those treated with fluoxetine.
After 28 days treatment there were more complex changes in [3H]AF-DX 384 binding. Compared with vehicle, rats treated with imipramine had higher levels of [3H]AF-DX 384 binding in all layers in the cortex except the middle layer of the parietal cortex (Figure 3D; Supplementary Table 4). By contrast, [3H]AF-DX 384 binding was only higher in the inner layer of the parietal cortex in rats treated with fluoxetine. Compared to vehicle, [3H]AF-DX 384 binding was lower in olfactory tubule from rats treated with fluoxetine and imipramine for 28 days. Finally, [3H]AF-DX 384 binding differed between rats treated with imipramine and fluoxetine in the outer and inner binding layer of the cingulate, frontal and parietal cortices and in the olfactory tubule.
Discussion
In this study we confirmed our earlier finding of lower [3H]AF-DX 384 binding in BA 46 from subjects with BPD and MDD (Gibbons et al., 2009) and extended that finding to show lower binding of that radioligand in BA 24 from the same subjects. Our findings in BA 24 are not consistent with a study that failed to show any changes in [3H]AF-DX 384 binding in that region from subjects with mood disorders (Zavitsanou et al., 2005). There is no clear reason why the outcome from these two studies differ but major depressive disorder can be differentiated into melancholic and non-melancholic (Parker, 2003), which means the different outcomes from the two studies may be because of different balances between melancholic and nonmelancholic in the MDD cohorts. However, in addition to [3H]AF-DX 384 binding, we also report lower levels of CHRM2 protein in BA 24, but not BA 46, from BPD and MDD. This brings a consistency to our data in BA 24 where lower levels of CHRM2 would be expected to contribute to lower levels of [3H]AF-DX 384 binding from subjects with mood disorders. By contrast, the absence of significant changes in levels of CHRM2 in BA 46 from BPD and MDD suggests that levels of other CHRMs that bind [3H]AF-DX 384, for example CHRM4, may be altered in that region. Finally, oxotremorine-M stimulated GTPγS binding was lower in BA 24 from subjects with BPD and MDD which is consistent with decreased recruitment of G proteins by CHRM2 and/or CHRM4.
In this study we also show complex effects of treating rats with mood stabilisers and antidepressant drugs. The effect of drug treatment is most obvious and most consistent following treatment with lithium where higher levels of [3H]AF-DX 384 binding are present in the cortex, striatum and nucleus accumbens. After treatment with valproate levels of [3H]AF-DX 384 binding are also higher in the cingulate and parietal cortex as well as the striatum. This class effect of mood stabilisers raises the possibility that some of their therapeutic efficacy may be due to increasing levels of CHRMs in the CNS. There has been little study of the effects of treatment of mood stabilisers on CHRMs levels, however one study reported treatment with lithium was associated with higher levels of [3H]NMS, a pan-muscarinic receptor antagonist (Waelbroeck et al., 1990), binding in rat hippocampus but not striatum (Marinho et al., 1998). However, another study reported higher levels of [3H]Quinuclidinyl benzilate ([3H]QNB), another pan-muscarinic receptor antagonist (Simon et al., 1990), binding in the striatum but not the cortex or hippocampus (Lerer and Stanley, 1985) from rats treated with lithium. It has been suggested that both lithium and valproate exert their effects on the cholinergic system by affecting receptor signalling, rather than changing receptor destiny (Bymaster and Felder, 2002). Our data, and data from others, using intact animals now challenges this hypothesis.
In this study we show complex changes in levels of [3H]AF-DX 384 binding in rats treated with fluoxetine and imipramine. After 10 days of treatment both drugs seem to result in higher levels of [3H]AF-DX 384 binding but after 28 days this effect was only apparent in rats treated with imipramine. Our data would therefore suggest that only treatment with imipramine gives long term increases in [3H]AF-DX 384 binding and it is therefore possible this differential effect may be associated with the different treatment outcomes with these drugs; tricyclic antidepressants being more effective in melancholic depression (Parker et al., 1999). This proposition deserves further investigation given the efficacy of the muscarinic receptor antagonist scopolamine as an antidepressant drug in some subjects with depression (Furey and Drevets, 2006). As far as we are aware this is the first report of antidepressant drug treatments being associated with an increase in [3H]AF-DX 384 binding.
Overall, when controls were included, we have shown that [3H]AF-DX 384 density in BA 24 and 46, as well as CHRM2 protein levels, were significantly lower in suicide-completers compared to non-completers; these differences were not apparent when only psychiatric suicide completers and other psychiatric cases were compared. Notably, our findings excluding the controls agree with a study that showed no differences in the binding of [3H]QNB in BA 10, the hypothalamus or pons from suicide completers (Kaufmann et al., 1984). By contrast, higher levels of [3H]QNB binding have been reported in the frontal cortex of suicide completers (Meyerson et al., 1982). Our data might suggest there are complex interactions, particularly in BA 24, between changes in CHRM2 and the aetiology of mood disorders and those that occur in suicide completers. Our data showing no change in oxotremorine-M stimulated [35S]GTPγS binding in BA 46 from suicide completers adds to that showing no change in carbachol-stimulated [3H]GTPγS binding in BA 9 (Gonzalez-Maeso et al., 2002) to suggested marked changes in CHRM signalling are not present in the dorsolateral prefrontal cortex from suicide completers. One limitation of our current study is that cohort sizes prevent reliable analysis of suicide completion within individual psychiatric diagnoses and thus it is difficult to be certain whether the effects we report are specific to suicide-completion. We did not see variation in [3H]AF-DX 384 binding with suicide completion in our study on schizophrenia (Gibbons et al., 2012) reinforcing a potential complex relationship between the cortical binding of that radioligand, mood disorders and suicide completion.
One confound in human postmortem studies is drug treatments prior to death. The absence of correlations between levels of [3H]AF-DX 384 binding, CHRM2 protein and oxotremorine-M stimulated [35S]GTPγS binding and final recorded doses of mood stabilisers, antidepressant and antipsychotic drug doses would argue against changes in the measures in the cortex of people with mood disorders being a straight forward drug effect. This argument is reinforced by our data showing that the main effect of treating with mood stabilisers and antidepressant drugs is to increase [3H]AF-DX 384 binding, not decrease it as occurs in mood disorders.
Our study, and a previous neuroimaging study (Cannon et al., 2006), suggest changes in CHRM2 are most prominent in BA 24, a cortical region with varying activity associated with changes of mood in BPD (Blumberg et al., 2000; Deckersbach et al., 2008; Hulvershorn et al., 2012). Significantly, the cortical CHRM2 acts as a pre-synaptic autoreceptor (Mrzljak et al., 1998; Bymaster et al., 2003) and therefore derangement in its function would impact on levels of acetylcholine, which in turn would change the activity of other muscarinic, as well as the nicotinic, receptors. Notably, the absence of CHRM2 in the mammalian cortex is associated with a hypercholinergic state (Bymaster et al., 2003), therefore it seems likely that low levels of CHRM2 in the human cortex would have the same outcome due to reduced negative feedback through the receptor. The proposition that there is reduced negative feedback through CHRM2 in BA 24 in mood disorders is supported our finding of reduced CHRM2 / 4 mediated G-protein signalling in BA 24 from subjects with those disorders as this is the first post-receptor event modulating CHRM2 negative feedback. Hence our data raises the possibility that the proven antidepressant effects of the pan-CHRM antagonist scopolamine (Furey and Drevets, 2006; Drevets and Furey, 2010), and the potential antidepressant effects of drugs that target the nicotinic receptors (X. Li et al., 2012), involves the modulation of a hypercholinergic state in the cortex of subjects with mood disorders.
Statement of Interest
None.
Supplementary Material
Acknowledgments
The authors would like to thank Geoff Pavey for his contribution to preparing the human CNS tissue, Nahed Tawadros for hers in drug treating the rats in this study and Chris Felder (Eli Lilly) for providing the CHRM2 knockout mice. The fluoxetine used in this study was gifted by Eli Lilly. The authors acknowledge the Victorian Brain Bank Network which is supported by the Florey Institute for Neuroscience and Mental Health, the Alfred Hospital, the Victorian Forensic Institute of Medicine, the University of Melbourne and funded by Australia’s National Health & Medical Research Council, Helen Macpherson Smith Trust, Parkinson’s Victoria and Perpetual Philanthropic Services.
This work was supported by the National Health and Medical research Council (BD Senior Research Fellow: APP1002240; Project Grants: 628699 and APP 1066144), the Australian Research Council (ES: ARC Future Fellowship FT100100689), the University of Melbourne (WJ: University of Melbourne International Postgraduate Award) and the Victorian Government’s Operational Infrastructure Support.
References
- American Psychiatric A (1994) Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C: American Psychiatric Association. [Google Scholar]
- Blumberg HP, Stern E, Martinez D, Ricketts S, de Asis J, White T, Epstein J, McBride PA, Eidelberg D, Kocsis JH, Silbersweig DA. (2000) Increased anterior cingulate and caudate activity in bipolar mania. BiolPsychiatr 48:1045–1052. [DOI] [PubMed] [Google Scholar]
- Buchli R, Ndoye A, Rodriguez JG, Zia S, Webber RJ, Grando SA. (1999) Human skin fibroblasts express m2, m4, and m5 subtypes of muscarinic acetylcholine receptors. J Cell Biochem 74:264–277. [PubMed] [Google Scholar]
- Bymaster FP, Felder CC. (2002) Role of the cholinergic muscarinic system in bipolar disorder and related mechanism of action of antipsychotic agents. MolPsychiatr 7 Suppl 1:S57-S63. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, McKinzie DL, Felder CC, Wess J. (2003) Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res 28:437–442. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Carson RE, Nugent AC, Eckelman WC, Kiesewetter DO, Williams J, Rollis D, Drevets M, Gandhi S, Solorio G, Drevets WC. (2006) Reduced muscarinic type 2 receptor binding in subjects with bipolar disorder. ArchGenPsychiatry 63:741–747. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Klaver JK, Gandhi SK, Solorio G, Peck SA, Erickson K, Js NA, Eckelman WC, Furey ML, Sahakian BJ, McMahon FJ, Drevets WC. (2011) Genetic variation in cholinergic muscarinic-2 receptor gene modulates M(2) receptor binding in vivo and accounts for reduced binding in bipolar disorder. MolPsychiatr 16:407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Wu S, Rostamkhani M, McGue M, Iacono WG, MacMurray JP. (2002) Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. AmJMedGenet 114:527–529. [DOI] [PubMed] [Google Scholar]
- Cook RD, Weisberg S. (1999) Applied Regression Including Computing and Graphics. Hoboken: Wiley. [Google Scholar]
- Crook JM, Dean B, Pavey G, Copolov D. (1999) The binding of [3H]AF-DX 384 is reduced in the caudate-putamen of subjects with schizophrenia. Life Sci 64:1761–1771. [DOI] [PubMed] [Google Scholar]
- Dean B, Gray L, Scarr E. (2006) Regionally specific changes in levels of cortical S100beta in bipolar 1 disorder but not schizophrenia. AustNZJPsychiatry 40:217–224. [DOI] [PubMed] [Google Scholar]
- Dean B, Pereira A, Pavey G, Singh B. (1997) Repeated antidepressant drug treatment, time of death and frequency of handling do not affect [3H]paroxetine binding in rat cortex. Psychiatry Res 73:173–179. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Rauch SL, Buhlmann U, Ostacher MJ, Beucke JC, Nierenberg AA, Sachs G, Dougherty DD. (2008) An fMRI investigation of working memory and sadness in females with bipolar disorder: a brief report. Bipolar disorders 10:928–942. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Furey ML. (2010) Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. BiolPsychiatr 67:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton SL, Roche SL, Llavero HM, Oldknow KJ, Farquharson C, Gillingwater TH, Wishart TM. (2013) Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoSONE 8:e72457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Drevets WC. (2006) Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. ArchGenPsychiatry 63:1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons AS, Scarr E, McLean C, Sundram S, Dean B. (2009) Decreased muscarinic receptor binding in the frontal cortex of bipolar disorder and major depressive disorder subjects. J Affect Disord 116:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons AS, Scarr E, Boer S, Money T, Jeon WJ, Felder C, Dean B. (2012) Widespread decreases in cortical muscarinic receptors in a subset of people with schizophrenia. Int J Neuropsychopharmacol:1–10. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. (1999) Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A 96:1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Rodriguez-Puertas R, Meana JJ, Garcia-Sevilla JA, Guimon J. (2002) Neurotransmitter receptor-mediated activation of G-proteins in brains of suicide victims with mood disorders: selective supersensitivity of alpha(2A)-adrenoceptors. MolPsychiatr 7:755–767. [DOI] [PubMed] [Google Scholar]
- Hill C, Keks N, Roberts S, Opeskin K, Dean B, Mackinnon A, Copolov D. (1996) Problem of diagnosis in postmortem brain studies of schizophrenia. Am J Psychiatry 153:533–537. [DOI] [PubMed] [Google Scholar]
- Hulvershorn LA, Karne H, Gunn AD, Hartwick SL, Wang Y, Hummer TA, Anand A. (2012) Neural activation during facial emotion processing in unmedicated bipolar depression, euthymia, and mania. BiolPsychiatr 71:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM. (1974) Acetylcholine and depression. PsychosomMed 36:248–257. [DOI] [PubMed] [Google Scholar]
- Kaufmann CA, Gillin JC, Hill B, O’Laughlin T, Phillips I, Kleinman JE, Wyatt RJ. (1984) Muscarinic binding in suicides. Psychiatry Res 12:47–55. [DOI] [PubMed] [Google Scholar]
- Kingsbury AE, Foster OJ, Nisbet AP, Cairns N, Bray L, Eve DJ, Lees AJ, Marsden CD. (1995) Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain ResMolBrain Res 28:311–318. [DOI] [PubMed] [Google Scholar]
- Klein D, Kern RM, Sokol RZ. (1995) A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem Mol Biol Int 36:59–66. [PubMed] [Google Scholar]
- Lerer B, Stanley M. (1985) Effect of chronic lithium on cholinergically mediated responses and [3H]QNB binding in rat brain. Brain Res 344:211–219. [DOI] [PubMed] [Google Scholar]
- Li R, Shen Y. (2013) An old method facing a new challenge: re-visiting housekeeping proteins as internal reference control for neuroscience research. Life Sci 92:747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Frye MA, Shelton RC. (2012) Review of pharmacological treatment in mood disorders and future directions for drug development. Neuropsychopharmacol 37:77–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Wong JY, Krenus D, Jeffreys BJ, Porritt MJ, Howells DW. (1999) Tissue fixation prevents contamination of tritium-sensitive storage phosphor imaging plates. Biotechniques 26:432–434. [DOI] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, Gelernter J. (2005) CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: results from an extended case-control structured association study. Hum Mol Genet 14:2421–2434. [DOI] [PubMed] [Google Scholar]
- Marinho MM, de Sousa FC, de Bruin VM, Vale MR, Viana GS. (1998) Effects of lithium, alone or associated with pilocarpine, on muscarinic and dopaminergic receptors and on phosphoinositide metabolism in rat hippocampus and striatum. Neurochem Int 33:299–306. [DOI] [PubMed] [Google Scholar]
- McKinney M. (2006) Practical Aspects of Radioligand Binding. Curr Protoc Pharmacol Supplement 33:1.3.1–1.3.42. [DOI] [PubMed] [Google Scholar]
- Meyerson LR, Wennogle LP, Abel MS, Coupet J, Lippa AS, Rauh CE, Beer B. (1982) Human brain receptor alterations in suicide victims. PharmacolBiochemBehav 17:159–163. [DOI] [PubMed] [Google Scholar]
- Miller JH, Gibson VA, McKinney M. (1991) Binding of [3H]AF-DX 384 to cloned and native muscarinic receptors. J Pharmacol Exp Ther 259:601–607. [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Belcher S, Goldman-Rakic PS. (1998) Localization of the m2 muscarinic acetylcholine receptor protein and mRNA in cortical neurons of the normal and cholinergically deafferented rhesus monkey. JComp Neurol 390:112–132. [PubMed] [Google Scholar]
- Naudon L, Hotte M, Jay TM. (2007) Effects of acute and chronic antidepressant treatments on memory performance: a comparison between paroxetine and imipramine. Psychopharmacology (Berl) 191:353–364. [DOI] [PubMed] [Google Scholar]
- Parker G. (2003) Modern diagnostic concepts of the affective disorders. Acta PsychiatrScandSuppl:24–28. [DOI] [PubMed] [Google Scholar]
- Parker G, Mitchell P, Wilhelm K, Menkes D, Snowdon J, Schweitzer I, Grounds D, Skerritt P, Roy K, Hadzi-Pavlovic D. (1999) Are the newer antidepressant drugs as effective as established physical treatments? Results from an Australasian clinical panel review. Aust N Z J Psychiatry 33:874–881. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Hill CA, Dean B, Keks NA, Opeskin K, Copolov DL. (1998) Confirmation of the diagnosis of schizophrenia after death using DSM-IV: a Victorian experience. AustNZJPsychiatry 32:73–76. [DOI] [PubMed] [Google Scholar]
- Rowntree DW, Nevin S, Wilson A. (1950) The effects of diisopropylfluorophosphonate in schizophrenia and manic depressive psychosis. J Neurol Neurosurg Psychiatry 13:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr E, Keriakous D, Crossland N, Dean B. (2006) No change in cortical muscarinic M2, M3 receptors or [35S]GTPgammaS binding in schizophrenia. Life Sci 78:1231–1237. [DOI] [PubMed] [Google Scholar]
- Simon G, Filep J, Zelles T. (1990) Alpha adrenergic drugs inhibit [3H]-QNB binding to muscarinic receptors of rat heart, brain and parotid gland membranes. Life Sci 47:2021–2025. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. (2006) Human postmortem tissue: what quality markers matter? Brain Res 1123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelbroeck M, Tastenoy M, Camus J, Christophe J. (1990) Binding of selective antagonists to four muscarinic receptors (M1 to M4) in rat forebrain. Mol Pharmacol 38:267–273. [PubMed] [Google Scholar]
- Wang JC, et al. (2004) Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. HumMolGenet 13:1903–1911. [DOI] [PubMed] [Google Scholar]
- Wess J. (2003) Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends PharmacolSci 24:414–420. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Katsifis A, Yu Y, Huang XF. (2005) M2/M4 muscarinic receptor binding in the anterior cingulate cortex in schizophrenia and mood disorders. Brain ResBull 65:397–403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.