Abstract
Background:
Several recent trials indicate low-dose ketamine produces rapid antidepressant effects. However, uncertainty remains in several areas: dose response, consistency across patient groups, effects on suicidality, and possible biases arising from crossover trials.
Methods:
A systematic search was conducted for relevant randomized trials in Medline, Embase, and PsycINFO databases up to August 2014. The primary endpoints were change in depression scale scores at days 1, 3 and 7, remission, response, suicidality, safety, and tolerability. Data were independently abstracted by 2 reviewers. Where possible, unpublished data were obtained on treatment effects in the first period of crossover trials.
Results:
Nine trials were identified, including 201 patients (52% female, mean age 46 years). Six trials assessed low-dose ketamine (0.5mg/kg i.v.) and 3 tested very low-dose ketamine (one trial assessed 50mg intra-nasal spray, another assessed 0.1–0.4mg/kg i.v., and another assessed 0.1–0.5mg/kg i.v., intramuscular, or s.c.). At day 3, the reduction in depression severity score was less marked in the very low-dose trials (P homogeneity <.05) and among bipolar patients. In analyses excluding the second period of crossover trials, response rates at day 7 were increased with ketamine (relative risk 3.4, 95% CI 1.6–7.1, P=.001), as were remission rates (relative risk 2.6, CI 1.2–5.7, P=.02). The absolute benefits were large, with day 7 remission rates of 24% vs 6% (P=.02). Seven trials provided unpublished data on suicidality item scores, which were reduced on days 1 and 3 (both P<.01) but not day 7.
Conclusion:
Low-dose ketamine appears more effective than very low dose. There is substantial heterogeneity in clinical response, with remission among one-fifth of patients at 1 week but most others having benefits that are less durable. Larger, longer term parallel group trials are needed to determine if efficacy can be extended and to further assess safety.
Keywords: Ketamine, major depression, meta-analysis
Introduction
Aside from electro-convulsive therapy (ECT), there are no widely used treatments that provide large or rapid benefits for patients suffering from severe depression (Martinowich et al., 2013). This presents a major clinical challenge, especially for patients who have not responded fully to existing therapy or if symptoms include suicidality. In 2000 a small crossover trial suggested that a single subanaesthetic dose of ketamine could provide a large antidepressant benefit, starting within a few hours and lasting for at least several days (Berman et al., 2000). Ketamine appeared to improve specific depressive symptoms such as sadness, suicidality, and helplessness, rather than induce a nonspecific mood-elevating effect. Since then, several more trials have been published (Zarate et al., 2006; Diazgranados et al., 2010a; Zarate et al., 2012; Murrough et al., 2013; Sos et al., 2013), but existing reports and reviews (aan het Rot et al., 2010; Aan Het Rot et al., 2012; Katalinic et al., 2013; Brittner et al., 2014; Caddy et al., 2014; McGirr et al., 2014) do not easily allow a direct comparison of how treatment effects persist in the days following treatment, given differences in graphical and tabular reporting methods and outcome scales. Three very recently reported trials (Lai et al., 2014; Lapidus et al., 2014) (C. K. Loo, V. Galvez, E. O’Keefe, unpublished data) evaluated lower doses of ketamine than previously tested, providing an opportunity to compare treatment efficacy and tolerability of different doses. One other key issue is the extent of bias resulting from the use of a crossover design in all but one previous trial. Potential biases from crossover designs, such as carryover of treatment effect into the second treatment period, can be addressed by restricting analyses to the first treatment period but often requires unpublished data. We therefore conducted a systematic review of trials of ketamine in patients with treatment-resistant depression to evaluate antidepressant efficacy, suicidality, safety, and tolerability.
Methods
Types of Trials
We considered all relevant randomized trials (either crossover or parallel) in which ketamine was used specifically for the treatment of a Major Depressive Episode (DSM-IV diagnosis) and was compared with placebo, including active placebo. Eligible trials included participants with either unipolar or bipolar affective disorder. We excluded trials conducted in the context of ECT and surgery, but there were no restrictions on concomitant pharmacological or psychological treatments.
Types of Interventions
We included any trial that attempted to evaluate a comparison between single administration of ketamine and placebo for the treatment of major depressive disorder. There was no restriction on ketamine regimen used (eg, dose or route).
Types of Outcome Measures
The primary endpoint was change in depression severity scores from baseline on depression scales such as the Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960) and/or the Montgomery Åsberg Depression Rating Scale [MADRS]) at the following times: day 1 (24 hours after first dose), day 3, and day 7. Other outcomes were: clinical remission (defined as HAM-D <7 or MADRS <10); clinical response (defined as ≥50% reduction in depression severity score); suicidality measures, including the suicidality item of HAM-D or MADRS; safety and tolerability as assessed by reported adverse events and dropouts.
Search Methods for Identification of Trials
Reports were restricted to English language publications. Publications for this review were identified by searching Medline, Embase, and PsycINFO databases before August 2014 using the following search terms as free text or subject headings as appropriate for each database: (“ketamine” OR “NMDA receptor antagonist”) AND (“depression” OR “major depressive disorder” OR “bipolar depression” OR “depressive disorder” OR “dysthymic disorder” OR “treatment resistant depression”) AND (“ randomized controlled trial” OR “controlled clinical trial” OR “randomized” OR “placebo”) (full details available in supplementary Table 1 in the online data supplement). If additional studies cited in these articles met with these criteria, then they were also included.
Selection of Studies
Two authors (A.R. and Y.X.) reviewed the search strategy and selected studies for inclusion in the review. In case of disagreement, M.H. arbitrated.
Data Extraction and Analysis
When relevant publications were selected, 2 authors (A.R. and Y.X.) independently extracted information on year of publication, geographic location, sample size, loss to follow-up, mean age, study design, major inclusion and exclusion criteria, concomitant treatments, and outcome measures as well as safety and tolerability.
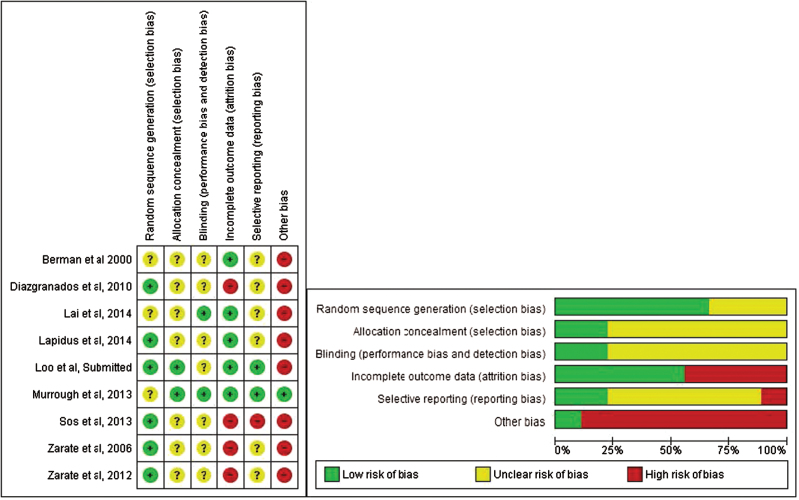
Potential sources of bias were identified for each trial using criteria recommended in the Cochrane Handbook (Higgins and Green, 2009). For random sequence generation, we categorized trials as “low risk” if random-number charts or coin tossing was used, as “unclear” if there were no details of sequence generation, and as “high risk” if sequence was generated by odd/even date of birth. For allocation concealment, we categorized trials as low risk if opaque or sealed envelopes were used, as unclear if there was no detailed information and as high risk if assignments could possibly be foreseen. For blinding, we categorized studies as low risk if blinding was ensured and unlikely to be broken, as unclear if an inactive placebo was used, and as high risk if outcome measurement was likely to be influenced by lack of blinding. For incomplete outcome data, we categorized trials as low risk if missing outcome data balanced in numbers and with similar reasons across intervention groups, as unclear if reports of drop- outs were insufficient, and as high risk if there were imbalances in numbers or reasons for missing data across intervention groups. For selective reporting, we categorized trials as low risk if there was a published protocol and the outcome measures listed in the protocol were reported, as unclear if protocol could not be found and as high risk if a described outcome measure in the protocol or methods was not reported in results. Assessment of period and carryover effects were listed under “other sources of bias.” We categorized crossover trials as high risk and parallel trials as low risk.
Depression severity score (HAM-D and/or MADRS) at each follow-up time point was calculated for each trial, for ketamine or placebo groups, and a placebo-corrected value for each time point was calculated. If not available in tabular form, HAM-D and/or MADRS results at different time points were estimated independently from Figureures in the articles by 2 authors. Placebo-corrected HAM-D scores were plotted separately for each trial with that outcome measure, and placebo-corrected MADRS scores for all trials with that outcome measure. In addition, we plotted a percentage reduction in depression severity score for all trials based on MADRS score where available and HAM-D for other trials. We conducted a meta-analysis of mean MADRS score at day 1, 3, and 7, with day of administration defined as day 0. These analyses were conducted initially to include the data from the single parallel trial and the first period of all crossover trials. We also compared the relative risk (RR) during the first period against the second period in crossover trials in order to examine the direction and magnitude of carryover effects. Meta-analysis of continuous outcomes was conducted using Stata13 (www.stata.com), Windows 7. Meta-analyses of response rates and clinical remission at days 1, 3, and 7 were conducted using Review Manager Version 5.3. software (RevMan, 2014) to estimate RR and number needed to treat (NNT). All meta-analyses were done using a random effects model. The I2 statistic was used to assess heterogeneity of trial results, and subgroup heterogeneity was tested using a chi-squared statistic (Higgins and Green, 2009). Data were available only on day 4 for one trial (Sos et al., 2013), and this was included in the day 3 category. Data were sought from the crossover trials for each treatment period separately.
Results
Search Results
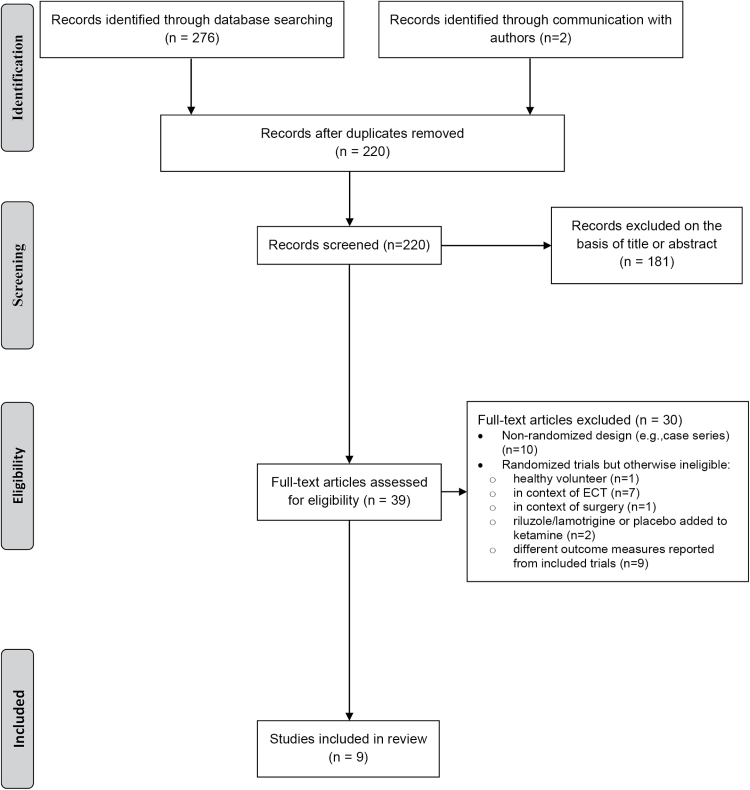
The search results and study selection process are summarized in a PRISMA flowchart (Figure 1) showing the number of unique references identified by the search, the number of records excluded, and the number of full-text records retrieved. One placebo-controlled trial was not included because the sequence was not randomized (Valentine et al., 2011) and one randomized trial was not included because ketamine was compared with ECT (Ghasemi et al., 2014). One unpublished trial of the effects of intra-operative ketamine on depression was identified, but data were not available (M. Bastos, personal communication).
Figure 1.
Flow diagram for systematic review.
Baseline Characteristics
Nine randomized, placebo-controlled trials were identified, and a total of 201 patients (105 females and 96 males) were included (Table 1) (Berman et al., 2000; Zarate et al., 2006, 2012; Diazgranados et al., 2010a; Murrough et al., 2013; Sos et al., 2013; Lai et al., 2014; Lapidus et al., 2014) (C. K. Loo, V. Galvez, E. O’Keefe, unpublished data). Eight studies (Berman et al., 2000; Zarate et al., 2006, 2012; Diazgranados et al., 2010a; Sos et al., 2013; Lai et al., 2014; Lapidus et al., 2014) (C. K. Loo, V. Galvez, E. O’Keefe, unpublished data) were crossover trials with a 1- to 2-week washout period between treatments, and one was a parallel group design with 1 week of follow-up (Murrough et al., 2013). Two trials (Diazgranados et al., 2010a; Zarate et al., 2012) included only patients with bipolar disorder (BD), and 7 trials (Berman et al., 2000; Zarate et al., 2006; Murrough et al., 2013; Sos et al., 2013; Lai et al., 2014; Lapidus et al., 2014) (C. K. Loo, V. Galvez, E. O’Keefe, unpublished data) included patients with only unipolar depression (although one trial [Berman et al., 2000] included one patient with BD). All patients were treatment resistant, variously defined (Table 1). Patients with history of psychosis or recent substance use disorders were generally excluded. Patients in the 2 BD trials (Diazgranados et al., 2010a; Zarate et al., 2012) were drug free except for lithium or valproate. In the other 7 trials, patients were drug free in 2 (Berman et al., 2000; Zarate et al., 2006), allowed to take only a nonbenzodiazepine hypnotic in one (Murrough et al., 2013), and taking stable doses of other psychotropic medications in 4 trials (Sos et al., 2013; Lai et al., 2014; Lapidus et al., 2014) (C. K. Loo, V. Galvez, E. O’Keefe, unpublished data). Six trials (Berman et al., 2000; Zarate et al., 2006, 2012; Diazgranados et al., 2010a; Murrough et al., 2013; Sos et al., 2013) assessed a single 40-minute infusion of i.v. ketamine at a subanaesthetic low dose of 0.5mg/kg (defined here as low dose). Three trials tested lower doses (defined here as very low-dose trials): 50mg intranasally (as 10mg every 5 minutes), estimated to achieve plasma concentrations equivalent to about 0.3mg/kg i.v. (Lapidus et al., 2014); 0.1 to 0.4mg/kg i.v. (Lai et al., 2014), or 0.1 to 0.5mg/kg i.v., intramuscular, or s.c. in an ascending dose design (with placebo randomly inserted) (C. K. Loo, V. Galvez, E. O’Keefe, unpublished data). Seven trials (Berman et al., 2000; Zarate et al., 2006, 2012; Diazgranados et al., 2010a; Sos et al., 2013; Lai et al., 2014; Lapidus et al., 2014) used 0.9% saline as placebo and 2 used 0.045mg/kg (Murrough et al., 2013) or 0.01mg/kg (C. K. Loo, V. Galvez, E. O’Keefe, unpublished data) midazolam as an active placebo. Potential sources of bias are summarized in Figure 2.
Table 1.
Characteristics of Previous Trials of One-Off Ketamine in Patients with Severe Mood Disorder
| Trial |
Region,
Country |
Sample Size
b
(N) |
Mean Age (SD),
(y) |
Male (N) | Design | Major Inclusion Criteria | Major Exclusion Criteria | Intervention | Control | Concomitant Treatments | Loss to Follow-Up | Outcome Measures† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berman et al., 2000 | Connecticut | 9 | 37 (10) | 4 | Crossover (washout ≥1 week) |
Recurrent unipolar major depression (N=8); Bipolar disorder (N=1) |
Recent alcohol or substance abuse; any other Axis I disorders |
Ketamine i.v. 0.5mg/kg |
Saline placebo i.v. |
Nil and drug free 2 weeks prior |
2 before last treatment (1 placebo, 1 ketamine), attended all previous follow-ups. | HAM-D-25, BDI, (VAS-high), (BPRS) |
| Zarate et al., 2006 | Bethesda, Maryland | 18 | 47 (11) | 6 | Crossover (washout 1 week) |
Major depression disorder, DSM-IV; failed ≥2 antidepressant trials; HAMD-21 ≥ 18 |
Psychotic features; bipolar disorder; history of antidepressant- or substance- induced hypomania/mania |
Ketamine i.v. 0.5mg/kg |
Saline placebo i.v. |
Nil and drug free 2 weeks prior |
4 before last treatment (placebo), attended all previous follow-ups; 1 after first treatment (placebo), didn`t attend follow-up after Day 2. |
HAM-D-21a, BDI, (BPRS), (YMRS), VAS-depression |
| Diazgranados et al., 2010a | Bethesda, Maryland | 18 | 48 (13) | 6 | Crossover (washout 2 weeks) |
Bipolar I or II depression, DSM-IV, failed antidepressant trial and mood stabilizer; MADRS ≥ 20; current major depressive episode ≥4 weeks | Psychotic features; substance abuse or dependence in last 3 months; unstable medical condition; serious risk of suicide; previous treatment with ketamine | Ketamine i.v. 0.5mg/kg |
Saline placebo i.v. |
Lithium/ valproate no other drugs during trial nor in prior 2 weeks (5 for fluoxetine); no psychotherapy |
3 after first treatment (2 ketamine, 1 placebo), didn`t finish follow-ups. 2 after last treatment (both ketamine), didn`t finish follow-ups. |
MADRSa, HAM-D-17, BDI, VAS-depression, VAS-anxiety, HAM-A, (BPRS), (YMRS) |
| Zarate et al., 2012 | Bethesda, Maryland |
15 | 47 (10) | 7 | Crossover (washout 2 weeks) |
Bipolar I or II depression, DSM-IV, failed antidepressant trial and mood stabilizer; MADRS ≥ 20; current major depressive episode ≥4 weeks | Psychotic features; unstable medical condition; substance abuse or dependence in last 3 months; previous treatment with ketamine | Ketamine i.v. 0.5mg/kg |
Saline placebo i.v. |
Lithium/ valproate no other drugs during trial nor in prior 2 weeks (5 for fluoxetine); no psychotherapy |
4 after first treatment (3 ketamine, 1 placebo), didn`t finish follow-ups. | MADRSa
HAM-D-17, BDI, VAS-depression, VAS-anxiety, HAM-A, (BPRS), (CADSS), (YMRS) |
| Murrough et al., 2013 | Houston, Texas and New York City | 72 | 47 (13) Ketamine 43 (12) Midazolam |
35 | Parallel | Major depressive disorder, DSM-IV; failed ≥3 antidepressant trials; ≥1 previous major depressive episode or lasting ≥2 years; ≥32 IDS-C | Psychotic illness or bipolar disorder; alcohol/substance abuse in the previous 2 years; unstable medical illness; serious and imminent suicidal or homicidal risk; <27 on MMSE | Ketamine i.v. 0.5mg/kg (N=47) |
Midazolam i.v. 0.045mg/ kg (N=25) |
Only prn nonbenzodiazepine hypnotic; drug free 1 week before (4 weeks for fluoxetine); no psychotherapy |
2 from ketamine arm: both after 24-hour evaluation; 3 from placebo arm: all after 24-hour evaluation |
MADRSa, QIDS-SR, CGI, (CADSS), (BPRS), (YMRS); |
| Sos et al., 2013 | Prague, Czech Republic | 30 | 42 (15) Ketamine first 45 (11) placebo first |
15 | Crossover (washout 1 week) |
Major depressive disorder, DSM- IV; score of ≥20 on MADRS at baseline | Suicide risk, any other Axis I or II disorders; unstable medical illness or neurological disorder; psychotic symptom/ disorder in 1st/2nd degree relatives; electroconvulsive therapy within 3 months | Ketamine i.v. Loading: 0.27mg/kg Maintenance: 0.27mg/kg |
Saline placebo i.v. |
Remained on antidepressant medications and dosages maintained throughout the duration of the study |
1 before last treatment (ketamine), attended all previous follow-ups. 2 after first treatment (ketamine), didn`t finish follow-ups. 5 after last treatment (3 ketamine, 2 placebo), didn`t finish follow-ups. |
MADRSa
(BPRS) |
| Lapidus et al., 2014 | New York City, USA | 20 | 48 (13) | 10 | Crossover (washout ≥1 week) |
Major depressive disorder, DSM-IV; failed ≥1 trial of adequate dose and duration of antidepressant; IDS-C ≥ 30 | Any other Axis I disorders; suicide risk; substance abuse or dependence in last 6 months; psychotic disorder; bipolar disorder; developmental disorder; previous abuse/ dependence on ketamine | Ketamine intranasal 50 mg | Saline placebo intranasal |
Stable doses of psychotropic medication, including antidepressant treatment | 2 before first treatment (1 ketamine, 1 placebo). | MADRSa, QIDS-SR, HAM-A, (BPRS), (CADSS), (YMRS), (SAFTEE) |
| Lai et al., 2014 | Sydney, Australia | 4 | 51 (17) | 2 | Crossover (washout 1 week) 4 dosages of ketamine |
Major depressive episode ≥4 weeks, DSM-IV; MADRS ≥ 20; failed ≥ 1 trial of antidepressant during current episode | Comorbid Axis I or II disorders (aside from one subject with phobic disorder); | Ketamine i.v. 0.1, 0.2, 0.3, 0.4mg/Kg |
Saline placebo i.v. |
Remain on stable doses of psychotropic medications; no changes in medication dosage and no ECT 4 weeks prior to trial entry |
1 after placebo, 0.1, and 0.2mg/kg (attended all previous follow-ups); 1 after placebo, 0.1, 0.2 and 0.3mg/kg (attended all previous follow-ups). |
MADRSa, CGI, QIDS-SR, (BPRS), (YMRS), (CADSS), (SAFTEE). |
| C. K. Loo, V. Galvez, E. O’Keefe, unpublished data | Sydney Australia | 15 | 49 (11) | 11 | Crossover (washout 1 week) 5 dosages of ketamine (i.v., N=4); (IM, N=5); (SC, N=6) |
Major Depressive Disorder, depression episode of duration ≥ 4 weeks, DSM IV; MADRS ≥ 20; failed to ≥1 trial of antidepressant during current episode | Schizophrenia, rapid cycling bipolar disorder or any current psychotic symptoms | Ketamine i.v., IM or SC; 0.1, 0.2, 0.3, 0.4, 0.5mg/kg |
Midazolam i.v., SC or IM 0.01mg/kg |
Remain on stable doses of psychotropic medications; no changes in medication dosage and no ECT 4 weeks prior to trial entry | 1 after placebo, 0.1, and 0.2mg/kg i.v.; 1 after placebo, 0.1, and 0.2mg/kg IM; 1 after placebo, 0.1, 0.2, and 0.3mg/kg IM; 1 after placebo, 0.1, 0.2, 0.3 and 0.4mg/kg IM; 1 after placebo, 0.1 and 0.2mg/kg SC; 1 after placebo SC; | MADRSa, (BPRS), (YMRS - item 1), (CADSS), (SAFTEE) |
| Total/ average |
201 | 46 | 96(48%) | 129 (64%) patients in crossover | Ketamine:145 (82%) i.v.; 20 (11%) intranasal; 5 (3%) intramuscular; 6 (3%) s.c. | Saline:94 (61%) i.v.; 20 (13%) intranasal; Midazolam:29 (19%) i.v.; 5 (3%) intramuscular; 6 (4%) s.c. |
27 (13%) no concomitant medications;33 (16%) on Lithium/valproate; 72 (36%) only nonbenzodiazepine hypnotic; 69 (34%) on other psychotropic medications |
40 |
Abbreviations: MMSE: Mini-Mental State Examination; IDS-C, the Inventory of Depressive Symptomatology—Clinician Rated. †HAM-D, Hamilton Depression Rating Scale scores; BDI, Beck Depression Inventory; VAS(-high), Visual Analog Scales score (for intoxication “high”); BPRS, Brief Psychiatric Rating Scale;YMRS, Young Mania Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; HAM-A, Hamilton Anxiety Rating Scale; CADSS, Clinician Administered Dissociative States Scale; QIDS-SR,Quick Inventory of Depressive Symptomatology-Self Report; CGI, Clinical Global Impression; SAFTEE, Systematic Assessment for Treatment Emergent Effects.Measures in parentheses indicate the safety and tolerability measures.
aPrimary outcome measures.
bSample size was identified at the time of randomization, with an exception for Murrough et al., 2013. There were 73 patients at the time of randomization and 72 remained after allocation. One patient in the ketamine group did not receive intervention. Characteristics (eg, age and sex) were reported based on the 72 patients.
Figure 2.
Potential sources of bias in included trials. See Methods for explanation of potential biases.
Effects on Depression Severity Scores over Time
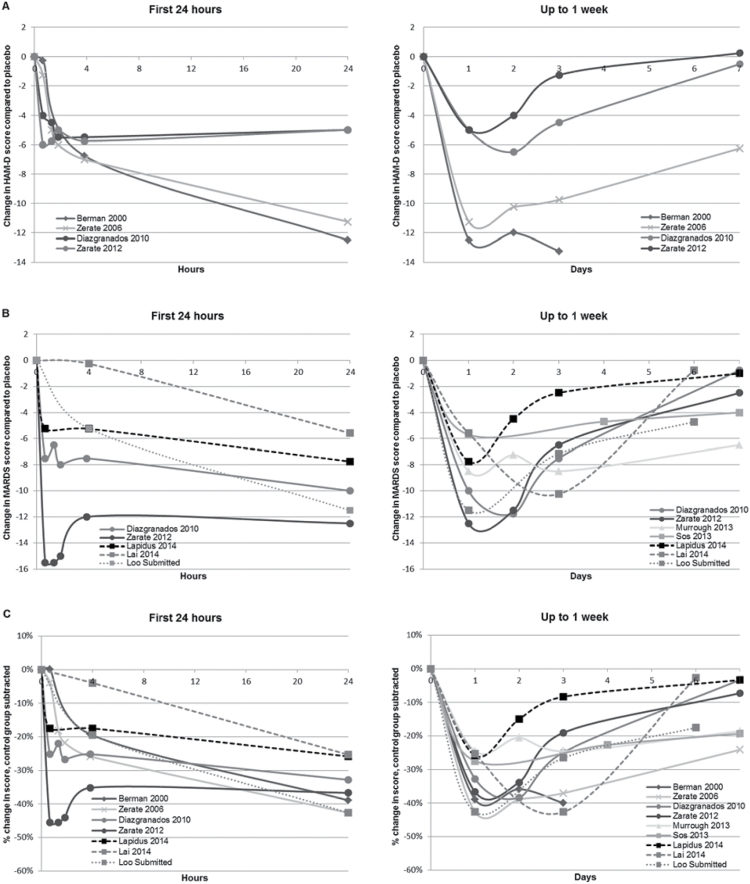
Effects on depression severity over time are presented in Figure 3, with data redrawn from original trials and placed on a uniform linear scale, and shown in Figure 4 with data from the single parallel trial and from the first period of crossover trials. A large reduction in depression severity was evident within 4 hours in all but one small trial of very low-dose ketamine, and treatment effects were largest at day 1. In the trials of very low-dose ketamine (Lai et al., 2014; Lapidus et al., 2014) (C. K. Loo, V. Galvez, E. O’Keefe, unpublished data), the reduction in overall severity appeared smaller and shorter lived. A test for heterogeneity indicated a significant difference in treatment effects at day 3 for low-dose compared with very low-dose trials (P=.02). In the 4 trials conducted with ketamine 0.5mg/kg i.v. for patients with unipolar depression (Berman et al., 2000; Zarate et al., 2006; Murrough et al., 2013; Sos et al., 2013), the large reduction in average mood score at 24 hours remained evident, though moderately attenuated, up to day 7. In the 2 trials among patients with BD, the treatment effect largely dissipated by days 4 to 7 (Diazgranados et al., 2010a; Zarate et al., 2012). A smaller treatment effect in BD patients compared with unipolar patients on the HAM-D was evident by 24 hours. There was no evidence of greater benefit in trials that aimed to attain high peak concentrations, either with an i.v. push during a few minutes (Lai et al., 2014) or an initial loading dose of 0.27mg/kg during the first 10 minutes (Sos et al., 2013).
Figure 3.
Reductions in depression severity scores following single-dose ketamine in patients with major depression. (A) Placebo-corrected changes in Hamilton Depression Rating Sclae Scores (HAM-D). (B) Placebo-corrected changes in Montgomery-Åsberg Depression Rating Scale (MADRS). (C) Placebo-corrected percentage changes in HAM-D/MADRS. Data are placebo-corrected, and axes redrawn on a linear scale to avoid a visual misrepresentation of how the treatment effect evolves over time. Lines with circular markers represent trials among patients with bipolar disorder depression. Other trials were among patients with unipolar depression. Lines with square markers represent patients remained on pretrial antidepressant treatment. Solid lines represent low dose (i.v. ketamine 0.5mg/kg). Dashed lines represent very low dose (intranasal ketamine 50mg or i.v. 0.3mg/kg; Lai et al. and Loo et al. used an ascending dose design and the data for 0.3mg/kg are shown).
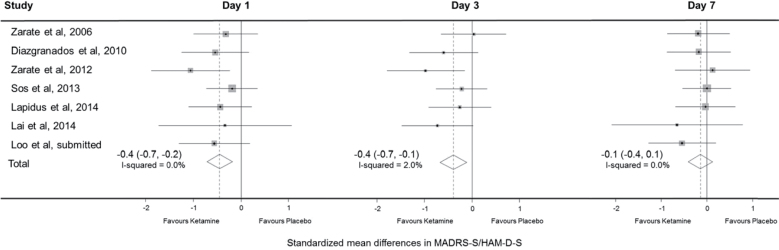
Figure 4.
Difference in standardized mean mood score on days 1, 3, and 7 for crossover trials, first period only. Data are shown only for the first period in crossover trials. Hamilton Depression Rating Sclae Scores (HAM-D) was reported by Zarate et al., 2006 with the remainder reporting Montgomery-Åsberg Depression Rating Scale (MADRS). 95% CIs of treatment effects are represented by horizontal lines for individual trials, by grey diamonds for trial subgroups, and by black diamonds for all trials. A vertical dashed line goes through the overall pooled result for all trials, with a result to the left of the vertical line indicating a reduction in mood score in the ketamine group.
Effects on Response and Remission Rates over Time
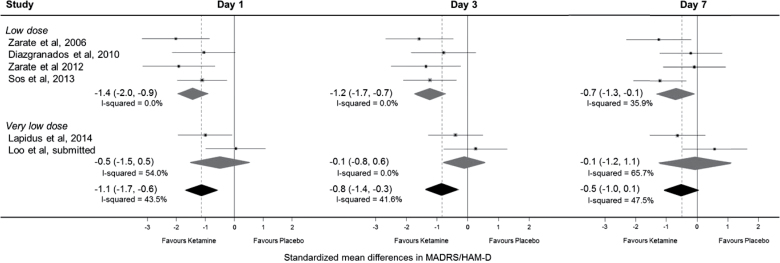
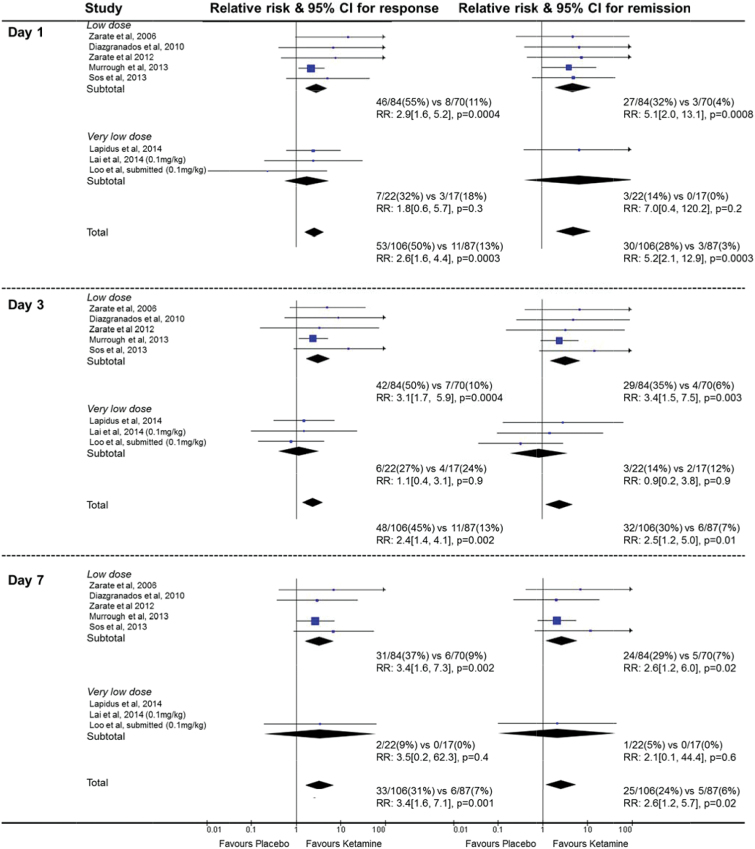
Analyses of treatment effects on response and remission, including only the single parallel group trial and the first treatment period of crossover trials, are summarized in Figure 5. At day 1, there was a large treatment effect, with 50% of those in the ketamine group compared with 13% in the placebo group meeting criteria for response (RR 2.6, 95% CI 1.6 to 4.4, P=.0003; NNT 2.9, 1.9–7.1). There was a similarly large effect on remission of symptoms at day 1 (RR 5.2, 95% CI 2.1–12.9, P=.0003; NNT 4.5, 3.1–8.3) among those receiving ketamine. At day 7, response rates were substantially increased in the ketamine group and to a larger degree (RR 3.4, 95% CI 1.6 to 7.1, P=.001; NNT 6.3, 3.4–25). Remission rates were still substantially increased in the ketamine group, although to a lesser degree (RR 2.6, 95% CI 1.2–5.7, P=.02; NNT 9.1, 5–50). While the pooled results of 3 trials of very low-dose ketamine showed no significant effect on response or remission on days 1, 3, or 7 and the treatment effect appeared smaller compared to low-dose ketamine, formal subgroup heterogeneity tests were not significant (P=.09 for response and P=.12 for remission on day 3).
Figure 5.
Effects of single-dose ketamine on response and remission rates at days 1, 3, and 7. Data included from the parallel trial (Murrough et al.) and from the first period of crossover trials. 95% CIs of treatment effects are represented by horizontal lines for individual trials and by diamonds for subgroups or all trials. Results on the right side of the vertical line indicated benifit in the ketamine group.
One of the major potential biases in crossover trials is dropout after the first treatment, and this occurred in 12/46 patients who received ketamine initially compared with 5/55 who received placebo initially in 5 trials (Zarate et al., 2006, 2012; Diazgranados et al., 2010a; Sos et al., 2013; Lapidus et al., 2014). Dropout was for improved mood for 4 patients in the ketamine group and 2 in the placebo group. In terms of treatment effects in the second period of the crossover trials, overall 0/34 patients responded at day 1 who received placebo as their second treatment compared with 22/50 patients responding at day 1 after receiving ketamine as their second treatment. Remission was achieved in 0/34 patients at day 1 who received placebo as their second treatment compared with 8/50 patients after receiving ketamine as their second treatment. Thus, it did appear that carryover effects were not marked, although this cannot be assessed reliably since fewer patients in the ketamine groups proceeded to the second period.
Effects on Suicidality
Measures of suicidality were published in 4 trials (Berman et al., 2000; Zarate et al., 2006, 2012; Murrough et al., 2013) and these are summarized in Table 2. Each trial reported a significant reduction in suicidality assessed using different measures. Seven trials provided unpublished data on the suicide item component of a depression scale, 1 for HAM-D (Zarate et al., 2006), and 6 for MADRS (Diazgranados et al., 2010a; Zarate et al., 2012; Sos et al., 2013; Lai et al., 2014; Lapidus et al., 2014) (C. K. Loo, V. Galvez, E. O’Keefe, unpublished data). Overall, reported suicidality scores were low at baseline, with an average of 1.6 on the MADRS suicidality item (which ranges from 0 to 6) for trials that reported this outcome, as shown in Table 3. Nonetheless, a significant reduction in suicidality severity score was observed for the ketamine group at days 1 and 3, as seen in Figure 6.
Table 2.
Published Measures of Suicidality
| Trial | Measure Used | Outcome Reported in Publication |
|---|---|---|
| Berman et al., 2000 | HAM-D suicidality item | While undergoing active treatment, significant decreases were observed for suicidality (P < .02). Control treatment was not associated with significant improvement in any of the HAM-D items. |
| Zarate et al., 2006 | HAM-D suicidality item | Significant main effect for drug |
| Zarate et al., 2012 | Changes in suicide item scores on the MADRS, HAM-D, and BDI | Within 40min, suicidal ideation significantly improved in subjects receiving ketamine compared with placebo (Cohen’s d 0.98, 95% CI 0.64 –1.33); this improvement remained significant through day 3. Reductions in suicide item scores for each of the MADRS, HAM-D, and BDI using linear mixed models (each P <.001) |
| Murrough et al., 2013 | Composite index of explicit suicidal ideation (Beck Scale for Suicidal Ideation, MADRS suicide item, Quick Inventory of Depressive Symptoms suicide item) | Fifty-three percent of ketamine-treated patients scored 0 on all 3 explicit suicide measures at 24h compared with 24% of the midazolam group (χ = 4.6; P = .03) |
Abbreviations: BDI, Beck Depression Inventory; HAM-D, Hamilton Depression Rating Scale; MADRS, Montgomery Åsberg Rating Scale.
Table 3.
Unpublished Data on Suicidality Scores at Baseline
| Trial | Suicidality Item Subscale Used | Ketamine | Placebo | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Zarate et al., 2006 | HAM-D, item 4* | 1.9 | 1.1 | 1.2 | 1.1 |
| Diazgranados et al., 2010a | MADRS, item 10** | 2.0 | 1.9 | 2.4 | 1.9 |
| Zarate et al., 2012 | MADRS, item 10** | 2.3 | 1.6 | 2.5 | 1.5 |
| Sos et al., 2013 | MADRS, item 10** | 0.4 | 0.7 | 0.4 | 0.7 |
| Lapidus et al., 2014 | MADRS, item 10** | 1.7 | 1.4 | 1.7 | 1.7 |
| Lai et al., 2014 | MADRS, item 10** | 1.8 | 1.0 | 1.8 | 1.0 |
| Loo et al., unpublished | MADRS, item 10** | 1.9 | 1.2 | 2.3 | 1.0 |
Abbreviations: MADRS, Montgomery Åsberg Rating Scale.
*Ranges from 0 to 4; **ranges from 0 to 6.
Figure 6.
Standardized mean differences in suicide item scores at days 1, 3, and 7. Suicide item score from Hamilton Depression Rating Sclae Scores (HAM-D) provided by Zarate et al., 2006, and from Montgomery-Åsberg Depression Rating Scale (MADRS) for other trials. 95% CIls of treatment effects are represented by horizontal lines for individual trials and by diamonds for all trials. A vertical dashed line goes through the overall pooled result for all trials, with a result to the left of the vertical line indicating a reduction in mood suicide item score in the ketamine group.
Tolerability and Side Effects
Measures of tolerability and side effects were summarized in Table 4. Low-dose ketamine at 0.5mg/kg, infused over 40 minutes, was generally well tolerated, with transient, mild-to-moderate dissociative symptoms and blood pressure and/or heart rate increases in a minority of patients. For example, in the largest trial, 17% of patients had significant dissociative symptoms immediately after ketamine infusion, but these all resolved within 2 hours and no severe psychotic symptoms (paranoia, hallucinations, or delusions) occurred in either arm (Murrough et al., 2013). The characteristic symptomatic side effects (eg, confusion, blurred vision) and the increases in heart rate and blood pressure resolved within 4 hours of administration in all trials. These side effects were less marked with very low-dose ketamine delivered intranasally for 20 minutes (Lapidus et al., 2014) but were more marked with very low-dose ketamine given as an i.v. push for a few minutes (Lai et al., 2014). Among trials that reported a full listing of adverse events in both groups, there was no excess in the ketamine vs placebo after administration day (Murrough et al., 2013; Lai et al., 2014; Lapidus et al., 2014) (C. K. Loo, V. Galvez, E. O’Keefe, unpublished data).
Table 4.
Measures of Safety and Tolerability in Included Trials
| Trial | VAS-High | BPRS | CADSS | YMRS | SAEs |
|---|---|---|---|---|---|
| Berman et al., 2000 | Ketamine produced markedly greater scores. Scores are significantly different between two groups till 40min and return to baseline by 110min. | Ketamine produced significantly greater scores, especially the positive symptoms. Scores are significantly different between 2 groups until 40min and return to baseline by 120min. | Not mentioned | Not mentioned | Not mentioned |
| Zerate et al., 2006 | Not mentioned | The positive symptoms subscale scores were worse for participants receiving ketamine than those receiving placebo only at 40min. | Not mentioned | Worse for participants receiving ketamine than placebo at 40 minutes, but they were significantly better from days 1 to 2. | Nil |
| Diazgranados et al., 2010a | Not mentioned | Ketamine and placebo differed only at 40min, and this difference was due to a small, nonsignificant decrease with placebo and an even smaller increase with ketamine. | A ketamine/placebo difference was seen at 40min only (large increase on ketamine). | Patients receiving ketamine had higher scores at 40min but significantly lower scores at days 2 and 14. Compared with baseline, there was no significant change in manic symptoms in patients receiving placebo. | One patient developed manic symptoms in the ketamine group that resolved within 80min and an affective switch occurred for one patient in the placebo group. |
| Zarate et al., 2012 | Not mentioned | No significant drug effect or interaction. | Higher values in patients receiving ketamine only at 40min. | No significant drug effect or interaction. | Nil |
| Murrough et al., 2013 | Not mentioned | The positive symptoms subscale scores for ketamine patients beyond the 40min ranged from 4.02 to 4.04. | At 40min, the average score for the ketamine group was 14.7 (10.6–18.8), and 2.28 (0.0–4.8) for the midazolam group. Average scores for the ketamine group beyond 40min ranged between 0.065 and 0.533. | The first item of the YMRS at 40min was 0.6 for ketamine and 0.12 for midazolam group. | Patient 1: BP=73/40 (1min) HR <30 bpm (30sec), spontaneous recovery; Patient 2: Suicide attempt while tapering off of psychotropic medication, patient was hospitalized . |
| Lapidus et al., 2014 | Not mentioned | No relationship between ketamine associated changes in dissociative or psychotomimetic symptoms and antidepressant response was found | No relationship between ketamine associated changes in dissociative or psychotomimetic symptoms and antidepressant response was found | Measured, but not reported | Nil |
| Lai et al., 2014 | Not mentioned | Clear dose–response relationship for psychotomimetic symptoms occurring within 40min. Scores returned to pre- treatment levels within 4h for all subjects. | Clear dose–response relationship for psychotomimetic symptoms occurring within 40min. Scores returned to pre-treatment levels within 4h for all subjects. | Not mentioned | During 0.4mg/kg dosage Two patients: tachycardia (150 bpm); One patient: BP increase (140/80 to 195/105). Spontaneous recovery within 15 min |
| C. K. Loo, V. Galvez, E. O’Keefe, unpublished data | Not mentioned | No evidence of treatment emergent mania, at any time point, across routes of administration and doses | Dose-response relationship between psychotomimetic effects and ketamine treatment for all routes, with higher peak scores in the i.v. group. Scores resolved without intervention by 40 minutes post- injection for all routes. | No evidence of treatment emergent mania, at any time point, across routes of administration and doses | Across groups, MAP elevations did not exceed >20% from baseline, except for 4 patientsa (i.v., N=2; IM, N=2). These effects resolved by 30min without intervention. Increases in heart rate did not exceed 120% of baseline, except in three participants (N=1, i.v.; N=1 intramuscular; N=1, S.C.). |
Abbreviations: AE, adverse event; BPRS, Brief Psychiatric Rating Scale; CADSS, Clinician Administered Dissociative States Scale; CGI, Clinical Global Impression; MAP, mean arterial pressure; QIDS-SR, Quick Inventory of Depressive Symptomatology-Self Report; SAE, serious adverse event; SAFTEE, Systematic Assessment for Treatment Emergent Effects; VAS-high, Visual Analog Scales score for intoxication “high”; YMRS, Young Mania Rating Scale.
aIn the i.v. group, 1 participant experienced a 25% increase 1 hour posttreatment (0.1 mg/kg) and a 24% MAP increase 5 min posttreatment (0.2–0.5 mg/kg). A second participant experienced a 44% MAP increase 10 min posttreatment (0.1 mg/kg). In the IM group, 1 participant experienced a 30% MAP increase 10 min posttreatment (0.4 mg/kg) and a second experienced a 39% MAP increase 5 min posttreatment (0.3 mg/kg).
Eleven events were reported as serious adverse events in the ketamine group: hypotension and bradycardia occurred in 1 person, which resolved in <1 minute and was considered to be due to a vaso-vagal episode (Murrough et al., 2013); suicide attempt occurred in 1 patient while tapering off of psychotropic medication (Murrough et al., 2013); tachycardia (>150 bpm) occurred in 2 patients (Lai et al., 2014); and mean arterial pressure elevations >20% from baseline in 5 patients (Lai et al., 2014) (C. K. Loo, V. Galvez, E. O’Keefe, unpublished data). Also, while not reported as serious adverse events, one ketamine-treated patient developed manic symptoms that resolved within 80 minutes, and an affective switch occurred for one patient in the placebo group (Diazgranados et al., 2010a). All the hemodynamic side effects resolved within a few hours with no lasting effects. Thus, it could be summarized that 3 major psychiatric events (suicide attempt before treatment, transient manic symptoms, and affective switch) were reported in the studies, of which only one was potentially attributable to ketamine (transient manic symptoms). No major medical events occurred in the trials.
Diverse measures were used to test possible psychotomimetic, dissociative, or mood elevation symptoms, including Visual Analogue Scale score for intoxication “high” (Aitken, 1969), Brief Psychiatric Rating Scale (Overall and Gorham, 1962), Young Mania Rating Scale (Young et al., 1978), and Clinician Administered Dissociative States Scale (Bremner et al., 1998). The results showed that Visual Analogue Scale score for intoxication “high” scores returned to baseline within 110 minutes of the infusion (Berman et al., 2000). In no case did euphoria, derealization, or depersonalization persist beyond 110 minutes (Zarate et al., 2006).
Continuation into the second phase of the crossover trial was also assessed as a proxy of tolerability, and most dropouts were reported as being due to changes in mood rather than adverse events. In 5 crossover trials with first-phase data available (Zarate et al., 2006, 2012; Diazgranados et al., 2010a; Sos et al., 2013; Lapidus et al., 2014), 12 of 46 patients did not proceed to placebo treatment after receiving ketamine first. For 4 patients, this was due to improved mood (Zarate et al., 2006), for 4 it was due to worsening mood (Diazgranados et al., 2010a; Sos et al., 2013), and other reasons were involved for an additional 4 (Zarate et al., 2012; Lapidus et al., 2014).
Discussion
Summary of Findings
This systematic review of trials of single-dose ketamine compared with placebo for treatment-resistant patients in a major depressive episode confirms a large reduction in depression severity and suicidality that is apparent within 4 hours after ketamine administration. The data suggest benefits are smaller and shorter lived with very low-dose ketamine. There is also a suggestion of short-lived effects in patients with BD. There is substantial heterogeneity in clinical response, with benefits lasting <1 week for most patients, but approximately one-fifth of patients remaining in remission at 1 week.
Limitations
This systematic review has a number of methodological limitations. Most important is the small sample size (average of only 23 patients per trial) and 201 patients in total. The use of a crossover design in all but one trial is a potential issue. While a crossover design improves study power to partly mitigate problems associated with small sample size, it can have limitations for mood disorder trials. Crossover designs are most reliable when assessing a fully reversible treatment, with outcomes that return to baseline levels after a suitable washout period (ie, short-lived interventions that do not affect the natural history of a stable illness). For treatments that do not have these criteria, one can observe period effects (ie, the treatment effect in the first period is systematically different from that in the second period) and/or carryover effects (ie, the treatment effect in the first period is still operating during the second period). Statistical tests to detect period and carryover effects are relatively insensitive, and analytic approaches to overcome these issues are generally unsatisfactory (Senn, 2002). However, these issues can be addressed with a separate analysis of first period data, which was possible in this meta-analysis. Finally, all trials involved one-off treatments and varying measures of side effects and had relatively short follow-up; hence, the safety and efficacy of long-term treatment remain uncertain.
Strengths of this review include the reporting of additional unpublished data from numerous trials and analyses of only the first treatment period, increasing the reliability. It is also the first review to compare results of low-dose and very low-dose ketamine, suggesting larger benefits with the former. The consistency of the findings described in this review suggest a true treatment effect in improving depressive symptoms, albeit temporarily for most. Several additional strands of evidence indicate that the benefit observed in these trials is a result of depression treatment, rather than nonspecific mood elevation or a ‘high’ following ketamine administration: there is improvement in core depressive symptoms such as sadness, suicidality, and helplessness; the time course of benefits is in the following days and sometimes weeks, whereas mood elevation with drugs of abuse is generally limited to the time of intoxication; the response pathway can be interrupted in animal models of depression treatment (Autry et al., 2011); and postoperative mood improvement occurs among patients given ketamine intra-operatively, who would have been unaware of immediate subjective effects (Kudoh et al., 2002; Argiriadou et al., 2004). Recent trials have also suggested possible benefits among patients with posttraumatic stress disorder (Whiteford et al., 2015) and in obsessive compulsive disorder (Rodriguez et al., 2013), and there is encouraging initial evidence comparing ketamine with ECT (Ghasemi et al., 2014).
Relevance for Clinical Practice and Research
Most clinicians, drug regulators, and health funders will require further research evidence before low-dose ketamine is adopted in clinical practice. The results of this review have several implications for future research.
First, research is urgently required on efficacy and safety of longer treatment regimens. A key issue is whether the ketamine response can be maintained or even enhanced by repeated dosing, such as the 2 to 3 times weekly schedule that has been assessed in some case series with promising findings (Price et al., 2009; aan het Rot et al., 2010; DiazGranados et al., 2010b; Larkin and Beautrais, 2011; Rasmussen et al., 2013; Shiroma et al., 2014). Safety, tolerability, and abuse potential of repeated dosing is a critical issue for future trials. While some reassurance can be taken from the much more extensive use of i.v., oral, and sublingual ketamine in chronic pain control (Chong et al., 2009), future longer term trials must assess safety in this patient population. Trials should assess the likelihood of abuse potiential in subjects with major depression, while incorporating strategies to mitigate this.
Second, trials should adopt parallel designs rather than crossover designs, since benefits remain for more than 1 week in a significant proportion of patients; hence, this situation does not meet the fundamental criterion for crossover trials to have a stable disease baseline and short-lived treatment effect in all patients. Third, trials should recruit many times more patients, so that the effects in different subgroups can be assessed reliably given the considerable heterogeneity in response. Fourth, trial designs should reflect the possibility of different effects among patients with bipolar and unipolar disorder. Fifth, there is no evidence concerning first-line treatment of major depressive disorder: ketamine may have a role to play in patients with severe presenting symptoms, including suicidality, and could conceivably cover the lag phase before SSRI efficacy onset (and reduction of suicidal ideation may be particularly important in young adults). Further evidence of reduction in suicidality was recently provided by an additional trial published after our search cut-off date, conducted among 27 patients with mood and anxiety spectrum disorders who presented with clinically significant suicidal ideation.(Murrough et al., 2015) Ketamine may also augment the response from standard treatments. For example, while ECT is indicated in severe depression with acute suicidality it may take a week or longer to alleviate symptoms and ketamine may play an important role by acting more quickly in this setting. Finally, further data on safety and efficacy of use in ‘real world’ clinical settings is clearly required, given that many of the trials were conducted in highly medically controlled environments. While i.v. infusions may be practical in service settings used for ECT, other formulations and delivery routes, such as intranasal spray, s.c., intramuscular, or oral, are likely to be more broadly applicable to clinical practice. Minimizing dissociative and hemodynamic side effects is important for widespread applicability, and this is likely to involve avoiding high peak plasma levels (Lai et al., 2014). However, very low doses are associated with lower efficacy, suggesting multiple low doses delivered gradually or sequentially may be optimal. Future trials could also assess ketamine enantiomers (Paul et al., 2009), metabolites (Zarate Jr et al., 2012), or other glutamatergic agents.
Conclusions
This systematic review confirmed a large, rapid benefit in response and remission following a single dose of ketamine in patients with treatment resistant depression. There was also a reduction in suicidality. Transient psychotomimetic and heemodynamic effects occurred. There were no major medical events. Collectively, these data suggest ketamine has considerable promise for the acute treatment of major depression and provide the rationale for a large, parallel group, randomized, placebo-controlled trial to assess safety and efficacy of a longer course of ketamine in patients with severe mood disorders.
Statement of Interest
Dr. Lapidus has received consulting fees from LCN consulting and serves on the advisory board for Halo Neuro Inc. In addition to performing ketamine research, he provides clinical treatments with ketamine. Until September 2014, Dr. Lapidus was at the Icahn School of Medicine at Mount Sinai, which has been named on a use patent on ketamine for the treatment of depression. The Icahn School of Medicine has entered into a licensing agreement for the use of ketamine as therapy for treatment-resistant depression and could potentially benefit if ketamine were to gain approval for the treatment of depression. Dr. Loo has received an honorarium from Lundbeck to speak at independent educational sessions organised by psychiatrists, for which Lundbeck provided funding. Dr. Glozier has received speaking honoraria from Lundbeck, Janssen, and Servier Laboratories, and advisory board fees from Lundbeck.
Supplementary Material
Acknowledgments
Unpublished data were provided by Veronica Galvez, Kyle Lapidus, Colleen Loo, David A. Luckenbaugh, Alexander McGirr, Peter Sos, and Carlos A. Zarate.
National Heart Foundation Future Leader Fellowship (grant number 100034) (Level 2) to M.H. National Institutes of Health (grant number K23 MH104465), the Brain and Behavior Research Foundation, APIRE/Janssen, and the Le Foundation to K.L. National Health and Medical Research Council (NHMRC) Centre for Research Excellence (grant number 1061043), Janssen, and Lundbeck to N.G. National Health and Medical Research Council (NHMRC) (grant number 1037196) to P.M. NHMRC Principal Fellowship (grant number 1066280) and NHMRC Program Grant (grant number 1052555) to A.R. These funding bodies had no role in the conduct or reporting of the review.
References
- aan het Rot M, Collins Ka, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145. [DOI] [PubMed] [Google Scholar]
- Aan Het Rot M, Zarate Ca, Charney DS, Mathew SJ. (2012) Ketamine for depression: where do we go from here? Biol Psychiatry 72:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RC. (1969) Measurement of feelings using visual analogue scales. Proc R Soc Med 62:989. [PMC free article] [PubMed] [Google Scholar]
- Argiriadou H, Himmelseher S, Papagiannopoulou P, Georgiou M, Kanakoudis F, Giala M, Kochs E. (2004) Improvement of pain treatment after major abdominal surgery by intravenous S+-ketamine. Anesth Analg 98:1413–1418, table of contents. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P-f, Kavalali ET, Monteggia LM. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello a, Anand a, Oren Da, Heninger GR, Charney DS, Krystal JH. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. (1998) Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). J Trauma Stress 11:125–136. [DOI] [PubMed] [Google Scholar]
- Brittner M, Micoulaud-Franchi JA, Richieri R, Boyer L, Adida M, Lancon C, Fond G. (2014) [Ketamine in acute and severe major depressive disorder]. Presse Med 43:492–500. [DOI] [PubMed] [Google Scholar]
- Caddy C, Giaroli G, White TP, Shergill SS, Tracy DK. (2014) Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Ther Adv Psychopharmacol 4:75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C, Schug SA, Page-Sharp M, Jenkins B, Ilett KF. (2009) Development of a sublingual/oral formulation of ketamine for use in neuropathic pain: preliminary findings from a three-way randomized, crossover study. Clin Drug Investig 29:317–324. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr (2010a) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim La, Brutsche NE, Ameli R, Henter ID, Luckenbaugh Da, Machado-Vieira R, Zarate Ca. (2010b) Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi M, Kazemi MH, Yoosefi A, Ghasemi A, Paragomi P, Amini H, Afzali MH. (2014) Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res 215:355–361. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) Rating scale for depression. J Neurol Neurosur PS 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S. (editors) (2009) Cochrane handbook for systematic reviews of interventions version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane-handbook.org.
- Katalinic N, Lai R, Somogyi A, Mitchell PB, Glue P, Loo CK. (2013) Ketamine as a new treatment for depression: a review of its efficacy and adverse effects. Aust N Z J Psychiatry 47:710–727. [DOI] [PubMed] [Google Scholar]
- Kudoh A, Takahira Y, Katagai H, Takazawa T. (2002) Small-dose ketamine improves the postoperative state of depressed patients. 114–118. [DOI] [PubMed]
- Lai R, Katalinic N, Glue P, Somogyi AA, Mitchell PB, Leyden J, Harper S, Loo CK. (2014) Pilot dose–response trial of i.v. ketamine in treatment-resistant depression. World J Biol Psychiatry 0:1–6. [DOI] [PubMed] [Google Scholar]
- Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW. (2014) A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry 76:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin GL, Beautrais AL. (2011) A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol 14:1127–1131. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Jimenez DV, Zarate CA, Manji HK. (2013) Rapid antidepressant effects: moving right along. Mol Psychiatry 18:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. (2014) A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychological Medicine FirstView:1–12. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Murrough J, Soleimani L, DeWilde K, Collins K, Lapidus K, Iacoviello B, Lener M, Kautz M, Kim J, Stern J. (2015) Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychological medicine 1–10. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812. [Google Scholar]
- Paul R, Schaaff N, Padberg F, Möller H-J, Frodl T. (2009) Comparison of racemic ketamine and S-ketamine in treatment-resistant major depression: report of two cases. World J Biol Psychiatry 10:241–244. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, Palmer BA, Ritter MJ, Schak KM, Sola CL, Hanson AJ, Frye MA. (2013) Serial infusions of low-dose ketamine for major depression. J Psychopharmacol 27:444–450. [DOI] [PubMed] [Google Scholar]
- RevMan (2014) The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, Flood P, Simpson HB. (2013) Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology 38:2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn S. (2002) Crossover trials in clinical research: John Wiley & Sons.
- Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, Lim KO. (2014) Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord 155:123–129. [DOI] [PubMed] [Google Scholar]
- Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. (2013) Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett 34:287–293. [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [1H]-MRS. Psychiatry Res. 2011. February 28; 191(2): 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. (2015) The global burden of mental, neurological and substance use disorders: an analysis from the global burden of disease study 2010. PLoS ONE 10:e0116820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. (1978) A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–435. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt Ca, Liberty V, Luckenbaugh Da. (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SLV, Ramamoorthy A, Moaddel R, Wainer IW. (2012) Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.