Abstract
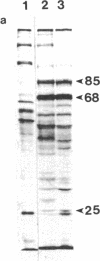
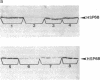
The synthesis of heat shock proteins (hsps) at normal physiological and elevated temperatures has been correlated with the natural adaptation of an organism to heat in nine lizard species studied. These species differ drastically by their adaptation to elevated temperature and represent a spectrum of forms isolated from various geographical regions of the Union of Soviet Socialist Republics. The synthesis of hsps belonging to the hsp70 family and their correspondent mRNAs have been compared at different temperature regimes. This analysis has shown that lizards inhabiting the Middle Asia deserts are characterized by a higher content of hsp70-like proteins at normal physiological temperatures (2- to 5-fold differences) when compared with the forms from central and northern regions of the European part of the Union of Soviet Socialistic Republics. Analysis of hsp70 mRNA at different temperatures substantiated these observations, showing evident correlation between adaptation of a given form to hyperthermia and the quantity of hsp70 mRNA in the cells under non-heat-shock conditions. The results obtained with a wide spectrum of ecologically different lizard species, coupled with other relevant data, enable us to propose a general rule applicable to poikilothermic organisms. This rule postulates the direct correlation between the characteristic temperature of the ecological niche of a given species and the amount of hsp70-like proteins in the cells at normal temperature.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienz M. Developmental control of the heat shock response in Xenopus. Proc Natl Acad Sci U S A. 1984 May;81(10):3138–3142. doi: 10.1073/pnas.81.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. Xenopus hsp 70 genes are constitutively expressed in injected oocytes. EMBO J. 1984 Nov;3(11):2477–2483. doi: 10.1002/j.1460-2075.1984.tb02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bosch T. C., Krylow S. M., Bode H. R., Steele R. E. Thermotolerance and synthesis of heat shock proteins: these responses are present in Hydra attenuata but absent in Hydra oligactis. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7927–7931. doi: 10.1073/pnas.85.21.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfoleded state depends on two chaperonin proteins and Mg-ATP. Nature. 1989 Dec 21;342(6252):884–889. doi: 10.1038/342884a0. [DOI] [PubMed] [Google Scholar]
- Kimpel J. A., Key J. L. Presence of Heat Shock mRNAs in Field Crown Soybeans. Plant Physiol. 1985 Nov;79(3):672–678. doi: 10.1104/pp.79.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Heat shock and the sorting of luminal ER proteins. EMBO J. 1989 Nov;8(11):3171–3176. doi: 10.1002/j.1460-2075.1989.tb08475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Gething M. J. Protein structure. Chaperones, paperones. Nature. 1989 Nov 16;342(6247):224–225. doi: 10.1038/342224a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ul'masov Kh A., Ovezmukhammedov A., Karaev K. K., Evgen'ev M. B. Molekuliarnye mekhanizmy adaptatsii k gipertermii u vysshikh organizmov. III. Induktsiia belkov teplovogo shoka u dvukh vidov leishmanii. Mol Biol (Mosk) 1988 Nov-Dec;22(6):1583–1589. [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]