Abstract
Metastasis is a complex process that is regulated by multiple signaling pathways, with the focal adhesion kinase (FAK)/paxillin pathway playing a major role in the formation of focal adhesions and cell motility. N-myc downstream regulated gene-1 (NDRG1) is a potent metastasis suppressor in many solid tumor types, including prostate and colon cancer. Considering the antimetastatic effect of NDRG1 and the crucial involvement of the FAK/paxillin pathway in cellular migration and cell-matrix adhesion, we assessed the effects of NDRG1 on this important oncogenic pathway. In the present study, NDRG1 overexpression and silencing models of HT29 colon cancer and DU145 prostate cancer cells were used to examine the activation of FAK/paxillin signaling and the formation of focal adhesions. The expression of NDRG1 resulted in a marked and significant decrease in the activating phosphorylation of FAK and paxillin, whereas silencing of NDRG1 resulted in an opposite effect. The expression of NDRG1 also inhibited the formation of focal adhesions as well as cell migration and cell-collagen adhesion. Incubation of cells with novel thiosemicarbazones, namely di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone and di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone, that upregulate NDRG1 also resulted in decreased phosphorylation of FAK and paxillin. The ability of these thiosemicarbazones to inhibit cell migration and metastasis could be mediated, at least in part, through the FAK/paxillin pathway.
Introduction
N-myc downstream regulated gene 1 (NDRG1) is a predominantly cytoplasmic 43-kDa protein that is upregulated by cellular iron depletion (Le and Richardson, 2004; Kovacevic et al., 2008; Fang et al., 2014). A number of studies examining the role of NDRG1 in vivo and in patient specimens have demonstrated that NDRG1 acts as a potent metastasis suppressor in a number of different tumor types (Bandyopadhyay et al., 2003, 2004; Shah et al., 2005; Maruyama et al., 2006; Chen et al., 2012; Dixon et al., 2013; Kovacevic et al., 2013, 2016; Sun et al., 2013a, b; Jin et al., 2014; Liu et al., 2015).
In terms of cell migration, NDRG1 inhibits F-actin polymerization and organization into stress fibers, which are critical for cell locomotion (Sun et al., 2013b). This latter effect was mediated through inhibition of the Rho-associated, coiled-coil containing protein kinase 1/phosphorylated myosin light chain 2 (pMLC2) signaling pathway (Sun et al., 2013b). However, despite these advances in understanding the role of NDRG1 in cell migration and metastasis, further studies are required to elucidate the detailed mechanisms regarding how NDRG1 inhibits these processes.
A significant driver of cellular migration and metastasis is the focal adhesion kinase (FAK), also known as protein tyrosine kinase 2, which is an important non-receptor tyrosine kinase (RTK) (Gabarra-Niecko et al., 2003). Elevated FAK expression has been demonstrated in colorectal cancer, breast cancer, liver cancer, prostate cancer, etc. (Tremblay et al., 1996; Cance et al., 2000; Su et al., 2002; Gabarra-Niecko et al., 2003). There are several sites of tyrosine phosphorylation on FAK, including: 1) the major site of auto-phosphorylation (Tyr397); 2) the activation domain of FAK (Tyr576/7); and 3) the focal adhesion targeting sequence (Tyr925), which is important for its discrete linking to the focal adhesion complex (McLean et al., 2005; Du et al., 2014).
When FAK is autophosphorylated on Tyr397 in response to stimuli, it enables Src recruitment (Richardson et al., 1997; McLean et al., 2005). This interaction between FAK and Src provides high-affinity binding sites for the Src homology 2 domain of different proteins (Gabarra-Niecko et al., 2003). These binding sites on FAK reside in the activation loop (Tyr576/7) and the focal adhesion targeting sequence (Tyr925) (Panetti, 2002; Gabarra-Niecko et al., 2003; McLean et al., 2005). The adaptor protein paxillin is one of the main phosphorylation targets of the phosphorylated FAK-Src complex (Burridge et al., 1992; Sieg et al., 2000). Paxillin is a scaffolding protein containing several domains involved in protein-protein interactions (Panetti, 2002). Paxillin colocalizes with F-actin in focal adhesions and links the extracellular matrix (ECM) to the membrane-attached cytoskeleton (Turner, 2000; Zouq et al., 2009).
The FAK/paxillin pathway plays a crucial role in cytoskeletal remodeling, cell migration, and cell adhesion (Deakin and Turner, 2008; Shan et al., 2009). Activated FAK enhances paxillin phosphorylation (Tyr118), allowing this latter molecule to promote membrane protrusions and focal adhesions through complex protein-protein interactions (Chen and Gallo, 2012). Tyrosine phosphorylation of paxillin can also activate small Rho GTPases, including RhoA, PAK1, Rho-associated, coiled-coil containing protein kinase 1, Rac1, and Cdc42, which are involved in cytoskeletal assembly and reorganization (Tsubouchi et al., 2002; Raftopoulou and Hall, 2004). Collectively, targeting the FAK/paxillin phosphorylation signaling pathway is a potential therapeutic strategy for cancer treatment.
Considering recent findings demonstrating the inhibitory effect of NDRG1 on cancer cell migration and metastasis, this study aimed to examine if NDRG1 affects FAK/paxillin signaling. Moreover, a novel class of thiosemicarbazone anticancer agents, namely di-2-pyridylketone 4, 4-dimethyl-3-thiosemicarbazone (Dp44mT; Fig. 1A) and di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC; Fig. 1A) that markedly upregulate NDRG1 in cancer cells (Le and Richardson, 2004; Yuan et al., 2004; Kovacevic et al., 2008, 2011a, 2016; Sun et al., 2013b) were also examined to determine their effects on the FAK/paxillin signaling pathway. This was crucial, because these agents exert antiproliferative and antimigratory activity in vitro as well as antitumor and antimetastatic activity in vivo (Whitnall et al., 2006; Liu et al., 2012; Lovejoy et al., 2012; Kovacevic et al., 2016). Moreover, the lead compound, DpC, is set to enter clinical trials in 2016 and may offer a new approach to the treatment of metastatic cancers (Jansson et al., 2015a).
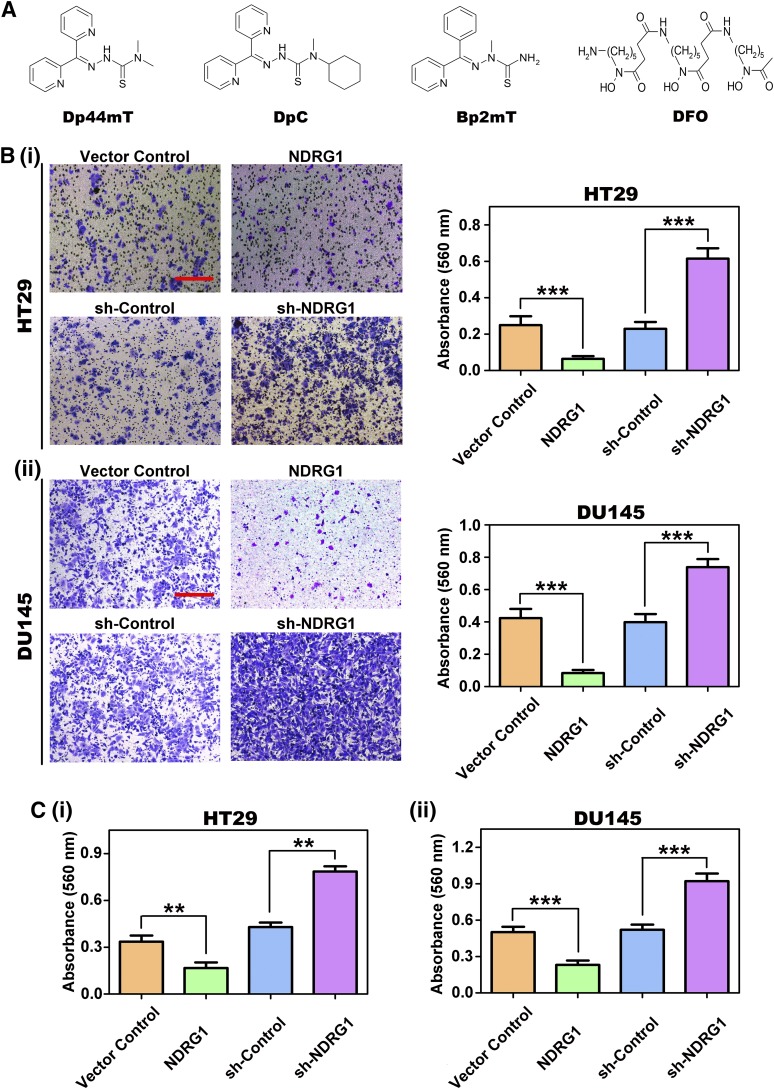
Fig. 1.
(A) Line drawings of the chemical structures of Dp44mT, DpC, Bp2mT, and DFO. (B) NDRG1 inhibits cell migration in HT29 colorectal cancer cells and DU145 cells. Representative images of cell migratory activity in transwell chambers of NDRG1 overexpressing (NDRG1) and silencing (sh-NDRG1) (i) HT29 and (ii) DU145 cells. Scale bars: 200 μm. (C) NDRG1 inhibits cell-collagen I adhesion in (i) HT29 and (ii) DU145 cells. All data are shown as mean ± S.D. (3–5 experiments). **P < 0.01; ***P < 0.001.
Herein, we demonstrate that NDRG1 overexpression or treatment with Dp44mT and DpC leads to reduced formation of focal adhesions and inhibited cell migration and cell-collagen adhesion via FAK/paxillin signaling. This investigation further highlights the potent anticancer activity of Dp44mT and DpC. This is mediated, at least in part, through NDRG1 upregulation, which subsequently downregulates the FAK/paxillin pathway.
Materials and Methods
Reagents.
The thiosemicarbazones, Dp44mT (Fig. 1A) and DpC (Fig. 1A), and the negative control compound, Bp2mT (Fig. 1A), were synthesized and characterized using standard methods (Richardson et al., 2006; Lovejoy et al., 2012). Desferrioxamine (DFO; Fig. 1A) was purchased from Sigma-Aldrich (St. Louis, MO). The thiosemicarbazone ligands, Dp44mT, DpC, and their respective control, Bp2mT, were dissolved in dimethyl sulfoxide (DMSO) and further diluted to a final concentration of 5 μM in culture media, whereas DFO was diluted in culture media to a final concentration of 250 μM. The FAK phosphorylation inhibitor, PF-562271 (Formula: C21H20F3N7O3S), was purchased from Selleckchem (Houston, TX). PF-562271 was dissolved in DMSO and further diluted to a working concentration of 10 μM in culture media. Cells were incubated with either: control media [containing DMSO at 0.05% (v/v); to match the concentration of the dissolved thiosemicarbazones and other agents], DFO, Dp44mT, DpC, or the FAK inhibitor for 24 hours/37°C before protein extraction for Western analysis.
Cell Culture.
The human colon cancer and prostate cancer cells, HT29 and DU145, were obtained from the American Type Culture Collection (ATCC, Manassas, VA). NDRG1 overexpressing and silenced clones of DU145 and HT29 cells, and their respective control cells were generated as described previously and grown under established conditions (Chen et al., 2012; Sun et al., 2013b).
Transwell Migration Assay and Cell-ECM Adhesion Assay.
The transwell migration assay was performed using Corning 24-well transwell chambers (6.5 mm diameter, 8.0 μm pore size; Corning Inc., Lowell, MA). Briefly, 5 × 104 cells in 200 µl serum-free medium were placed into the top chamber and 600 µl of 10% FBS containing medium was placed into the bottom chamber. After an incubation of 12 hours (HT29) or 20 hours (DU145), the cells that did not invade to the lower chamber were removed. The chambers were then stained with crystal violet and eluted with extraction solution (33% acetic acid). The relative migration abilities were quantified by optical absorbance at 560 nm using a PerkinElmer 1420 multilabel plate reader (Perkin Elmer, Waltham, MA). Notably, to avoid the confounding effects of proliferation on cell migration results, the incubation times used were markedly less than the doubling times for these cells, namely HT29 (24 hours) and DU145 (42 hours).
The cell-collagen I adhesion assay was performed using CytoSelect 48-Well Adhesion Assay (Cell Biolabs, San Diego, CA) according to the manufacturer’s protocol. Briefly, a cell suspension (5 × 105 cells/200 µl FBS-free medium) was added to the inside of each well (collagen I-coated wells) and incubated for 2 hours/37°C for DU145 cells and 3 hours/37°C for HT29 cells. After 4 or 5 washes with phosphate-buffered saline, the wells were stained with crystal violet and then eluted with extraction solution. The relative adhesion abilities were quantified by optical absorbance at 560 nm using the plate reader above.
Protein Extraction and Immunoblots.
Whole cell lysates were extracted using lysis buffer with proteinase inhibitor (Cat. 11836170001; Roche, Basel, Switzerland) and PhosSTOP (Cat. 04906845001; Roche) and Western blotting performed as described previously (Kovacevic et al., 2008). Briefly, equal amounts of protein (50 μg) were loaded and separated on a 10% SDS-PAGE gel, and then transferred to polyvinylidene fluoride membranes. The membranes were incubated with primary antibodies at 4°C overnight, and then incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 hours. The primary antibodies used (diluted at 1:1000–1:2000) included: NDRG1, paxillin from Abcam (Cambridge, UK); p-FAK (Tyr397), p-FAK (Tyr576/7), p-FAK (Tyr925), p-paxillin (Tyr118), FAK were from Cell Signaling Technology (Beverly, MA); β-actin (diluted at 1:10,000) was purchased from Sigma-Aldrich. The secondary antibodies implemented (diluted 1:10,000) include horseradish peroxidase-conjugated anti-goat, anti-rabbit, and anti-mouse antibodies from Sigma-Aldrich.
Immunofluorescence.
Cells seeded on coverslips were fixed with 4% paraformaldehyde (Sigma-Aldrich) for 10 minutes and permeabilized with 0.2% Triton X-100 for 5 minutes at room temperature. The coverslips were then incubated overnight with primary antibodies at 4°C, followed by incubation with fluorescent secondary antibody for 1 hour at room temperature. After washing with phosphate-buffered saline, the coverslips were stained with anti-fade mounting solution containing 4′,6-di-amidino-2-phenylindole (DAPI; Invitrogen) and images were examined and captured using a Zeiss LSM 510 Meta Spectral Confocal Microscope (Carl Zeiss, Oberkochen, Germany) with a 63× oil objective. Raw images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Gene Silencing by Small Interfering RNA.
Silencing FAK expression using FAK siRNA was performed following the manufacturer’s instructions. Briefly, at 60% confluence, sh-NDRG1 and sh-Control cells were transfected with FAK Silencer Select siRNA duplexes (si-FAK; 10 nM; Ambion, Waltham, MA), or the Silencer Negative Control siRNA (si-Con) at 10 nM using Lipofectamine 2000 (Invitrogen, Waltham, MA). After a 6 hour/37°C siRNA incubation, fresh medium was then added for an additional 60 hour/37°C incubation and then whole cell lysates were extracted and immunoblots were performed.
Statistical Analysis.
Data are expressed as mean ± S.D. of at least three independent experiments. Analysis was performed using Student’s t test and ANOVA (GraphPad Prism 5.0; GraphPad Software, San Diego, CA), with P < 0.05 being considered statistically significant.
Results
NDRG1 Overexpression in HT29 and DU145 Cells Decreases Migration and Cell-Collagen I Adhesion.
Considering the important role of NDRG1 in inhibiting tumor cell metastasis (Bandyopadhyay et al., 2003, 2004; Shah et al., 2005; Maruyama et al., 2006; Chen et al., 2012; Kovacevic et al., 2013, 2016; Dixon et al., 2013; Sun et al., 2013b; Jin et al., 2014; Liu et al., 2015), the current study has assessed its role in suppressing tumor cell migration and cell-collagen I adhesion through FAK/paxillin signaling.
In these studies, we used two well characterized cell types, namely DU145 prostate cancer cells and HT29 colon cancer cells that stably overexpress exogenous human NDRG1 (denoted “NDRG1”) and compared the results to cells transfected with the vector alone (denoted “Vector Control”) (Chen et al., 2012). As additional models to investigate NDRG1 function, NDRG1-silenced clones (denoted “sh-NDRG1”) of these two cell-types were generated and compared with cells transfected with an empty control plasmid (denoted “sh-Control”) (Chen et al., 2012). These cell lines were specifically used because: 1) they are representative models of tumor-types where NDRG1 has been shown to have an anti-metastatic role in vitro and in vivo (Liu et al., 2012) and 2) we have extensively characterized these cells in previous studies examining the function of NDRG1 (Chen et al., 2012; Sun et al., 2013b; Jin et al., 2014).
Initially, because cancer cell migration and ECM adhesion are key factors in metastasis and NDRG1 is a metastasis suppressor (Ellen et al., 2008; Kitowska and Pawełczyk, 2010; Melotte et al., 2010), we investigated the effect of NDRG1 on cell migration and ECM adhesion using the transwell migration (Fig. 1B) and cell-collagen I adhesion assay (Fig. 1C), respectively. By using the transwell migration assay, NDRG1 overexpression in HT29 and DU145 cells resulted in a significant (P < 0.001) reduction (3.1–4.2-fold) of migration relative to their respective Vector Control cells [Fig. 1B, (i) and (ii)]. On the other hand, NDRG1 silencing in both these cell types resulted in a significant (P < 0.001) increase (1.8–2.6-fold) of cellular migration relative to the sh-Control [Fig. 1B, (i) and (ii)]. These studies again support the role of NDRG1 as an inhibitor of cellular migration (Hickok et al., 2011; Chen et al., 2012).
Cell adhesion assays in HT29 and DU145 cells demonstrated that NDRG1 overexpression resulted in a significant (P < 0.001-0.01) decrease (1.7–2.0-fold) in cellular-ECM adhesion relative to the Vector Control [Fig. 1C, (i) and (ii)]. In contrast, NDRG1 silencing led to a significant (P< 0.001–0.01) increase (1.6–1.9-fold) in cellular-ECM adhesion versus the sh-Control in both HT29 and DU145 cells ([Fig. 1C, (i) and (ii)].
NDRG1 Overexpression in HT29 and DU145 Cells Decreases Activation of FAK and Paxillin, while Silencing of NDRG1 Increases FAK and Paxillin Activation.
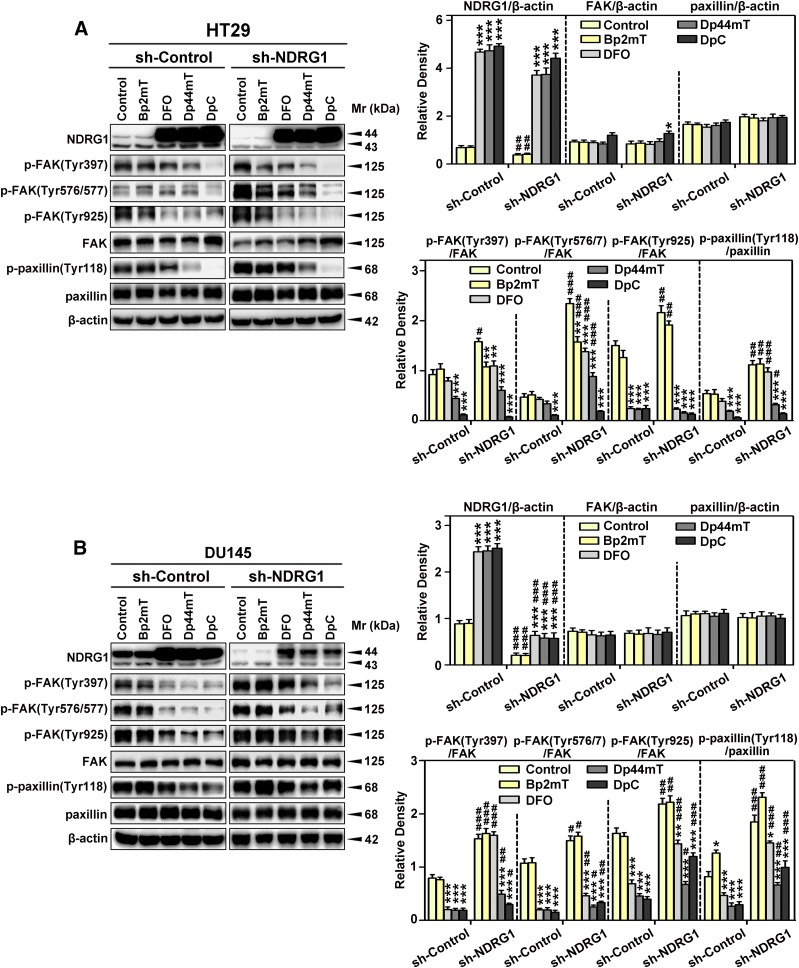
Considering the marked effect of NDRG1 expression on cellular-migration and -ECM adhesion (Fig. 1, B and C), studies then assessed the effect of this metastasis suppressor on the phosphorylation and total protein levels of FAK and paxillin in the NDRG1 overexpressing and silenced clones of HT29 (Fig. 2A) and DU145 cells (Fig. 2B). In both NDRG1 overexpressing cell types, exogenous expression of Flag-tagged NDRG1 was demonstrated by immunoblotting, where a band at ∼45 kDa was detected (Fig. 2, A and B). In addition, endogenously expressed NDRG1 was demonstrated at ∼43 and/or ∼44 kDa, suggesting potential phosphorylation or other posttranslational modifications (Murray et al., 2004; Kovacevic et al., 2011a; Ghalayini et al., 2013). Considering this, it is notable that the densitometric analysis shown throughout this study represents the total of all NDRG1 bands. In both HT29 and DU145 cells, NDRG1 expression was markedly and significantly (P < 0.001) greater in the overexpression clones relative to the Vector Controls, whereas the shNDRG1 clone demonstrated a pronounced and significant (P < 0.001) decrease in NDRG1 levels relative to the sh-Control (Fig. 2, A and B).
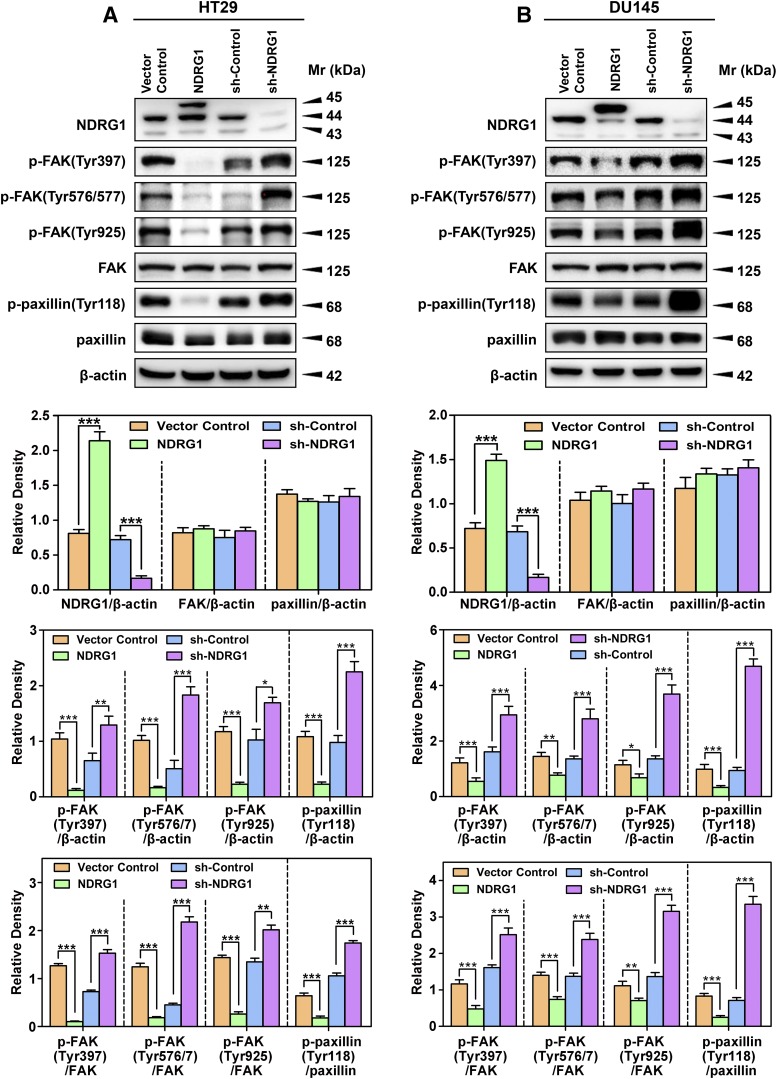
Fig. 2.
NDRG1 expression inhibits phosphorylation of FAK and paxillin using HT29 (A) and DU145 (B) cells. Immunoblotting was conducted to examine NDRG1 expression, phosphorylation of FAK (Tyr397, Tyr567/7, and Tyr925), total FAK, phosphorylation of paxillin (Tyr118), and total paxillin using NDRG1 overexpressing and silencing (sh-NDRG1) models and their respective controls in both cell types. Immunoblots shown are representative of three independent experiments. Densitometry for NDRG1 and total FAK/paxillin expression are expressed relative to the loading control, β-actin, whereas the phosphorylation levels for FAK and paxillin are displayed both relative to β-actin and as a ratio of their respective total protein levels, as shown on separate graphs. Densitometry data are shown as mean ± S.D. relative to the respective vector control or sh-Control cells as appropriate (3–5 experiments). *P < 0.05; **P < 0.01; ***P < 0.001.
Once activated, both FAK and paxillin play crucial roles in migration and invasion (Lauffenburger and Horwitz, 1996; Turner, 2000). Initial studies assessed the effect of NDRG1 expression on the phosphorylation of FAK at Tyr397, Tyr576/7, and Tyr925 (Fig. 2, A and B), because these are the key sites responsible for activation and subsequent downstream signaling (Calalb et al., 1995; Panetti, 2002). In most blots assessing FAK phosphorylation, only one band was demonstrated at 125 kDa, although in others, two closely migrating bands were observed, as reported by others (McLean et al., 2005; Du et al., 2014).
Interestingly, NDRG1 overexpression in both cell types resulted in a significant (P < 0.001–0.05) decrease of phosphorylated FAK at all phosphorylation sites examined (Tyr397, Tyr576/7, and Tyr925), relative to Vector Control cells, with there being no significant alteration in total FAK levels (Fig. 2, A and B). Furthermore, NDRG1 overexpression in both cell types resulted in a significant (P < 0.001–0.01) decrease of the ratio of phosphorylated FAK(Tyr397, Tyr576/7, and Tyr925) to total FAK, relative to Vector Control cells (Fig. 2, A and B). In contrast, NDRG1 silencing (sh-NDRG1) resulted in a significant (P < 0.001–0.05) increase in phosphorylated FAK at all three sites compared with the sh-Control, with no significant alteration in total FAK levels. The silencing of NDRG1 also resulted in a marked and significant (P < 0.001–0.01) increase in the ratio of phosphorylated FAK to total FAK at all three phosphorylation sites compared with the sh-Control, with no significant alteration in total FAK levels (Fig. 2, A and B).
An important downstream target of activated FAK is paxillin, which becomes activated when phosphorylated at Tyr118 (Azuma et al., 2005). Paxillin is a focal adhesion-associated protein that plays a role in cell adhesion to the extracellular matrix, cell spreading, and migration, and hence, was important to examine (Schaller, 2001). Overexpression of NDRG1 in both cell types resulted in a significant (P < 0.001) decrease in p-paxillin (Tyr118) levels relative to Vector Control cells, whereas there was no significant alteration in total paxillin expression (Fig. 2, A and B). Similarly, NDRG1 overexpression in both cell types resulted in a significant (P < 0.001) decrease in the p-paxillin(Tyr118)/paxillin ratio relative to Vector Control cells (Fig. 2, A and B). On the other hand, examining HT29 and DU145 sh-NDRG1 cells, there was a significant (P < 0.001) increase in p-paxillin (Tyr118) levels relative to sh-Control cells, with no significant alteration in total paxillin expression (Fig. 2, A and B). Silencing of NDRG1 in HT29 and DU145 cells also resulted in a significant (P < 0.001) increase in p-paxillin(Tyr118)/paxillin ratio relative to sh-Control cells (Fig. 2, A and B).
In summary, NDRG1 expression inhibits FAK-paxillin signaling, which may be critical in terms of the ability of this metastasis suppressor to diminish tumor cell migration and adhesion to the substratum.
NDRG1 Expression Inhibits the Formation of Focal Adhesions.
To further investigate the mechanism of NDRG1 activity in inhibiting cell migration and attachment, immunofluorescence studies were performed to assess p-paxillin and F-actin expression and distribution. This was important because both p-paxillin and F-actin are integral components of focal adhesions, which play an important role in cellular adhesion, migration, and metastasis (Nobes and Hall, 1995; Deakin and Turner, 2008).
In these studies, NDRG1 overexpressing and silenced HT29 and DU145 cells were used to examine p-paxillin (Tyr118) levels and localization. This latter molecule was found to be present as punctate staining in Vector Control and sh-Control HT29 [Fig. 3A(i)] and DU145 cells [(Fig. 3A(ii)]. These puncta of fluorescence are consistent with the formation of focal adhesions on the cell surface (Wang et al., 1993; Keselowsky et al., 2004). Upon NDRG1 overexpression in both HT29 and DU145 cells, there was a significant (P < 0.01–0.05) decrease in the p-paxillin fluorescence density relative to the Vector Control cells (Fig. 3, A and B). On the other hand, in both cell types, there was a marked and significant (P< 0.001) increase in p-paxillin fluorescence intensity in sh-NDRG1 cells relative to the sh-Control cells (Fig. 3, A and B). In fact, under these conditions, and particularly in DU145 cells, the p-paxillin puncta increased in size to form fluorescent plaques (Fig. 3A).
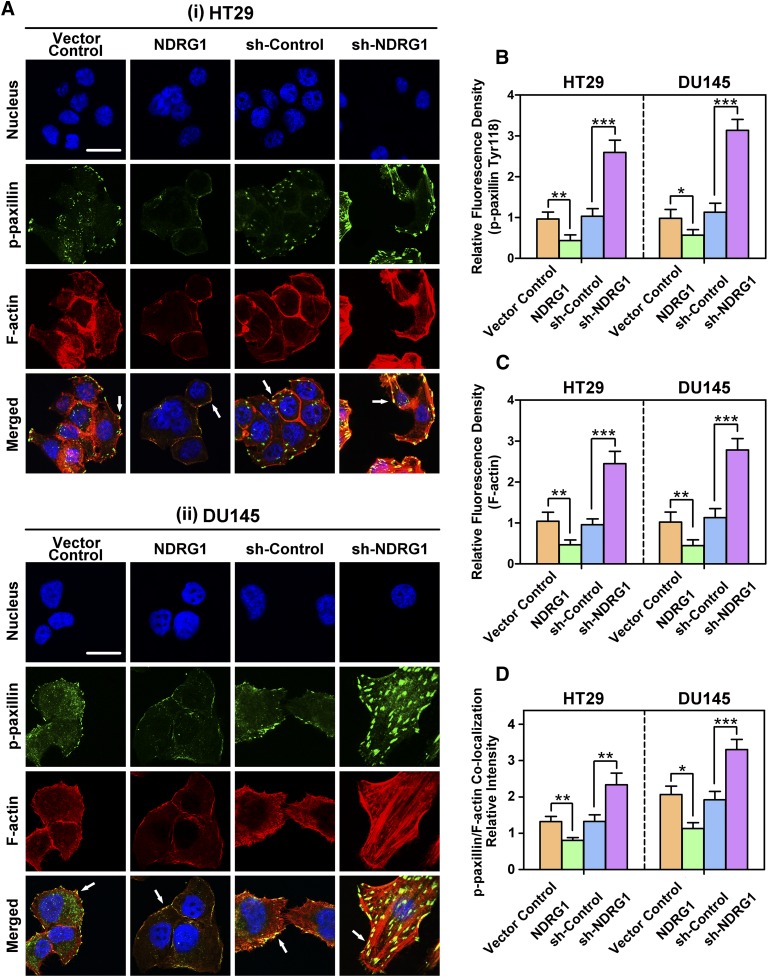
Fig. 3.
(A) NDRG1 expression suppresses the formation of focal adhesions and stress fibers in both HT29 (i) and DU145 (ii) cells. Representative immunofluorescence images demonstrate the inhibitory effect of NDRG1 expression on p-paxillin (Tyr118; green) and F-actin (red; stained with rhodamine-phalloidin) levels and their colocalization in HT29 and DU145 cells. The cell nuclei (blue) were stained with DAPI. The presence of yellow staining upon the electronic merge indicates the colocalization of paxillin and stress fibers (see white arrows), showing the formation of focal adhesions on the cell surface. Scale bar: 10 μm. Expression of p-paxillin (B) and F-actin (C) was quantified by calculating the relative fluorescence density (i.e., fluorescence intensity/area) value in NDRG1 overexpressing or silenced cells compared with their respective controls. (D) The p-paxillin/F-actin colocalization intensity was calculated in HT29 and DU145 cells using Image J software. The histogram values in (B–D) are shown as mean ± S.D. (3–5 images from different fields). *P < 0.05; **P < 0.01; ***P < 0.001 relative to the respective control cells.
Assessment of F-actin fluorescence demonstrated diffuse cytoplasmic staining in Vector Control and sh-Control cells that was predominantly distributed peripherally at the cell membrane (Fig. 3A). As observed in previous studies (Sun et al., 2013b), NDRG1 overexpression in HT29 and DU145 cells resulted in a significant (P < 0.01) decrease in the intensity of F-actin expression, which appeared confined predominately to the plasma membrane (Fig. 3, A and C). At the same time, NDRG1 overexpression in HT29 and DU145 cells led to a more rounded and less aggressive cellular morphology relative to the relevant Vector Control cells (Fig. 3A). In contrast, the silencing of NDRG1 in both cell types resulted in a significant (P < 0.001) increase in F-actin expression (Fig. 3, A and C) and the formation of distinct stress fibers (particularly in DU145 cells) relative to the sh-Control (Fig. 3A). Furthermore, the sh-NDRG1 cells became more spindle or angular-shaped and had a more aggressive cellular morphology than their sh-Control counterparts.
It is well known that p-paxillin (green) can co-localize with F-actin (red) on the plasma membrane to form focal adhesions [yellow (Sattler et al., 2000; Schaller, 2001; Deakin and Turner, 2008)]. In the Vector Control and sh-Control cells, colocalization between p-paxillin and F-actin appeared as very faint particulate staining on the cell membrane in HT29 cells [Fig. 3A(i)], while being slightly more widely distributed through the cell and on the plasma membrane in DU145 cells [Fig. 3A(ii)]. Examining cells with NDRG1 overexpression, the colocalization intensity formed between p-paxillin and F-actin in the merged image was significantly (P < 0.01–0.05; Fig. 3D) reduced relative to the Vector Control cells (Fig. 3A). This led to the yellow fluorescence being confined predominantly to isolated segments of the cell (Fig. 3A). In contrast, sh-NDRG1 cells demonstrated a marked and significant (P < 0.001–0.01; Fig. 3D) increase in the colocalization intensity between p-paxillin and F-actin in both cell types, with yellow plaques being observed particularly throughout DU145 cells (Fig. 3A). In summary, expression of the metastasis suppressor, NDRG1, in both cell types decreases colocalization between p-paxillin and F-actin, which are involved in focal adhesion function, including tumor cell adhesion and migration (Nagano et al., 2012).
NDRG1 Regulates Paxillin through Modulating FAK.
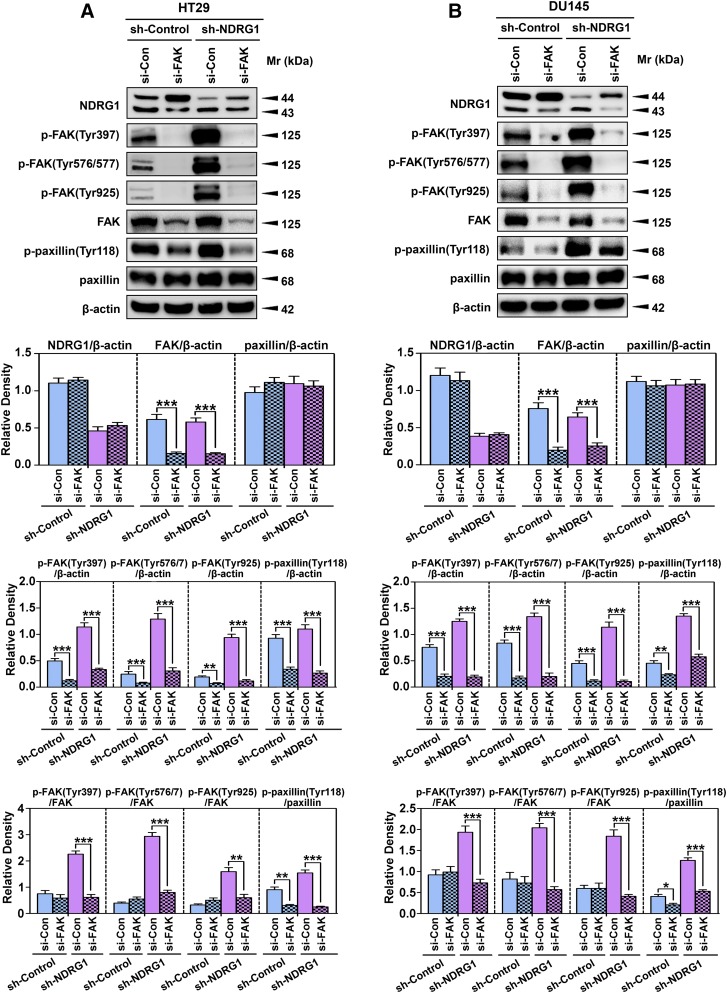
The investigations above demonstrated that NDRG1 overexpression decreased, whereas NDRG1 silencing activated the FAK/paxillin pathway (Figs. 2 and 3). To investigate whether NDRG1 directly regulates paxillin phosphorylation, or if it exerts this effect by inhibiting FAK phosphorylation, further studies were performed with the well-characterized FAK inhibitor PF-562271 [(Roberts et al., 2008) Fig. 4] or FAK siRNA (Fig. 5).
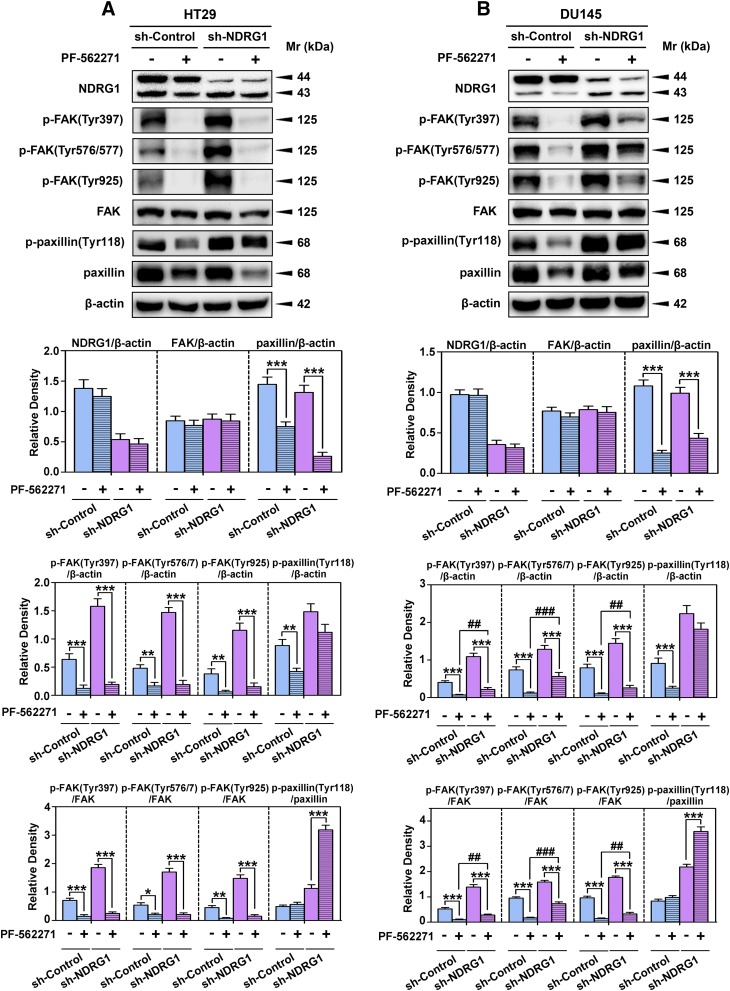
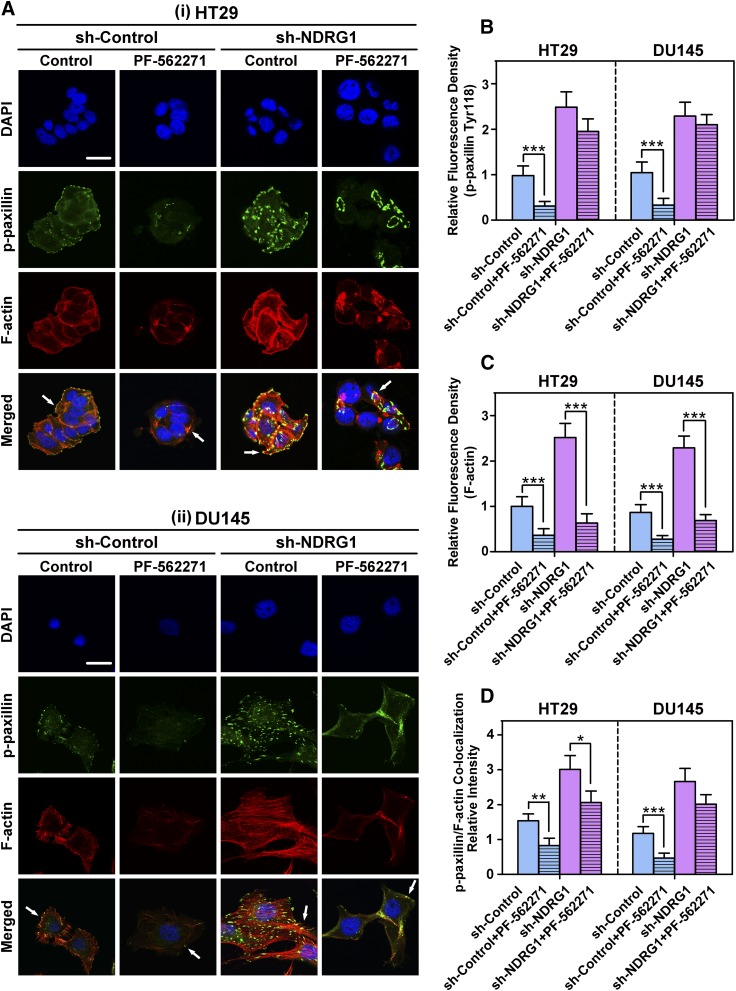
Fig. 4.
Examination of the effect of the FAK phosphorylation inhibitor, PF-562271, on p-paxillin and total paxillin levels in HT29 and DU145 sh-Control and sh-NDRG1 cells. The sh-Control and sh-NDRG1 HT29(A) and DU145 (B) cells were incubated with the FAK phosphorylation inhibitor PF-562271 (10 μM; 24 hours/37°C) and levels of p-FAK (Tyr397, Tyr576/7, Tyr925), total FAK, p-paxillin (Tyr118), and total paxillin were examined by immunoblot analysis. Immunoblots are representative of three independent experiments. Densitometry for NDRG1 and total FAK and paxillin expression are expressed relative to the loading control, β-actin, whereas the phosphorylation levels for FAK and paxillin are displayed both relative to β-actin and as a ratio of their respective total protein levels as shown on separate graphs. Densitometry data are shown as mean ± S.D. relative to the untreated sh-Control or sh-NDRG1 cells as appropriate. *P < 0.05; **P < 0.01; ***P < 0.001. ##P < 0.01; ###P < 0.001 relative to cells treated with PF-562271 in the sh-Control group.
Fig. 5.
NDRG1 mediates paxillin phosphorylation via a FAK-dependent mechanism as demonstrated using FAK siRNA in HT29 and DU145 cells. The sh-Control and sh-NDRG1 HT29 (A) and DU145 (B) cells were incubated with FAK-specific siRNA (si-FAK) or negative control siRNA (si-Con). Immunoblots shown are representative of three independent experiments. Densitometry for NDRG1 and total FAK/paxillin expression are expressed relative to the loading control, β-actin, whereas the phosphorylation levels for FAK and paxillin are displayed both relative to β-actin and as a ratio of their respective total protein levels as shown on separate graphs. Densitometry data are shown as mean ± S.D. relative to si-Con for both sh-NDRG1 and sh-Control cells (3–5 experiments). *P < 0.05; **P < 0.01; ***P < 0.001.
The HT29 and DU145 sh-Control and sh-NDRG1 cells were incubated with PF-562271 (10 μM) for 24 hours/37°C. These incubation conditions were previously shown to effectively inhibit FAK phosphorylation (Du et al., 2014). After incubation with PF-562271, NDRG1 expression was not significantly affected in either sh-Control or sh-NDRG1 cells relative to the untreated control (Fig. 4, A and B). In contrast, the phosphorylation of FAK at three different sites (i.e., Tyr397, Tyr576/7, and Tyr925) was significantly (P < 0.001–0.01) decreased by PF-562271 treatment, regardless of NDRG1 expression (Fig. 4, A and B). However, PF-562271 did not significantly affect total FAK expression in both cell types (Fig. 4, A and B). Because of this, the ratios of p-FAK (i.e., Tyr397, Tyr576/7, and Tyr925) to total FAK were significantly (P < 0.001–0.05) decreased by PF-562271 treatment, regardless of NDRG1 expression (Fig. 4, A and B).
The silencing of NDRG1 in DU145 cells (but not HT29 cells; Fig. 4, A and B) led to a significant (P < 0.001–0.01) increase in FAK phosphorylation at each site examined even in the presence of PF-562271 compared with the PF-562271 treated sh-Control cells. Similarly, NDRG1 silencing in DU145 cells (but not HT29 cells; Fig. 4, A and B) significantly (P < 0.001–0.01) increased p-FAK(Tyr397, Tyr576/7, and Tyr925)/FAK ratios even in the presence of PF-562271 compared with the PF-562271 treated sh-Control cells. Although, it was notable that this latter increase in FAK phosphorylation was markedly (P < 0.001) lower than that of the untreated sh-NDRG1 cells (Fig. 4B).
Furthermore, PF-562271 also significantly (P < 0.001–0.01) decreased the phosphorylation of paxillin (Tyr118) in sh-Control HT29 and DU145 cells relative to the untreated sh-Controls (Fig. 4, A and B). However, this latter agent also markedly and significantly (P < 0.001) reduced total paxillin levels in both cell-types (Fig. 4, A and B), suggesting a possible nonspecific effect of PF-562271. The net result of the decrease in phosphorylated and total paxillin levels after PF-562271 treatment was that the p-paxillin/paxillin ratio did not significantly change in the sh-Control cells (Fig. 4, A and B). Interestingly, NDRG1 silencing led to a significant (P < 0.001) increase in the p-paxillin/paxillin ratio in both HT29 and DU145 cells relative to the sh-Control group in the presence of PF-562271 (Fig. 4, A and B).
To further investigate if inhibition of FAK modulates the effect of NDRG1 on paxillin phosphorylation, a more specific strategy using FAK siRNA (si-FAK) was used in comparison with a negative control siRNA (si-Con; Fig. 5, A and B). Importantly, si-FAK did not significantly affect total NDRG1 expression relative to the si-Con in either the sh-Control or sh-NDRG1 HT29 or DU145 cells (Fig. 5, A and B). However, it was notable that an alteration in the relative proportions of the two NDRG1 isoforms was observed in both cell types upon incubation with si-FAK (Fig. 5, A and, B). Indeed, si-FAK increased the upper NDRG1 band at the expense of the lower band when compared with si-Con treated HT29 and DU145 cells (Fig. 5, A and B). Considering this, treatment with FAK activity inhibitor PF-562271 did not alter the relative ratios of the NDRG1 bands (Fig. 4, A and B). These results could suggest that the cellular protein level of FAK, but not its activity, may regulate the expression of NDRG1 isoforms through a feedback mechanism.
Examining the effect of si-FAK on HT29 and DU145 sh-Control cells, it was notable that silencing this molecule displayed significantly (P < 0.001–0.01) lower pFAK (Tyr397, Tyr576/7, and Tyr925) and total FAK levels (P < 0.001; Fig. 5, A and B). These changes resulted in no significant alteration in the p-FAK/FAK ratios in the sh-Control cells after incubation of si-FAK relative to si-Con (Fig. 5, A and B). In the sh-NDRG1 cells, si-FAK was also able to significantly (P < 0.001) reduce the levels of total and phosphorylated FAK for both HT29 and DU145 cells compared with the si-Con (Fig. 5, A and B). However, examining the ratio of p-FAK to total FAK, there was a marked and significant (P < 0.001–0.01) decrease of FAK phosphorylation (at all 3 sites) for both HT29 and DU145 sh-NDRG1 cells in response to si-FAK compared with si-Con sh-NDRG1 cells (Fig. 5, A and B).
Similarly, silencing of FAK also resulted in a significant (P < 0.001–0.01) inhibition of p-paxillin (Tyr118) levels relative to the si-Con, while having no significant effect on total paxillin levels in both sh-Control and sh-NDRG1 HT29 and DU145 cells (Fig. 5, A and B). This effect resulted in a significant (P < 0.001–0.05) decrease in the p-paxillin (Tyr118)/paxillin ratio in both sh-Control and sh-NDRG1 HT29 and DU145 cells. Taken together, the results above indicate that NDRG1 decreases p-paxillin levels, at least in part, via its inhibitory effects on FAK activation.
NDRG1 Inhibits Tumor Cell Migration and Adhesion via Inhibition of FAK/Paxillin Signaling.
Our studies above demonstrate that NDRG1 expression reduced p-paxillin levels, at least in part, through its inhibitory effect on FAK activation (Figs. 2–5). To further examine this, we investigated whether inhibition of FAK/paxillin signaling can reverse cell migration and adhesion that is induced by NDRG1 silencing in HT29 and DU145 cells (Fig. 6). Hence, cell migration assays and cell-collagen I adhesion assays were performed using both sh-NDRG1 and sh-Control cells after treatment with PF-562271 or FAK siRNA transfection.
Fig. 6.
NDRG1 expression inhibits HT29 and DU145 tumor cell migration (A and B) and cell-collagen I adhesion (C and D) via inhibition of FAK/paxillin signaling. (A and B) The HT29 and DU145 cells (i.e., sh-Control and sh-NDRG1 clones) were incubated with either the FAK inhibitor PF-562271 (10 µM) (A) or si-FAK (B) and displayed markedly inhibited migratory ability, relative to untreated or si-Con groups. (C and D) The inhibitory effect of the FAK phosphorylation inhibitor PF-562271 (C) or si-FAK (D) on cell-collagen I adhesion in both HT29 and DU145 cell-types (sh-Control and sh-NDRG1). All data are shown as mean ± S.D. (n = 3). Scale bars: 200 μm. **P < 0.01; ***P < 0.001.
Initial studies examined cell migration in sh-Control and sh-NDRG1 HT29 and DU145 cells in the presence and absence of PF-562271 (10 μM; Fig. 6A) or si-FAK versus si-Con (Fig. 6B). Compared with the control groups, treatment with PF-562271 (10 μM) or si-FAK significantly (P < 0.001–0.01) decreased the migration of both sh-Control and sh-NDRG1 HT29 and DU145 cells by approximately 60–80% (Fig. 6, A and B).
Furthermore, cell-collagen I adhesion assays were also performed to explore whether the inhibition of FAK phosphorylation or silencing of FAK expression could affect the adhesive ability of the sh-Control and sh-NDRG1 cells (Fig. 6C). In these studies, the adhesive ability of HT29 and DU145 sh-Control and sh-NDRG1 cells treated with 10 μM PF-562271 was significantly (P < 0.001) inhibited (3.5–4.0-fold) relative to the untreated control (Fig. 6C). Similarly, the adhesive ability of HT29 and DU145 sh-Control and sh-NDRG1 cells transfected with si-FAK was also found to be significantly (P < 0.001) decreased (approximately 2.8-fold) compared with the si-Con-transfected cells (Fig. 6D). Together, these results further demonstrate that the silencing of NDRG1 leads to increased cell migration and cell-collagen I adhesion via a FAK-mediated mechanism, as the inhibition of FAK using PF-562271 or si-FAK is able to markedly reduce these latter effects.
NDRG1-Mediated Inhibition of Focal Adhesions Occurs via FAK Activation.
Because NDRG1 silencing could significantly enhance F-actin remodeling into stress fibers and focal adhesion formation (Fig. 3), we further assessed the formation of focal adhesions and distribution of F-actin in sh-NDRG1/sh-Control cells after treatment with the FAK inhibitor PF-562271 (Fig. 7) or transfection with FAK siRNA (Fig. 8). These studies were performed to determine whether the NDRG1-mediated effects on focal adhesion formation were dependent on the ability of NDRG1 to inhibit FAK activation.
Fig. 7.
(A) The FAK phosphorylation inhibitor PF-562271 inhibits the formation of focal adhesions in both HT29 (i) and DU145 (ii) cells. Representative immunofluorescence images demonstrate the effect of PF-562271 on p-paxillin (Tyr118; green) and F-actin (red; stained with rhodamine-phalloidin) levels and their colocalization (yellow) in HT29 and DU145 cells (i.e., sh-Control and sh-NDRG1 cells). Cell nuclei (blue) were stained with DAPI. The yellow color after the electronic merge indicates colocalization of paxillin and stress fibers (see white arrows), indicating the formation of focal adhesions. Scale bar: 20 μm. Histograms show the relative fluorescence density for both p-paxillin (B) and F-actin (C) as well as the colocalization intensity of p-paxillin and F-actin (D). The histogram values in (B–D) are shown as mean ± S.D. (3–5 images from different fields). *P < 0.05; **P < 0.01; ***P < 0.001 relative to the respective control cells.
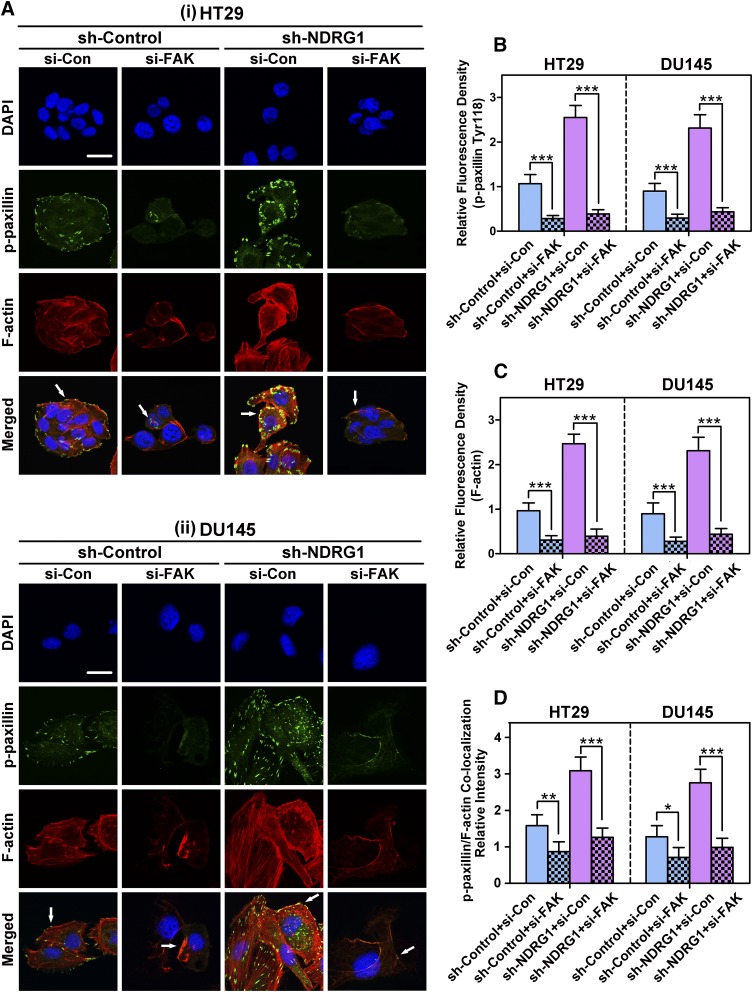
Fig. 8.
(A) The inhibitory effect of FAK siRNA on the formation of focal adhesions in both HT29 (i) and DU145 (ii) cells. (A) Representative immunofluorescence images indicate the effect of FAK-specific siRNA on p-paxillin (Tyr118; green) and F-actin (red; stained with rhodamine-phalloidin) levels and their colocalization in HT29 and DU145 cells (i.e., sh-Control and sh-NDRG1 cells). The cell nuclei (blue) were stained with DAPI. The yellow color in the merge indicates colocalization of paxillin and stress fibers (see white arrows), showing the formation of focal adhesions. Scale bar: 20 μm. Histograms show the relative fluorescence density for p-paxillin (B) and F-actin (C), as well as the colocalization intensity of p-paxillin and F-actin (D). The histogram values in (B–D) are shown as mean ± S.D. (3–5 images from different fields). *P < 0.05; **P < 0.01; ***P < 0.001 relative to the respective control cells.
As is shown in Fig. 7A, (i) and (ii), after incubation of HT29 and DU145 cells with PF-562271 (10 μM), sh-Control cells showed significantly decreased p-paxillin levels (green; P < 0.001; Fig. 7, A and B), F-actin expression (red; P < 0.001; Fig. 7, A and C) and colocalization of these proteins (yellow; P < 0.001–0.01; Fig. 7, A and D) compared with the untreated controls. However, the HT29 and DU145 sh-NDRG1 cells treated with PF-562271 (10 μM) demonstrated no significant change in p-paxillin level (Fig. 7B), but a significant (P < 0.001) decrease in F-actin density (Fig. 7C). The p-paxillin/F-actin colocalization intensity in HT29 sh-NDRG1 cells treated with PF-562271 was significantly (P < 0.05) decreased compared with the respective untreated sh-NDRG1 cells (Fig. 7D). In DU145 sh-NDRG1 cells treated with PF-562271, a slight but not significant decrease of p-paxillin/F-actin colocalization was also observed compared with untreated cells.
Notably, apart from its effects described above, PF-562271 markedly affected the cellular distribution of p-paxillin, F-actin, and focal adhesions (shown as yellow plaques in the merged images), which was particularly prominent in the sh-NDRG1 cells (Fig. 7A). In fact, in the presence of PF-562271, focal adhesions were no longer observed as discreet plaques and appeared more coalesced, particularly in DU145 cells (Fig. 7A).
As found with the FAK inhibitor PF-562271, silencing of FAK using siRNA (si-FAK) also markedly reduced the levels of p-paxillin [green; P < 0.001; Fig. 8,A, (i) and (ii), and B] and F-actin stress fibers [red; P < 0.001; Fig. 8A, (i) and (ii), and C] in both HT29 and DU145 sh-Control and sh-NDRG1 cells. This led to a significant (P < 0.001–0.05) reduction of the colocalization intensity of focal adhesions (yellow in merged images) in HT29 and DU145 cells incubated with si-FAK compared with si-Con cells (Fig. 8D). Notably, these effects were more apparent in sh-NDRG1 HT29 and DU145 cells. As observed for PF-562271 [Fig. 7A, (i) and (ii)], there was a tendency for the focal adhesions to become coalesced after incubation with si-FAK rather than appearing as distinct plaques in the si-Con cells [Fig. 8A, (i) and (ii)].
Taken together, the results in Figs. 7 and 8 demonstrate that NDRG1 silencing leads to increased focal adhesions and formation of stress fibers, which occur, at least in part, through FAK/paxillin signaling. Hence, these effects could account for the increased migratory and adhesive ability observed in these cells when NDRG1 is silenced (Figs. 1 and 6).
Novel Thiosemicarbazones, Particularly DpC, Inhibit the Phosphorylation of FAK and Paxillin.
Previous studies from our laboratory and others have indicated that novel di-2-pyridylketone thiosemicarbazones (i.e., Dp44mT and DpC) markedly inhibit tumor growth and metastasis both in vitro and in vivo (Yuan et al., 2004; Kovacevic et al., 2011b; Liu et al., 2012; Lovejoy et al., 2012; Wang et al., 2014). Intriguingly, these agents also markedly upregulate NDRG1 expression, with this effect being mediated by iron depletion via HIF-1α-dependent and -independent mechanisms (Le and Richardson, 2004; Richardson, 2005; Kovacevic et al., 2011a; Lovejoy et al., 2012; Fang et al., 2014). Considering this enhanced NDRG1 expression induced by thiosemicarbazones and also the inhibitory effect of NDRG1 on FAK/paxillin phosphorylation (Fig. 2), we further examined whether thiosemicarbazones regulate FAK/paxillin phosphorylation via up-regulation of NDRG1.
To assess the roles of Dp44mT and DpC in regulating FAK/paxillin signaling, the analog Bp2mT (Fig. 1A) was used as a negative control as it cannot bind cellular iron. This thiosemicarbazone has a similar chemical structure to Dp44mT and DpC (Fig. 1A) but cannot chelate metals because the methyl group on the thiosemicarbazone bridge prevents electron delocalization and, thus, ligation (Yuan et al., 2004). Also, the effects of Dp44mT and DpC were compared with DFO, which is the “gold-standard” iron chelator for the treatment of iron-overload disease (Olivieri and Brittenham, 1997). Furthermore, NDRG1 overexpressing cells (i.e., HT29 and DU145; labeled “NDRG1”) were also included as positive controls. This was done as a comparison to evaluate the effect of DFO, Dp44mT, and DpC in terms of their ability to upregulate NDRG1 via iron depletion (Le and Richardson 2004; Kovacevic et al., 2011a).
In these studies, HT29 and DU145 cells were incubated for 24 hours/37°C with either the Control (medium containing 0.05% DMSO), DFO (250 μM), Dp44mT (5 μM), DpC (5 μM), or the negative control compound Bp2mT (5 μM). With the exception of Bp2mT, the concentrations of all agents used above have been demonstrated in previous reports to induce NDRG1 expression (Liu et al., 2015). Compared with Dp44mT and DpC, a much higher concentration of DFO (250 μM) was used because of its poor membrane permeability and thus, lower chelation efficacy (Merlot et al., 2013).
As shown in Fig. 9, A and B, HT29 and DU145 cells that were incubated with DFO, Dp44mT, or DpC displayed markedly and significantly (P < 0.001) increased NDRG1 expression (especially for HT29 cells) relative to the Controls. In fact, the expression level was similar to cells hyperexpressing the NDRG1 vector. In contrast, HT29 or DU145 cells incubated with the negative control agent Bp2mT showed no significant effect on NDRG1 expression, relative to the Control (Fig. 9, A and B). Of the chelators assessed, DFO showed the least activity, significantly (P < 0.001) decreasing the p-FAK(Tyr925)/FAK ratio only in HT29 cells (Fig. 9A), while significantly (P < 0.001) decreasing p-FAK(Tyr397, Tyr576/7, and Tyr925)/FAK ratios and p-paxillin(Tyr118)/paxillin ratio in DU145 cells (Fig. 9B). Hence, DFO appeared more effective in terms of influencing the phosphorylation of FAK and paxillin in DU145 cells relative to HT29 cells.
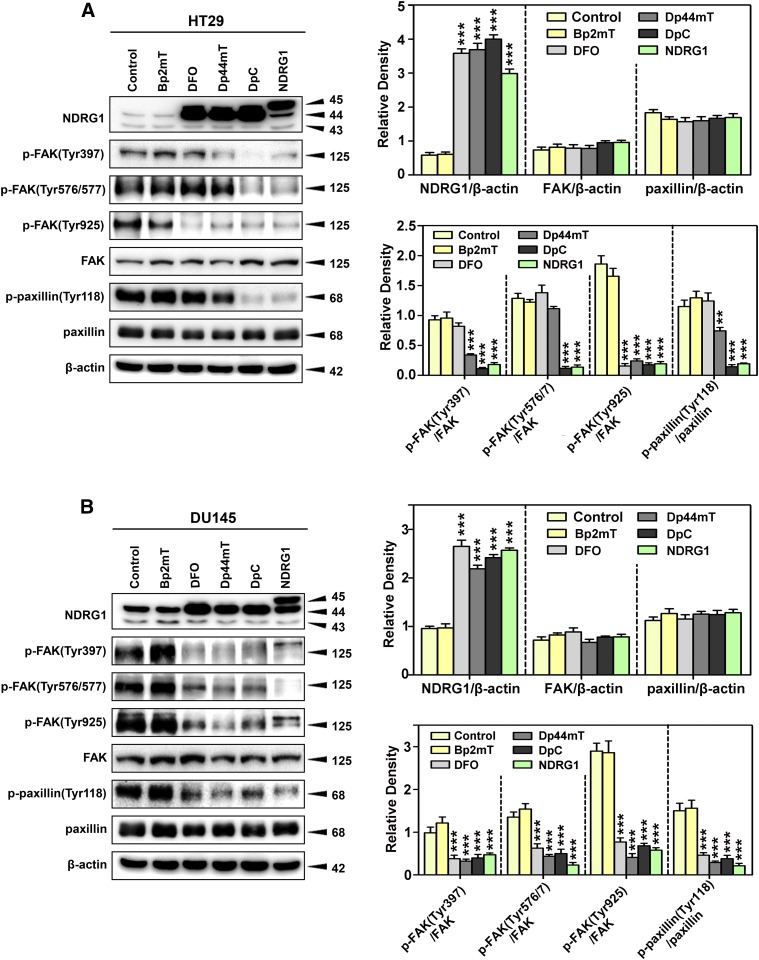
Fig. 9.
Di-2-pyridylketone thiosemicarbazones (i.e., Dp44mT and DpC) markedly upregulate NDRG1 and decrease FAK/paxillin phosphorylation levels in both HT29 (A) and DU145 (B) cells. The HT29 and DU145 cells were incubated with control medium containing 0.05% of DMSO (Control), Bp2mT (5 μM), DFO (250 μM), Dp44mT (5 μM), or DpC (5 μM) for 24 hours/37°C and NDRG1 expression as well as phosphorylation levels of FAK and paxillin were detected by immunoblot analysis. Bp2mT (5 μM) has close structural similarity to Dp44mT and DpC but cannot bind metal ions. Hence, it is a negative control. Cells stably overexpressing NDRG1 were used as a positive control for the effects of increasing NDRG1 levels (labeled as “NDRG1”). Densitometry for NDRG1 and total FAK and paxillin levels are expressed relative to the loading control, β-actin, whereas the phosphorylation levels for FAK and paxillin are displayed as the ratio of the phosphorylated compared with the total proteins. Densitometry is shown as mean ± S.D. (3–5 experiments). **P < 0.01 and ***P < 0.001 relative to the Control.
The activity of Dp44mT in HT29 was more broad than DFO, significantly (P < 0.001–0.01) inhibiting the p-FAK(Tyr397 and Tyr925)/FAK ratios and p-paxillin(Tyr118)/paxillin ratio (Fig. 9A). As observed for DFO, Dp44mT demonstrated a more extensive effect in DU145 relative to HT29 cells, significantly (P < 0.001) inhibiting the p-FAK(Tyr397, Tyr576/7, and Tyr925)/FAK ratios and p-paxillin(Tyr118)/paxillin ratio (Fig. 9B). The activity of DpC in terms of its inhibitory effect on phosphorylation of FAK and paxillin was the greatest among the three chelators, having efficacy that approached cells overexpressing NDRG1 (Fig. 9, A and B). In fact, DpC significantly (P < 0.001) inhibited p-FAK(Tyr397, Tyr576/7, and Tyr925)/FAK ratios and p-paxillin(Tyr118)/paxillin ratio in both cell-types (Fig. 9, A and B). None of the agents tested significantly affected the expression of total FAK or paxillin.
Overall, these results indicate that Dp44mT and DpC had a potent effect on inhibiting FAK/paxillin phosphorylation at different sites, an effect that is consistent with their ability to upregulate NDRG1 expression. However, it is of interest to note that despite the ability of DFO, Dp44mT, and DpC to similarly upregulate NDRG1 in both cell types, for each agent, a different spectrum of activity was observed in terms of inhibiting FAK and paxillin phosphorylation (Fig. 9, A and B). Hence, the effect of these agents on other molecular effectors additional to NDRG1 cannot be excluded.
Examination of the Role of NDRG1 in the Inhibitory Activity of Thiosemicarbazones on the Phosphorylation of FAK and Paxillin.
Because both thiosemicarbazones and NDRG1 overexpression had similar effects in terms of reducing FAK/paxillin phosphorylation (Fig. 9), we further investigated whether these agents inhibited FAK/paxillin signaling through upregulating NDRG1 (Fig. 10). In these studies, NDRG1-silenced HT29 and DU145 cells (sh-NDRG1), and their corresponding control cells (sh-Control) were incubated with DFO, Dp44mT, and DpC as well as the negative control Bp2mT for 24 hours/37°C using the same concentrations as described above (Fig. 9).
Fig. 10.
Di-2-pyridylketone thiosemicarbazones (i.e., Dp44mT and DpC) markedly upregulate NDRG1 and decrease FAK/paxillin phosphorylation levels in HT29 (A) and DU145 (B). Both the NDRG1-silencing clones (sh-NDRG1) and their respective sh-Control HT29 and DU145 cells were incubated with: control medium containing 0.05% of DMSO (Control), Bp2mT (5 μM), DFO (250 μM), Dp44mT (5 μM), or DpC (5 μM) for 24 hours/37°C. Immunoblotting was then used to detect the levels of p-FAK (Tyr397, Tyr576/7, Tyr925), total FAK, p-paxillin (Tyr118), and total paxillin in response to these agents in sh-Control and sh-NDRG1 cells. Densitometry for NDRG1 and total FAK and paxillin levels are expressed relative to the loading control, β-actin, whereas the phosphorylation levels for FAK and paxillin are displayed as the ratio of the phosphorylated compared with the total proteins. Densitometry is shown as mean ± S.D. (3–5 experiments). *P < 0.05; **P < 0.01; ***P < 0.001 relative to Control cells. #P < 0.05; ##P < 0.01; ###P < 0.001 relative to cells incubated with the same treatment in the sh-Control group.
Assessing HT29 and DU145 sh-Control cells, incubation with DFO, Dp44mT, or DpC led to a marked and significant (P < 0.001) increase in NDRG1 expression (approximately 8- to 10-fold in HT29; 2- to 3-fold in DU145), relative to Control cells or those treated with Bp2mT (Fig. 10, A and B). In HT29 sh-NDRG1 cells, DFO, Dp44mT, and DpC could still markedly and significantly (P < 0.001) upregulate NDRG1 expression, but to a slightly lesser degree than that of HT29 sh-Control cells (Fig. 10A). In contrast, in the DU145 sh-NDRG1 cells, the expression of NDRG1 induced by these chelators was markedly and significantly (P < 0.001) lower compared with the DU145 sh-Control cells (Fig. 10B). The different effects on NDRG1 expression in these sh-NDRG1 cells could be due to the varied response of these different cell types to NDRG1 silencing and to the agents examined.
As was shown in Fig. 9, A and B, DFO was only able to reduce the pFAK(Tyr925)/FAK ratio in HT29 sh-Control cells (Fig. 10A). In contrast, for DU145 sh-Control cells, DFO significantly (P < 0.001) reduced the p-FAK/FAK ratio at all sites examined (i.e., Tyr397, Tyr576/7, and Tyr925), in addition to the p-paxillin/paxillin ratio (Fig. 10B). Furthermore, Dp44mT and DpC significantly (P < 0.001) decreased the p-FAK(Tyr397 and Tyr925)/FAK ratio and p-paxillin(Tyr118)/paxillin ratio in both HT29 and DU145 sh-Control cells, whereas only DpC significantly (P < 0.001) decreased the p-FAK(Tyr576/7)/FAK ratio in HT29 sh-Control cells (Fig. 10, A and B), as demonstrated in Fig. 9, A and B.
After chelator treatment, the NDRG1 levels in sh-NDRG1 HT29 cells were only slightly reduced, relative to the sh-Control HT29 (Fig. 10A). Hence, the effect of the chelators on p-FAK/FAK and p-paxillin/paxillin ratio in the sh-NDRG1 cells was not markedly different when compared with the sh-Control cells (Fig. 10A). An exception to this was that the p-FAK(Tyr576/7)/FAK and p-paxillin(Tyr118)/paxillin levels remained significantly (P < 0.001–0.05) higher in the sh-NDRG1 cells compared with the sh-Control cells in response to DFO and Dp44mT in HT29 cells (Fig. 10, A and B). It is also important to note that Bp2mT significantly (P < 0.01) reduced the p-FAK(Tyr397 and Tyr576/7)/FAK ratio in the sh-NDRG1 HT29 cells, although the reason for this effect of the negative control was unclear (Fig. 10A).
Interestingly, despite a marked and significant (P < 0.001) reduction of the p-FAK(Tyr397, Tyr576/7, and Tyr925)/FAK ratio, DpC significantly (P < 0.05) increased total FAK levels in the HT29 sh-NDRG1 cells relative to the Control, whereas it had no significant effect in HT29 sh-Control cells (Fig. 10A). The reason for this observation is unclear and may involve a compensatory response due to the reduced activation of FAK.
In contrast to HT29 cells (Fig. 10A), in DU145 sh-NDRG1 cells, DFO, Dp44mT, and DpC induced significantly (P < 0.001) lower levels of NDRG1 compared with the sh-Control cells (Fig. 10B). Moreover, the effects of these agents on downregulating p-FAK/FAK and p-paxillin/paxillin ratios were significantly (P < 0.001–0.05) reduced compared with the sh-Control cells (Fig. 10B). This observation suggested the ability of the iron chelators to inhibit FAK/paxillin phosphorylation can be influenced by changes in NDRG1 expression. Hence, these data indicate that Dp44mT and DpC can inhibit the phosphorylation of FAK and paxillin, at least in part, through NDRG1 upregulation.
Discussion
In this study we investigated the mechanisms that underlie the ability of NDRG1 to decrease cellular migration and cell-collagen I adhesion in both colorectal and prostate cancer cells. Our results demonstrate that NDRG1 can markedly inhibit cell migration, cell-collagen I adhesion, and the formation of focal adhesions. In fact, for the first time, we demonstrate that NDRG1 inhibits the FAK/paxillin signaling pathway, which plays a critical role in tumor cell metastasis.
Tumor cell migration and cell-ECM adhesion are two key factors for the process of tumor metastasis (Yamaguchi et al., 2005). Moreover, there is a close relationship between tumor cell migration and cell-ECM adhesion (Lester and McCarthy, 1992; Lock et al., 2008). The interaction between membrane receptors (i.e., integrins, EGFR, etc.) and ECM substrates leads to changes in cell morphology and regulates cell migration, which is driven by F-actin polymerization and stress fiber formation (Girard and Nerem, 1995). A recent study examining the function of NDRG1 in colon and prostate cancer cells demonstrated that this metastasis suppressor inhibited cell migration and that this was accompanied by a marked reduction in stress fiber formation (Sun et al., 2013b). Moreover, this latter investigation also demonstrated that the ROCK/pMLC2 pathway, which directly promotes stress fiber polymerization and contraction, was inhibited by NDRG1 in these cells (Sun et al., 2013b). The upstream mechanisms driving the regulation of the ROCK/pMLC2 pathway by NDRG1 were, until now, elusive. However, the current report demonstrated for the first time that NDRG1 negatively regulates the activation of FAK, which lies upstream of the Rho A/ROCK pathway and is directly linked to the RTKs at the cell membrane (Pirone et al., 2006).
The ability of NDRG1 to regulate downstream signaling cascades at the receptor level was further highlighted in a recent study demonstrating the suppression of the ErbB family of RTKs, namely EGFR, HER2, and HER3 by this metastasis suppressor (Liu et al., 2015; Kovacevic et al., 2016). In these studies, NDRG1 inhibited the dimerization and activation of these latter receptors (Liu et al., 2015; Kovacevic et al., 2016). Although FAK has typically been associated with receptors such as integrins, studies have also shown that FAK is required for efficient EGF-stimulated cell motility (Schlaepfer and Mitra, 2004; Hwang et al., 2011). In fact, FAK was found to associate with EGFR, with this binding being mediated by Src-3Δ4, which binds directly to FAK and links this latter molecule to EGFR (Long et al., 2010). Hence, it is possible that the NDRG1-mediated effects on EGFR and FAK are linked, with the inhibition of one of these latter molecules hindering the activity of the other. Further evidence that Src is involved in the NDRG1-mediated inhibition of FAK comes from our recent report that Src activity is inhibited by NDRG1, and the downstream effects of this metastasis suppressor on cell migration and invasion are directly due to this effect (Liu et al., 2015). Src directly activates FAK by phosphorylating it at Tyr576/577, which is the site we identified to be inhibited by NDRG1 in this study. Hence, the effect of NDRG1 on FAK is likely to be due to its effect on Src.
The activation of FAK signaling is initiated once the cell surface interacts with the ECM, resulting in the induction of FAK autophosphorylation at Tyr397 (Guan, 1997; Mitra and Schlaepfer, 2006). This leads to its binding with the Src homology 2 domain of Src, which phosphorylates additional sites on FAK, leading to its full activation (Guan, 1997; Mitra and Schlaepfer, 2006). Paxillin is a cytoskeletal adaptor protein and is a major substrate of the FAK/Src complex and can also be phosphorylated at Tyr31 and Tyr118 (Turner, 2000; Deakin and Turner, 2008). Paxillin phosphorylation has long been associated with the coordinate formation of focal adhesions and stress fibers (Nakamura et al., 2000; Webb et al., 2004).
In the current investigation, NDRG1 overexpression significantly reduced the phosphorylation of FAK and paxillin, leading to marked inhibition of focal adhesions (Fig. 3). Moreover, the effects of NDRG1 on paxillin phosphorylation were found to be, at least in part, dependent on its inhibition of FAK phosphorylation. This was demonstrated by using the FAK inhibitor PF-562271 as well as FAK-specific siRNA, both of which markedly reduced the oncogenic effects observed in the sh-NDRG1 HT29 and DU145 cells, including: 1) increased paxillin phosphorylation (Figs. 4 and 5); 2) increased cell migration (Fig. 6); and 3) increased formation of focal adhesions (Figs. 7 and 8).
It is also well known that FAK regulates cell adhesion/motility by mediating the phosphorylation of paxillin and p130Cas as well as the activity of small GTPases (RhoA, Cdc42, and Rac1) (Pirone et al., 2006; Myers et al., 2012). Our laboratory showed that NDRG1 inhibits the phosphorylation of p130Cas (Liu et al., 2015) and also the activity of ROCK/Rac1 (Sun et al., 2013b). Herein, we demonstrate that NDRG1 overexpression inhibits the phosphorylation of FAK and paxillin, which are directly upstream of p130Cas and ROCK/Rac1 (Tsubouchi et al., 2002; Raftopoulou and Hall, 2004; Zouq et al., 2009). It is likely that NDRG1 inhibits p130Cas and ROCK/Rac1 through its effects on FAK/paxillin, leading to the inhibition of cell adhesion/motility.
Considering the marked inhibitory effect of NDRG1 on FAK/paxillin signaling, this metastasis suppressor may be a promising therapeutic target for the treatment of metastatic cancers. To this end, we further examined a novel class of potent and selective anticancer agents that significantly upregulate NDRG1 expression in cancer cells, namely the thiosemicarbazones, Dp44mT and DpC (Le and Richardson, 2004; Kovacevic et al., 2011a; Lovejoy et al., 2012; Quach et al., 2012). These agents have been demonstrated to block the epithelial mesenchymal transition and cell metastasis (Chen et al., 2012; Liu et al., 2012; Sun et al., 2013b; Lui et al., 2015), largely because of their ability to increase NDRG1 expression, which occurs through HIF-1α-dependent and -independent mechanisms (Le and Richardson, 2004; Lane et al., 2013). In addition, these agents are reported to inhibit tumor growth and metastasis via the oral and/or intravenous routes in different cancer xenograft models and, notably, are able to overcome resistance to currently used chemotherapeutics (Whitnall et al., 2006; Kovacevic et al., 2011a; Liu et al., 2012; Lovejoy et al., 2012; Wang et al., 2014; Jansson et al., 2015b). Hence, the ability of these agents to inhibit FAK/paxillin signaling was important to examine and may lead to more effective targeting of these pathways.
We demonstrate that FAK/paxillin signaling was markedly suppressed upon treatment with both thiosemicarbazones, which also markedly upregulated NDRG1. Furthermore, NDRG1-silencing induced a pronounced reduction in the inhibitory effects of Dp44mT and DpC on FAK and paxillin phosphorylation in DU145 prostate cancer cells. On the other hand, examining HT29 cells, the silencing of NDRG1 did not markedly perturb the activity of Dp44mT and DpC. This effect may be due to the marked increase of NDRG1 expression in response to these agents, which was not inhibited by NDRG1 siRNA in these cells. It was notable that incubation with DFO, Dp44mT, and DpC induced robust expression of NDRG1 in HT29 and DU145 cells, but the effects of these agents on p-FAK (Tyr397, Tyr576/577, and Tyr925) and p-paxillin (Tyr118) were different, particularly for DpC. This observation may indicate that different chelators could target multiple effectors, as demonstrated in previous reports (Kovacevic et al., 2016), and this could explain the differential activity.
It is of interest that FAK inhibitors, such as PF-562271, are currently being assessed in preclinical models and clinical trials (Golubovskaya, 2014). However, because the FAK pathway is integrated with other oncogenic signaling pathways, a more effective therapeutic strategy could be the combination of specific FAK inhibitors with other agents that target associated pathways (Golubovskaya, 2014). The novel thiosemicarbazones examined in this study may potentially enhance the efficacy of current FAK inhibitors.
Overall, these results indicate that NDRG1 plays an important role in the antimetastatic activity of Dp44mT and DpC. This is in agreement with earlier studies, which demonstrated that NDRG1 is important for the antitumor effects of these agents in vitro (Dixon et al., 2013; Sun et al., 2013b; Kovacevic et al., 2016) and for Dp44mT antimetastatic activity in vivo (Liu et al., 2012). This report further highlights the potential of these novel agents against metastatic cancers.
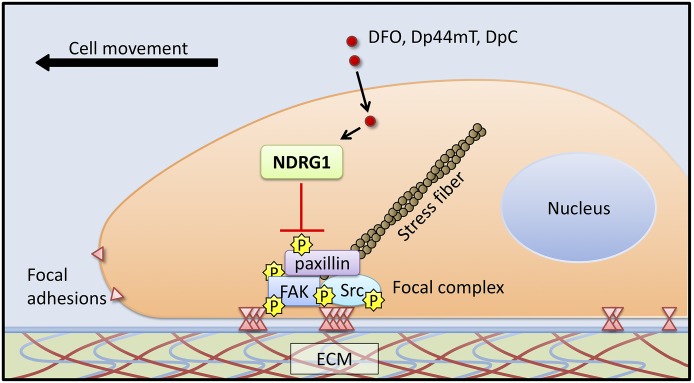
In summary, this investigation indicates that NDRG1 could inhibit tumor cell migration, cell-ECM attachment, and focal adhesion formation through regulation of FAK/paxillin signaling (Fig. 11). Moreover, novel thiosemicarbazones could also suppress FAK/paxillin phosphorylation by, in part, the upregulation of NDRG1 (Fig. 11). These new insights into the antimetastatic effects of NDRG1, as well as the novel thiosemicarbazones on FAK/paxillin phosphorylation could lead to promising new anticancer strategies.
Fig. 11.
Schematic illustration summarizing the inhibitory effect of NDRG1 on cell migration, cell-ECM attachment, and focal adhesion formation. The di-2-pyridylketone thiosemicarbazones, namely Dp44mT and DpC, inhibit FAK/paxillin phosphorylation, at least in part, via their ability to upregulate NDRG1.
Abbreviations
- DAPI
4',6-diamidino-2-phenylindole
- DFO
desferrioxamine
- DMSO
dimethyl sulfoxide
- Dp44mT
di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone
- DpC
di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- NDRG1
N-myc downstream regulated gene-1
- PF-562271
N-methyl-N-[3-[[[2-[(2-oxo-1,3-dihydroindol-5-yl)amino]-5-(trifluoromethyl)pyrimidin-4-yl]amino]methyl]pyridin-2-yl]methanesulfonamide
- pMLC2
phosphorylated myosin light chain 2
- RTK
receptor tyrosine kinase
- siRNA
small interfering RNA
Authorship Contributions
Participated in research design: Wangpu, Yue, Zheng, and Richardson.
Conducted experiments: Wangpu, Lu, and Xi.
Contributed new reagents or analytic tools: Wangpu, Park, Menezes, and Richardson.
Performed data analysis: Wangpu, Lu, Xi, Huang, Kovacevic, and Richardson.
Wrote or contributed to the writing of the manuscript: Wangpu, Yue, Sahni, Zheng, Kovacevic, and Richardson.
Footnotes
This work was supported by a PhD Scholarship from the China Scholarship Council, National Health and Medical Research Council of Australia (NHMRC) Senior Principal Research Fellowship [APP1062607], NHMRC Project Grant [APP1060482], NHMRC Australian Training Fellowship [APP1037323], Cancer Institute New South Wales Early Career Development Fellowship [12/ECF/2-17], National Natural Science Foundation of China Project Grants [81201539, 81201625, 81402423], Science and Technology Commission of Shanghai Municipality Project Grants [13JC1404100, 11411950700], and National High Technology Research and Development Program 863 Project Grant [2012AA021103].
D. R. R. consults and is a stakeholder in the companies, Oncochel Therapeutics LLC and Pty. Ltd, which are developing DpC for the treatment of advanced and resistant solid tumors.
References
- Azuma K, Tanaka M, Uekita T, Inoue S, Yokota J, Ouchi Y, Sakai R. (2005) Tyrosine phosphorylation of paxillin affects the metastatic potential of human osteosarcoma. Oncogene 24:4754–4764. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, Saito K, Commes T, Hayashi S, Watabe M, et al. (2003) The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res 63:1731–1736. [PubMed] [Google Scholar]
- Bandyopadhyay S, Pai SK, Hirota S, Hosobe S, Takano Y, Saito K, Piquemal D, Commes T, Watabe M, Gross SC, et al. (2004) Role of the putative tumor metastasis suppressor gene Drg-1 in breast cancer progression. Oncogene 23:5675–5681. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. (1992) Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol 119:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calalb MB, Polte TR, Hanks SK. (1995) Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol 15:954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. (2000) Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res 6:2417–2423. [PubMed] [Google Scholar]
- Chen J, Gallo KA. (2012) MLK3 regulates paxillin phosphorylation in chemokine-mediated breast cancer cell migration and invasion to drive metastasis. Cancer Res 72:4130–4140. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhang D, Yue F, Zheng M, Kovacevic Z, Richardson DR. (2012) The iron chelators Dp44mT and DFO inhibit TGF-β-induced epithelial-mesenchymal transition via up-regulation of N-Myc downstream-regulated gene 1 (NDRG1). J Biol Chem 287:17016–17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin NO, Turner CE. (2008) Paxillin comes of age. J Cell Sci 121:2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon KM, Lui GY, Kovacevic Z, Zhang D, Yao M, Chen Z, Dong Q, Assinder SJ, Richardson DR. (2013) Dp44mT targets the AKT, TGF-β and ERK pathways via the metastasis suppressor NDRG1 in normal prostate epithelial cells and prostate cancer cells. Br J Cancer 108:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T, Qu Y, Li J, Li H, Su L, Zhou Q, Yan M, Li C, Zhu Z, Liu B. (2014) Maternal embryonic leucine zipper kinase enhances gastric cancer progression via the FAK/Paxillin pathway. Mol Cancer 13:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen TP, Ke Q, Zhang P, Costa M. (2008) NDRG1, a growth and cancer related gene: regulation of gene expression and function in normal and disease states. Carcinogenesis 29:2–8. [DOI] [PubMed] [Google Scholar]
- Fang BA, Kovačević Ž, Park KC, Kalinowski DS, Jansson PJ, Lane DJ, Sahni S, Richardson DR. (2014) Molecular functions of the iron-regulated metastasis suppressor, NDRG1, and its potential as a molecular target for cancer therapy. Biochim Biophys Acta 1845:1–19. [DOI] [PubMed] [Google Scholar]
- Gabarra-Niecko V, Schaller MD, Dunty JM. (2003) FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev 22:359–374. [DOI] [PubMed] [Google Scholar]
- Ghalayini MK, Dong Q, Richardson DR, Assinder SJ. (2013) Proteolytic cleavage and truncation of NDRG1 in human prostate cancer cells, but not normal prostate epithelial cells. Biosci Rep 33:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard PR, Nerem RM. (1995) Shear stress modulates endothelial cell morphology and F-actin organization through the regulation of focal adhesion-associated proteins. J Cell Physiol 163:179–193. [DOI] [PubMed] [Google Scholar]
- Golubovskaya VM. (2014) Targeting FAK in human cancer: from finding to first clinical trials. Front Biosci (Landmark Ed) 19:687–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JL. (1997) Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol 29:1085–1096. [DOI] [PubMed] [Google Scholar]
- Hickok JR, Sahni S, Mikhed Y, Bonini MG, Thomas DD. (2011) Nitric oxide suppresses tumor cell migration through N-Myc downstream-regulated gene-1 (NDRG1) expression: role of chelatable iron. J Biol Chem 286:41413–41424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG, Song GY, Kwon KI, Jeong TC, Jeong HG. (2011) Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol Nutr Food Res 55:594–605. [DOI] [PubMed] [Google Scholar]
- Jansson PJ, Kalinowski DS, Lane DJ, Kovacevic Z, Seebacher NA, Fouani L, Sahni S, Merlot AM, Richardson DR. (2015a) The renaissance of polypharmacology in the development of anti-cancer therapeutics: Inhibition of the “Triad of Death” in cancer by Di-2-pyridylketone thiosemicarbazones. Pharmacol Res 100:255–260. [DOI] [PubMed] [Google Scholar]
- Jansson PJ, Yamagishi T, Arvind A, Seebacher N, Gutierrez E, Stacy A, Maleki S, Sharp D, Sahni S, Richardson DR. (2015b) Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes multidrug resistance by a novel mechanism involving the hijacking of lysosomal P-glycoprotein (Pgp). J Biol Chem 290:9588–9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Liu W, Menezes S, Yue F, Zheng M, Kovacevic Z, Richardson DR. (2014) The metastasis suppressor NDRG1 modulates the phosphorylation and nuclear translocation of β-catenin through mechanisms involving FRAT1 and PAK4. J Cell Sci 127:3116–3130. [DOI] [PubMed] [Google Scholar]
- Keselowsky BG, Collard DM, García AJ. (2004) Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 25:5947–5954. [DOI] [PubMed] [Google Scholar]
- Kitowska A, Pawełczyk T. (2010) N-myc downstream regulated 1 gene and its place in the cellular machinery. Acta Biochim Pol 57:15–21. [PubMed] [Google Scholar]
- Kovacevic Z, Chikhani S, Lovejoy DB, Richardson DR. (2011a) Novel thiosemicarbazone iron chelators induce up-regulation and phosphorylation of the metastasis suppressor N-myc down-stream regulated gene 1: a new strategy for the treatment of pancreatic cancer. Mol Pharmacol 80:598–609. [DOI] [PubMed] [Google Scholar]
- Kovacevic Z, Chikhani S, Lui GY, Sivagurunathan S, Richardson DR. (2013) The iron-regulated metastasis suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the PI3K and Ras signaling pathways. Antioxid Redox Signal 18:874–887. [DOI] [PubMed] [Google Scholar]
- Kovacevic Z, Fu D, Richardson DR. (2008) The iron-regulated metastasis suppressor, Ndrg-1: identification of novel molecular targets. Biochim Biophys Acta 1783:1981–1992. [DOI] [PubMed] [Google Scholar]
- Kovacevic Z, Menezes SV, Sahni S, Kalinowski DS, Bae DH, Lane DJ, Richardson DR. (2016) The metastasis suppressor, N-myc downstream regulated gene-1 (NDRG1), down-regulates the ErbB family of receptors to inhibit downstream oncogenic signaling pathways. J Biol Chem 291:1029–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic Z, Sivagurunathan S, Mangs H, Chikhani S, Zhang D, Richardson DR. (2011b) The metastasis suppressor, N-myc downstream regulated gene 1 (NDRG1), upregulates p21 via p53-independent mechanisms. Carcinogenesis 32:732–740. [DOI] [PubMed] [Google Scholar]
- Lane DJ, Saletta F, Suryo Rahmanto Y, Kovacevic Z, Richardson DR. (2013) N-myc downstream regulated 1 (NDRG1) is regulated by eukaryotic initiation factor 3a (eIF3a) during cellular stress caused by iron depletion. PLoS One 8:e57273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. (1996) Cell migration: a physically integrated molecular process. Cell 84:359–369. [DOI] [PubMed] [Google Scholar]
- Le NT, Richardson DR. (2004) Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: a link between iron metabolism and proliferation. Blood 104:2967–2975. [DOI] [PubMed] [Google Scholar]
- Lester BR, McCarthy JB. (1992) Tumor cell adhesion to the extracellular matrix and signal transduction mechanisms implicated in tumor cell motility, invasion and metastasis. Cancer Metastasis Rev 11:31–44. [DOI] [PubMed] [Google Scholar]
- Liu W, Xing F, Iiizumi-Gairani M, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Kobayashi A, Mo YY, et al. (2012) N-myc downstream regulated gene 1 modulates Wnt-β-catenin signalling and pleiotropically suppresses metastasis. EMBO Mol Med 4:93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Yue F, Zheng M, Merlot A, Bae DH, Huang M, Lane D, Jansson P, Lui GY, Richardson V, et al. (2015) The proto-oncogene c-Src and its downstream signaling pathways are inhibited by the metastasis suppressor, NDRG1. Oncotarget 6:8851–8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, Wehrle-Haller B, Strömblad S. (2008) Cell-matrix adhesion complexes: master control machinery of cell migration. Semin Cancer Biol 18:65–76. [DOI] [PubMed] [Google Scholar]
- Long W, Yi P, Amazit L, LaMarca HL, Ashcroft F, Kumar R, Mancini MA, Tsai SY, Tsai MJ, O’Malley BW. (2010) SRC-3Delta4 mediates the interaction of EGFR with FAK to promote cell migration. Mol Cell 37:321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy DB, Sharp DM, Seebacher N, Obeidy P, Prichard T, Stefani C, Basha MT, Sharpe PC, Jansson PJ, Kalinowski DS, et al. (2012) Novel second-generation di-2-pyridylketone thiosemicarbazones show synergism with standard chemotherapeutics and demonstrate potent activity against lung cancer xenografts after oral and intravenous administration in vivo. J Med Chem 55:7230–7244. [DOI] [PubMed] [Google Scholar]
- Lui GY, Kovacevic Z, Richardson V, Merlot AM, Kalinowski DS, Richardson DR. (2015) Targeting cancer by binding iron: Dissecting cellular signaling pathways. Oncotarget 6:18748–18779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y, Ono M, Kawahara A, Yokoyama T, Basaki Y, Kage M, Aoyagi S, Kinoshita H, Kuwano M. (2006) Tumor growth suppression in pancreatic cancer by a putative metastasis suppressor gene Cap43/NDRG1/Drg-1 through modulation of angiogenesis. Cancer Res 66:6233–6242. [DOI] [PubMed] [Google Scholar]
- McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. (2005) The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer 5:505–515. [DOI] [PubMed] [Google Scholar]
- Melotte V, Qu X, Ongenaert M, van Criekinge W, de Bruïne AP, Baldwin HS, van Engeland M. (2010) The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J 24:4153–4166. [DOI] [PubMed] [Google Scholar]
- Merlot AM, Kalinowski DS, Richardson DR. (2013) Novel chelators for cancer treatment: where are we now? Antioxid Redox Signal 18:973–1006. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 18:516–523. [DOI] [PubMed] [Google Scholar]
- Murray JT, Campbell DG, Morrice N, Auld GC, Shpiro N, Marquez R, Peggie M, Bain J, Bloomberg GB, Grahammer F, et al. (2004) Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem J 384:477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JP, Robles E, Ducharme-Smith A, Gomez TM. (2012) Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. J Cell Sci 125:2918–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Hoshino D, Koshikawa N, Akizawa T, Seiki M. (2012) Turnover of focal adhesions and cancer cell migration. Int J Cell Biol 2012:310616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Yano H, Uchida H, Hashimoto S, Schaefer E, Sabe H. (2000) Tyrosine phosphorylation of paxillin alpha is involved in temporospatial regulation of paxillin-containing focal adhesion formation and F-actin organization in motile cells. J Biol Chem 275:27155–27164. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. (1995) Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53–62. [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Brittenham GM. (1997) Iron-chelating therapy and the treatment of thalassemia. Blood 89:739–761. [PubMed] [Google Scholar]
- Panetti TS. (2002) Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front Biosci 7:d143–d150. [DOI] [PubMed] [Google Scholar]
- Pirone DM, Liu WF, Ruiz SA, Gao L, Raghavan S, Lemmon CA, Romer LH, Chen CS. (2006) An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J Cell Biol 174:277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach P, Gutierrez E, Basha MT, Kalinowski DS, Sharpe PC, Lovejoy DB, Bernhardt PV, Jansson PJ, Richardson DR. (2012) Methemoglobin formation by triapine, di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT), and other anticancer thiosemicarbazones: identification of novel thiosemicarbazones and therapeutics that prevent this effect. Mol Pharmacol 82:105–114. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. (2004) Cell migration: Rho GTPases lead the way. Dev Biol 265:23–32. [DOI] [PubMed] [Google Scholar]
- Richardson A, Malik RK, Hildebrand JD, Parsons JT. (1997) Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol 17:6906–6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DR. (2005) Molecular mechanisms of iron uptake by cells and the use of iron chelators for the treatment of cancer. Curr Med Chem 12:2711–2729. [DOI] [PubMed] [Google Scholar]
- Richardson DR, Sharpe PC, Lovejoy DB, Senaratne D, Kalinowski DS, Islam M, Bernhardt PV. (2006) Dipyridyl thiosemicarbazone chelators with potent and selective antitumor activity form iron complexes with redox activity. J Med Chem 49:6510–6521. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, Richter D, Emerson E, Lin J, Kath J, et al. (2008) Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res 68:1935–1944. [DOI] [PubMed] [Google Scholar]
- Sattler M, Pisick E, Morrison PT, Salgia R. (2000) Role of the cytoskeletal protein paxillin in oncogenesis. Crit Rev Oncog 11:63–76. [PubMed] [Google Scholar]
- Schaller MD. (2001) Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20:6459–6472. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK. (2004) Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev 14:92–101. [DOI] [PubMed] [Google Scholar]
- Shah MA, Kemeny N, Hummer A, Drobnjak M, Motwani M, Cordon-Cardo C, Gonen M, Schwartz GK. (2005) Drg1 expression in 131 colorectal liver metastases: correlation with clinical variables and patient outcomes. Clin Cancer Res 11:3296–3302. [DOI] [PubMed] [Google Scholar]
- Shan Y, Yu L, Li Y, Pan Y, Zhang Q, Wang F, Chen J, Zhu X. (2009) Nudel and FAK as antagonizing strength modulators of nascent adhesions through paxillin. PLoS Biol 7:e1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. (2000) FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2:249–256. [DOI] [PubMed] [Google Scholar]
- Su JM, Gui L, Zhou YP, Zha XL. (2002) Expression of focal adhesion kinase and alpha5 and beta1 integrins in carcinomas and its clinical significance. World J Gastroenterol 8:613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhang D, Bae DH, Sahni S, Jansson P, Zheng Y, Zhao Q, Yue F, Zheng M, Kovacevic Z, et al. (2013a) Metastasis suppressor, NDRG1, mediates its activity through signaling pathways and molecular motors. Carcinogenesis 34:1943–1954. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang D, Zheng Y, Zhao Q, Zheng M, Kovacevic Z, Richardson DR. (2013b) Targeting the metastasis suppressor, NDRG1, using novel iron chelators: regulation of stress fiber-mediated tumor cell migration via modulation of the ROCK1/pMLC2 signaling pathway. Mol Pharmacol 83:454–469. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S. (1996) Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer 68:164–171. [DOI] [PubMed] [Google Scholar]
- Tsubouchi A, Sakakura J, Yagi R, Mazaki Y, Schaefer E, Yano H, Sabe H. (2002) Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration. J Cell Biol 159:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE. (2000) Paxillin and focal adhesion signalling. Nat Cell Biol 2:E231–E236. [DOI] [PubMed] [Google Scholar]
- Wang J, Yin D, Xie C, Zheng T, Liang Y, Hong X, Lu Z, Song X, Song R, Yang H, et al. (2014) The iron chelator Dp44mT inhibits hepatocellular carcinoma metastasis via N-Myc downstream-regulated gene 2 (NDRG2)/gp130/STAT3 pathway. Oncotarget 5:8478–8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260:1124–1127. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. (2004) FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 6:154–161. [DOI] [PubMed] [Google Scholar]
- Whitnall M, Howard J, Ponka P, Richardson DR. (2006) A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci USA 103:14901–14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Wyckoff J, Condeelis J. (2005) Cell migration in tumors. Curr Opin Cell Biol 17:559–564. [DOI] [PubMed] [Google Scholar]
- Yuan J, Lovejoy DB, Richardson DR. (2004) Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: in vitro and in vivo assessment. Blood 104:1450–1458. [DOI] [PubMed] [Google Scholar]
- Zouq NK, Keeble JA, Lindsay J, Valentijn AJ, Zhang L, Mills D, Turner CE, Streuli CH, Gilmore AP. (2009) FAK engages multiple pathways to maintain survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J Cell Sci 122:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]