Abstract
Landmark studies describing the effect of microbial infection on the expression and activity of hepatic CYP3A used bacterial lipopolysaccharide as a model antigen. Our efforts to determine whether these findings were translatable to viral infections led us to observations suggesting that engagement of integrin receptors is key in the initiation of processes responsible for changes in hepatic CYP3A4 during infection and inflammation. Studies outlined in this article were designed to evaluate whether engagement of integrins, receptors commonly used by a variety of microbes to enter cellular targets, is vital in the regulation of CYP3A in the presence and absence of virus infection. Mice infected with a recombinant adenovirus (AdlacZ) experienced a 70% reduction in hepatic CYP3A catalytic activity. Infection with a mutant virus with integrin-binding arginine-glycine-aspartic acid (RGD) sequences deleted from the penton base protein of the virus capsid (AdΔRGD) did not alter CYP3A activity. CYP3A mRNA and protein levels in AdlacZ-treated animals were also suppressed, whereas those of mice given AdΔRGD were not significantly different from uninfected control mice. Silencing of the integrin β-subunit reverted adenovirus-mediated CYP3A4 suppression in vitro. Silencing of the α-subunit did not. Suppression of integrin subunits had a profound effect on nuclear receptors pregnane X receptor and constitutive androstane receptor, whereas retinoid X receptor α was largely unaffected. To our knowledge, this is the first time that extracellular receptors, like integrins, have been indicated in the regulation of CYP3A. This finding has several implications owing to the important role of integrins in normal physiologic process and in many disease states.
Introduction
The CYP3A enzyme family is unique in its capability of metabolizing a variety of compounds that span 38 different therapeutic indications in addition to natural biomolecules (steroids, cholesterol), toxins, and carcinogens (Sevior et al., 2012). The performance of this rather diverse group of enzymes is also highly sensitive to microbial infection (Croyle, 2009; Gandhi et al., 2012). Although the primary paradigm of the field attributed this effect to the production of interferons, cytokines, and chemokines (Zanger and Schwab, 2013), we found that the expression and function of hepatic CYP3A2 in the rat is suppressed for 14 days after a single dose of a recombinant adenovirus, long after these inflammatory mediators dissipate (Callahan et al., 2005). Additional studies revealed that reducing the immunogenicity of the virus by chemical and physical means did not mitigate this effect (Callahan et al., 2008a). These findings (coupled with a clinical report detailing minimal changes in hepatic CYP3A in patients given exogenous cytokines in the absence of infection; Reiss and Piscitelli, 1998) suggest that the presence of the microbial pathogen itself plays a role in the process that alters CYP3A during infection.
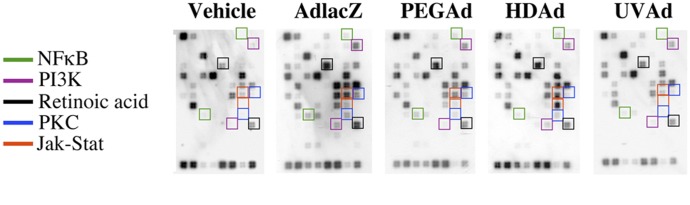
In an effort to identify the underlying mechanism by which virus infection alters CYP3A expression and function, gene expression patterns in the liver of animals given a single dose of different live and inactivated recombinant adenoviruses were analyzed over time using signal transduction microarrays (Fig. 1; Table 1). Global evaluation of data identified five pathways that were consistently upregulated for 14 days after infection. The nuclear factor κB pathway was the most profoundly affected by all viruses compared with saline-treated controls. Other pathways upregulated by the presence of virus (in descending order of intensity) were the phosphoinositide 3-kinase (PI3K), Janus kinase/signal transducers and activators of transcription (Jak-Stat), protein kinase C, and retinoic acid pathways. Each of these pathways has been documented to alter the expression and function of hepatic CYP3A in some capacity (Ding and Staudinger, 2005; Wang et al., 2008; Zangar et al., 2008; Pondugula et al., 2009; Kacevska et al., 2013). Further evaluation of these data with that from a pilot study in which CYP3A activity was suppressed in primary hepatocytes treated with an arginine-glycine-aspartic acid (RGD) peptide known to engage integrins in the absence of virus infection (Callahan et al., 2008a) solidified our hypothesis that engagement of integrin receptors by the virus at the start of infection may be sufficient to initiate processes that suppress hepatic CYP3A.
Fig. 1.
Live and inactivated recombinant adenoviruses activate certain signal transduction pathways in the liver. Gene profiling was performed on liver tissue isolated from male Sprague-Dawley rats 4 days after a single dose (5.7 × 1012 viral particles per kilogram) of virus. Representative gene expression arrays and densitometric profiles for each treatment group at this time point are illustrated here. Highlighted boxes represent genes associated with five different pathways that were significantly elevated in three of the four treatment groups with respect to saline-treated controls. Housekeeping genes are located in the bottom horizontal row of each array and were used for normalization and analysis. The primary virus used in this study was a first-generation adenovirus, expressing the E. coli β-galactosidase transgene (AdlacZ). The early region 1 (E1, involved in virus replication) and early region 3 (E3, involved in evasion of the host immune response) in the genome were removed in this vector to accommodate the β-galactosidase transgene cassette. A PEGylated version of this virus (PEGAd), which has a significantly lower immunologic profile (Croyle et al., 2001, 2002), and an inactive control, AdlacZ inactivated by exposure to riboflavin and UV light (UVAd; Callahan et al., 2008b), were included to study the effect of the immune response against virus capsid proteins and virus receptor interactions on signal transduction patterns during infection. A HDAd vector, devoid of all viral genes and containing the β-galactosidase transgene (Croyle et al., 2005), was also included to fully study the effect of viral gene expression on signal transduction in the liver. NFκB, nuclear factor κB; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C.
TABLE 1.
Differential expression patterns of genes associated with various signal transduction pathways in the liver 4 days after administration of recombinant adenovirus
Descriptions of vectors are provided in the legend for Figure 1 . Numbers represent the ratio of the intensity of gene expression in a sample obtained from an animal given virus with respect to an animal given saline (vehicle control). Gene expression was normalized based on minimal background subtraction and interquartile normalization using ScanAlyze software and the GEArray Expression Analysis Suite.
| Gene | Pathway | AdlacZ | PEG | HDAd | UVAd |
|---|---|---|---|---|---|
| A2m | Jak-Stat | 0.754 | 3.232 | 0.710 | 1.705 |
| Bax | p53 | 0.697 | 1.634 | 2.745 | 2.361 |
| Bcl2a1a | NFκB | 1.815 | 6.477 | 15.443 | 2.761 |
| Ccl2 | LDL | 0.865 | 1.971 | 0.944 | 1.074 |
| Cend1a | Wnt, PI3K | 3.012 | 6.543 | 0.225 | 5.267 |
| Cdkn1a | p53 and androgen | 0.685 | 1.573 | 0.044 | 2.682 |
| Cebpb | Insulin | 0.943 | 2.290 | 0.154 | 3.584 |
| Fos | Stress, CREB, PKC, PLC | 1.061 | 1.662 | 0.436 | 0.718 |
| Ctsda | Retinoic acid, estrogen | 1.533 | 4.141 | 0.096 | 2.956 |
| Egfr | Estrogen, androgen | 0.940 | 0.939 | 0.144 | 2.617 |
| Egfr1 | Mitogenic, CREB | 3.146 | 6.875 | 0.289 | 0.937 |
| Ei24 | p53 | 0.647 | 2.132 | 1.023 | 1.634 |
| Fasn | Insulin | 0.641 | 1.169 | 0.170 | 1.606 |
| Fn1 | PI3K/AKT | 0.628 | 1.480 | 0.029 | 1.954 |
| Gadd45a | p53 | 1.119 | 0.865 | 0.238 | 1.045 |
| Gys2 | Insulin | 0.746 | 1.614 | 0.059 | 1.563 |
| Hnf3b | Hedgehog | 1.223 | 1.532 | 0.400 | 1.363 |
| Hspb1 | Stress | 1.482 | 2.556 | 0.227 | 2.398 |
| Hspca | Stress | 1.575 | 3.839 | 0.144 | 3.279 |
| Icam1 | NFκB and phospholipase C | 1.134 | 5.012 | 0.614 | 1.137 |
| Igfbp3 | p53 | 0.382 | 0.877 | 1.708 | 2.834 |
| Il4r | Jak-Stat | 1.085 | 1.674 | 0.211 | 1.814 |
| Irf1a | Jak-Stat | 2.368 | 4.078 | 3.186 | 2.265 |
| Juna | Wnt, PI3K/AKT, PKC | 3.362 | 5.572 | 0.360 | 2.663 |
| Klk3 | Androgen | 0.417 | 1.481 | 0.633 | 1.177 |
| Tnfrsf6 | p53, NFAT | 1.646 | 0.756 | 0.794 | 0.781 |
| Lta | NFκB | 1.297 | 0.396 | 0.497 | 0.837 |
| Mdm2 | p53 | 4.117 | 4.066 | 3.854 | 2.040 |
| Miga | Jak-Stat | 3.984 | 3.395 | 7.851 | 1.862 |
| Myc | Wnt, stress, PKC | 0.922 | 1.774 | 0.923 | 1.773 |
| Nfkb1a | NFκB | 1.782 | 3.501 | 3.788 | 1.429 |
| Nfkbia | NFκB | 2.209 | 1.775 | 0.592 | 1.479 |
| Nos2 | Jak-Stat, NFκB, PLC | 2.699 | 0.510 | 2.267 | 1.517 |
| Odc1a | PKC | 3.527 | 2.822 | 4.258 | 1.311 |
| Ptena | PI3K/AKT | 3.605 | 1.400 | 8.479 | 1.975 |
| Rbp1a | Retinoic acid | 1.443 | 2.236 | 1.935 | 2.271 |
| Vcam | NFκB, PKC, LDL | 1.804 | 0.879 | 1.461 | 0.258 |
CREB, cAMP response element-binding protein; LDL, low-density lipoprotein; NFAT, nuclear factor of activated T cells; NFκB, nuclear factor κB; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; PLC, phospholipase C.
Row corresponds with signal transduction pathways in arrays depicted in Figure 1.
The integrin family consists of 24 membrane-spanning receptors. Each receptor consists of a heterodimeric, noncovalently associated combination of 1 of the 18 α protein subunits and 1 of the 8 β protein subunits (Campbell and Humphries, 2011). In humans, integrins are expressed in almost every cell type and primarily mediate adhesion to the extracellular matrix but they also play a role in embryonic development and cell differentiation (Desgrosellier and Cheresh, 2010). All integrin subunits have a single membrane-spanning helix and usually a short, unconstructed cytoplasmic tail (Shattil et al., 2010). Integrins are also employed by a variety of enveloped and nonenveloped viruses (Stewart and Nemerow, 2007), fungi (Forsyth et al., 1998), and bacteria (Hauck et al., 2012) to gain entry and establish infection in host cells. Adenoviruses bind to several extracellular receptors, including integrins. Well characterized integrins responsible for cellular entry of human adenovirus are integrin receptors αvβ3 and αvβ5. Both integrin subtypes promote virus internalization through interaction with a conserved RGD motif in the adenovirus penton base (Wolfrum and Greber, 2013). Integrin receptors are attractive targets for pathogens in that they play a key role in endocytosis and intracellular trafficking throughout the cytoplasm (Caswell et al., 2009). Integrins are also involved in numerous disease states, including cancer, thrombosis, multiple sclerosis, psoriasis, asthma, ulcerative colitis, and acute coronary syndromes (Cox et al., 2010). Hence, integrin receptors have evolved during the last few years as exciting therapeutic targets, with approximately 300 anti-integrin drugs in various stages of clinical testing (Goodman and Picard, 2012).
Given the importance of integrins in infection and other noninfectious disease states, the primary goal of the studies summarized in this article was to further solidify the hypothesis that integrin receptors influence the expression and function of hepatic CYP3A in vivo. This was achieved by infecting mice with two recombinant adenoviruses that differ only in their ability to bind integrin receptors (Shayakhmetov et al., 2005a). A secondary goal of these studies was to identify specific integrin receptor subunits responsible for changes in CYP3A. In vitro assessment of CYP3A activity and expression after knockdown of various α- and β-subunits of integrin receptors in a novel human-derived hepatocyte cell line (Wonganan et al., 2014) allowed us to prioritize the role that each plays in the regulation of CYP3A and several transcription factors known to moderate this enzyme. To our knowledge, this is the first report describing a role for these ubiquitous receptors in the regulation of drug metabolism in vivo and in human hepatocytes.
Materials and Methods
Reagents.
Acetopromazine was purchased from Fort Dodge Laboratories (Atlanta, GA). Ketamine was purchased from Pfizer (New York, NY). Phosphate-buffered saline (PBS), EDTA, glucose-6-phosphate, glucose-6-phosphate dehydrogenase, β-NADP sodium salt hydrate, 11α-hydroxyprogesterone, and testosterone were purchased from Sigma-Aldrich (St. Louis, MO). Protogel acrylamide was purchased from National Diagnostics (Atlanta, GA). Testosterone metabolite standards were purchased from Steraloids Inc. (Wilton, NH). Oligonucleotide primers were custom synthesized by Sigma Life Science (Woodlands, TX). All other chemicals were of analytical reagent grade and were purchased from EMD Chemicals (Gibbstown, NJ) unless specified otherwise.
Recombinant Adenovirus Production.
All viruses were amplified in human embryonic kidney 293 cells except for the helper-dependent adenovirus (HDAd), which was amplified in 293Cre cells with the use of the helper virus AdLC8Luc1, and purified from secondary lysates as previously described (Croyle et al., 2005). Viruses were purified by banding twice on cesium chloride gradients and were desalted on Econo-Pac 10 DG disposable chromatography columns (BioRad, Hercules, CA) equilibrated with sterile 100 mM PBS (pH 7.4). Positive fractions were collected and the number of virus particles determined using the method of Maizel et al. (1968) with the following formula: virus particles/ml = (absorbance at 260 nm) × (dilution factor) × 1.1 × 1012.
Modification of Recombinant Adenoviruses.
Virus capsid proteins were covalently modified with monomethoxypoly(ethylene) glycol, activated by tresyl chloride (Sigma-Aldrich) according to an established protocol (Croyle et al., 2000). Approximately 15,445 polyethylene glycol (PEG) molecules were associated with each virus particle in the studies outlined here as determined by a PEG-biotin assay (Croyle et al., 2005). Inactive control virus was produced by mixing freshly purified virus with riboflavin (50 μM, final concentration) and subsequent exposure of the mixture to UV light for 1 hour according to a protocol established in our laboratory (Callahan et al., 2008b). Virus inactivation was confirmed by limiting dilution and infection of HeLa cells.
Administration of Adenoviral Vectors.
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas at Austin and are in accordance with the guidelines established by the National Institutes of Health for the humane treatment of animals.
Rat Studies.
For microarray analysis, catheters were surgically implanted in the right jugular vein of male Sprague-Dawley rats (aged 9 to 10 weeks; Harlan Sprague-Dawley, Inc. Indianapolis, IN). Twenty-four hours later, rats were given a single intravenous dose of 5.7 × 1012 virus particles per kilogram in a 0.5-ml volume or vehicle alone (PBS, 0.5 ml). Four days later, animals were euthanized and the liver was immediately excised and stored in RNAlater (Qiagen, Valencia, CA) at 4°C for microarray analysis.
Mouse Studies.
Male C57BL/6 mice (aged 6–8 weeks; Jackson Laboratories, Bar Harbor, ME) were maintained in a 12-hour light/dark cycle environment with free access to standard mouse chow (Harlan, Indianapolis, IN) and deionized water. After a 7-day acclimation period, a single dose of 1.5 × 1011 virus particles in a volume of 100 μl or vehicle (PBS, 100 μl) was given to mice by tail vein injection. Animals (n = 5 per group) were euthanized 24 and 48 hours after treatment. At this time, serum was collected and a portion of the liver was placed in Tissue-Tek optimal cutting temperature compound (Sakura Finetek, Torrance, CA) for histologic analysis. Remaining liver tissues were excised, rinsed in saline, snap frozen in liquid nitrogen, and stored at −80°C prior to preparation of microsomes and isolation of nucleic acids for reverse-transcription polymerase chain reaction (RT-PCR) and quantitative reverse-transcription polymerase chain reaction (qRT-PCR).
Microarray Evaluation of Hepatic Gene Expression In Vivo.
The relative expression of genes associated with various signal transduction pathways after virus infection was assessed using oligo GEArray Rat Signal Transduction Pathway Finder microarrays (ORN-014; SuperArray, Frederick, MD). Array-grade RNA was isolated using the RNeasy kit (Qiagen). Biotin-labeled cDNA probes were synthesized using 3 μg total isolated RNA and biotin-16-uridine-5′-triphosphate (Roche, Indianapolis, IN) for each array. The membrane was incubated with GEAhyb Hybridization Solution (SuperArray) for 3 hours at 60°C prior to adding 10 μg denatured cDNA probe for overnight hybridization at 60°C. The membrane was then washed twice with 2× saline sodium citrate buffer containing 1% SDS followed by two additional washes with 0.1× saline sodium citrate containing 0.5% SDS for 15 minutes each at 60°C. Arrays were blocked with GEArray Blocking Solution Q (SuperArray) for 40 minutes at room temperature. Two milliliters of binding buffer (Buffer F; SuperArray), containing a 1:8000 dilution of alkaline phosphatase-conjugated streptavidin (SuperArray), was added for 10 minutes at room temperature. The array image was developed using the CDP-Star chemiluminescent substrate (SuperArray) and was recorded on X-ray film after a 30-minute exposure. The image was scanned using a flatbed scanner (Microtek, Carson, CA).
Arrays were analyzed with ScanAlyze software (Michael Eisen, Lawrence Berkeley National Laboratory, Berkeley, CA) and the GEArray Expression Analysis Suite (SuperArray). Expression levels for each gene on a given array were calculated using minimal background subtraction and interquartile normalization to an internal standard (lactate dehydrogenase A) on each membrane. Data in Table 1 reflect the average ratios of the intensities for a given gene from a given treatment group to that of control animals given saline.
Histologic Analysis of Transgene Expression.
Liver tissues were harvested and immersed in disposable peel-away molds containing Tissue-Tek optimal cutting temperature compound and were stored at −80°C prior to analysis. Sections were fixed with 0.5% glutaraldehyde (Sigma-Aldrich), and β-galactosidase activity was determined by incubation with the substrate X-gal (5-bromo-4-chloro-3-indolyl-β-galactoside; Gold Biotechnology, St. Louis, MO) for 4 hours at 37°C in the dark. Staining medium was removed, and blue-colored, positive cells were visually tallied. For green fluorescent protein (GFP) analysis, sections of liver tissues were stained with a rabbit anti-GFP antibody (Molecular Probes, Eugene, OR) and with a horseradish peroxidase (HRP)–conjugated anti-rabbit IgG antibody (Abcam, Cambridge, UK). Spots were developed with 3-amino-9-ethylcarbazole and H2O2 substrate buffer (Sigma-Aldrich) and counterstained with hematoxylin (Sigma-Aldrich). Tissues were examined with a Leica DM LB microscope (Leica Microsystems Inc., Buffalo Grove, IL) and were photographed using a Leica DFC 320 camera.
Testosterone Hydroxylation Assay: Microsomes.
Hepatic microsomes were prepared by ultracentrifugation according to established methods (Coon et al., 1978). Two hundred micrograms of microsomal protein was incubated with testosterone, a NADPH regenerating system (5 mM NADP, 100 mM glucose-6-phosphate, and 100 mM magnesium chloride), and 5 units of glucose-6-phosphate dehydrogenase for 15 minutes at 37°C with gentle agitation. The reaction was quenched with dichloromethane and 11α-hydroxyprogesterone was added as an internal standard. The organic phase was evaporated under a constant stream of filtered air and samples were dissolved in methanol. Testosterone and its metabolites were separated and quantified by high-performance liquid chromatography as described (van der Hoeven, 1984). Peak areas of the hydroxylated metabolites were measured and compared with peak areas of the internal standard within the same experimental run.
Western Blot Analysis: Microsomal Proteins.
Hepatic microsomes (50 μg) were separated on 8% (CYP3A) or 12% SDS polyacrylamide gels by electrophoresis and transferred to nitrocellulose membranes (BioRad). Protein blots were blocked overnight at 4°C in blocking buffer containing 5% nonfat dry milk in 0.05% (v/v) Tween-20 and Tris-buffered saline. After blocking, membranes were incubated with polyclonal rabbit anti–retinoid X receptor (RXR) α antibody (D20, sc-553), anti–pregnane X receptor (PXR) antibody (H-160, sc-25381), or constitutive androstane receptor (CAR) 1/2 antibody (M-127, sc-13065), each at 1:800 dilution. They were then incubated with goat anti-rabbit IgG-HRP secondary antibody (sc-2004, 1:8000 dilution; all from Santa Cruz Biotechnology, Dallas, TX). To evaluate changes in CYP3A protein, a polyclonal rabbit anti-CYP3A1 antibody (1:3000 dilution; BD Gentest, Woburn, MA) was used together with a polyclonal anti-rabbit IgG-HRP–conjugated secondary antibody (1:3000; Cell Signaling Technology, Danvers, MA).
HC-04
Cells were washed twice with ice-cold PBS prior to the addition of 500 μl ice-cold lysis buffer (radioimmunoprecipitation assay buffer; Thermo Fisher Scientific, Waltham, MA) containing 1% protease inhibitors (Halt Protease and Phosphatase Inhibitor Cocktail; Pierce, Rockford, IL). After incubation on ice for 5 minutes, cells were lysed by extrusion 25 times through a 20-gauge needle (Becton Dickinson, Franklin Lakes, NJ) attached to a 1-ml syringe (Becton Dickinson) and were placed on ice for an additional 40 minutes. Lysates were then cleared by centrifugation at 14,000g for 15 minutes at 4°C and stored at −80°C. Proteins (50 μg) were separated on a 12% SDS polyacrylamide gel by electrophoresis and transferred to a nitrocellulose membrane (BioRad). Protein blots were blocked overnight at 4°C in blocking buffer containing 3% bovine serum albumin in 0.05% Tween-20 in Tris-buffered saline. Membranes were then incubated for 2 hours with mouse anti-CYP3A4 antibody (1:2000 dilution, HL3, sc-53850) followed by 1 hour of incubation with a goat anti-mouse IgG-HRP antibody (1:10,000 dilution, sc-2005; all from Santa Cruz Biotechnology). Membranes with small interfering RNA (siRNA)–treated samples were incubated for 1 hour with the same anti-PXR, anti-CAR, and anti-RXR antibodies used for microsome blots, each at 1:800 dilution, followed by 1 hour of incubation with a goat anti-rabbit IgG-HRP antibody (1:10,000 dilution, sc-2004; Santa Cruz Biotechnology). Immune complexes for microsomal proteins and isolated protein from HC-04 cells were detected by chemiluminescence (SuperSignal West Pico Chemiluminescence Substrate; Thermo Fisher Scientific). The intensity of protein bands was quantified relative to the signal obtained for an internal standard, β-actin (mouse anti–β-actin monoclonal antibody, AC-15, sc-69879, 1:1000 dilution; Santa Cruz Biotechnology), run on the same gel using Kodak 1D image analysis software (Eastman Kodak Co., Rochester, NY).
RNA Isolation.
RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Isolated RNA (1 μg) was reverse transcribed with random hexamers using the SuperScript III first-strand synthesis system (Invitrogen) in a Master Cycler Pro thermal cycler (Eppendorf AG, Hamburg, Germany). Samples were then subjected to RT-PCR or qRT-PCR as described below.
Microsomes (RT-PCR/qRT-PCR).
Changes in CYP3A mRNA expression in mouse liver microsomes were assessed using an AmpliTaq Gold PCR Master Mix kit (Applied Biosystems, Foster City, CA) with the following cycling conditions: 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 1 minute for a total of 32 cycles. Cycling was initiated at 94°C for 3 minutes and terminated at 72°C for 10 minutes. Primer sequences for mouse CYP3A11 were 5′-CTC AAT GGT GTG TAT ATC CCC C-3′ (forward) and 5′-CCG ATG TTC TTA GAC ACT GCC-3′ (reverse) (Xu et al., 2004). QuantumRNA 18S internal standards (Applied Biosystems/Ambion, Austin, TX) were coamplified using proprietary competimer technology in individual reaction tubes. Reaction products were visualized on a 2% agarose gel containing ethidium bromide, and the intensity of each band determined by densitometric analysis using Kodak 1D image analysis software (Eastman Kodak Co.). For qRT-PCR, PXR, RXRα, and CAR mRNA expression was assessed using SYBR GreenER qPCR SuperMix (Invitrogen) analyzed on a ViiA7 Real-Time PCR system (Applied Biosystems) with the following cycling conditions: 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. Primer sequences for mouse PXR, RXRα, and CAR were 5′-GTT CAA GGG CGT CAT CAA CT-3′ (forward) and 5′-TTC TGG AAG CCA CCA TTA GG-3′ (reverse); 5′-ACA AGG ACT GCC TGA TCG AC-3′ (forward) and 5′-CAT GTT TGC CTC CAC GTA TG-3′ (reverse); and 5′-CTC AAG GAA AGC AGG GTC AG-3′ (forward) and 5′-AGT TCC TCG GCC CAT ATT CT-3′ (reverse), respectively (Down et al., 2007).
Hepatocytes (qRT-PCR).
Changes in gene expression during silencing and virus infection were assessed using a SYBR Green qPCR SuperMix kit (Invitrogen). An aliquot of SuperMix was added to cDNA isolated from HC-04 cells before thermocycling on a ViiA7 Real-Time PCR system utilizing the same conditions as described above. Primer sequences for human CYP3A4, PXR, RXRα, CAR, integrin αv, integrin β3, and integrin β5 were as follows: 5′-GAT TGA CTC TCA GAA TTC AAA AGA AAC TGA-3′ (forward) and 5′-GGT GAG TGG CCA GTT CAT ACA TAA TG-3′ (reverse) (Yoshitsugu et al., 2006); 5′-AGA AGG AGA TGA TCA TGT CCG A-3′ (forward) and 5′-GTT TGT AGT TCC AGA CAC TGC C-3′ (reverse) (Gardner-Stephen et al., 2004); 5′-CCT TTC TCG GTC ATC AGC TC-3′ (forward) and 5′-CTC GCA GCT GTA CAC TCC AT-3′ (reverse) (Lim et al., 2007); 5′-GCA AGG GTT TCT TCA GGA GAA C-3′ (forward) and 5′-CTT CAC AGC TTC CAG CAA AGG-3′ (reverse) (Tanii et al., 2013); 5′-AAT CTT CCA ATT GAG GAT ATC AC-3′ (forward) and 5′-AAA ACA GCC AGT AGC AAC AAT-3′ (reverse); 5′-CCG TGA CGA GAT TGA GTC A-3′ (forward) and 5′-AGG ATG GAC TTT CCA CTA GAA-3′ (reverse); and 5′-GGA GCC AGA GTG TGG AAA CA-3′ (forward) and 5′-GAA ACT TTG CAA ACT CCC TC-3′ (reverse), respectively (Dingemans et al., 2010).
All qRT-PCR data were analyzed by the established comparative threshold cycle (CT) method using the following equation: fold change due to treatment = 2^–[(CT gene of interest – CT internal control) infected animals/cells – (CT gene of interest – CT internal control) uninfected animals/cells] (Schmittgen and Livak, 2008).
Serum Cytokines and Transaminases.
Cytokines, interleukin (IL)-6, and IL-12, were quantitated with commercially available enzyme-linked immunosorbent assay kits according to the manufacturer's protocols (Invitrogen). Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were determined using Vitros AST/SGOT and ALT/SGPT DT slides on a Vitros DTSC Autoanalyzer (Ortho-Clinical Diagnostics, Rochester, NY).
Cell Culture.
HC-04 cells (human hepatocytes: MRA-975, MR4; American Type Culture Collection, Manassas, VA) were maintained in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium (50/50 ratio; Mediatech, Manassas, VA) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen) and 2 mM l-glutamine (Hyclone, Logan, UT).
RNA Interference: HC-04 Cells.
HC-04 cells were seeded in either six-well tissue culture dishes at a density of 1 × 105 cells per well or in 96-well tissue culture plates (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ) at a density of 6 × 103 cells per well in complete HC-04 culture medium 48 hours prior to transfection. Cells in each well were then transfected with 100 pmol (6 wells) or 5 pmol (96 wells) of siRNA targeting integrin αv (sc-29373), β3 (sc-29375), and/or β5 (sc-35680) (all from Santa Cruz Biotechnology) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. For double-silencing studies, 50 pmol (6 wells) or 2.5 pmol (96 wells) of siRNA was used for each target. Efficiency of the knockdown was confirmed by qRT-PCR and compared with expression levels in cells treated with a nonspecific siRNA control (sc-37007; Santa Cruz Biotechnology). Forty-eight hours after silencing, cells were infected with either AdlacZ at a multiplicity of infection of 500 or an equivalent volume of saline as a control for 72 hours prior to assessing CYP3A4 activity using a P450-Glo CYP3A4 Luciferin-IPA assay kit (Promega, Madison, WI). Data were generated through comparison of CYP3A4 activity in virus-infected cells to activity in uninfected, saline-treated cells in which the same integrin subunit was silenced. Activity in the uninfected cells was normalized to 100% and data are reported as activity that remained after virus infection (percentage of remaining activity). The activity of CYP3A4 in control cells treated with nonspecific siRNA was ±4.65% of the activity of untreated HC-04 cells, which is similar to the range found in a normal human control population (Hirth et al., 2000).
Statistical Analyses.
Statistical analyses of data were performed using SigmaStat software (Systat Software Inc., San Jose, CA). Differences with respect to treatment were calculated using a one-way analysis of variance followed by a Bonferroni/Dunn post hoc test. Differences were determined to be significant when the probability of chance explaining the results was reduced to less than 5% (P < 0.05).
Results
Hepatic Microarray Analysis of Signal Transduction Pathways After a Single Dose of Adenovirus.
During some of the initial studies conducted in our laboratory in which we characterized CYP3A expression and function in the rat (Callahan et al., 2005, 2006, 2008a), samples of liver tissue were taken and processed for assessment of changes in genes associated with different signal transduction patterns. Samples were taken 4 days after administration of four different live and inactivated recombinant adenovirus vectors: AdlacZ (a first-generation E1/E3 deleted adenovirus serotype 5 virus expressing the Escherichia coli β-galactosidase transgene under the control of a cytomegalovirus promoter), PEGAd (the AdlacZ virus modified by covalent attachment of PEG to the virus capsid, which reduces the immune response to the virus; Croyle et al., 2001, 2002), HDAd (a third-generation adenovirus in which all elements of the viral genome except the inverted terminal repeats and the packaging signal [Ψ] are removed and which also contains the constitutive androstane receptor-galactosidase transgene; Croyle et al., 2005), and UVAd (the AdlacZ vector inactivated by exposure to UV light; Callahan et al., 2008b). Data from these samples were compared with saline-treated controls (vehicle). Significant increases in gene expression in adenovirus-treated samples are summarized in Table 1. Figure 1 shows representative microarray images in which five signal transduction pathways linked to CYP3A expression are highlighted. These were consistently upregulated in all virus-treated animals. Two genes associated with the nuclear factor κB pathway, B-cell leukemia/lymphoma 2 related protein A1 (Bcl2a1), and nuclear factor κ light chain gene enhancer in B-cells 1 (Nfkb1), were significantly altered by all of the vectors. Levels of Bcl2a1 were increased 1.8-, 6.5-, 15.4-, and 2.8-fold after AdlacZ, PEGAd, HDAd, and UVAd treatment, respectively. Nfkb1 was increased by a factor of 1.8, 3.5, 3.8, and 1.4 after treatment with AdlacZ, PEGAd, HDAd, and UVAd, respectively. Two genes involved in the protein kinase C pathway, c-Jun N-terminal kinase (JNK/Jun) and ornithine decarboxylase 1 (Odc1), were increased in the AdlacZ (3.4- and 3.5-fold) and PEGAd (5.6- and 2.8-fold) groups. Odc1 was elevated 4.3-fold in HDAd-treated animals and Jun was increased 2.7-fold after UVAd treatment. Administration of the HDAd vector caused the largest induction (7.9-fold) in the expression of monokine induced by interferon-γ (Mig), a gene associated with the Jak-Stat pathway. Another gene associated with the Jak-Stat pathway, interferon regulatory factor 1 (Irf1), was elevated 2.4-, 4.1-, 3.2-, and 2.3-fold in AdlacZ-, HDAd-, and UVAd-treated animals compared with saline-treated control animals. Expression of cyclin D1 (Cend1) and phosphatase and tensin homolog (Pten), two genes associated with the phosphoinositide 3-kinase signaling pathways, resulted in the largest increase after PEGAd administration (6.5-fold) and HDAd-treatment (8.5-fold), respectively. The last highlighted pathway is the retinoic acid pathway, in which expression of cathepsin D (Ctsd) was increased by a factor of 1.5, 4.1, and 3.0 in AdlacZ-, PEGAd-, and UVAd-treated animals, respectively. Another gene associated with this pathway, retinol binding protein 1, cellular (Rbp1), was induced 1.4-, 2.2-, 1.9-, and 2.3-fold after treatment with AdlacZ, PEGAd, HDAd, and UVAd, respectively. These results (in conjunction with in vitro studies in which CYP3A was suppressed after treatment with a peptide rich in RGD sequences, known to engage integrin receptors; Campbell and Humphries, 2011) served as the basis for the studies outlined in this article.
Effect of Integrin-Independent Virus Infection on Hepatic CYP3A Expression and Function In Vivo.
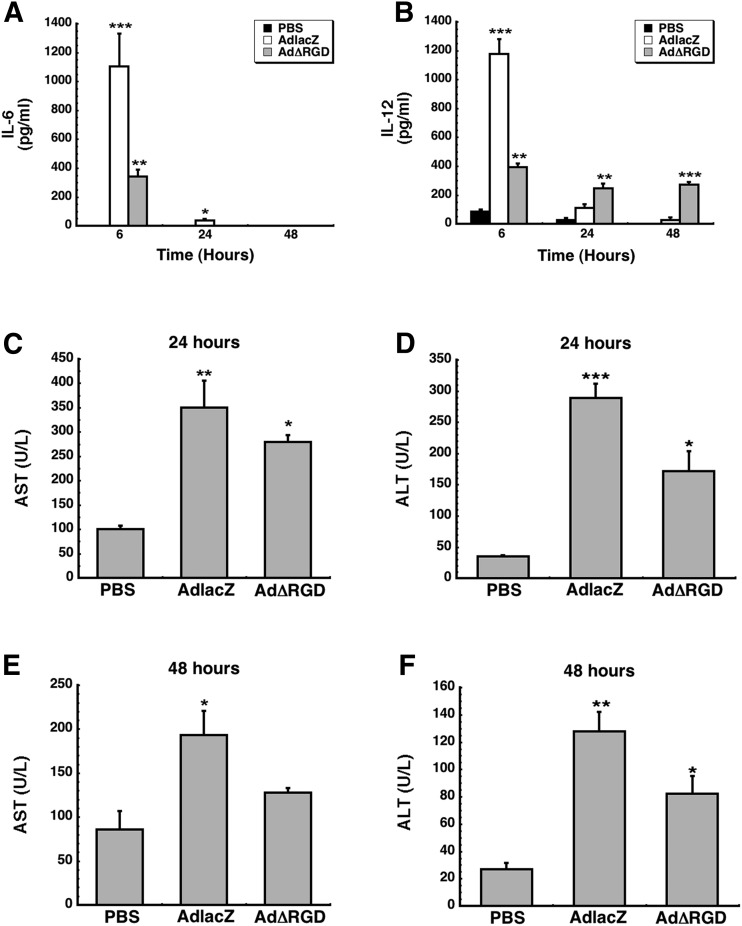
To date, all of our studies evaluating the expression and function of hepatic CYP3A during adenovirus infection were conducted in the rat model. The in vivo experiments summarized below were conducted in the mouse model so that if a connection between integrin silencing/engagement and CYP3A expression was made, additional mechanistic experiments could be conducted in knockout models with similar backgrounds. Thus, to validate our hypothesis that virus–integrin interactions are required for infection-induced changes in CYP3A in vivo, mice were given a single dose of either AdlacZ or a similar virus in which the RGD motif within the penton capsid protein was deleted via polymerase chain reaction–directed mutagenesis (AdΔRGD; Shayakhmetov et al., 2005a). Twenty-four hours after administration, mice given the AdlacZ vector experienced a notable reduction in CYP3A catalytic activity, (approximately 70% compared with saline-treated mice; PBS) (Fig. 2A, P < 0.01). Forty-eight hours after treatment, CYP3A activity continued to be suppressed in the AdlacZ group (56.4% of control) (Fig. 2B, P < 0.01). Conversely, the AdΔRGD vector did not alter CYP3A activity at either time point. A similar trend was noted with respect to CYP3A protein expression with reductions of 38% and 23% of control noted 24 and 48 hours after administration of AdlacZ, respectively (Fig. 2, C and D, P < 0.05), whereas protein levels were unaltered by the AdΔRGD virus. CYP3A11 mRNA levels were also reduced by the AdlacZ vector (32.8% of control) 24 hours after treatment (Fig. 2E, P < 0.05) and returned to baseline levels 48 hours after infection (Fig. 2F, P = 0.67). CYP3A11 mRNA levels were unaffected by AdΔRGD throughout the study period. The observation that CYP3A activity was suppressed to a greater extent than protein and gene expression after infection with AdlacZ is not unique to these studies because it has previously been seen in vitro and could be attributable to post-translational modifications of the CYP3A enzyme (Wonganan et al., 2014).
Fig. 2.
Systemic administration of a RGD-deleted recombinant adenovirus does not alter hepatic CYP3A expression and function in mice. (A–F) Catalytic activity, protein, and mRNA levels of hepatic CYP3A11 were evaluated 24 hours (A, C, and E, respectively) and 48 hours (B, D, and F, respectively) after systemic administration of 1 × 1011 particles of either a first-generation adenovirus expressing β-galactosidase (AdlacZ) or a vector of the same generation expressing GFP with all RGD residues removed from capsid proteins (AdΔRGD). Data are reported as the means ± S.E. of values obtained from five mice per treatment group at each time point. *P < 0.05; **P < 0.01 with respect to vehicle control (PBS).
Histochemical Evaluation of Transgene Expression.
To determine that differences in CYP3A expression between the AdlacZ and the AdΔRGD virus were not simply due to disparities in the ability of each virus to infect the liver, tissue sections from mice given the AdlacZ vector were stained with the histochemical substrate for the β-galactosidase transgene, X-gal, whereas livers from mice given AdΔRGD were stained with an antibody against the GFP transgene (Fig. 3). Staining of tissues obtained from animals given a saline bolus (vehicle, negative control) revealed minimal endogenous levels of either transgene in the liver (Fig. 3, A, E, and I). Twenty-four hours after administration of AdlacZ, approximately 80% of the cells were positive for β-galactosidase (Fig. 3B), whereas 15% of the cells in sections taken from mice given AdΔRGD were positive for GFP (Fig. 3G). GFP expression increased in these animals at the 48-hour time point, with approximately 80% of hepatocytes expressing the transgene (Fig. 3K). Sections from animals given an adenovirus expressing GFP without the RGD mutation demonstrated similar transgene expression patterns at 24 and 48 hours (Fig. 3, H and L). Endogenous staining for β-galactosidase was not present in any of the sections obtained from animals given viruses expressing GFP (Fig. 3, C and D.) GFP was also absent in sections obtained from animals given AdlacZ (Fig. 3, F and J).
Fig. 3.
Hepatic Transduction Efficiency of a RGD-Deleted Recombinant Adenovirus in the Mouse. Histological localization of transgene expression after a single dose of saline (first column), 1 × 1011 particles of a first generation adenovirus expressing beta-galactosidase (AdlacZ, second column) or 1 × 1011 particles of a vector of the same generation expressing green fluorescent protein (GFP) with RGD residues removed from capsid proteins (AdRGD, third column) or a GFP expressing first generation adenovirus (AdGFP, fourth column). Transgene expression from the AdlacZ vector was detected 24 hours after infection (Panel B). Moderate transgene expression was found 24 hours after infection for the (AdRGD and AdGFP) vectors (Panels G and H) and increased at the 48 hour time point (Panels H and L). Background GFP and beta-galactosidase expression was not detected in sections from animals given saline (E and I) nor was beta-galactosidase found in samples from animals given vectors expressing GFP (Panels C and D). Magnification for all panels: 200 ×.
Effect of Integrin-Independent Entry on the Toxicology Profile of Recombinant Adenovirus.
Cytokines IL-6 and IL-12 were selected for evaluation in our studies since IL-6 is a hallmark indicator of macrophage activation and IL-12 is indicative of dendritic cell activation in response to adenovirus infection (Schnell et al., 2001; Zhang et al., 2001). In each of these antigen presenting cells, integrins are required for transcription and for functional activation of each cytokine protein via the endosomal escape pathway (Di Paolo et al., 2009). In a manner that has been noted previously in our laboratory and those of others, serum IL-6 was significantly elevated 6 hours after administration of the AdlacZ vector (1105 ± 226.1 pg/ml, Fig. 4A). IL-6 levels were significantly lower in samples taken from animals given AdΔRGD at the same time point (340.4 ± 50.1 pg/ml). IL-6 declined to 38.2 ± 11.7 pg/ml by 24 hours after infection with AdlacZ. It was not detectable in samples taken from animals given AdΔRGD at this time point and in any sample collected 48 hours after treatment. The IL-12 profile for AdlacZ was similar to that for IL-6 in that levels spiked to 1175.7 ± 103.1 pg/ml at 6 hours and declined to 109.2 ± 28.6 pg/ml and 25.6 ± 17.9 pg/ml at the 24- and 48-hour time points, respectively (Fig. 4B). IL-12 levels in samples obtained 6 hours after administration of AdΔRGD were three times lower than those from mice given AdlacZ (392.1 ± 26.1 pg/ml); however, the concentration of this cytokine was significantly higher than that detected in the AdlacZ group at the 24-hour (247.6 ± 30.3 pg/ml) and 48-hour (270.0 ± 18.6 pg/ml) time points. This finding was somewhat unexpected; however, early studies with AdΔRGD and the native wild-type virus described notable differences in intracellular trafficking and endosomal escape patterns (Shayakhmetov et al., 2005a), processes that significantly affect development of the innate immune response to the virus (Leopold and Crystal, 2007; Teigler et al., 2014). Other studies in which AdΔRGD was given to mice in the same manner as described in our studies have reported notable suppression of a variety of proinflammatory cytokines (Di Paolo et al., 2009); however, levels of cytokines with dual inflammatory and protective roles after administration of the virus have not previously been described. When looking at the IL-6 data, it is clear that the inability of AdΔRGD to stimulate the cells through an integrin-dependent pathway suppressed production of this cytokine. By contrast, integrin-independent interaction of the virus with dendritic cells induced production of IL-12 in a potentially inactive form and/or in a form incapable of escalating the immune response to this modified virus (Zundler and Neurath, 2015).
Fig. 4.
Removal of RGD epitopes from the adenovirus capsid alters the cytokine secretion profile and elicits a mild increase in serum transaminases in the mouse. (A) Serum IL-6 levels measured 6, 24, and 48 hours after a single dose of saline (PBS) or 1 × 1011 particles of either a first-generation adenovirus expressing β-galactosidase (AdlacZ) or a vector of the same generation expressing GFP with RGD residues removed from capsid proteins (AdΔRGD). (B) Forty-eight hour IL-12 secretion profile of mice given saline, AdlacZ, or AdΔRGD. (C) Serum AST profile 24 hours after administration of recombinant vectors. (D) Serum ALT profile 24 hours after a single dose of virus. (E) Serum AST levels 48 hours after administration of virus. (F) Serum ALT 48 hours after administration of virus. For each panel, data represent the means ± S.E. of values obtained from five mice per treatment group at each time point. *P < 0.05; **P < 0.01; ***P < 0.001 with respect to vehicle control (PBS).
Serum AST and ALT levels are often used as indicators for determining liver function during inflammation, injury, or disease (Ozer et al., 2008). Twenty-four hours after virus administration, AST levels were 4 (AdlacZ) and 3 (AdΔRGD) times that of saline-treated animals (Fig. 4C). At this time, serum ALT was also 9 and 5 times above baseline in samples from mice given the AdlacZ and AdΔRGD vectors, respectively (Fig. 4D). At the 48-hour time point, serum AST levels in mice given the AdΔRGD vector were not significantly different from those given saline (127.7 ± 5.2 versus 85.7 ± 20.9 U/L, P = 0.18), whereas those from mice given AdlacZ were still notably elevated (193.3 ± 27.1 U/L, Fig. 4E). ALT levels were still significantly higher than baseline in samples taken from each treatment group at the 48-hour time point (Fig. 4F, P < 0.05).
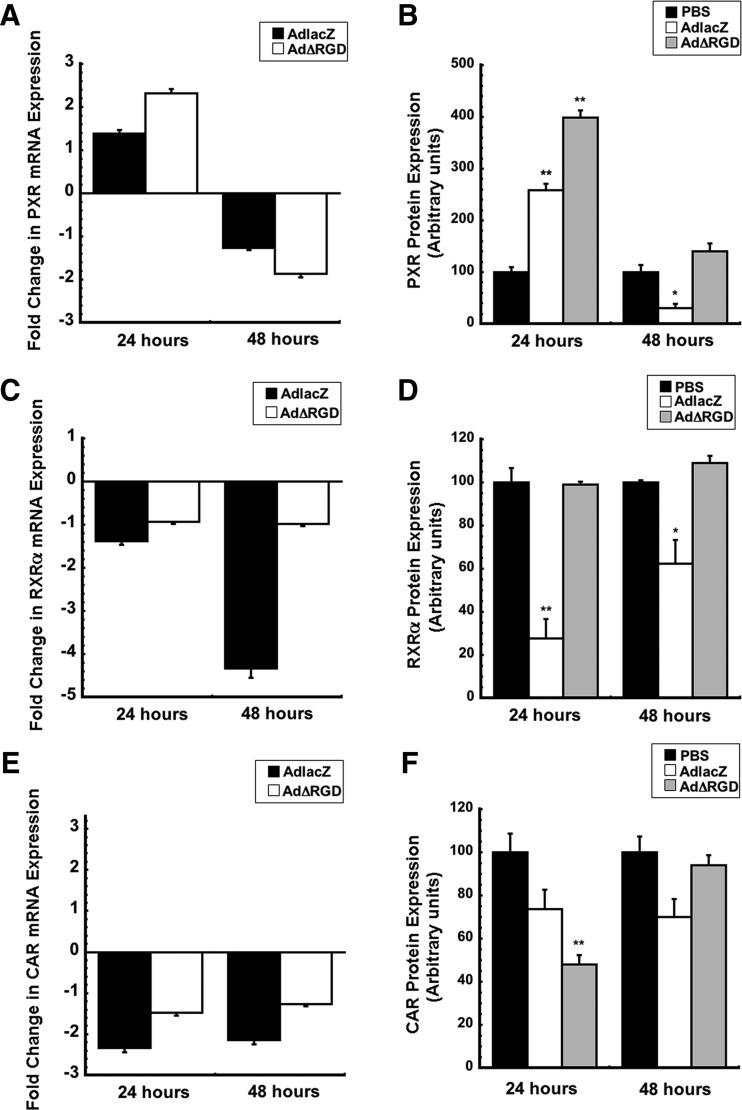
Effect of Integrin-Independent Entry on Nuclear Receptors Regulating CYP3A Expression.
On a transcriptional level, hepatic CYP3A is constitutively expressed in the liver, but several nuclear receptors, including PXR, CAR, and RXR, are primarily responsible for regulating CYP3A gene expression (Croyle, 2009). However, it remains to be known which is predominantly influenced by virus infection and is ultimately responsible for our observed changes in CYP3A (Callahan et al., 2008a; Wonganan et al., 2014). In an effort to determine how integrin engagement changes each of these key regulators of CYP3A in vivo, gene and protein expression was determined 24 and 48 hours after infection with each virus (Fig. 5). PXR gene expression was unaffected by AdlacZ 24 and 48 hours after infection. By contrast, a 2-fold increase was seen at 24 hours and a 2-fold decrease was observed at 48 hours after infection with AdΔRGD with respect to uninfected saline controls (Fig. 5A). PXR protein levels increased 24 hours after administration of both viruses and declined to baseline (AdΔRGD) and below baseline (AdlacZ) levels at 48 hours (Fig. 5B, P < 0.05 and P < 0.01). RXRα gene expression was unaffected for animals treated with AdΔRGD for 24 and 48 hours. However, samples collected 48 hours after AdlacZ infection showed a 5-fold reduction in RXRα mRNA (Fig. 5C). RXRα protein expression was reduced 27% (24 hours) and 63% (48 hours) for AdlacZ-infected animals with respect to saline controls (Fig. 5D, P < 0.05 and P < 0.01). A 2-fold reduction was seen in CAR gene expression in animals treated with AdlacZ for 24 and 48 hours, whereas treatment with AdΔRGD did not have any effect on this nuclear receptor (Fig. 5E). A general trend of suppressed protein levels was observed for CAR in animals infected with either virus (Fig. 5F, P < 0.01).
Fig. 5.
Virus-mediated changes in gene and protein expression of nuclear receptors regulating CYP3A4. (A–F) Gene expression of nuclear receptors PXR, RXRα, and CAR (A, C, and E, respectively) and protein levels (B, D, and F, respectively) in mice after 24 or 48 hours of infection with either AdlacZ or AdΔRGD. Mice were given saline (PBS) or 1 × 1011 particles of either AdlacZ or AdΔRGD and gene and protein expression of nuclear receptors regulating CYP3A4 was assessed 24 and 48 hours postinfection using qRT-PCR and Western blot techniques, respectively. Data are reported as the means ± S.E. of values obtained from five mice per treatment group at each time point. *P < 0.05; **P < 0.01 with respect to vehicle control (PBS).
Treatment with an Integrin-Specific Peptide Inhibits CYP3A4 Activity in the HC-04 Cell Line.
To confirm that a human hepatic cell line (HC-04) can be used for mechanistic studies involving virus–integrin and CYP3A interactions, an initial pilot experiment was performed in which HC-04 cells were treated with a linear peptide containing a RGD sequence, a known integrin receptor ligand, or a control RGE (1.5 mg/ml) peptide that does not engage integrins. Treatment with the RGD peptide significantly suppressed CYP3A4 activity in HC-04 cells by 40% with respect to untreated controls, whereas the RGE peptide did not effect CYP3A catalytic activity (Fig. 6A) in the absence of virus infection. The amount of peptide used was sufficient to cover the majority of integrin receptors on the cells because, in a separate experiment, it reduced the transduction efficiency of AdlacZ by approximately 70% (Fig. 6, B and C). Results from this study confirm results from previous studies performed in primary rat hepatocytes (Callahan et al., 2008a), making this cell line useful for further mechanistic studies to determine how integrins regulate CYP3A4 in humans.
Fig. 6.
Engagement of integrin receptors with a noninfectious peptide suppresses CYP3A4 activity in HC-04 cells. (A) Catalytic activity of CYP3A after treatment with peptides containing either RGD or RGE sequences. Results are reported as the means ± S.E. of values obtained from three culture plates per condition. *P < 0.05 with respect to saline-treated cells (control). (B) Histochemical staining for β-galactosidase expression in hepatocytes infected with AdlacZ at a MOI of 100. Similar staining patterns were detected for cells treated with a control peptide containing an RGE sequence at a concentration of 1.5 mg/ml for 2 hours prior to infection. (C) Histochemical staining for β-galactosidase expression in hepatocytes treated with a RGD peptide (1.5 mg/ml). Peptide was added to culture media for 2 hours at 4°C prior to infection with AdlacZ at a MOI of 100. MOI, multiplicity of infection; RGE, arginine-glycine-glutamic acid. Original magnification, ×200.
Effect of Silencing of Integrin Expression on the Catalytic Activity of Hepatic CYP3A4 In Vitro.
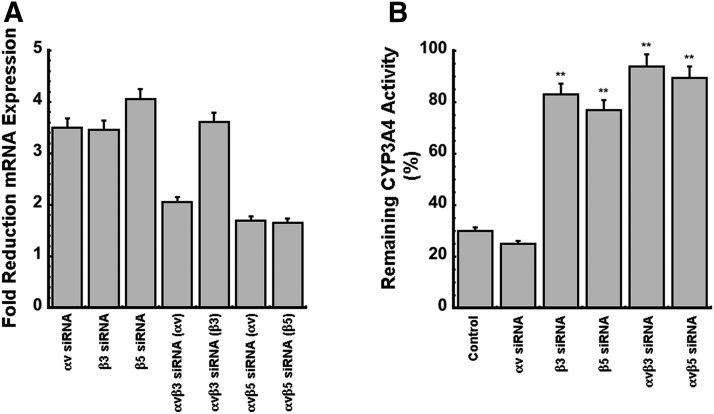
To determine which part of the integrin receptor regulates CYP3A4, expression of αv, β3, and/or β5 subunits were knocked down via siRNA targeting in the HC-04 cell line. Forty-eight hours after transfection with optimized sequences, gene expression was confirmed to be reduced by 3.5-fold for integrin αv and β3 and 4-fold for integrin β5 (Fig. 7A). When silencing both subunits at the same time, the αvβ3 combination allowed for the most maximal suppression (3.6-fold below cells transfected with a nontargeting siRNA sequence, Fig. 7A). At this time (48 hours postsilencing), cells were infected with virus (AdlacZ, multiplicity of infection of 500) or saline and CYP3A4 activity was assessed 72 hours later. Adenovirus-mediated suppression of CYP3A4 activity was still seen in cells treated with negative control siRNA and αv siRNA (reduced by 70% and 75% with respect to uninfected controls, respectively, Fig. 7B). Very minimal reductions in CYP3A4 activity were detected when the β3 or β5 subunit was silenced (7% and 23% reduction, respectively). Less of an effect (6%–10% reduction) was seen when both αv and β subunits were silenced together (Fig. 7B).
Fig. 7.
Silencing of β3 and/or β5 integrin subunits reverses adenovirus-mediated changes in CYP3A4. (A) Degree of silencing achieved for each integrin subunit in HC-04 cells. Cells were transfected for 48 hours with either a nontargeting control siRNA (control) or siRNA targeting the αv, β3, or β5 subunit of the integrin receptor. Silencing was confirmed by qRT-PCR using primers specific for the different integrins. (B) CYP3A4 catalytic activity in HC-04 cells infected with adenovirus (MOI of 500) for 72 hours after integrin silencing. Data depicted in (B) were generated through comparison of CYP3A4 activity in virus-infected cells to activity in uninfected, saline-treated cells in which the same integrin subunit was silenced. Activity in the uninfected cells was normalized to 100% and data are reported as activity that remained after virus infection (percentage of remaining activity). Results are reported as the means ± S.E. of values obtained from three culture plates per condition. **P < 0.01 with respect to siRNA control-treated cells, labeled as control on the figure. MOI, multiplicity of infection.
Silencing of Integrin Subunits Alters CAR and PXR Gene Expression In Vitro.
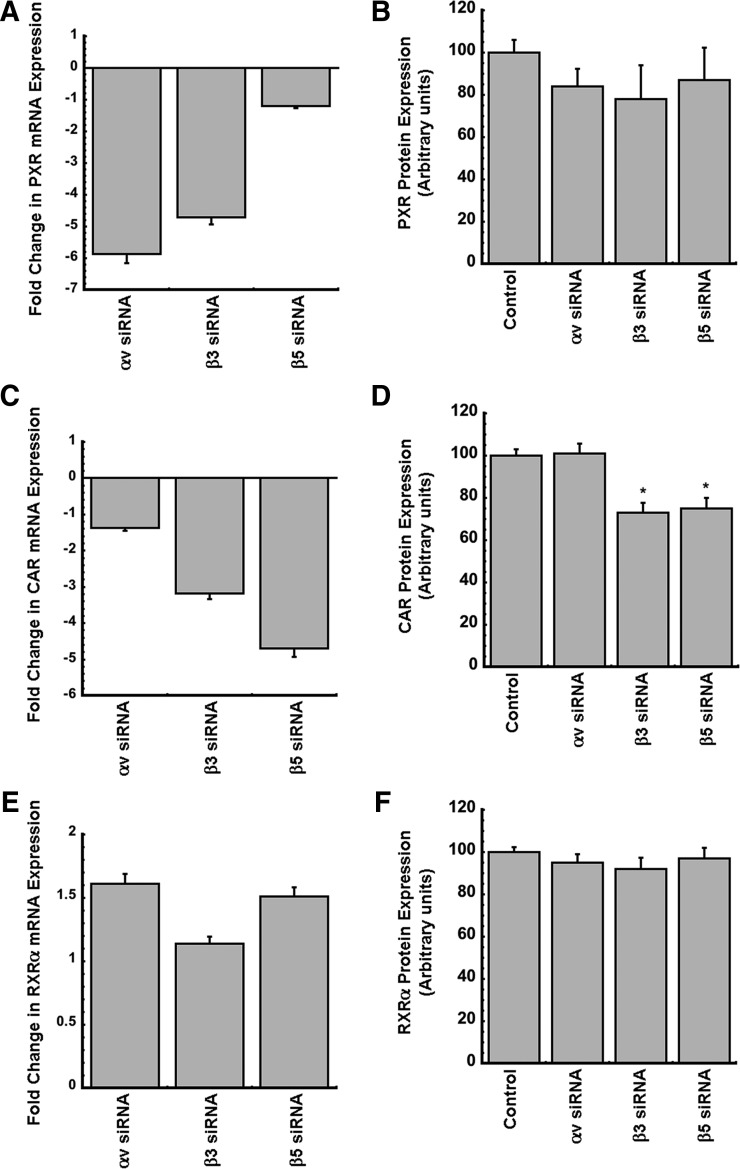
Baseline gene and protein expression levels of the nuclear receptors PXR, CAR, and RXRα was determined in HC-04 cells 48 hours after knockdown of each integrin subunit (Fig. 8). Silencing of subunits αv and β3 decreased gene expression of PXR by 6- and 5-fold, respectively, whereas silencing of the β5 subunit had no effect (Fig. 8A). A general trend of reduction in PXR protein expression after integrins were silenced was observed; however, this was not found to be statistically significant (Fig. 8B). Silencing of either β-subunit significantly decreased the gene and protein expression level of CAR, whereas silencing of the αv subunit did not alter CAR at the mRNA or protein level (Fig. 8, C and D, respectively). Silencing with either αv, β3, or β5 siRNA had no significant effect on RXRα, although the general trend was a small increase in the gene expression level of RXRα when silencing the αv and β5 subunits (Fig. 8E). RXRα protein levels were not affected by silencing of any of the integrin receptor subunits (Fig. 8F).
Fig. 8.
Silencing of αv, β3, or β5 integrin subunits in the absence of virus infection decreases gene expression and protein levels of PXR and CAR but not RXRα. (A, C, and E) Gene expression levels of nuclear receptors PXR (A), CAR (C), and RXRα (E) were assessed in HC-04 cells 48 hours after treatment with siRNA targeting the αv, β3, or β5 subunit of the integrin receptor. (B, D, and F) Protein expression levels of PXR (B), CAR (D), and RXRα (F) in samples treated the same way as described above but harvested for protein and analyzed by Western blot analysis. Values for control samples in these panels represent protein levels in HC-04 cells treated with nontargeting siRNA. Results are reported as the means ± S.E. of values obtained from three culture plates per condition.
Discussion
Like many other pathogens, human adenovirus serotype 5 utilizes additional receptors like the coxsackie- and adenovirus receptor (AdCAR) and heparan sulfate proteoglycans (HSPGs) to enter cells (Arnberg, 2012). Although integrins facilitate internalization of virus particles, AdCAR and HSPGs are responsible for viral attachment to the hepatocyte (Wolfrum and Greber, 2013). Until recently, HSPGs were considered to be the primary hepatic receptor for adenovirus binding in vivo (Kalyuzhniy et al., 2008; Zaiss et al., 2015). However, studies in knockout mice suggest that the low-density lipoprotein receptor–related protein may be responsible for adenovirus-mediated transduction of the human liver and in various animal models of disease (Shayakhmetov et al., 2005b; Zaiss et al., 2015). AdCAR is poorly expressed in the liver in vivo but has been shown to mediate infection in cultured cells (Zaiss et al., 2015). AdΔRGD transduced the liver of mice in the outlined studies through these alternative receptors. The role of these receptors in the regulation of CYP3A seems minimal since our data clearly show that elimination of the adenovirus–integrin interaction reverses virus-induced changes in cytochrome P450 previously noted with wild-type virus.
Integrins lack intrinsic enzymatic signaling; instead, they are activated by binding of extracellular ligands (like viruses and other microbes), which results in clustering and changes in ligand-binding affinity of integrins on the cell surface (Goodman and Picard, 2012). Activation in this manner also changes the tertiary and quaternary structures of these receptors, which propagate across the cell membrane to activate cytoplasmic kinase- and cytoskeletal-signaling cascades. This outside-in response initiates changes in cell polarity, survival, proliferation, cytoskeletal structure, and gene expression (Campbell and Humphries, 2011). Integrins are also regulated by internal stimuli, initiating intracellular signals, which convert the extracellular domains into a high-affinity ligand-binding state. This inside-out response (Ye et al., 2011) affects cell adhesion, migration, and extracellular matrix assembly (Goodman and Picard, 2012). Given that adenovirus infection is a complex, multistage process, we believe that the virus induces a sustained effect on CYP3A expression and function by first inducing pathways stimulated by the outside-in response during internalization, followed by induction of additional pathways stimulated by the inside-out response during nuclear transit and expression of virus genes throughout the cell. We also realize that although simple engagement of integrins with the RGD peptide elicited a comparable reduction in CYP3A activity to that seen with adenovirus, this effect may be induced by a different mechanism because monomeric RGD peptides are internalized via a fluid-phase endocytic pathway (Sancey et al., 2009). Additional in vitro and in vivo studies with RGD-based integrin ligands and other integrin- and nonintegrin-dependent pathogens are currently underway in our laboratory to further define the role of the outside-in and the inside-out response on hepatic CYP3A expression and to confirm that our observations are not unique to the adenovirus.
The most interesting finding of these studies was that silencing of the β3- and β5-integrin subunits reversed the effect of adenovirus-mediated suppression of CYP3A4 in human hepatocytes (Fig. 7). This is important since the β subunit is largely responsible for the inside-out response. The key activator of the inside-out response is the intracellular protein talin. Talin binds specifically to the tail of the β-subunit of the integrin receptor (Calderwood et al., 1999), resulting in separation of the α- and β-tail ultimately leading to increased binding affinity of the integrin receptor (Morse et al., 2014). Several viral proteins interact with talin in vivo in various acts of self-preservation (Brown et al., 2011; Stanton et al., 2014). Disruption of the β-subunit and talin interaction by pUL135 protein suppresses integrin function and protects human cytomegalovirus from natural killer and T cells (Stanton et al., 2014). By contrast, knockdown of talin enhances susceptibility to retroviral infection in human cells (Brown et al., 2011). To date, adenovirus–talin interactions have not been described. Hence, in vitro studies in which talin is silenced and in vivo studies utilizing talin knockout animal models (Calderwood et al., 2013) should be preformed to determine what role talin plays in the regulation of CYP3A4.
Nuclear receptors, including PXR, CAR, and RXR, are primarily responsible for regulating hepatic CYP3A expression (Croyle, 2009). Analysis of changes in each of these receptors in vivo revealed that RXRα, essential for binding of nuclear receptors to the dXREM and prPXRE regions of the CYP3A4 5′ promoter (Wang et al., 2008), was involved in the observed downregulation of CYP3A via integrin–virus interactions (Fig. 5). This observation was not congruent with those made in cultured human hepatocytes in which RXRα gene and protein expression was not significantly affected by silencing of β integrins (Fig. 8). An interesting biphasic response of PXR was induced in vivo by both viruses, indicating that it was affected in an integrin-independent manner. Silencing experiments did not reflect these observations because PXR was notably suppressed when the β3 integrin subunit was silenced. It is not clear at this time whether these disparities are due to species-specific differences or differences in inside-out/outside-in responses between in vivo and in vitro systems. Studies using both the AdlacZ- and AdΔRGD viruses in RXRα and PXR knockout mice, primary hepatocytes isolated from these animals and human hepatocytes in which each nuclear receptor is silenced will assist us in making these distinctions. Although notable reductions in CAR were observed in animals given AdlacZ only, they were less profound than RXRα, suggesting that CAR is less responsive to virus-induced changes in CYP3A initiated through integrin engagement. By contrast, CAR was more responsive to silencing of integrins, especially the β subunits, than RXRα (Fig. 8). This suggests that β3 holds its influence over CYP3A by downregulating PXR and CAR.
Although silencing of integrin subunits clearly influenced CYP3A activity and mRNA levels of nuclear receptors, protein levels of each remained largely unaffected. This, coupled with the fact that CYP3A activity was suppressed to a greater extent than protein and mRNA levels in our in vivo studies, suggests that post-translational modification of CYP3A and/or various nuclear receptors may be responsible for integrin-induced changes in hepatic CYP3A (Fig. 2). Microarray studies conducted early in our assessment of how CYP3A responds to adenovirus infection (Fig. 1; Table 1) demonstrated that several signal transduction pathways known to phosphorylate RXR, PXR, and CAR and alter formation of transcriptional complexes to drive CYP3A expression (Shao and Lazar, 1999; Brändlin et al., 2002; Pimienta et al., 2007) were upregulated in the liver. These pathways and others initiated by the engagement of integrin receptors may also alter CYP3A in a similar manner. Data in Fig. 8 also suggest that specific integrin subunits may uniquely affect CYP3A activity and expression through different mechanisms/pathways. We are currently developing assays for studying changes in post-translational modifications of CYP3A4, CAR, PXR, and RXRα during virus infection using mass spectrometry. These assays paired with evaluation of promoter occupancy by each nuclear receptor during virus infection will be paramount to further study the role of each integrin subunit on hepatic CYP3A expression and function in the future.
Integrin receptors have been implicated in the regulation of several functions in tumor cells, such as migration, invasion, proliferation, and survival. The diversity in these processes suggests that pathology of cancer as well as other noninfectious diseases in which integrins play a role (e.g., cardiovascular disease, diabetes, and wound healing) may not be attributed to a single integrin receptor, but instead are the end result of crosstalk between several integrin receptors. Thus, drugs targeting single as well as multiple integrin receptors have been developed for treatment of cancer and other diseases (Desgrosellier and Cheresh, 2010; Sheldrake and Patterson, 2014). In this context, data generated in our studies suggest that hepatic drug metabolism patterns can be significantly altered in response to these medications, which will most likely be given with other chemotherapeutic agents that are metabolized by CYP3A4, resulting in either an enhanced therapeutic effect or severe toxicities due to accumulation of unmetabolized by-products over time. Various expression patterns of integrins have been shown to correlate with increased tumor progression and decreased patient survival in breast, cervical, and colon cancers (Desgrosellier and Cheresh, 2010). Recent findings have shown that cancer cells change their integrin repertoire in response to drug treatment (Sheldrake and Patterson, 2014). Given the link between integrin expression and CYP3A4 activity illustrated here, integrin receptors may play a notable role in the development of drug resistance. Experiments to further explore the correlation between integrin expression, CYP3A4 activity, and tumor resistance are underway in our laboratory. Understanding this interplay could potentially be used to regulate integrin expression during the early stages of cancer development to mitigate severity of disease or to modulate drug metabolism to improve therapeutic outcomes in patients through personalized drug therapies directed at integrins.
We have shown, for the first time, a direct correlation between engagement of integrin receptors and the regulation of hepatic CYP3A activity. Although engagement of other intracellular receptors, including Toll-like receptors, has been implicated in the regulation of CYP3A during infection (Shah et al., 2014), we now provide evidence supporting the notion that extracellular receptors influence hepatic drug metabolism under infectious and noninfectious conditions. These results may translate beyond CYP3A activity because several nuclear receptors that regulate it play notable roles in the regulation of other metabolic enzymes and drug transporters. We are currently assessing the function of several hepatic efflux and uptake transporters in vivo and in vitro in response to integrin expression.
Acknowledgments
The authors thank MR4 for providing HC-04 cells contributed by Jetsumon Sattabongkot Prachumsri as well as Dr. Dmitry Shayakhmetov (Emory University) for providing the AdΔRGD construct. They also thank Dahlia Astone and Dr. Lucio Pastore (University of Naples Federico II) for assistance in establishing the helper-dependent adenovirus system in their laboratory. Finally, the authors acknowledge Stephen C. Schafer for preparation of histochemical images depicted in this article.
Abbreviations
- AdCAR
coxsackie- and adenovirus receptor
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CAR
constitutive androstane receptor
- CT
threshold cycle
- GFP
green fluorescent protein
- HDAd
helper-dependent adenovirus
- HRP
horseradish peroxidase
- HSPG
heparan sulfate proteoglycan
- IL
interleukin
- Jak-Stat
Janus kinase/signal transducers and activators of transcription
- PEG
polyethylene glycol
- PXR
pregnane X receptor
- qRT-PCR
quantitative reverse-transcription polymerase chain reaction
- RGD
arginine-glycine-aspartic acid
- RGE
arginine-glycine-glutamic acid
- RT-PCR
reverse-transcription polymerase chain reaction
- RXR
retinoid X receptor
- siRNA
small interfering RNA
- X-gal
5-bromo-4-chloro-3-indolyl-β-galactoside
Authorship Contributions
Participated in research design: Jonsson-Schmunk, Wonganan, Choi, Callahan, Croyle.
Conducted experiments: Jonsson-Schmunk, Wonganan, Choi, Callahan.
Performed data analysis: Jonsson-Schmunk, Wonganan, Choi, Callahan, Croyle.
Wrote or contributed to the writing of the manuscript: Jonsson-Schmunk, Wonganan, Choi, Croyle.
Footnotes
This research was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R21GM69870 (to M.A.C.)], the National Institutes of Health National Institute of Allergy and Infectious Diseases [Grant U01AI078045 (to M.A.C.)], and a University of Texas at Austin [James W. McGinity Graduate Fellowship and Williams and McGinity Graduate Fellowship (both to K.J.-S.)].
References
- Arnberg N. (2012) Adenovirus receptors: implications for targeting of viral vectors. Trends Pharmacol Sci 33:442–448. [DOI] [PubMed] [Google Scholar]
- Brändlin I, Eiseler T, Salowsky R, Johannes FJ. (2002) Protein kinase C(mu) regulation of the JNK pathway is triggered via phosphoinositide-dependent kinase 1 and protein kinase C(epsilon). J Biol Chem 277:45451–45457. [DOI] [PubMed] [Google Scholar]
- Brown C, Morham SG, Walsh D, Naghavi MH. (2011) Focal adhesion proteins talin-1 and vinculin negatively affect paxillin phosphorylation and limit retroviral infection. J Mol Biol 410:761–777. [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Campbell ID, Critchley DR. (2013) Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol 14:503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. (1999) The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem 274:28071–28074. [DOI] [PubMed] [Google Scholar]
- Callahan SM, Boquet MP, Ming X, Brunner LJ, Croyle MA. (2006) Impact of transgene expression on drug metabolism following systemic adenoviral vector administration. J Gene Med 8:566–576. [DOI] [PubMed] [Google Scholar]
- Callahan SM, Ming X, Lu SK, Brunner LJ, Croyle MA. (2005) Considerations for use of recombinant adenoviral vectors: dose effect on hepatic cytochromes P450. J Pharmacol Exp Ther 312:492–501. [DOI] [PubMed] [Google Scholar]
- Callahan SM, Wonganan P, Croyle MA. (2008a) Molecular and macromolecular alterations of recombinant adenoviral vectors do not resolve changes in hepatic drug metabolism during infection. Virol J 5:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan SM, Wonganan P, Obenauer-Kutner LJ, Sutjipto S, Dekker JD, Croyle MA. (2008b) Controlled inactivation of recombinant viruses with vitamin B2. J Virol Methods 148:132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ID, Humphries MJ. (2011) Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 3:a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Vadrevu S, Norman JC. (2009) Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 10:843–853. [DOI] [PubMed] [Google Scholar]
- Coon MJ, van der Hoeven TA, Dahl SB, Haugen DA. (1978) Two forms of liver microsomal cytochrome P-450, P-450lm2 and P-450LM4 (rabbit liver). Methods Enzymol 52:109–117. [DOI] [PubMed] [Google Scholar]
- Cox D, Brennan M, Moran N. (2010) Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov 9:804–820. [DOI] [PubMed] [Google Scholar]
- Croyle MA. (2009) Long-term virus-induced alterations of CYP3A-mediated drug metabolism: a look at the virology, immunology and molecular biology of a multi-faceted problem. Expert Opin Drug Metab Toxicol 5:1189–1211. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Chirmule N, Zhang Y, Wilson JM. (2001) “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J Virol 75:4792–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle MA, Chirmule N, Zhang Y, Wilson JM. (2002) PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther 13:1887–1900. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Le HT, Linse KD, Cerullo V, Toietta G, Beaudet A, Pastore L. (2005) PEGylated helper-dependent adenoviral vectors: highly efficient vectors with an enhanced safety profile. Gene Ther 12:579–587. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Yu QC, Wilson JM. (2000) Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum Gene Ther 11:1713–1722. [DOI] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, Papayannopoulou T, Shayakhmetov DM. (2009) Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity 31:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. (2005) Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochem Pharmacol 69:867–873. [DOI] [PubMed] [Google Scholar]
- Dingemans AM, van den Boogaart V, Vosse BA, van Suylen RJ, Griffioen AW, Thijssen VL. (2010) Integrin expression profiling identifies integrin alpha5 and beta1 as prognostic factors in early stage non-small cell lung cancer. Mol Cancer 9:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Down MJ, Arkle S, Mills JJ. (2007) Regulation and induction of CYP3A11, CYP3A13 and CYP3A25 in C57BL/6J mouse liver. Arch Biochem Biophys 457:105–110. [DOI] [PubMed] [Google Scholar]
- Forsyth CB, Plow EF, Zhang L. (1998) Interaction of the fungal pathogen Candida albicans with integrin CD11b/CD18: recognition by the I domain is modulated by the lectin-like domain and the CD18 subunit. J Immunol 161:6198–6205. [PubMed] [Google Scholar]
- Gandhi A, Moorthy B, Ghose R. (2012) Drug disposition in pathophysiological conditions. Curr Drug Metab 13:1327–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Stephen D, Heydel JM, Goyal A, Lu Y, Xie W, Lindblom T, Mackenzie P, Radominska-Pandya A. (2004) Human PXR variants and their differential effects on the regulation of human UDP-glucuronosyltransferase gene expression. Drug Metab Dispos 32:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SL, Picard M. (2012) Integrins as therapeutic targets. Trends Pharmacol Sci 33:405–412. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Borisova M, Muenzner P. (2012) Exploitation of integrin function by pathogenic microbes. Curr Opin Cell Biol 24:637–644. [DOI] [PubMed] [Google Scholar]
- Hirth J, Watkins PB, Strawderman M, Schott A, Bruno R, Baker LH. (2000) The effect of an individual’s cytochrome CYP3A4 activity on docetaxel clearance. Clin Cancer Res 6:1255–1258. [PubMed] [Google Scholar]
- Kacevska M, Mahns A, Sharma R, Clarke SJ, Robertson GR, Liddle C. (2013) Extra-hepatic cancer represses hepatic drug metabolism via interleukin (IL)-6 signalling. Pharm Res 30:2270–2278. [DOI] [PubMed] [Google Scholar]
- Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, Shayakhmetov DM. (2008) Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci USA 105:5483–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold PL, Crystal RG. (2007) Intracellular trafficking of adenovirus: many means to many ends. Adv Drug Deliv Rev 59:810–821. [DOI] [PubMed] [Google Scholar]
- Lim PL, Tan W, Latchoumycandane C, Mok WC, Khoo YM, Lee HS, Sattabongkot J, Beerheide W, Lim SG, Tan TM, et al. (2007) Molecular and functional characterization of drug-metabolizing enzymes and transporter expression in the novel spontaneously immortalized human hepatocyte line HC-04. Toxicol In Vitro 21:1390–1401. [DOI] [PubMed] [Google Scholar]
- Maizel JV, Jr, White DO, Scharff MD. (1968) The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 36:115–125. [DOI] [PubMed] [Google Scholar]
- Morse EM, Brahme NN, Calderwood DA. (2014) Integrin cytoplasmic tail interactions. Biochemistry 53:810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. (2008) The current state of serum biomarkers of hepatotoxicity. Toxicology 245:194–205. [DOI] [PubMed] [Google Scholar]
- Pimienta G, Ficarro SB, Gutierrez GJ, Bhoumik A, Peters EC, Ronai Z, Pascual J. (2007) Autophosphorylation properties of inactive and active JNK2. Cell Cycle 6:1762–1771. [DOI] [PubMed] [Google Scholar]
- Pondugula SR, Dong H, Chen T. (2009) Phosphorylation and protein-protein interactions in PXR-mediated CYP3A repression. Expert Opin Drug Metab Toxicol 5:861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss WG, Piscitelli SC. (1998) Drug-cytokine interactions: mechanisms and clinical implications. BioDrugs 9:389–395. [DOI] [PubMed] [Google Scholar]
- Sancey L, Garanger E, Foillard S, Schoehn G, Hurbin A, Albiges-Rizo C, Boturyn D, Souchier C, Grichine A, Dumy P, et al. (2009) Clustering and internalization of integrin alphavbeta3 with a tetrameric RGD-synthetic peptide. Mol Ther 17:837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. [DOI] [PubMed] [Google Scholar]
- Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, Chen SJ, Varnavski AN, LeClair C, Raper SE, et al. (2001) Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther 3:708–722. [DOI] [PubMed] [Google Scholar]
- Sevior DK, Pelkonen O, Ahokas JT. (2012) Hepatocytes: the powerhouse of biotransformation. Int J Biochem Cell Biol 44:257–261. [DOI] [PubMed] [Google Scholar]
- Shah P, Guo T, Moore DD, Ghose R. (2014) Role of constitutive androstane receptor in Toll-like receptor-mediated regulation of gene expression of hepatic drug-metabolizing enzymes and transporters. Drug Metab Dispos 42:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D, Lazar MA. (1999) Modulating nuclear receptor function: may the phos be with you. J Clin Invest 103:1617–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Kim C, Ginsberg MH. (2010) The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol 11:288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Eberly AM, Li ZY, Lieber A. (2005a) Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. J Virol 79:1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. (2005b) Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol 79:7478–7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrake HM, Patterson LH. (2014) Strategies to inhibit tumor associated integrin receptors: rationale for dual and multi-antagonists. J Med Chem 57:6301–6315. [DOI] [PubMed] [Google Scholar]
- Stanton RJ, Prod’homme V, Purbhoo MA, Moore M, Aicheler RJ, Heinzmann M, Bailer SM, Haas J, Antrobus R, Weekes MP, et al. (2014) HCMV pUL135 remodels the actin cytoskeleton to impair immune recognition of infected cells. Cell Host Microbe 16:201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PL, Nemerow GR. (2007) Cell integrins: commonly used receptors for diverse viral pathogens. Trends Microbiol 15:500–507. [DOI] [PubMed] [Google Scholar]
- Tanii H, Shitara Y, Torii M, Sekine S, Iwata H, Horie T. (2013) Induction of cytochrome P450 2A6 by bilirubin in human hepatocytes. Pharmacol Pharm 4:182–190. [Google Scholar]
- Teigler JE, Kagan JC, Barouch DH. (2014) Late endosomal trafficking of alternative serotype adenovirus vaccine vectors augments antiviral innate immunity. J Virol 88:10354–10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven T. (1984) Assay of hepatic microsomal testosterone hydroxylases by high-performance liquid chromatography. Anal Biochem 138:57–65. [DOI] [PubMed] [Google Scholar]
- Wang K, Chen S, Xie W, Wan YJ. (2008) Retinoids induce cytochrome P450 3A4 through RXR/VDR-mediated pathway. Biochem Pharmacol 75:2204–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum N, Greber UF. (2013) Adenovirus signalling in entry. Cell Microbiol 15:53–62. [DOI] [PubMed] [Google Scholar]
- Wonganan P, Jonsson-Schmunk K, Callahan SM, Choi JH, Croyle MA. (2014) Evaluation of the HC-04 cell line as an in vitro model for mechanistic assessment of changes in hepatic cytochrome P450 3A during adenovirus infection. Drug Metab Dispos 42:1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DX, Wei W, Sun MF, Wu CY, Wang JP, Wei LZ, Zhou CF. (2004) Kupffer cells and reactive oxygen species partially mediate lipopolysaccharide-induced downregulation of nuclear receptor pregnane X receptor and its target gene CYP3a in mouse liver. Free Radic Biol Med 37:10–22. [DOI] [PubMed] [Google Scholar]
- Ye F, Kim C, Ginsberg MH. (2011) Molecular mechanism of inside-out integrin regulation. J Thromb Haemost 9 (Suppl 1):20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitsugu H, Nishimura M, Tateno C, Kataoka M, Takahashi E, Soeno Y, Yoshizato K, Yokoi T, Naito S. (2006) Evaluation of human CYP1A2 and CYP3A4 mRNA expression in hepatocytes from chimeric mice with humanized liver. Drug Metab Pharmacokinet 21:465–474. [DOI] [PubMed] [Google Scholar]
- Zaiss AK, Foley EM, Lawrence R, Schneider LS, Hoveida H, Secrest P, Catapang AB, Yamaguchi Y, Alemany R, Shayakhmetov DM, et al. (2015) Hepatocyte heparan sulfate is required for adeno-associated virus 2 but dispensable for adenovirus 5 liver transduction in vivo. J Virol 90:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangar RC, Bollinger N, Verma S, Karin NJ, Lu Y. (2008) The nuclear factor-kappa B pathway regulates cytochrome P450 3A4 protein stability. Mol Pharmacol 73:1652–1658. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Schwab M. (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138:103–141. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, Tazelaar J, Wilson JM. (2001) Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther 3:697–707. [DOI] [PubMed] [Google Scholar]
- Zundler S, Neurath MF. (2015) Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev 26:559–568. [DOI] [PubMed] [Google Scholar]