Abstract
A promising approach in treating cocaine abuse is to metabolize cocaine in the blood using a mutated butyrylcholinesterase (BChE) that functions as a cocaine hydrolase (CocH). In rats, a helper-dependent adenoviral (hdAD) vector–mediated delivery of CocH abolished ongoing cocaine use and cocaine-primed reinstatement of drug-seeking for several months. This enzyme also metabolizes ghrelin, an effect that may be beneficial in maintaining healthy weights. The effect of a single hdAD-CocH vector injection was examined in rats on measures of anxiety, body weight, cocaine self-administration, and cocaine-induced locomotor activity. To examine anxiety, periadolescent rats were tested in an elevated-plus maze. Weight gain was then examined under four rodent diets. Ten months after CocH-injection, adult rats were trained to self-administer cocaine intravenously and, subsequently, cocaine-induced locomotion was tested. Viral gene transfer produced sustained plasma levels of CocH for over 13 months of testing. CocH-treated rats did not differ from controls in measures of anxiety, and only showed a transient reduction in weight gain during the first 3 weeks postinjection. However, CocH-treated rats were insensitive to cocaine. At 10 months postinjection, none of the CocH-treated rats initiated cocaine self-administration, unlike 90% of the control rats. At 13 months postinjection, CocH-treated rats showed no cocaine-induced locomotion, whereas control rats showed a dose-dependent enhancement of locomotion. CocH vector produced a long-term blockade of the rewarding and behavioral effects of cocaine in rats, emphasizing its role as a promising therapeutic intervention in cocaine abuse.
Introduction
Over 1.5 million people in the United States habitually use cocaine (SAMHSA, 2014), but there are no effective treatments for those trying to break the habit (Somoza et al., 2013). This leads to a prolonged cycle of abuse, treatment, and relapse (e.g., Griffin et al., 1989; Vonmoos et al., 2013). Neurologic decrements from long-term cocaine intake may persist a year beyond cessation of use (review by Hanlon et al., 2013). These can include impaired attention, working memory, and executive function (Vonmoos et al., 2013, 2014), possibly owing to decreased frontal lobe activity (Volkow et al., 1992; Connolly et al., 2012). Thus, preventing the development of cocaine abuse in vulnerable populations and promoting abstinence in current users is an important public health goal.
One promising therapy for cocaine addiction involves the natural cocaine-hydrolyzing activity of butyrylcholinesterase (BChE; Inaba et al., 1978), a key to cocaine metabolism. Early work demonstrated that administration of BChE could slightly reduce the physiologic and behavioral effects of cocaine (see review by Gorelick, 1997). More recently, collective efforts to mutate BChE into an efficient cocaine hydrolase (CocH) progressively raised its catalytic efficiency by up to 4400-fold (Sun et al., 2001, 2002a,b; Pan et al., 2005; Gao and Brimijoin, 2006; Zheng et al., 2008, 2014; Xue et al., 2013). A near-optimal enzyme rescued rats from a lethal 100 mg/kg injection of cocaine and blocked cocaine-primed reinstatement, a model of relapse into cocaine-seeking behavior (Brimijoin et al., 2008). Later CocH significantly reduced progressive-ratio responding (i.e., motivation) for intravenous cocaine self-administration (Carroll et al., 2011), suggesting that CocH substantially dampened its reinforcing effects.
The therapeutic potential of CocH for treating cocaine abuse increased further when CocH cDNA was incorporated into a helper-dependent adenoviral gene transfer vector (hdAD; Parks et al., 1996). This vector localizes in liver tissue, where its payload cDNA drives hepatocytes to synthesize and release CocH into the blood stream at high levels for long intervals. CocH vector-treated rodents showed enhanced cocaine metabolism for several months (Gao et al., 2005; Gao and Brimijoin, 2004, 2006) to 2 years (Geng et al., 2013). In preclinical models of drug abuse, CocH-treated rats displayed no locomotor response to stimulatory doses of cocaine 30 days postinjection (Carroll et al., 2012). In another study, CocH-treated rats failed to resume cocaine seeking during 6-monthly probes examining cocaine-primed reinstatement (Anker et al., 2012). Recently, Zlebnik et al. (2014) showed that high-dose CocH vector even extinguished ongoing cocaine self-administration for several months, and cocaine-seeking only reappeared when the CocH enzyme was blocked by a long-lived inhibitor, iso-OMPA. Altogether these findings suggest that CocH vector might blunt ongoing cocaine use and block relapse after cessation of cocaine use in high-risk individuals.
One purpose of our study was to examine the hypothesis that CocH vector would reduce the initiation of intravenous cocaine self-administration in rats, even when tested long after treatment. Chen et al. (2015) recently found that elevated levels of native BChE or CocH also reduced aggression in mice, tracing such an effect to enzyme-driven inactivation of plasma ghrelin. This peptide hormone is better known for its role in hunger and feeding (Kojima et al., 1999), but it also plays a role in stress and anxiety (Carlini et al., 2002). Therefore, our second purpose was to test the hypothesis that CocH vector treatment, by affecting ghrelin, would lower anxiety in an elevated-plus maze, and/or reduce food intake and weight gain in rats exposed to diets differing in palatability and macronutrient content.
Methods
Animals
Twenty-four male Wistar rats (Harlan Laboratories Inc., Indianapolis, IN) weighing ∼200 g (52 days old) served as subjects. Rats were initially housed in rooms maintained at 24°C (40–50% humidity) under a 12/12-hour light/dark cycle (lights on at 0600) in polycarbonate cages with ad libitum access to water and various types of chow (see Dietary Manipulation later). Later, during cocaine self-administration, rats lived in their operant-conditioning chambers and were food-restricted to 85% of their free-feeding weight to facilitate drug-taking (see Carroll et al., 1979). After self-administration testing, rats were returned to polycarbonate tubs with ad lib access to water and chow (Harlan Teklad 2018; Harlan Laboratories Inc., Madison, WI). The experimental protocol complied with the Guide for the Care and Use of Animals (National Research Council, 2011) and was approved by the University of Minnesota Institutional Animal Care and Use Committee.
Apparatus
Elevated-Plus Maze.
The elevated-plus maze, of compressed plastic board (3/4” floor and 1/2” walls), rested on legs 50 cm above the floor. All four maze arms were 10 cm wide by 50 cm long and intersected at 90° angles in a 10 cm–by–10 cm central square. The closed arms opposed each other, with sides 40 cm high. MED-PC IV software (v. 4.3; Med Associates, St. Albans, VT), in conjunction with an infrared beam in the closed arm, was used to record time in the open arms and the numbers of open-arm entries.
Drug Self-Administration Chambers.
Drug self-administration occurred in custom-built eight-sided operant-conditioning chambers equipped with response levers, stimulus lights, and a drug pump that were all configured as previously described by Zlebnik et al. (2014). A computer running MED-PC IV (v. 4.3) collected data.
Locomotor Activity Chamber.
The locomotor activity chamber consisted of a circular track with an inner diameter of 35.6 cm and an outer diameter of 59.6 cm (Model ENV-580; Med Associates). Four infrared sensors were mounted 5 cm above the floor, spaced evenly at one-quarter-turn intervals around the track. Beam breaks were recorded with Med-PC IV software (v. 4.1). Other details were as previously reported (Carroll et al. (2012).
Viral Vector Encoding CocH
On postnatal day 62, twelve rats received a single tail-vein injection of hdAD vector (1012 particles) containing cDNA for CocH, a human butyrylcholinesterase mutated for cocaine hydrolysis (Zheng et al., 2008) under regulation by a human ApoE hepatic control region with a bovine growth hormone polyadenylation sequence. The CocH vector dose (1012 particles) was the highest employed in rats and it was selected on the basis of previous work (Zlebnik et al., 2014) showing that it blunted ongoing cocaine self-administration, unlike a lower dose (1011 particles). This high dose was also expected to produce a therapeutic range of CocH plasma levels after a year, some levels sufficient enough to block acquisition of intravenous cocaine self-administration, thus allowing an examination of the dose-response effects when apparent.
To facilitate transduction, all rats (including controls) were mildly immunosuppressed with dexamethasone (10 mg/kg, i.p.) 15 and 2 hours before vector injection, and again at (5 mg/kg, i.p.) 24 hours after the vector injection. Twelve control rats were treated likewise with the same volume of saline instead of vector.
Blood Collection and Measurement of Plasma CocH Activity
Blood draws were taken for long-term assessment of CocH and ghrelin levels at critical points (postinjection weeks: 1, 6, 8, 25, 44, and 58) spaced long enough to avoid loss of tail vein patency after repeated blood draws. For this process, rats were restrained in a polycarbonate tube and tails were cleaned in warm water. A 23-gauge butterfly syringe–mounted needle was inserted into a lateral tail vein approximately one-third the distance from the tip, and 0.2 ml of blood was drawn. Collected blood was centrifuged for 15 minutes (8000 g) in serum-separation tubes (Becton, Dickinson and Company, Franklin Lakes, NJ) and frozen before analysis of enzyme levels as previously described (Brimijoin et al., 2002).
Drugs
(–)-Cocaine HCl (National Institute of Drug Abuse, Research Triangle Institute, Research Triangle Park, NC) was dissolved in sterile saline, with heparin [5 United States Pharmacopeia units/ml]. The initial concentration of cocaine was 1.6 mg cocaine-HCl per milliliter of saline, and each cocaine infusion was a standard 0.4-mg/kg dose. In previous studies using the CocH vector, this dose was consistently self-administered by rats, and cocaine self-administration was reduced after vector injection (Zlebnik et al., 2014). The infusion length was determined on the basis of the weight of the rat (1 s/100 g, adjusted weekly) with an infusion rate of 0.025 ml/s. Cocaine injections to assess drug-induced locomotor activity were delivered at 2.5 ml/kg.
Jugular Catheterization Surgery
Ten months after injection, vector-treated (n = 10) and control rats (n = 12) were implanted with a chronic indwelling silastic catheter (15-cm long; Plastics One, Roanoke, VA) and then fit with an infusion harness following methods previously described (Zlebnik et al., 2014). After surgery, rats recovered for 3 days in the operant chamber and received antibiotics (enrofloxacin, 10 mg/kg, i.v.) and analgesics (buprenorphine, 0.05 mg/kg, s.c.; ibuprofen, 15 mg/kg, per os). Catheter patency was maintained with a daily flush (0.1 ml, i.v.) of heparinized saline (20 United States Pharmacopeia) units of heparin/ml saline]. Rats were weighed and tested for patency weekly by transcatheter infusion of 0.1 ml of ketamine (60 mg/ml) and midazolam (3 mg/ml) in saline. If the righting reflex was not inhibited within 10 seconds, then patency was considered lost and a new catheter was implanted in the left jugular using the aforementioned methods.
Elevated-Plus Maze
Control (N = 12) and vector-treated (N = 11) rats were placed in the center of the elevated-plus maze and allowed 10 minutes to explore under video observation. Between each test session, the maze was cleaned with 70% EtOH and allowed to dry before the next rat entered. Rats were tested weekly on the same day and time (∼1100 hours) for 5 weeks, starting 2 weeks after injection. Percentage time spent in the open-arm and the number of open-arm entries was recorded after each run.
Dietary Manipulation
From postinjection weeks 0 to 22, control (N = 12) and vector-treated (N = 11) rats enjoyed free access to water and four successive chow diets: 1) standard rodent chow (weeks 0–9; Teklad 2018), 2) a high-fat diet (weeks 10–13, D12492; Research Diets Inc., New Brunswick, NJ,), 3) a high-fat + high-carbohydrate diet (weeks 14–18, D12451; Research Diets), and 4) the high-fat + high-carbohydrate diet plus 10% high-fructose corn syrup (w/v) in addition to water (weeks 19–21). Each week, the rats were weighed and food and water consumption was measured. Weight gain and food consumed on the different diets were compared across groups using the daily average consumption for each diet. At week 22, rats returned to a standard diet (Teklad 2018) with free access to water.
Acquisition of Cocaine Self-Administration
Cocaine self-administration sessions lasted 6 hours (starting at 0900), during which a response on the active lever produced a cocaine infusion 0.4 mg/kg, i.v., on an fixed-ratio 1 schedule. Drug delivery was accompanied by illumination of the stimulus lights above the active lever, followed by a 20-second timeout, after which responding had no consequences. A response on the inactive lever illuminated stimulus lights above it for the same length of time as the active lever, but with no other consequence. During training, rats received ground food on the active lever and three noncontingent cocaine infusions every 2 hours (nine infusions per day). This continued until 15 infusions were earned for two consecutive sessions (maximum = 40 infusions per session). After discontinuation of food primes and noncontingent infusions, a rat that earned ≥35 cocaine infusions in thee separate sessions met the acquisition criteria and was transitioned into the maintenance phase. In this phase, the upper limit on earned infusions was removed, and the rat was allowed to self-administer cocaine until it completed at least five sessions with >55 infusions. Control (n = 10) and CocH-treated (n = 6) rats were given up to 21 days of training to reach acquisition criteria (some attrition owing to patency and health issues). The main dependent measure was the number of sessions needed to meet cocaine acquisition criteria. The number of active and inactive lever responses were also recorded, as well as the number of earned infusions throughout training, acquisition, and maintenance.
Cocaine-Induced Locomotor Activity
Locomotor activity in control (n = 10) and vector-treated (n = 6) rats was quantified by the approximate distance traveled, determined by the number of quarter-turns completed in a circular track (a distance of ∼37.4 cm/turn) during daily 30-minute sessions. An initial acclimation session was conducted to reduce exploratory behavior produced by the testing chamber’s novelty. In subsequent sessions, rats were given equivalent volumes of saline or cocaine (5, 10, and 20 mg/kg, i.p., counterbalanced) 15 minutes prior to the locomotor session.
Data Analysis
For the elevated-plus maze, between-group differences in open-arm entries or percentage of time spent in the open arms across the five sessions were assessed using a two-way analysis of variance (ANOVA) (group X exposure-week). Between-group differences in weight gain and chow consumption across the four different diets was assessed identically. Between-group differences in acquiring cocaine self-administration across training sessions were assessed with a Mantel-Cox log-rank test. Between-group differences in cocaine infusions during self-administration training were assessed by two-way ANOVA across the first six sessions when all subjects were still in the training phase. Differences in locomotor activity across cocaine doses were assessed by two-way ANOVA (vector treatment X cocaine dose). If main effects were observed in an ANOVA, a Šidák post hoc analysis was used to test for significant differences across levels of factors (p < 0.05).
Results
Elevated-Plus Maze.
CocH-treated rats did not differ significantly in anxiety-like behavior in the elevated plus-maze compared with control rats. Across five test sessions, no consistent between-group differences emerged in open-arm entries or in the percentage of time spent in the open arm (data not shown).
Dietary Manipulations.
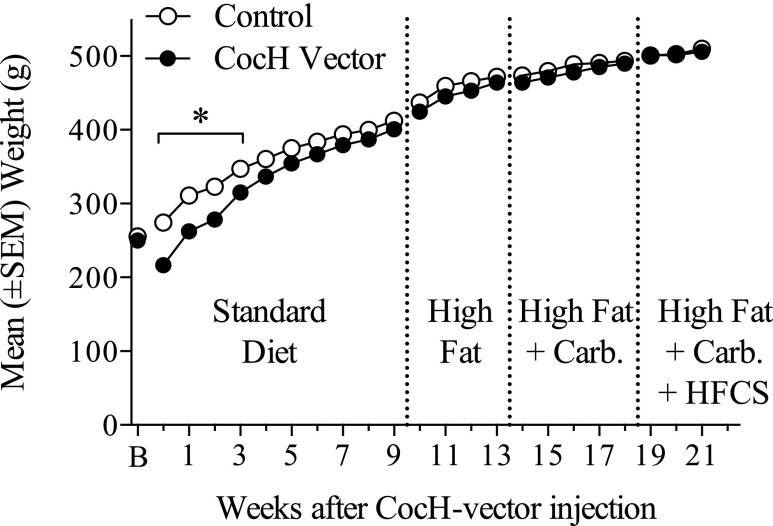
Body weights across the first 10 weeks after CocH-vector injection were analyzed using a two-way mixed-model ANOVA, which showed a significant main effect of CocH treatment (F1,21 = 10.13, p < .01), postinjection week (F9,189 = 712.9, p < .001), and a treatment X week interaction (F9,189 = 17.97, p < .001). A Šidák post hoc analysis indicated that CocH-treated rats weighed significantly less than controls during weeks 0 thru 3 (Fig. 1). Indeed, a two-way mixed-model ANOVA reported a significant interaction of standard chow consumed X postinjection week block (F2,42 = 10.63, p < .001). A post hoc analysis showed CocH-treated rats consumed less chow during the 0–2 weeks block (Table 1). In general, these findings indicate initially less chow consumption and slower weight gain in the CocH vector-treated rats, which gradually regained control levels by week 4. No significant between-group differences in body weight and food or water consumption reappeared during the three subsequent diet conditions [high fat, high fat + carb, high fat + carb + 10% high-fructose corn syrup (HFCS)], except for CocH rats consuming significantly less water during the High fat + carb condition [weeks 14–17) t(21) = 2.149, p < .05]. Control and vector-treated rats weighed the same on average at week 10 and gained weight at the same rate, regardless of diet. Furthermore, across the subsequent dietary manipulations, both groups of rats consumed similar amounts of kilocalories, chow, and 10% high-fructose corn syrup (Table 1).
Fig. 1.
Mean (±S.E.M.) weight (g) of control (n = 12) and vector-treated rats (n = 11) across post–vector injection weeks. Rats were given free access to water and various diets: standard, high fat, and high fat plus high carbohydrate sometimes supplemented with 10% HFCS in water (*p < 0.05). See Materials and Methods for details.
TABLE 1.
Mean daily consumption (in grams; S.E.M. in parenthesis) and kilocalories consumed during dietary manipulations
| Control | CocH Vector | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Weeks after CocH-Vector Injection | Chow Type | Chow | Water | 10% HFCS Waterd | Kcal/Day | Chow | Water | 10% HFCS Water | Kcal/Day |
| 0–2 | Standarda | 22.14* (0.35) | 27.29 (1.03) | — | 68.63 (1.09) | 20.71* (0.45) | 26.02 (0.84) | — | 64.20 (1.39) |
| 3–6 | Standard | 21.88 (0.34) | 26.08 (0.71) | — | 67.83 (1.05) | 21.52 (0.43) | 24.87 (1.10) | — | 66.71 (1.33) |
| 7–9 | Standard | 20.02 (0.25) | 24.11 (0.84) | — | 62.06 (0.81) | 19.70 (0.39) | 22.65 (0.86) | — | 61.07 (1.21) |
| 10–13 | High fatb | 17.00 (0.34) | 20.49 (0.78) | — | 89.08 (1.78) | 17.33 (0.47) | 18.71 (1.04) | — | 90.81 (2.46) |
| 14–17 | High fat + carb.c | 15.49 (0.27) | 18.39* (0.94) | — | 73.27 (1.28) | 15.43 (0.32) | 15.59* (0.90) | — | 72.98 (1.51) |
| 18–21 | High fat + carb. | 11.70 (0.42) | 0.62 (0.11) | 83.03 (6.40) | 78.66 (1.46) | 11.72 (0.25) | 1.74 (0.37) | 71.12 (5.79) | 75.43 (2.06) |
Standard, rat chow with 5.5% fat/41% carbohydrate, 3.1 kcal/g (Teklad 2018).
High fat, 60% fat/20% carbohydrate rodent chow, 5.24 kcal/g (Research Diets D12492).
High fat + carb.,45% fat/35% carbohydrate rat chow, 4.73 kcal/g (Research Diets D12451).
10% HFCS water, 10%, high fructose corn syrup in tap water, 0.28 kcal/g (*p < .05).
Cocaine Self-Administration.
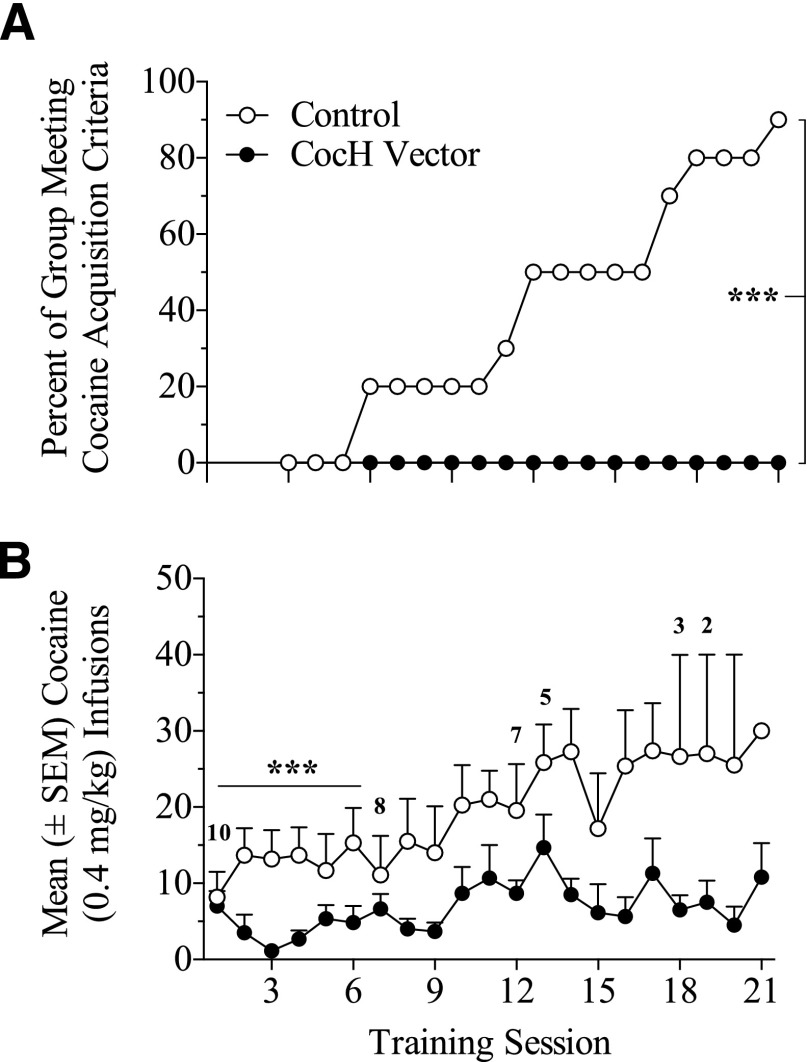
Figure 2 compares control and CocH-treated rats on intravenous cocaine self-administration at 10 months postinjection. (A) shows the percentage of rats reaching acquisition criteria within the 21 training sessions. None of the six CocH-treated rats that completed all training sessions met the acquisition criteria. In contrast, nine out of ten controls met the acquisition criteria. The difference between these groups was significant according to Mantel-Cox log-rank test [χ2 (1, 16) = 11.39, p < 0.001].
Fig. 2.
Cocaine self-administration in control and CocH vector-treated rats during 21 training sessions. (A) Percentage of rats reaching acquisition criteria for cocaine self-administration in the control (n = 10) and CocH groups (n = 6) across 21 training sessions (***p < 0.001). (B) Number of self-administered infusions (±S.E.M.) of cocaine (0.4 mg/kg, i.v.) earned across the 21 training sessions in the control and CocH groups (***p < 0.001). The numbers above the control group indicate the reduction in group-size as rats acquired cocaine self-administration.
Figure 2B displays mean self-administered infusions of cocaine in control and CocH rats across the 21 training sessions (numbers above the symbols indicate reductions in group size from rats meeting criteria to move into maintenance). CocH-treated rats earned consistently fewer infusions across the training sessions than did controls, which showed a rising trend of cocaine self-administration during the same time. Across the first six sessions, controls earned significantly more cocaine infusions than did CocH rats according to a two-way ANOVA (treatment X training session), and this revealed a main effect of treatment (F1, 84 = 14.98, p < 0.001).
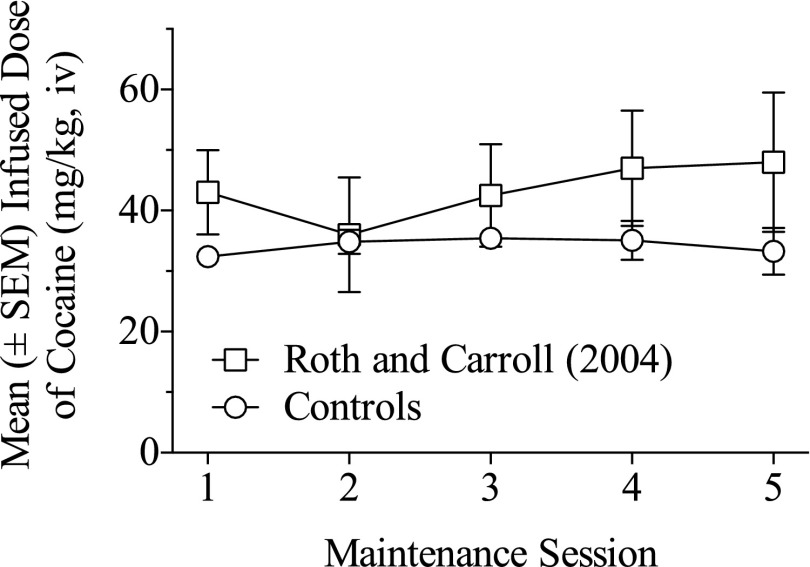
To determine whether rat age affected cocaine self-administration, cocaine self-administration by controls was compared with rats of equivalent ages under similar conditions in our laboratory (Roth and Carroll 2004). Figure 3 shows the dose of cocaine infused in the first five maintenance sessions by control rats (n = 8) and by the rats (n = 7) from a previous study by Roth and Carroll (2004) responding under similar conditions (6-hour sessions and 0.5 mg/kg i.v. infusion of cocaine). The pattern and amount of cocaine self-administered were not significantly different, indicating that the control rats behaved in a pattern typical for this cocaine dose and this rat strain in our laboratory.
Fig. 3.
Mean (±S.E.M.) dose of cocaine (mg/kg, i.v.) self-administered during the initial five 6-hour maintenance sessions for the control rats (n = 8) compared with a comparison control group (n = 7) of rats from a previous publication (Roth and Carroll, 2004).
Cocaine-Induced Locomotor Activity.
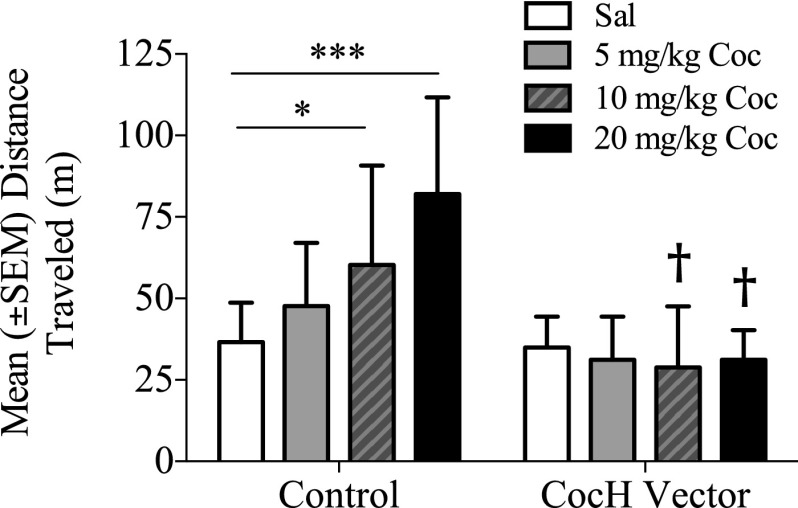
Figure 4 shows differences in cocaine-induced locomotor activity in control rats and CocH-treated rats 13 months post–vector injection. The latter traveled no further after doses of cocaine (5, 10, and 20 mg/kg) than after saline. In contrast, the control rats showed a clear, dose-dependent increase in locomotion. A two-way ANOVA on distance traveled revealed a significant main effect of CocH treatment (F1, 13 = 14.35, p < 0.01), cocaine dose (F3, 39 = 3.34, p < 0.05), and a treatment-by-dose interaction (F3, 39 = 4.49, p < 0.01). Post hoc testing of the controls found a significant increase in distance traveled after cocaine doses of 10 (p < 0.05) and 20 mg/kg (p < 0.001) compared with saline. The test also revealed significantly greater absolute locomotor activity in controls at the 10 and 20 mg/kg doses compared with CocH rats (p < 0.01).
Fig. 4.
Mean (±S.E.M.) distance traveled (m) by control (n = 10) and CocH-treated rats (n = 6) during 30-minute locomotor assessments following injections of saline and cocaine (Coc; 5, 10 and 20 mg/kg, i.p.) (*p < 0.05; ***p < 0.001; †p < 0.05).
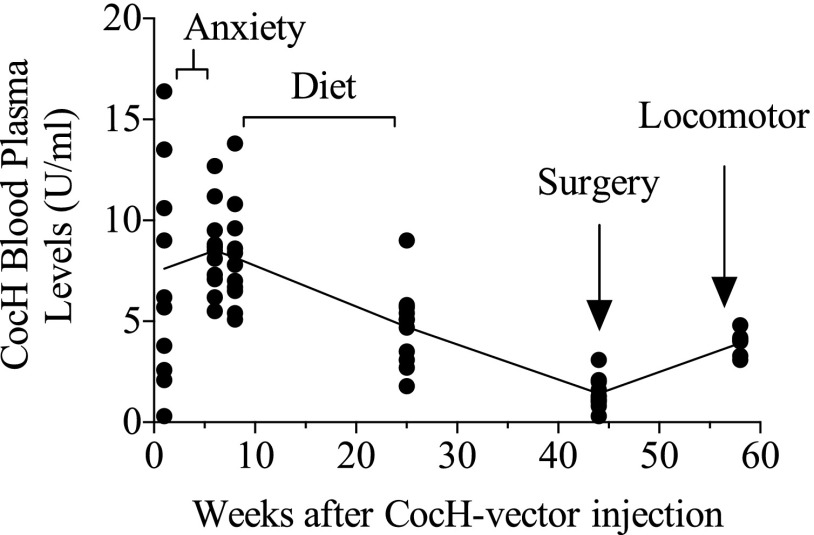
Measured Levels of CocH Activity.
Figure 5 shows CocH enzyme plasma levels in the vector-treated rats at many times between weeks 1 and 58 after the single hdAD-CocH vector injection. At week 1, there was a wide-range of individual differences in CocH activity. Higher and more consistent levels of enzyme activity were observed by 2 months postinjection. These enzyme levels were followed by a gradual decrease across the remaining 11 months.
Fig. 5.
Individual CocH enzyme levels (±S.E.M.) determined in vector-treated rats across weeks post–vector injection (line indicates group mean).
Discussion
Administration of a CocH vector served as a long-term preventive treatment of the rewarding and behavioral effects of cocaine, but it did not alter measures of anxiety. Initially, CocH also affected feeding behavior and reduced weight gain, but this did not persist after the first three weeks (Fig. 1 and Table 1). There is good reason to suspect that this transient effect reflected the human-enzyme basis of the CocH version used in these studies. In previous rodent studies, this enzyme has elicited an immune response that faded after a week and persisted as a low-grade immune reaction (Brimijoin, unpublished data). Therefore, the weight effect does not necessarily reflect BChE-driven reduction of plasma ghrelin levels. A further reason for this conclusion is that others have observed surprising disconnections between background ghrelin levels and feeding behavior. In particular, Lockridge and collaborators (Li et al., 2008) found that BChE knockout mice, with higher than normal plasma ghrelin and a propensity for obesity on fatty diets, consumed no more food than the wild-type controls.
The effects of CocH on cocaine-driven behaviors were stronger and more consistent. In contrast to controls, rats given a single hdAD-CocH vector injection (1012 viral particles) did not acquire intravenous cocaine self-administration at 10 months postinjection (Fig. 2), and they did not show cocaine-enhanced locomotor activity 13 months postinjection (Fig. 4). Senescence was not a cause of the failure to acquire cocaine self-administration, as the cocaine dose infused by controls in the present data is similar to that for rats of the same age in a previous study in our laboratory (Roth and Carroll, 2004, data in Fig. 3). Our findings indicate that a single CocH vector treatment at the present dosage produced a robust enzyme expression that was sustained at levels that blocked cocaine effects for over a year (Fig. 5). The long-term efficacy of this CocH-vector treatment has broad implications for the prevention of cocaine abuse.
The present study complements previous work from our laboratory demonstrating that CocH vector blocks reinstatement of cocaine-seeking behavior (Anker et al., 2012) and even blunts ongoing intravenous cocaine self-administration (Zlebnik et al., 2014). The findings confirm work showing that exogenously administered CocH reduces the physiologic and reinforcing effects of cocaine (e.g., Brimijoin et al., 2008; Carroll et al., 2011). These behavioral effects are achieved by the drastic reduction in the amount of cocaine reaching the brain as a result of CocH activity (Sun et al., 2002a). This has important implications for changing the development and escalation of adverse neuropsychological disorders resulting from chronic cocaine use (VonMoos et al., 2014). In the future, a safe and effective CocH vector therapy might not merely facilitate cocaine abstinence but also serve as a long-term preventive treatment of cocaine abuse in vulnerable populations.
The plasma level of CocH needed to block acquisition of cocaine self-administration appears to be much lower than what is necessary to block ongoing cocaine use. For example, Zlebnik et al. (2014) reported that a low CocH vector dose (1011 viral particles) produced CocH enzyme levels (0.7 IU/ml) that were insufficient to block the effect of cocaine and led to rats surmounting the enzymatic activity by doubling the amount of cocaine they self-administered. However, when a separate group was given a higher vector dose (1012 viral particles), cocaine-maintained responding escalated sharply at first but was then extinguished across 14 days as plasma CocH levels increased to 23.2 IU/ml. To verify the role of CocH activity, Zlebnik et al. (2014) blocked its enzymatic activity with iso-OMPA, and cocaine self-administration resumed. Here we add to those findings by showing that even at relatively low plasma levels (∼1.67 IU/ml), CocH activity sufficed to block acquisition of cocaine self-administration (Fig. 2). Thus, in naive subjects, low levels of CocH prevented initiation of cocaine use.
The prevention of cocaine-induced locomotor activity in the CocH-treated rats (Fig. 4) was somewhat consistent with Carroll et al. (2012). In that study, rats given a smaller dose of the hdAD-CocH vector (1011 viral particles) showed no cocaine-induced (15 m/kg, i.p.) increase in locomotor activity 1 month postinjection. However, Carroll and colleagues (2012) also reported that a separate group of rats treated with the same vector dose showed sensitization to the locomotor-enhancing effects of cocaine following repeated cocaine injections (10 mg/kg; 8 total). A likely explanation for the discrepancy is that CocH-treated rats produced CocH plasma levels (∼1.3 IU/ml) that were about one-third of those in the present study at 13 months (∼3.9 IU/ml).
The 13-month durability of CocH expression in our rats (Fig. 5) complements results of Geng et al. (2013), who reported that mice maintained substantial plasma levels of CocH for 2 years or more. The present results also concur with Zlebnik et al. (2014), who used the same form and dose (1012 particles) of vector and reported CocH enzyme expression levels across 10 weeks postinjection that were similar to the present study. In addition, we examined changes in weight under several different diets and observed only early differences in weight gain (e.g., postinjection weeks 0–3; Fig. 1 and Table 1) and consumption across a 21-week period. Hence, the vector was well tolerated. Collectively, these findings support the long-term safety and efficacy of the CocH vector as a selective anticocaine therapy.
A common concern with treatments that target a specific class of drugs (e.g., methadone for opioid abuse) is that individuals will switch to other drugs of abuse such as amphetamines (Tiihonen, et al., 2012). CocH vector treatment would be vulnerable to this issue. Anker et al. (2012) demonstrated that the CocH vector blocked reinstatement of cocaine-seeking in rats given priming doses of cocaine, but it did not prevent reinstatement primed by methamphetamine. Thus, like other therapies for polydrug abusers, the best treatment would be a combination of efficacious pharmacological and behavioral measures aimed at pre-existing drug abuse vulnerabilities (Stoops and Rush, 2014). In the future, the efficacy of the present viral vector-delivered treatment could serve as a prototype for the development of novel long-term interventions against an array of abused drugs.
In summary, rats treated once with a CocH vector failed to acquire cocaine self-administration when tested 10 months later (Fig. 2). Furthermore, at 13 months the CocH-treated rats did not display changes in cocaine-induced locomotor activity, unlike control rats (Fig. 4). At the present levels, the CocH vector did not substantially alter chow intake or weight gain across a 21-week period. Treatment with CocH also did not affect measures of anxiety in an elevated-plus maze. These findings indicate that the CocH-vector treatment served as a safe and effective long-term intervention to prevent initiation of cocaine self-administration. An advantage of this approach is that a single injection blocked cocaine effects for over a year, lending strong support for CocH vector as a targeted gene therapy for cocaine abuse, by itself or in combination with other established treatments. This model may also inform the development of related viral vector–delivered interventions for other forms of substance abuse.
Acknowledgments
The authors thank Dr. Natalie Zlebnik for thoughtful contributions, Seth Johnson for technical assistance, and Jared Mitchell and Heather Veghlan for assistance in conducting this research.
Abbreviations
- ANOVA
analysis of variance
- BChE
butyrylcholinesterase
- CocH
cocaine hydrolase
- hdAD
helper-dependent adenovirus
- HFCS
high-fructose corn syrup
- High fat + carb
high fat and high carbohydrate
- iso-OMPA
tetraisopropyl pyrophosphoramide
Authorship Contributions
Participated in research design: Smethells, Carroll, Swalve, Brimijoin.
Conducted experiments: Greer, Smethells, Swalve, Gao, Brimijoin.
Contributed new reagents or analytic tools: Parks.
Performed data analysis: Smethells, Gao, Greer, Swalve, Brimijoin, Carroll.
Wrote or contributed to the writing of the manuscript: Smethells, Swalve, Carroll, Brimijoin, Greer, Parks.
Footnotes
This study was supported by a grant from the Minnesota Partnership for Biotechnology and Medical Genomics [Grant 45505] to S.B. and M.E.C., and a National Institutes of Health National Institute of Drug Abuse training grant [Grant T32 DA007097] to J.R.S. (Dr. Thomas Molitor, P.I.).
References
- Anker JJ, Brimijoin S, Gao Y, Geng L, Zlebnik NE, Parks RJ, Carroll ME. (2012) Cocaine hydrolase encoded in viral vector blocks the reinstatement of cocaine seeking in rats for 6 months. Biol Psychiatry 71:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimijoin S, Shen ML, Sun H. (2002) Radiometric solvent-partitioning assay for screening cocaine hydrolases and measuring cocaine levels in milligram tissue samples. Anal Biochem 309:200–205. [DOI] [PubMed] [Google Scholar]
- Brimijoin S, Gao Y, Anker JJ, Gliddon LA, Lafleur D, Shah R, Zhao Q, Singh M, Carroll ME. (2008) A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology 33:2715–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini VP, Monzón ME, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR. (2002) Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun 299:739–743. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. (1979) Food deprivation increases oral and intravenous drug intake in rats. Science 205:319–321. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Gao Y, Brimijoin S, Anker JJ. (2011) Effects of cocaine hydrolase on cocaine self-administration under a PR schedule and during extended access (escalation) in rats. Psychopharmacology (Berl) 213:817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Zlebnik NE, Anker JJ, Kosten TR, Orson FM, Shen X, Kinsey B, Parks RJ, Gao Y, Brimijoin S. (2012) Combined cocaine hydrolase gene transfer and anti-cocaine vaccine synergistically block cocaine-induced locomotion. PLoS One 7:e43536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VP, Gao Y, Geng L, Parks RJ, Pang Y-P, Brimijoin S. (2015) Plasma butyrylcholinesterase regulates ghrelin to control aggression. Proc Natl Acad Sci USA 112:2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H. (2012) The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend 121:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Brimijoin S. (2004) An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther 310:1046–1052. [DOI] [PubMed] [Google Scholar]
- Gao Y, Brimijoin S. (2006) Viral transduction of cocaine hydrolase in brain reward centers. Cell Mol Neurobiol 26:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Atanasova E, Sui N, Pancook JD, Watkins JD, Brimijoin S. (2005) Gene transfer of cocaine hydrolase suppresses cardiovascular responses to cocaine in rats. Mol Pharmacol 67:204–211. [DOI] [PubMed] [Google Scholar]
- Geng L, Gao Y, Chen X, Hou S, Zhan C-G, Radic Z, Parks RJ, Russell SJ, Pham L, Brimijoin S. (2013) Gene transfer of mutant mouse cholinesterase provides high lifetime expression and reduced cocaine responses with no evident toxicity. PLoS One 8:e67446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA. (1997) Enhancing cocaine metabolism with butyrylcholinesterase as a treatment strategy. Drug Alcohol Depend 48:159–165. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. (1989) A comparison of male and female cocaine abusers. Arch Gen Psychiatry 46:122–126. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Beveridge TJR, Porrino LJ. (2013) Recovering from cocaine: insights from clinical and preclinical investigations. Neurosci Biobehav Rev 37 (9 Pt A):2037–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T, Stewart DJ, Kalow W. (1978) Metabolism of cocaine in man. Clin Pharmacol Ther 23:547–552. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660. [DOI] [PubMed] [Google Scholar]
- Li B, Duysen EG, Lockridge O. (2008) The butyrylcholinesterase knockout mouse is obese on a high-fat diet. Chem Biol Interact 175:88–91. [DOI] [PubMed] [Google Scholar]
- National Research Council (2011) Guidelines for the Care and Use of Laboratory Animals, 8th ed, The National Academic Press, Washington, DC. [Google Scholar]
- Pan Y, Gao D, Yang W, Cho H, Yang G, Tai H-H, Zhan CG. (2005) Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci USA 102:16656–16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. (1996) A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA 93:13565–13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. (2004) Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav 78:199–207. [DOI] [PubMed] [Google Scholar]
- Somoza EC, Winship D, Gorodetzky CW, Lewis D, Ciraulo DA, Galloway GP, Segal SD, Sheehan M, Roache JD, Bickel WK, et al. (2013) A multisite, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of vigabatrin for treating cocaine dependence. JAMA Psychiatry 70:630–637. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. (2014) Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research. Expert Rev Clin Pharmacol 7:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA (Substance Abuse and Mental Health Services Administration) (2014) Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863, Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Sun H, El Yazal J, Lockridge O, Schopfer LM, Brimijoin S, Pang Y-P. (2001) Predicted Michaelis-Menten complexes of cocaine-butyrylcholinesterase. Engineering effective butyrylcholinesterase mutants for cocaine detoxication. J Biol Chem 276:9330–9336. [DOI] [PubMed] [Google Scholar]
- Sun H, Pang Y-P, Lockridge O, Brimijoin S. (2002a) Re-engineering butyrylcholinesterase as a cocaine hydrolase. Mol Pharmacol 62:220–224. [DOI] [PubMed] [Google Scholar]
- Sun H, Shen ML, Pang Y-P, Lockridge O, Brimijoin S. (2002b) Cocaine metabolism accelerated by a re-engineered human butyrylcholinesterase. J Pharmacol Exp Ther 302:710–716. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Krupitsky E, Verbitskaya E, Blokhina E, Mamontova O, Föhr J, Tuomola P, Kuoppasalmi K, Kiviniemi V, Zwartau E. (2012) Naltrexone implant for the treatment of polydrug dependence: a randomized controlled trial. Am J Psychiatry 169:531–536. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang G-J, Fowler JS, Wolf AP, Dewey SL, Handlesman L. (1992) Long-term frontal brain metabolic changes in cocaine abusers. Synapse 11:184–190. [DOI] [PubMed] [Google Scholar]
- Vonmoos M, Hulka LM, Preller KH, Jenni D, Baumgartner MR, Stohler R, Bolla KI, Quednow BB. (2013) Cognitive dysfunctions in recreational and dependent cocaine users: role of attention-deficit hyperactivity disorder, craving and early age at onset. Br J Psychiatry 203:35–43. [DOI] [PubMed] [Google Scholar]
- Vonmoos M, Hulka LM, Preller KH, Minder F, Baumgartner MR, Quednow BB. (2014) Cognitive impairment in cocaine users is drug-induced but partially reversible: evidence from a longitudinal study. Neuropsychopharmacology 39:2200–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Hou S, Yang W, Fang L, Zheng F, Zhan C-G. (2013) Catalytic activities of a cocaine hydrolase engineered from human butyrylcholinesterase against (+)- and (-)-cocaine. Chem Biol Interact 203:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Yang W, Ko M-C, Liu J, Cho H, Gao D, Tong M, Tai HH, Woods JH, Zhan CG. (2008) Most efficient cocaine hydrolase designed by virtual screening of transition states. J Am Chem Soc 130:12148–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Xue L, Hou S, Liu J, Zhan M, Yang W, Zhan CG. (2014) A highly efficient cocaine-detoxifying enzyme obtained by computational design. Nat Commun 5:3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Brimijoin S, Gao Y, Saykao AT, Parks RJ, Carroll ME. (2014) Long-term reduction of cocaine self-administration in rats treated with adenoviral vector-delivered cocaine hydrolase: evidence for enzymatic activity. Neuropsychopharmacology 39:1538–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]