Abstract
Relapse to drug use is often cited as the major obstacle in overcoming a drug addiction. Whereas relapse can occur for a myriad of reasons, it is well established that complex neuroadaptations that occur over the course of addiction are major factors. Cocaine, as a potent dopamine transporter blocker, specifically induces alterations in the dopaminergic as well as other monoaminergic neurotransmissions, which lead to cocaine abuse and dependence. Evidence also suggests that adaptations in the endogenous opioids play important roles in pathophysiology of cocaine addiction. Following this evidence, we investigated a combination medication, levo-tetrahydropalmatine (l-THP) and low dose naltrexone (LDN), targeting primarily dopaminergic and endogenous opioid systems as a cocaine-relapse-prevention treatment. In the present study Wistar rats were used to assess the effects of l-THP and LDN on cocaine self-administration, drug-seeking behavior during cocaine reinstatement, spontaneous locomotion, and effects on the endogenous opioid system. We determined that the combination of l-THP and LDN reduces drug-seeking behavior during reinstatement more potently than l-THP alone. Additionally, the combination of l-THP and LDN attenuates the sedative locomotor effect induced by l-THP. Furthermore, we revealed that treatment with the combination of l-THP and LDN has an upregulatory effect on both plasma β-endorphin and hypothalamic POMC that was not observed in l-THP-treated groups. These results suggest that the combination of l-THP and LDN has great potential as an effective and well-tolerated medication for cocaine relapse prevention.
Introduction
Cocaine is a potent psychostimulant with a high risk for addiction and abuse. Despite the extensive research in the past decades, there remains no FDA-approved treatments for mitigating cocaine addiction (Nestler, 2005; Shorter and Kosten, 2011). Cocaine addiction induces alterations in the mesolimbic dopamine (DA) system, and considerable efforts were placed in the development of DA receptor antagonists as antiaddiction treatments (Dackis and O’Brien, 2001; Platt et al., 2002; Koob and Volkow, 2010). However, DA antagonists alone are often unsuccessful in clinical trials (Haney et al., 2001). Whereas DA plays a central role in cocaine addiction, many other neurotransmitters take part in the complex changes in the brain over the course of cocaine addiction (Trigo et al., 2010; Yoo et al., 2012). β-Endorphin is emerging as an important player in the development of addiction (Olive et al., 2001; Roth-Deri et al., 2003, 2004, 2008; Simmons and Self, 2009; Nguyen et al., 2012; Dikshtein et al., 2013). Attenuated β-endorphin has been demonstrated to play an important role in relapse to nicotine- or alcohol-seeking behavior (Marinelli et al., 2004; Shaw and al’Absi, 2008; Ueno et al., 2014). Pro-opiomelanocortin (POMC), also a part of the endogenous opioid system, is the precursor to β-endorphin, adrenocorticotropic hormone, or melanocyte-stimulating hormone. POMC is thought to play an important role in reinstatement to drug-seeking behavior, and in a recent study it was observed that animals with higher instances of reinstatement displayed a significant reduction in POMC expression (Zhou et al., 2015). Taken together these data suggest that the endogenous opioid system may be an important target in medication development for treatment of cocaine dependence.
Levo-tetrahydropalmatine (l-THP) is a purified alkaloid isolated from the herb Corydalis yanhusuo, native to China. It reduces the reward- and drug-seeking effects of cocaine, oxycodone, and heroin (Jin et al., 1986a,b; Marcenac et al., 1986; Ding, 1987; Mantsch et al., 2007; Liu et al., 2009; Mantsch et al., 2010; Figueroa-Guzman et al., 2011; Wang and Mantsch, 2012), suggesting a potential for l-THP in treatment of drug abuse and addiction. The dopamine receptor–blocking properties of l-THP may substantially contribute to its ability to attenuate drug-seeking behavior. Clinically, a pilot study conducted to assess the utility of l-THP for the treatment of heroin addiction determined that 30 mg l-THP given twice daily increased heroin abstinence over 3 months by 63% (Yang et al., 2008). However, l-THP also produces significant sedative effects in a dose-dependent manner (Xi et al., 2007; Xu et al., 2013), which may limit its utility in treatment of drug addiction.
Naltrexone, primarily a μ-opioid receptor antagonist, is FDA-approved for treatment of alcoholism and opioid detoxification. At therapeutic doses, naltrexone reduces drug-seeking behavior in rodents but is unsuccessful or ineffective in most human clinical studies owing to significant unwanted side effects, poor patient compliance, and high attrition rates (Schmitz et al., 2001; Grassi et al., 2007; Schmitz et al., 2009; Schmitz et al., 2014). However, the tolerability of naltrexone is significantly improved when given at subtherapeutic doses (Zagon et al., 2014). Low dose naltrexone (LDN), defined as <4 mg per day in humans (Brown and Panksepp, 2009; Mannelli et al., 2010; Wee et al., 2012; Cordery et al., 2014), is becoming commonly used as a long term treatment of autoimmune disorders (Rahn et al., 2011; Segal et al., 2014). Although the exact mechanisms underlying these actions are unknown, one possibility is that LDN may functionally upregulate endogenous opioid system through μ-opioid receptor antagonism.
We therefore propose here that the combination of l-THP and LDN may be safer than l-THP or LDN alone in prevention of cocaine relapse. This is on the basis of the following two assumptions: First, such a combination may produce additive therapeutics effects, which may significantly lower each drug dose and therefore attenuate unwanted side-effects produced by each individual drugs; and second, coadministration of LDN with l-THP may also antagonize l-THP-induced sedation by normalization or upregulation of the endogenous opioid system in cocaine-dependent subjects.
To test this hypothesis the present study was designed to determine whether the combination of l-THP and LDN more potently reduces relapse to drug-seeking behavior and with less unwanted sedative effect than l-THP alone in rat models. In addition, we also investigated whether coadministration of l-THP and LDN alters plasma β-endorphin concentrations and hypothalamic POMC expression.
Materials and Methods
Animals.
Experimentally naïve male Wistar rats (Charles River Laboratories, Raleigh NC) weighing 250–300g were used for all experiments. Animals were housed individually in a reverse light/dark cycle, with lights off at 7:00 and lights on at 19:00. Animals were given ad libitum access to food and water. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the U.S. National Academy of Sciences and were approved by the IACUC of University of Maryland and the National Institutes of Health National Institute of Drug Abuse.
Jugular Vein Catheterization.
The cocaine self-administration procedures, surgeries, and training schedules were the same as previously reported (Xi et al., 2007). Animals were prepared for self-administration experiments by surgical catheterization of right external jugular vein. Venous catheters, which were constructed of Micro-Renathane, inner diameter 0.012 inches, outer diameter 0.025 inches, (Braintree Scientific, Braintree, MA) was inserted 1.2 cm into the right jugular under sodium pentobarbital anesthesia (65 mg/kg i.p.) with standard aseptic surgical procedures. Catheters exited the jugular vein and passed subcutaneously to the top of the skull where they were connected to a modified 24-gauge cannula (Plastics One, Roanoke, VA) and mounted to the skull with jeweler’s screws and dental acrylic. During experimental procedures catheters were connected to an infusion pump via tubing encased in a protective metal spring from the head mount to the top of the chamber. Catheters were flushed directly prior to and directly following training sessions with a combination of heparin/gentamicin (5 IU + 10 mg/ml) in 0.9% saline solution to prevent blockage and infections.
Apparatus.
Intravenous self-administration experiments were conducted in operant test-response chambers 32 × 25 × 33cm (Med Associates Inc., St. Albans, VT). House lights were turned on at the beginning of each session. Every test chamber contained two test levers, one active and one inactive. Depression of the active lever activated infusion pump; depression of inactive lever was counted but had no consequence. The house light was turned on at the start of each 3-hour test session. To aid acquisition and maintenance of drug self-administration behavior, each drug infusion was always paired with a conditioned cue-light and a cue-sound (tone). The light and the speaker were installed 12 cm above the active lever. The light and tone were paired (on) during the 4.6-second drug infusion duration. There was no time-out after the fixed-ratio (FR) 1 or 2 drug infusion. All the animals were first trained on FR1 for approximately seven training sessions with 1 mg/kg per infusion of cocaine (to facilitate the acquisition of self-administered cocaine), and then switched to FR2 for 0.5 mg/kg per infusion of cocaine self-administration in the following experiments. Self-administration tests were completed during FR2 conditions. Scheduling of experimental events and data collection were accomplished using Med Associates software.
Cocaine Self-Administration Training.
Animals were allowed to recover from surgical catheterization. After recovery, they were placed into operant chamber and allowed to lever press for intravenous cocaine (1 mg/kg per infusion) delivered as 0.08 ml over an intertrial interval of 4.6 seconds on an FR1 reinforcement schedule. During the intertrial interval, additional lever presses were recorded but did not result in additional infusions. After achieving stable cocaine self-administration, animals were transitioned to 0.5 mg/kg per infusion on a FR1 schedule and transitioned to the final schedule of 0.5 mg/kg per infusion on an FR2 schedule. Each test session lasted 3 hours. After stable self-administration, pretreatments of 3 mg/kg, 5 mg/kg, or 10 mg/kg l-THP, 0.1 mg/kg LDN, and 3 mg/kg + 0.1 mg/kg, 5 mg/kg + 0.1 mg/kg, or 10 mg/kg + 0.1 mg/kg l-THP + LDN were administered intraperitoneally 30 minutes prior to the experimental session.
Cocaine Reinstatement.
Following stable self-administration experiments, seven consecutive training session in which animals demonstrated less that 10% change in drug-taking behavior, animals were transitioned to extinction, in which depression of the previously active lever resulted in no infusion, cue, or light signal. Infusion syringes were filled with physiologic saline. Animals were randomly assigned to one of the treatment groups described in Table 1. Immediately following each test session, animals were weighed and given an intraperitoneal treatment injection corresponding to their assigned group. Extinction continued until animals responded with a number of active lever presses no more than 10% of that achieved during cocaine self-administration over the 3-hour period. Twenty-four hours after extinction was achieved, animals were reinstated using i.p. 10 mg/kg cocaine (Amen et al., 2011). During cocaine reinstatement, light and environmental cues remained off.
TABLE 1 .
l-THP and LDN Treatments During Extinction
| Self-Administration | Extinction Treatment (Daily) | Reinstatement Treatment |
|---|---|---|
| Cocaine 0.5 mg/kg per inf. FR2 | Vehicle (n = 7) | Cocaine 10 mg/kg i.p. |
| None (n = 5) | 3 mg/kg l-THP and 0.1 mg/kg LDN 30 minutes prior | |
| Cocaine 10 mg/kg i.p. | ||
| None (n = 9) | 5 mg/kg l-THP and 0.1mg/kg LDN 30 minutes prior | |
| Cocaine 10 mg/kg i.p. | ||
| 0.1 mg/kg LDN (n = 6) | Cocaine 10 mg/kg i.p. | |
| 3 mg/kg l-THP (n = 9) | Cocaine 10 mg/kg i.p. | |
| 5 mg/kg l-THP (n = 10) | Cocaine 10 mg/kg i.p. | |
| 3 mg/kg l-THP and 0.1mg/kg LDN (n = 10) | Cocaine 10 mg/kg i.p. | |
| 5 mg/kg l-THP and 0.1mg/kg LDN (n = 11) | Cocaine 10 mg/kg i.p. |
Spontaneous Locomotor Activity.
Locomotor experiments were conducted in VersaMax open-field test chambers 40 cm × 40 cm (Omnitech Electronics Inc., Columbus, OH). Habituation and baseline were established over 3 days. Animals were weighed prior to each session and randomly assigned to treatment groups described in Table 2. During the drug treatment phase, animals were placed into open-field chambers for 1 hour. After the first hour, animals were removed, injected (intraperitoneally) with the assigned treatment, and placed back into the chamber for a second hour. Treatment continued for 10 days. Ten days was chosen as the typical extinction treatment period in the reinstatement experiment. Locomotion score was calculated by subtracting baseline average from total movements after injections.
TABLE 2 .
l-THP and LDN Treatment on Locomotion
| Habituation (Days 1–3) | Treatment (Days 4–13) |
|---|---|
| No Treatment | Vehicle |
| 0.1 mg/kg LDN | |
| 3 mg/kg l-THP | |
| 5 mg/kg l-THP | |
| 3 mg/kg l-THP and 0.1mg/kg LDN | |
| 5 mg/kg l-THP and 0.1mg/kg LDN |
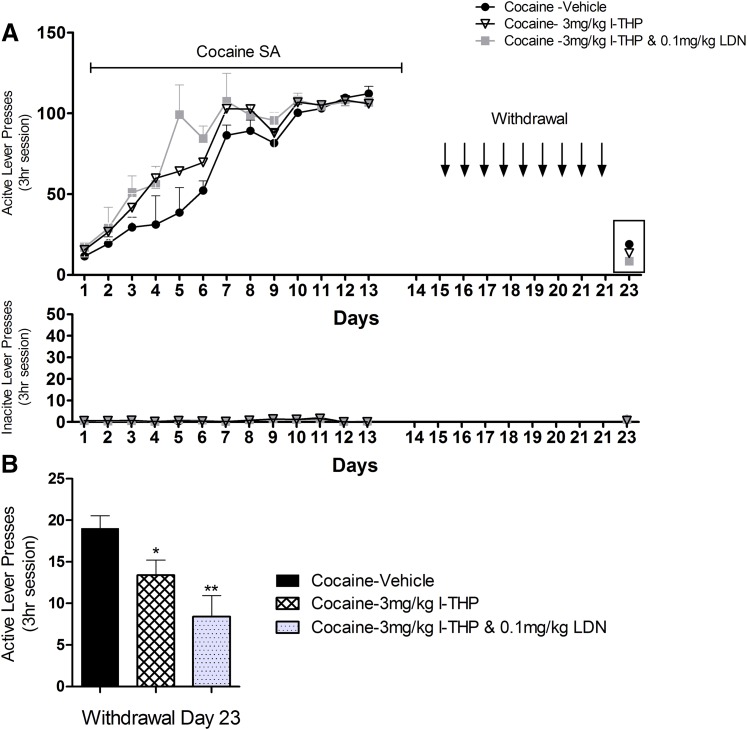
Cocaine Forced Abstinence.
After self-administering cocaine or sucrose for 13 sessions, animals were moved to forced abstinence. Forced abstinence differed from extinction in that animals remained in their home cages, were handled, administered injections every 24 hours, and did not return to the operant chambers until day 23. Groups are described in Table 3. On day 23, animals were placed back into operant chambers to measure drug craving, as determined by active lever presses. Animals were sacrificed 30 minutes following final session and tissue and plasma were harvested.
TABLE 3 .
l-THP and LDN Treatment on Cue Induced Cocaine-Seeking
| Self-Administration | Forced Abstinence Treatment (Daily) |
|---|---|
| Cocaine 0.5 mg/kg per inf. | Vehicle |
| 3 mg/kg l-THP | |
| 3 mg/kg l-THP and 0.1 mg/kg LDN |
Tissue Sample Collection.
Thirty-minutes after the final training session, animals were rapidly sacrificed via decapitation. Brains were quickly removed and immersed into dry ice-chilled methyl-butane for 10 seconds and stored in Tissue-Tek OCT medium (Sakura, Torrance, CA) at –80°C. Blood was collected from the trunk post-decapitation and stored in Lavender Vacutainer Tubes containing EDTA (Becton Dickinson, Franklin Lakes, NJ). Blood was transferred to centrifuge tubes containing aprotinin. Blood samples were centrifuged at 1600g for 15 minutes at 4°C and the plasma was removed and stored at –80°C.
Quantification of β-Endorphin Content.
β-Endorphin was quantified using a commercially available EIA kit (Phoenix Pharmaceuticals, Hayward CA). Plasma was acidified using equal amounts of buffer A and centrifuged at 10,000g for 20 minutes at 4°C. A SEP-column was equilibrated using washing buffer B followed by washing buffer A. Plasma was loaded onto the column and washed three times with buffer A. Peptides were eluted using buffer B and the eluent was collected into a polystyrene tube, evaporated, and stored at –20°C. Peptides were reconstituted with 125 μl of assay buffer. Reconstituted samples were further diluted to 1:50 with assay buffer. Assay was performed according to manufacturer’s specifications. Absorbance was read at 450 nm.
Visualization and Quantification of POMC by RNAscope.
Brains were rapidly harvested and snap-frozen 30 minutes after the completion of the above behavioral test on day 23 (see Cocaine Forced Abstinence). Brains were sliced 0.14-μm thick and mounted to slides. There were five animals in each of the treatment groups and six slices were analyzed from each animal, for a total of 30 analyzed slices per group. OCT-embedded brains were mounted on cryostat platform with OCT medium. Tissue was sectioned at the arcuate nucleus (Arc), beginning at approximately and –1.7 mm and –3.96 mm bregma as determined from the The Rat Brain in Stereotaxic Coordinates (Paxinos and Watson, 2006), and 0.14-μm tissue slices were mounted on Superfrost Plus Gold Slides (FisherScientific/ThermoFisher Scientific, Sunnyvale, CA).
Tissue sections were fixed in 10% formalin for 15 minutes at 4°C. Slides were dehydrated in ethanol baths of 50%, 70%, and 100% at room temperature. Tissues were then incubated in Pretreatment 4 from the RNAscope Pretreatment Kit (Advanced Cell Diagnostics, Hayward, CA) for 15 minutes at room temperature. Slides were dried and the probe for POMC was added for a 2-hour incubation in a hybridization oven. The hybridization oven was maintained at 40°C for all incubations. Probe hybridization was carried out in the hybridization oven in four amplification steps in alternating 30- and 15-minute incubations. After each amplification step, slides were washed two times in 1× wash buffer for 2 minutes. DAPI was added to slides for 10 seconds and cover-slipped with Fluoro-Gel (Electron Microscopy Services, Hatfield, PA).
Wide-field fluorescent images of the arcuate nucleus were captured using Nikon Eclipse Ti-E with an 20× objective. Images were deconvoluted using NIS Elements Software (Nikon Instruments, Melville, NY ) and pseudo-colored (POMC red, Ubiquitin (Ubc) green, and DAPI blue). Image Processing and Analysis by Java (ImageJ, NIH) was used to quantify total mRNA signal.
Drugs.
The drugs used in the present study were as follows: Cocaine hydrochloride, obtained from NIDA, was dissolved in physiologic saline. Levo-tetrahydropalmatine was obtained from the laboratory of Stephen Hoag at the University of Maryland, Baltimore. The l-THP was dissolved in 0.1 M H2SO4, brought to a pH of 5.5 ± 0.2 with 0.1 M NaOH and brought to correct volume with sterile water. l-THP concentrations of 3 mg/kg, 5 mg/kg, and 10 mg/kg were used in the study. For l-THP and LDN combinations, naltrexone hydrochloride (Sigma-Aldrich/SigmaMillipore, St. Louis, MO) was dissolved in the l-THP solution to a concentration of 0.1 mg/kg. The 0.1 M H2SO4/0.1 M NaOH/sterile water solution, pH of 5.5 ± 0.2, was used as vehicle and for the 0.1 mg/kg LDN dose. Additionally, each solution was syringe-passed through 0.22-μm filters to ensure the solutions were free of contaminates.
Data Analysis.
Statistical analyses were performed using GraphPad Prism 5.00 (La Jolla, CA) for Windows. Data are presented as mean ± S.E.M. Student’s t test. One-way and two-way analyses of variance (ANOVAs) followed by Bonferroni analysis were used for comparisons between groups where appropriate. Effect size was calculated using η2 for ANOVAs and Pearson’s r for correlations, and acceptable level of statistical significance for all tests was P < 0.05.
Results
Effects of l-THP and LDN on Cocaine Self-Administration.
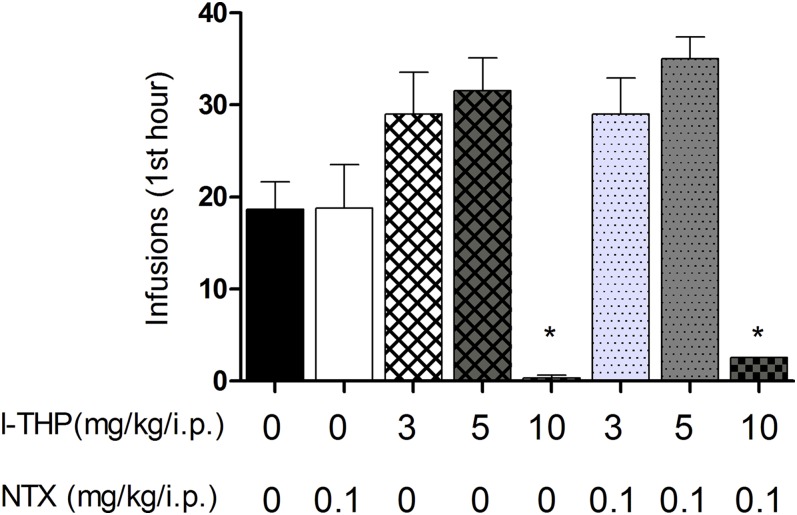
In our study setting, animals acquired stable cocaine self-administration over 14 experimental sessions (111.9 ± 3.975 lever presses per session). Pretreatment with l-THP produced a biphasic drug-taking effect. The low doses of l-THP produced an initial increase in self-administration, whereas the high dose of l-THP produced a reduction in cocaine self-administration (Fig. 1) One-way ANOVA revealed a significant drug treatment main effect [F(7,59) = 11.56, P < 0.001]. Effect-size calculations suggest a moderate to highly significant practical effect [η2 = 0.58]. Post-hoc Bonferroni analyses revealed a significant reduction of cocaine self-administration after 10 mg/kg l-THP and 10 mg/kg l-THP and 0.1 mg/kg LDN combination (P < 0.05). Animals administered LDN exhibited no change in self-administration (P > 0.05). Treatment with the combination l-THP and LDN had no significant effect, as with the respective doses of l-THP alone (P > 0.05).
Fig. 1.
Effect of pretreatment of l-THP and LDN on cocaine self-administration. After animals achieved stable cocaine self-administration, animals were administered l-THP, LDN, or l-THP and LDN 30 minutes prior to experimental session. Intraperitoneal administration of l-THP produced a biphasic effect: Low doses (3 mg/kg, and 5 mg/kg l-THP, n = 7) increased and a high dose (10 mg/kg, n = 7, and 10 mg/kg l-THP and 0.1 mg/kg LDN, n = 7) induced sedation, inhibiting cocaine self-administration. *P < 0.05 compared with vehicle treatment determined by one-way ANOVA. LDN neither altered cocaine self-administration by itself (0.1 mg/kg LDN, n = 7), nor altered l-THP’s effects on cocaine self-administration behavior (3 mg/kg l-THP and 0.1 mg/kg LDN, n = 7 and 5 mg/kg l-THP and 0.1 mg/kg LDN, n = 7). Values are expressed as mean ± S.E.M.
Effects of l-THP and LDN on Cocaine-Induced Reinstatement of Drug-Seeking Behavior.
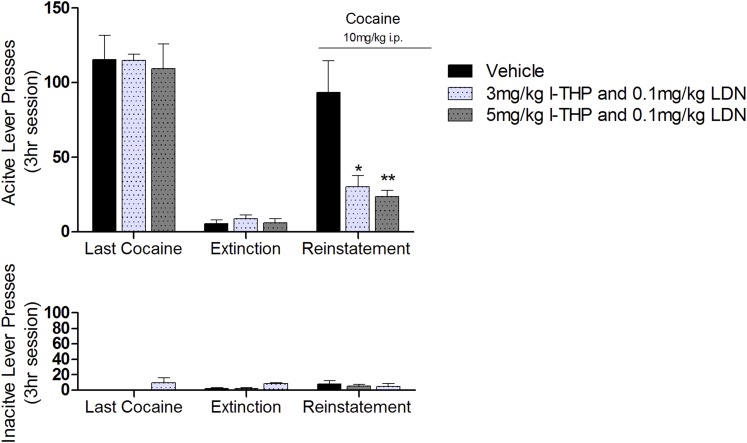
After stable self-administration was achieved, animals went to extinction until drug-seeking behavior was extinguished, defined as active lever responses less than 10% of the responses achieved over a stable cocaine self-administration experimental sessions (6.50 ± 1.42 lever presses per session). Pretreatment with l-THP and/or LDN, administered 30 minutes prior to reinstatement test, significantly attenuated cocaine-induced (10 mg/kg, i.p.) reinstatement of drug-seeking behavior [F(2,63) = 3.54, P < 0.05, two-way ANOVA] (Fig. 2). Effect size, however, was determined to be low to moderate [η2 = 0.38]. Bonferroni post-hoc analyses revealed a significant reduction in drug-seeking after 3 mg/kg l-THP and 0.1 mg/kg LDN (P < 0.05) and the 5 mg/kg l-THP and 0.1 mg/kg (P < 0.01) compared with the vehicle control group.
Fig. 2.
Effect of single pretreatment of l-THP and LDN on cocaine reinstatement. In the experimental session following extinction, animals were administered intraperitoneal injections of l-THP and LDN 30 minutes prior to a priming dose of 10 mg/kg cocaine. Two-way ANOVA showed that 3 mg/kg l-THP and 0.1 mg/kg LDN (n = 5) and 5 mg/kg l-THP and 0.1 mg/kg LDN (n = 9) dose-dependently and significantly inhibited reinstatement of cocaine-seeking behavior assessed by active lever presses. *P < 0.5, **P < 0.01 respectively, compared with vehicle group (n = 7). Values expressed as mean ± S.E.M.
Effects of Chronic l-THP and LDN Pretreatment on Cocaine-Induced Reinstatement of Drug-Seeking Behavior.
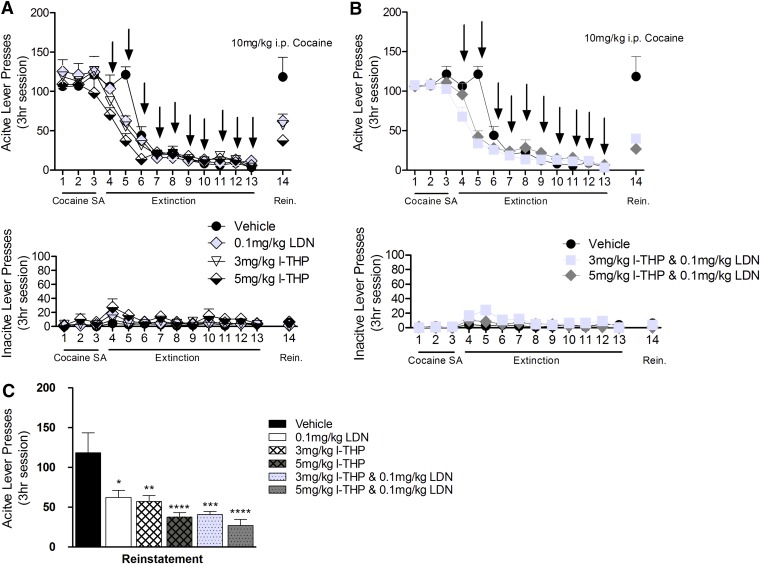
Animals in this experiment were exposed to the same self-administration conditions used in the previous experiment; however, during extinction training the animals were given l-THP and/or LDN daily for 10 consecutive days during extinction. At 24 hours after the last drug injection, cocaine-induced reinstatement of drug-seeking behavior was significantly lower in all drug-treatment groups than in the vehicle control group of rats (Fig. 3). One-way ANOVA for the summarized data in Fig. 3C revealed a significant treatment main effect [F(5,51) = 9.085, P < 0.001]. Effect-size calculation suggests a moderate applied effect [η2 = 0.49]. Bonferroni post-hoc analyses demonstrated a significant reduction in cocaine-seeking after 0.1 mg/kg LDN (P < 0.05), 3 mg/kg l-THP (P < 0.01), 3 mg/kg l-THP and 0.1 mg/kg LDN (P < 0.001), 5 mg/kg l-THP (P < 0.001), and 5 mg/kg l-THP and 0.1 mg/kg LDN (P < 0.001) administration.
Fig. 3.
Effect of 10 days administration of l-THP and LDN on cocaine reinstatement. Animals received intraperitoneal injections for 10 days until extinction was reached. In the experimental session following, animals were primed with 10 mg/kg cocaine and placed back into operant chambers. (A and B) Extinction timeline, arrows representing administration of l-THP (3 mg/kg, n = 9 and 5 mg/kg, n = 10), LDN (0.1 mg/kg, n = 6), or l-THP and LDN combinations (3 mg/kg and 0.1 mg/kg, n = 10, and 5 mg/kg and 0.1 mg/kg, n = 11). (C) Significantly inhibited reinstatement of drug-seeking behavior assessed by active lever responses, expressed as mean ± S.E.M. Bonferroni post-hoc analysis determined that both the combinations of 3 mg/kg l-THP and 0.1 mg/kg LDN and 5 mg/kg l-THP and 0.1 mg/kg LDN were more efficacious than any of the single doses. *P < 0.5, **P < 0.1, ***P < 0.001, ****P < 0.0001 compared with vehicle group (n = 7).
Effects of l-THP and LDN on Locomotion.
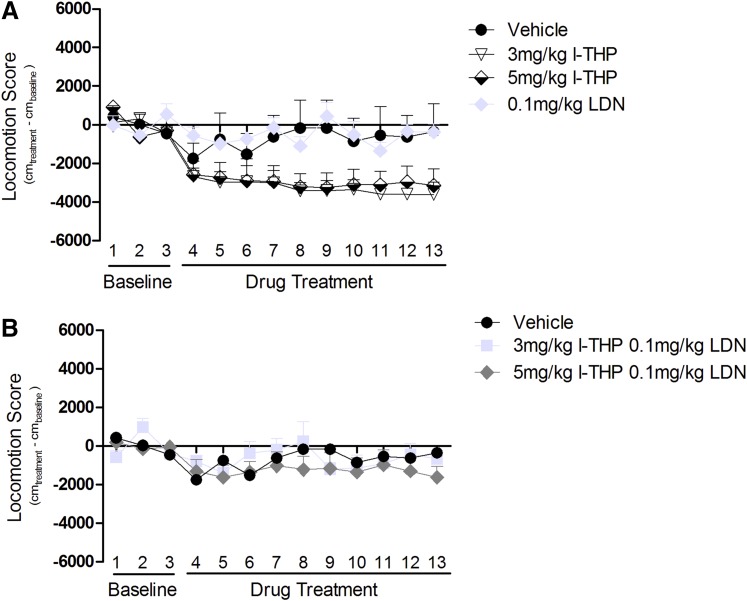
Animals were administered l-THP, LDN, or l-THP + LDN daily for 10 consecutive days. The timeline of 10 days was selected on the basis of the findings in the above reinstatement experiments (see previous section). The baseline was created using the average of the spontaneous locomotion measurements taken over the first 3 consecutive days (3381 ± 168.1 cm average baseline). Baseline was subtracted from total movements for each day. A dose of 3 mg/kg or 5 mg/kg l-THP alone significantly inhibited spontaneous locomotor activities over the 60 minutes (two-way ANOVA, [F(2,140) = 22.34, P < 0.001]), whereas 0.1 mg/kg LDN alone had no effect on spontaneous locomotion (Fig. 4A). Both combination doses of l-THP and LDN did not produce a significant reduction in spontaneous locomotion [F(2,195) = 0.2794, P = 0.2794, two-way ANOVA] (Fig. 4B). Effect size was determined to be moderate to highly significant [η2 = 0.51].
Fig. 4.
Effect of l-THP and LDN on spontaneous locomotion. Animals established a baseline over 3 consecutive days; following that they were given intraperitoneal injections of the assigned treatments for 10 days. Values are expressed as mean of the locomotor score ± S.E.M., n = 6 for all groups. Two-way ANOVA determined treatment had a significant effect on spontaneous locomotion. ****P < 0.0001. (A) l-THP (3 mg/kg and 5 mg/kg) produced a dose-dependent reduction on locomotion, and LDN (0.1 mg/kg) had no effect on locomotion. (B) Effect of coadministration of l-THP and LDN.
Effects of l-THP and LDN on Cue-Induced Cocaine-Seeking.
After stable cocaine self-administration was achieved, animals underwent forced abstinence in their home cages in the animal facility for 9 days, during which 3 mg/kg l-THP, 3 mg/kg l-THP, and 0.1 mg/kg LDN, or vehicle was given daily to three separate groups of rats (Fig. 5A). Animals were then placed back into the same self-administration chambers where they obtained cocaine self-administration before on day 23. Re-exposure to cocaine-associated contextual cues induced a significant cocaine-seeking behavior (i.e., active lever presses) in the absence of cocaine or cocaine-associated cues (Fig. 5B, the vehicle control group). Pretreatment with 3 mg/kg l-THP or 3 mg/kg l-THP and 0.1 mg/kg LDN produced a significant reduction in cue-induced drug-seeking compared with the vehicle control group and the combination group was more effective then l-THP alone. One-way ANOVA for the data shown in Fig. 5B revealed a significant treatment main effect [F(3,19) = 3.413, P = 0.043]. Moreover, effect-size calculations revealed a moderate practical effect [η2 = 0.40]. Student’s t tests revealed a significant reduction in cocaine-seeking after 3 mg/kg l-THP [t(8) = 2.354, P = 0.0464] or 3 mg/kg l-THP and 0.1 mg/kg LDN [t(8) = 3.581 P = 0.0072].
Fig. 5.
Self-administration and drug-seeking behavior. (A) Active and inactive lever presses over the course of the experimental timeline. There was no statistical difference in stable self-administration between the groups. (B) Comparison of drug-seeking behavior measured by active lever presses. After 13 self-administrations sessions, animals were moved to forced abstinence, in which they received treatment in their home cages for 10 days. On day 23 they were placed back into the operant chambers, with all cues and rugs removed; values are expressed as mean ± S.E.M.; n = 5 for all groups. There was a significant difference in active lever responses on day 23. *P = 0.0371. Student’s t test determined a significant difference between the cocaine-vehicle group and the cocaine- 3 mg/kg l-THP and 0.1 mg/kg LDN group. **P = 0.0073. There was also less significant difference between cocaine-vehicle group and cocaine 3 mg/kg l-THP group. *P = 0.0464.
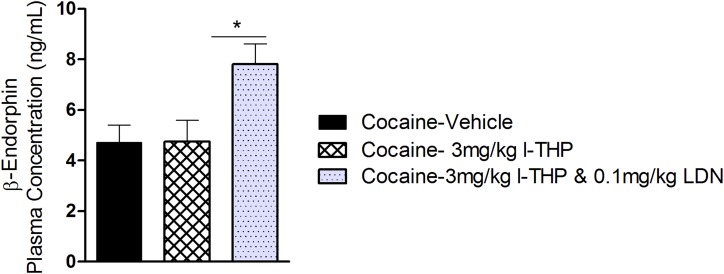
Effects of l-THP and LDN on Plasma Concentrations of β-Endorphin in Rats after Forced Cocaine Abstinence.
After the completion of the above behavioral test, plasma samples were collected 30 minutes via the implanted intravenous catheter. Coadministration of 3 mg/kg l-THP and 0.1 mg/kg LDN significantly elevated plasma β-endorphin level compared with the vehicle control group. A dose of 3 mg/kg l-THP alone had no effect (Fig. 6). One-way ANOVA for the data shown in Fig. 6 revealed a significant treatment main effect [F(3,39) = 3.92, P = 0.016]. However the effect-size calculations demonstrate a moderate to low significant effect [η2 = 0.24].
Fig. 6.
Mean ± S.E.M. concentration of plasma β-endorphin during forced abstinence. Plasma was obtained 30 minutes directly following last experimental session on day 23. There was a significant difference in β-endorphin concentration between the cocaine plus 3 mg/kg l-THP and 0.1 mg/kg LDN group and the cocaine plus vehicle group (*P < 0.05), as well as between the cocaine plus 3 mg/kg l-THP and 0.1 mg/kg LDN group and the cocaine plus 3 mg/kg l-THP group. *P < 0.05. Samples were analyzed in duplicates; n = 5 for all groups.
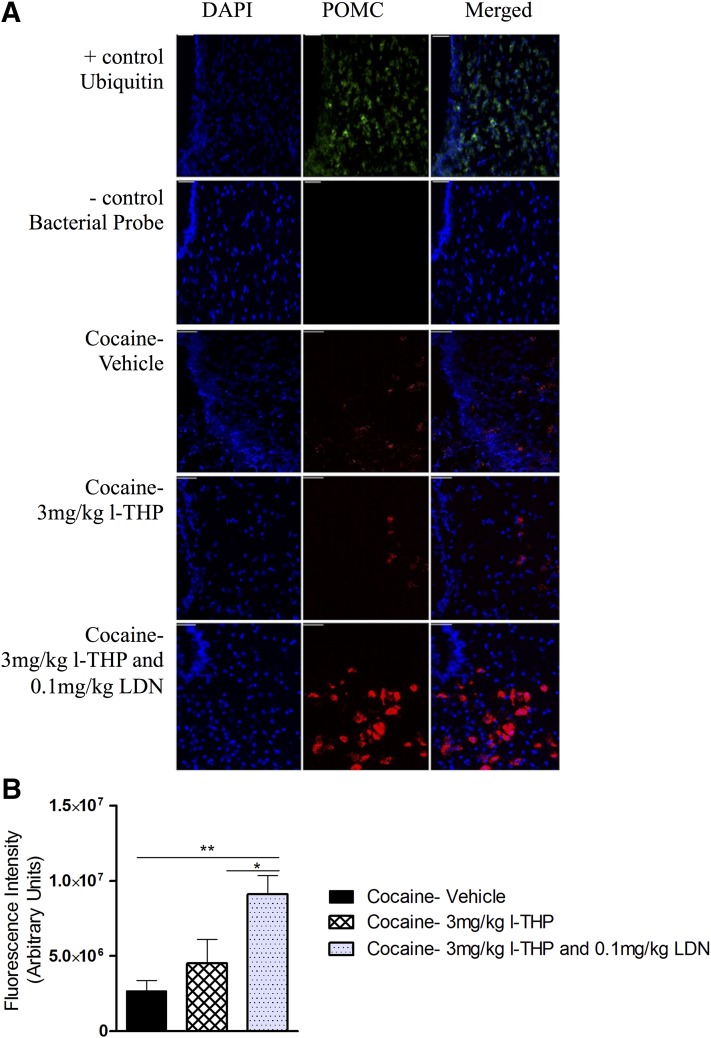
Effects of l-THP and LDN on POMC mRNA Expression in Rats after Forced Cocaine Abstinence.
Figure 7A shows the RNAscope in situ hybridization data, illustrating that coadministration of 3 mg/kg l-THP and 0.1 mg/kg LDN significantly elevated POMC expression in the hypothalamus, whereas 3 mg/kg l-THP alone had no effect. One-way ANOVA for the summarized data shown in Fig. 7B revealed a significant treatment main effect [F(3,119) = 6.529, P = 0.0004]. Effect-size calculations suggest only a minimal practical effect [η2 = 0.14]. Bonferroni post-hoc analyses indicated a significant increase in POMC expression after 3 mg/kg l-THP and 0.1 mg/kg LDN (P < 0.05) but not after 3 mg/kg l-THP alone (P > 0.05) compared with the vehicle group.
Fig. 7.
Fluorescence intensity of POMC mRNA during forced abstinence. (A) POMC mRNA, in red, detected by RNAscope. Brains were harvested and snap-frozen 30 minutes following the last experimental session on day 23. Six 0.14-μm coronal sections were taken from each animal (n = 5 for all groups, total of 30 sections per group) and analyzed for total POMC mRNA fluorescence between –2 mm and –3.5 mm bregma. 3V, third ventricle; scale bar 100 μm. (B) Fluorescence intensity of POMC mRNA, expressed as mean ± S.E.M. Treatment had a significant effect on POMC mRNA intensity P = 0.0004. Bonferroni post-hoc analysis revealed a significant difference between the cocaine plus vehicle and the cocaine plus 3 mg/kg l-THP and 0.1 mg/kg LDN group. **P < 0.01, between cocaine plus 3 mg/kg l-THP and cocaine plus 3 mg/kg l-THP and 0.1 mg/kg LDN. *P < 0.01.
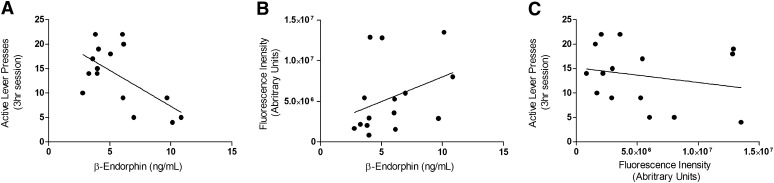
Correlation Between Cocaine-Seeking Behavior, β-Endorphin, and POMC Expression.
Figure 8A shows the strong significant negative correlation between active lever presses during reinstatement and plasma concentrations of β-endorphin [r(15) = –0.617, P = 0.0142]. Also shown in Fig. 8B is the weak positive correlation between POMC expression and plasma concentrations of β-endorphin [r(15) = 0.361, P = 0.187]. A correlation was not observed between POMC expression and active lever presses during reinstatement shown in Fig. 8C [r(15) = –0.214, P = 0.440].
Fig. 8.
Correlation between cocaine-seeking behavior, β-endorphin, and POMC expression. (A) Strong negative correlation between plasma concentrations of β-endorphin and cocaine-seeking behavior during reinstatement, r = –0.617, *P < 0.05. (B) Weak positive correlation, but nonsignificant, between plasma concentrations of β-endorphin and POMC expression, r = .361, P = 0.187 Finally, (C) demonstrates the lack of correlation between cocaine-seeking behavior during reinstatement and POMC expression, r = 0.214, P = 0.440; n = 15 for all correlation analysis.
Discussion
Combination medications are becoming increasingly commonplace in clinical practice and can be especially effective when used to treat individuals suffering from mental disorders and who are multisymptomatic (Tohen et al., 2003; Kupfer et al., 2012; Biler et al., 2014; Shorter et al., 2015). In the case of relapse prevention, drug cravings may be effectively managed when medications treat both behavioral and physiologic symptoms. However, the majority of pharmaceuticals developed for the treatment of substance abuse disorders target one pathway. The combination of l-THP and LDN targets both dopaminergic signaling and the regulation of endogenous opioids. In this report, we provide evidence that the combination of l-THP and LDN is more potent with fewer unwanted side effects than l-THP alone, on the basis of the following observations: 1) l-THP and LDN has demonstrated significantly greater effect in attenuating drug-seeking behavior than l-THP alone; 2) l-THP and LDN when administered together do not result in a reduction of spontaneous locomotion; 3) l-THP and LDN appears to increase peripheral concentrations of β-endorphin; 4) l-THP and LDN appears to upregulate expression of POMC in the arcuate nucleus.
l-THP has been investigated as a potential treatment of addiction by several other groups (Mantsch et al., 2007, 2010; Figueroa-Guzman et al., 2011; Wang and Mantsch, 2012). Presently, we determined that l-THP produced a biphasic effect and this is consistent with the results of previous study (Xi et al., 2007). The increase in cocaine self-administration is attributed to the partial blockade of postsynaptic DA receptors, which decrease the rewarding properties of cocaine and lead to a compensatory increase in drug-taking. Even as the higher doses of l-THP drastically reduced cocaine self-administration, we also noted a strong sedative effect, assessed by a decrease in locomotion, suggesting that nonspecific sedation or locomotion impairment is the main cause for reduced self-administration in the high l-THP dose group. In contrast to l-THP, LDN had no effect on cocaine self-administration. There was no difference in cocaine self-administration between animals receiving lower doses of l-THP or l-THP and LDN.
We observed that pretreatment of l-THP and LDN significantly attenuated cocaine- or cue-induced reinstatement of drug-seeking behavior in a dose-dependent manner. This is consistent with previous findings using l-THP alone (Mantsch et al., 2007, 2010; Figueroa-Guzman et al., 2011; Wang and Mantsch, 2012), suggesting that the combination of l-THP and LDN does not alter therapeutic effects of l-THP. In contrast, there is a trend of enhanced therapeutic effects in both cocaine self-administration and reinstatement tests compared with the effects produced by the same doses of l-THP alone.
Additionally, we extensively investigated the effect of a 10-day treatment on drug-seeking behavior during reinstatement. This dosing schedule was designed to follow a more clinically relevant timeline, as often a repeated daily treatment plan is used as opposed to single treatment prior to a relapse (Katz and Higgins, 2003; Epstein et al., 2006; Yahyavi-Firouz-Abadi and See, 2009; Bailey and Husbands, 2014). Cocaine reinstatement tests were performed about 18–20 hours after the final dosing of l-THP and LDN. Half-lives of l-THP and NTX are 10 hours and 4 hours, respectively, leaving less than 25% and 3.125% of the bolus doses of l-THP and LDN biologically available at the time of reinstatement test (Dunbar et al., 2006; Slawson et al., 2007; Wu et al., 2013), suggesting that repeated drug treatment produced a prolonged therapeutic effect in prevention of relapse to drug-seeking behavior. To our knowledge we are the first to report that the combination of l-THP and LDN as well as l-THP alone administered on this schedule significantly attenuates drug-seeking behavior. A previous study conducted by Gerrits et al. (2005) observed 3 mg/kg NTX attenuates cocaine reinstatement only when given repeatedly; the authors suggested that the endogenous opioid system, specifically β-endorphin, may be involved in relapse to cocaine-seeking behavior.
The 10-day administration of l-THP reduced spontaneous locomotion at both 3 mg/kg and 5 mg/kg doses. Previous experiments reported observing sedation at slightly higher doses; however, we attributed the observed differences to changes in the locomotion experimental design (Xi et al., 2007; Xu et al., 2013). Coadministration of l-THP with LDN prevented the reduction of locomotion produced by l-THP alone; yet LDN alone did not alter locomotor behavior at such a low dose, suggesting that LDN coadministration may block the sedative effects of l-THP. This effect may be mediated through brief opioid receptor antagonism leading to a transient β-endorphin release (Corder et al., 2013). In turn, the β-endorphin induces the release of DA in the nucleus accumbens via activation of μ-opioid receptors on GABAergic neurons that inhibits ventral tegmental area GABAergic neurons (Simmons and Self, 2009; Spanagel et al.1991), resulting in the overall increase of spontaneous locomotion.
To test this β-endorphin hypothesis, we further investigated the effect of l-THP and LDN on plasma β-endorphin and on the expression of the precursor polypeptide POMC and measured both β-endorphin and POMC in rats after 10 days of withdrawal from the last cocaine self-administration session in the absence or presence of l-THP and LDN pretreatment. The 3-mg/kg l-THP and 0.1-mg/kg LDN treatment significantly increased plasmas β-endorphin concentrations, supporting our hypothesis that treatment with l-THP and LDN in rats during forced abstinence may initially increase brain β-endorphin release, resulting in an increase in peripheral concentrations of β-endorphin. We found a significant reduction of POMC gene expression in the cocaine self-administration group compared with the sucrose self-administration group. Pretreatment with l-THP and LDN might renormalize (or increase) brain POMC expression. We do, however, acknowledge that there could be several other mediators at play whose activation could also elicit a similar effect to the one seen in the present study. POMC is also the precursor to adrenocorticotropic hormone and melanocyte-stimulating hormone, and therefore the effect on POMC could be unrelated to the result of one the factors outside the scope of these experiment.
Our findings solidify a connection between cocaine-seeking behavior and the endogenous opioids and corroborate the findings of many other groups (Olive et al., 2001; Kiefer et al., 2002; Marinelli et al., 2003, 2004; Roth-Deri et al., 2003, 2008; Shaw and al’Absi, 2008; Seo et al., 2009; Nguyen et al., 2012; Dikshtein et al., 2013; Zhou et al., 2013). β-endorphin is known to play an important role in addictions. In recovering smokers, attenuated concentrations of β-endorphin directly contribute to smoking relapse (Marinelli et al., 2003; Roth-Deri et al., 2008; Shaw and al’Absi, 2008). In addition to being a contributing factor in cocaine, nicotine, and heroin craving, a β-endorphin deficit is hypothesized as a contributing factor in relapse of alcohol use (Roth-Deri et al., 2004; Dikshtein et al., 2013). Hypothalamic POMC expression is significantly reduced in animals that return to heroin-seeking behavior during relapse (Zhou, Y et al., 2015).
We believe the evidence presented supports a β-endorphin-deficit hypothesis. Other groups have demonstrated a correlation between attenuated β-endorphin release in the brain and cocaine-seeking behavior (Dikshtein et al., 2013); however, to our knowledge we are the first to demonstrate a strong negative correlation between reinstatement plasma β-endorphin. From a more meaningful and practical point of view, post-hoc analysis demonstrated weak positive correlation between plasma levels of β-endorphin and brain POMC expression. Although this correlation was not significant, we believe that this result was limited by of our relatively small sample size and hypothesize that future experiments conducted with a larger sample size would demonstrate a significant positive correlation between plasma β-endorphin and POMC expression. Alternatively, the weak correlation may be the result of other modulations of POMC expression, which are beyond the scope of the present study.
LDN is used as an off-label treatment of autoimmune disorders; however, there is no conclusive study examining its mechanism (Brown and Panksepp, 2009). As l-THP is well documented not to possess affinity for opioid receptors, the changes in β-endorphin and POMC expression are believed to be the result of treatment with LDN. However, the complete mechanism by which l-THP or the combination of l-THP and LDN exert their effects remains elusive. L-THP is reported to facilitate DA release in the nucleus accumbens, normalizing DA signaling. DA changes combined with increase of hypothalamic POMC and β-endorphin during forced abstinence presumably reduce drug cravings, attenuating drug-seeking behavior. Furthermore, the β-endorphin release increases spontaneous locomotion in rodents, which explains spontaneous locomotion observed between animals treated with l-THP and l-THP and LDN (Spanagel et al., 1991; Marquez et al., 2008).
In summary, the data presented is evidence of the therapeutic potential of the combination of l-THP and LDN as an efficacious treatment for prevention of cocaine relapse. We hypothesize l-THP and LDN mediates release of endogenous opioids and dopamine, allowing dopaminergic signaling in the brain to approach preaddiction homeostasis. This treats both the behavioral symptoms and physiologic symptoms observed during recovery and reduces drug cravings and relapses. Future studies should explore the proposed mechanism of LDN as well as validate efficacy of l-THP and LDN in human trials.
Acknowledgments
The authors would like to thank Guo-Hua Bi, Haying Zhang, and Xiao-Fei Wang for technical assistance.
Abbreviations
- ANOVA
analysis of variance
- DA
dopamine
- DAPI
2-(4-amidinophenyl)-1H-indole-6-carboxamidine
- FR
fixed-ratio
- LDN
low dose naltrexone
- l-THP
levo-tetrahydropalmatine
- NTX
naltrexone
- POMC
pro-opiomelanocortin
- Ubc
Ubiquitin
Authorship Contributions
Participated in research design: Xi, Wang, Sushchyk.
Conducted experiments and preformed data analysis: Sushchyk.
Wrote or contributed to writing of the manuscript: Xi, Wang, Sushchyk.
Footnotes
The National Institutes of Health National Institute on Drug Abuse [Grant DA-031401 (J.B.W.)] and the National Institute on Drug Abuse Intramural Research Program supported this work. The authors declare no finical conflict.
References
- Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, Baker DA. (2011) Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology 36:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Husbands SM. (2014) Novel approaches for the treatment of psychostimulant and opioid abuse - focus on opioid receptor-based therapies. Expert Opin Drug Discov 9:1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Panksepp J. (2009) Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses 72:333–337. [DOI] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, et al. (2013) Constitutive μ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 341:1394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordery SF, Taverner A, Ridzwan IE, Guy RH, Delgado-Charro MB, Husbands SM, Bailey CP. (2014) A non-rewarding, non-aversive buprenorphine/naltrexone combination attenuates drug-primed reinstatement to cocaine and morphine in rats in a conditioned place preference paradigm. Addict Biol 19:575–586. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. (2001) Cocaine dependence: a disease of the brain’s reward centers. J Subst Abuse Treat 21:111–117. [DOI] [PubMed] [Google Scholar]
- Dikshtein Y, Barnea R, Kronfeld N, Lax E, Roth-Deri I, Friedman A, and Yadid G (2013) Beta-endorphin via the delta opioid receptor is a major factor in the incubation of cocaine craving. Neuropsychopharmacology 38:2508–2514 10.1038/npp.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding GS. (1987) Important Chinese herbal remedies. Clin Ther 9:345–357. [PubMed] [Google Scholar]
- Dunbar JL, Turncliff RZ, Dong Q, Silverman BL, Ehrich EW, Lasseter KC. (2006) Single- and multiple-dose pharmacokinetics of long-acting injectable naltrexone. Alcohol Clin Exp Res 30:480–490. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Guzman Y, Mueller C, Vranjkovic O, Wisniewski S, Yang Z, Li SJ, Bohr C, Graf EN, Baker DA, Mantsch JR. (2011) Oral administration of levo-tetrahydropalmatine attenuates reinstatement of extinguished cocaine seeking by cocaine, stress or drug-associated cues in rats. Drug Alcohol Depend 116:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits, M. A., Kuzmin, A. V., and van Ree, J. M. (2005). Reinstatement of cocaine-seeking behavior in rats is attenuated following repeated treatment with the opioid receptor antagonist naltrexone. European neuropsychopharmacology, 15(3):297–303. [DOI] [PubMed] [Google Scholar]
- Grassi MC, Cioce AM, Giudici FD, Antonilli L, Nencini P. (2007) Short-term efficacy of Disulfiram or Naltrexone in reducing positive urinalysis for both cocaine and cocaethylene in cocaine abusers: a pilot study. Pharmacol Res 55:117–121. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. (2001) Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 155:330–337. [DOI] [PubMed] [Google Scholar]
- Jin GZ, Wang XL, Shi WX. (1986a) Tetrahydroprotoberberine–a new chemical type of antagonist of dopamine receptors. Scientia Sinica Series B 29:527–534. [PubMed] [Google Scholar]
- Katz JL, Higgins ST. (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 168:21–30. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Horntrich M, Jahn H, Wiedemann K. (2002) Is withdrawal-induced anxiety in alcoholism based on beta-endorphin deficiency? Psychopharmacology (Berl) 162:433–437. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Yan LD, Zhou PL, Wu CF, Gong ZH. (2009) Levo-tetrahydropalmatine attenuates oxycodone-induced conditioned place preference in rats. Eur J Pharmacol 602:321–327. [DOI] [PubMed] [Google Scholar]
- Mannelli, P., Peindl, K., Patkar, A. A., Wu, L. T., Pae, C. U., and Gorelick, D. A. (2010). Reduced cannabis use after low-dose naltrexone addition to opioid detoxification. J Clin Psychopharmacol 30:476-478. DOI:10.1097/JCP.0b013e3181e5c168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Li SJ, Risinger R, Awad S, Katz E, Baker DA, Yang Z. (2007) Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology (Berl) 192:581–591. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Wisniewski S, Vranjkovic O, Peters C, Becker A, Valentine A, Li SJ, Baker DA, Yang Z. (2010) Levo-tetrahydropalmatine attenuates cocaine self-administration under a progressive-ratio schedule and cocaine discrimination in rats. Pharmacol Biochem Behav 97:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcenac F, Jin GZ, Gonon F. (1986) Effect of l-tetrahydropalmatine on dopamine release and metabolism in the rat striatum. Psychopharmacology (Berl) 89:89–93. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. (2003) A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 169:60–67. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. (2004) An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience 127:777–784. [DOI] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Dabaja I, Gajawada N, Lutfy K. (2008) The role of beta-endorphin in the acute motor stimulatory and rewarding actions of cocaine in mice. Psychopharmacology (Berl) 197:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2005). The neurobiology of cocaine addiction. Sci Pract 3:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Marquez P, Hamid A, Kieffer B, Friedman TC, Lutfy K. (2012) The rewarding action of acute cocaine is reduced in β-endorphin deficient but not in μ opioid receptor knockout mice. Eur J Pharmacol 686:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. (2001) Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci 21:RC184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos, G., Watson, C. (2006). The Rat Brain in Stereotaxic Coordinates (6th ed.). Cambridge, MA: Academic Press. [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. (2002) Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 163:265–282. [DOI] [PubMed] [Google Scholar]
- Rahn KA, McLaughlin PJ, Zagon IS. (2011) Prevention and diminished expression of experimental autoimmune encephalomyelitis by low dose naltrexone (LDN) or opioid growth factor (OGF) for an extended period: Therapeutic implications for multiple sclerosis. Brain Res 1381:243–253. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Zangen A, Aleli M, Goelman RG, Pelled G, Nakash R, Gispan-Herman I, Green T, Shaham Y, Yadid G. (2003) Effect of experimenter-delivered and self-administered cocaine on extracellular beta-endorphin levels in the nucleus accumbens. J Neurochem 84:930–938. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Schindler CJ, Yadid G. (2004) A critical role for beta-endorphin in cocaine-seeking behavior. Neuroreport 15:519–521. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Green-Sadan T, Yadid G. (2008) Beta-endorphin and drug-induced reward and reinforcement. Prog Neurobiol 86:1–21. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. (2001) Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav 26:167–180. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Lindsay JA, Green CE, Herin DV, Stotts AL, Moeller FG. (2009) High-dose naltrexone therapy for cocaine-alcohol dependence. Am J Addict 18:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Green CE, Stotts AL, Lindsay JA, Rathnayaka NS, Grabowski J, Moeller FG. (2014) A two-phased screening paradigm for evaluating candidate medications for cocaine cessation or relapse prevention: modafinil, levodopa-carbidopa, naltrexone. Drug Alcohol Depend 136:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D, Macdonald JK, Chande N. (2014) Low dose naltrexone for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2:CD010410. [DOI] [PubMed] [Google Scholar]
- Seo YJ, Kwon MS, Choi SM, Lee JK, Park SH, Jung JS, Sim YB, Suh HW. (2009) Possible involvement of the hypothalamic pro-opiomelanocortin gene and beta-endorphin expression on acute morphine withdrawal development. Brain Res Bull 80:359–370. [DOI] [PubMed] [Google Scholar]
- Shaw D, and al’Absi M (2008). Attenuated beta endorphin response to acute stress is associated with smoking relapse. Pharmacol Biochem Behav 90:357–362. DOI:10.1016/j.pbb.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter D, Kosten TR. (2011) Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med 9:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, Self DW. (2009) Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology 34:1946–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawson MH, Chen M, Moody D, Comer SD, Nuwayser ES, Fang WB, Foltz RL. (2007) Quantitative analysis of naltrexone and 6beta-naltrexol in human, rat, and rabbit plasma by liquid chromatography-electrospray ionization tandem mass spectrometry with application to the pharmacokinetics of Depotrex in rabbits. J Anal Toxicol 31:453–461. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Bals-Kubik R, Shippenberg TS. (1991) Beta-endorphin-induced locomotor stimulation and reinforcement are associated with an increase in dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 104:51–56. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Martin-García E, Berrendero F, Robledo P, Maldonado R. (2010) The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend 108:183–194. [DOI] [PubMed] [Google Scholar]
- Ueno K, Kiguchi N, Kobayashi Y, Saika F, Wakida N, Yamamoto C, Maeda T, Ozaki M, Kishioka S (2014). Possible involvement of endogenous opioid system located downstream of α7 nicotinic acetylcholine receptor in mice with physical dependence on nicotine. J Pharmacol Sci 124:47–53. [DOI] [PubMed] [Google Scholar]
- Wang JB, Mantsch JR. (2012) l-tetrahydropalamatine: a potential new medication for the treatment of cocaine addiction. Future Med Chem 4:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Vendruscolo LF, Misra KK, Schlosburg JE, and Koob GF (2012). A combination of buprenorphine and naltrexone blocks compulsive cocaine intake in rodents without producing dependence. Sci Translat MedI 4:146ra110. DOI: 10.1126/scitranslmed.3003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Luo Y, Xu, J, Gu S, Luo X, Wu H, and Zhao G (2013) Determination of levo-tetrahydropalmatine in rat plasma by HPLC and its application to pharmacokinetics studies. Afr J Pharm Pharmacol 7:545–551. [Google Scholar]
- Xi ZX, Yang Z, Li SJ, Li X, Dillon C, Peng XQ, Spiller K, Gardner EL. (2007) Levo-tetrahydropalmatine inhibits cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropharmacology 53:771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wang Y, Ma Z, Chiu YT, Huang P, Rasakham K, Unterwald E, Lee DY, Liu-Chen LY. (2013) L-isocorypalmine reduces behavioral sensitization and rewarding effects of cocaine in mice by acting on dopamine receptors. Drug Alcohol Depend 133:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Shao YC, Li SJ, Qi JL, Zhang MJ, Hao W, Jin GZ. (2008) Medication of l-tetrahydropalmatine significantly ameliorates opiate craving and increases the abstinence rate in heroin users: a pilot study. Acta Pharmacol Sin 29:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Kitchen I, Bailey A. (2012). The endogenous opioid system in cocaine addiction. what lessons have opioid peptide and receptor knockout mice taught us? Br J Pharmacol 166:1993–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon I, Hammer L, and McLaughlin P (2014) Low dose naltrexone inhibits the progression of clinical disease in established relapse-remitting experimental autoimmune encephalomyelitis—a model for multiple sclerosis (651.3). FASEB J 28 (1 Supplement), 651.3.
- Zhou Y, Leri F, Cummins E, Kreek MJ. (2015) Individual differences in gene expression of vasopressin, D2 receptor, POMC and orexin: vulnerability to relapse to heroin-seeking in rats. Physiol Behav 139:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Niikura K, Carai MA, Femenía T, García-Gutiérrez MS, Manzanares J, Ho A, Gessa GL, Kreek MJ. (2013) Voluntary alcohol drinking enhances proopiomelanocortin gene expression in nucleus accumbens shell and hypothalamus of Sardinian alcohol-preferring rats. Alcohol Clin Exp Res 37 (Suppl 1):E131–E140. [DOI] [PMC free article] [PubMed] [Google Scholar]