Abstract
Strategies to develop a functional cure for HIV infection will likely require boosting of effector T cell responses to eliminate reactivated, latently infected cells. We have recently explored an assay for assessing antigen-specific regulation of T cell proliferation, which was related to clinical progression in untreated patients and to vaccine efficacy in two trials of therapeutic Gag-based vaccines. We here expand the same assay to further investigate regulation mediated by various inhibitory pathways. Peripheral blood mononuclear cells from 26 asymptomatic HIV-infected, antiretroviral therapy-naïve patients were stimulated with Gag and Env overlapping peptide panels for 5 days. Monoclonal antibodies (mAbs) blocking inhibitory mediators interleukin (IL) 10, transforming growth factor (TGF) β, programmed death ligand (PD–L) 1 and herpes virus entry mediator (HVEM) were added to parallel cultures. Functional T cell regulation (FTR) was defined as the difference in proliferation between stimulated cultures with and without blocking mAbs. FTR was detected in 54% of patients. Blockade of IL-10/PD-L1 and IL10/TGF-β detected all cases with Gag- and Env-associated FTR, respectively. In accordance with previous findings, isolated Env FTR was associated with higher plasma HIV RNA and lower CD4 counts, while patients with both Gag and Env FTR also had higher Gag- and Env-specific proliferative CD8+ T cell responses. There was no association between FTR and frequencies of activated regulatory T cells. In conclusion, we observed substantial heterogeneity in FTR between patients, inhibitory pathways and HIV antigens. FTR may help to individualize immunomodulation and warrants further assessment in clinical immunotherapy trials.
Introduction
Effective HIV-specific cytotoxic T lymphocyte (CTL) responses are central to immune control of HIV infection [1,2]. CD8+ T cell responses against HIV emerge during the course of acute infection, concurrently with falling plasma viremia [3,4]. The small minority of patients who naturally control HIV infection maintain highly effective HIV-specific CTL responses over time, exhibiting both polyfunctionality and potent HIV-suppressive effects [5,6]. On the other hand, most patients chronically infected with HIV progressively lose HIV-specific CD8+ T cell responses [7], through reduced CD4+ T cell help [8], clonal T cell loss [9], immune exhaustion [10] and other negative regulatory mechanisms. Importantly, these defects in HIV-specific T cell immunity are not fully restored by antiretroviral therapy (ART) [11,12].
Despite its ability to durably suppress HIV replication, ART does not eradicate the latent viral reservoir, and lifelong therapy is necessary to avoid rapid viral rebound [13]. This has sparked efforts to develop therapeutic strategies able to establish durable viral control in the absence of ART, a so-called functional cure [14,15]. Many of these approaches will require an induction or boosting of the patients’ impaired HIV-specific CTL function in order to eliminate reactivated, latently infected cells [16] and maintain viral control. This may be attained by therapeutic vaccination or other immunomodulatory therapy.
Antigen-induced T cell activation and proliferation are subject to negative regulation through a variety of signalling pathways, including the anti-inflammatory cytokines interleukin (IL) 10 and transforming growth factor (TGF) β as well as negative co-signalling molecules programmed death (PD) 1 and CD160. We have recently explored an assay for assessing negative regulation of HIV-specific T cell function mediated by IL-10 and TGF-β. This regulation parameter was associated with clinical progression in untreated HIV infection [17]. Moreover, pre-existing and evolving regulation of HIV vaccine-specific CD8+ T cell responses coincided with low final responses against therapeutic Gag peptide-vaccines in ART-treated patients [18,19]. Thus, such an approach of assessing antigen-induced T cell regulation may prove clinically useful and these data suggest that regulation should be taken into account when considering patients for immunomodulatory therapy as part of a functional cure. In addition, quantifying the contribution of various pathways in suppressing T cell function may allow individually tailored interventions directed at these mechanisms [20–23].
The aim of this study was to further explore mechanisms of functional T cell regulation (FTR) of CD8+ T cell responses against HIV Gag and Env antigens, mediated by not only IL-10 and TGF-β, but also PD-1/PD-L1 and CD160/HVEM pathways. We observed substantial heterogeneity in FTR between patients, inhibitory pathways and HIV antigens, and an apparent detrimental effect on clinical parameters of isolated Env-related FTR.
Methods
Study participants
Twenty-six asymptomatic HIV-1 seropositive patients from the Department of Infectious Diseases, University Oslo Hospital were included in the study. All included patients were above 18 years of age, ART-naïve and none fulfilled the criteria of elite or viremic controllers. No patients were co-infected with hepatitis B or C virus and none had symptoms or findings of intercurrent or opportunistic infections. CD4+ and CD8+ T cell counts and plasma HIV RNA were determined by routine clinical assays. Clinical characteristics are shown in Table 1.
Table 1. Clinical characteristics (n = 26).
| Age (years) | 42.5 (40.0–49.0) * |
| Gender (male/female) | 23/3 |
| Time since HIV diagnosis (years) | 2.0 (1.0–3.9) |
| CD4+ T cell counts (cells/uL) | 416 (337–514) |
| CD8+ T cell counts (cells/uL) | 1 325 (1 054–1 611) |
| Plasma HIV RNA (copies/mL) | 34 500 (10 000–81 000) |
*: Data given as median (lower—upper quartile)
The study was approved by The Norwegian South-Eastern Regional Committee for Medical and Health Research Ethics. Written, informed consent was obtained from all study participants.
Samples
Peripheral blood mononuclear cells (PBMC) were isolated using Vacutainer Cell Preparation Tubes (Becton Dickinson (BD), NJ, USA). Cells were frozen in RPMI containing 40% FCS and 10% DMSO and stored at -145°C. CD4+ and CD8+ T cell counts and plasma HIV RNA loads were determined by routine clinical assays.
Proliferation/Regulation assays
Cells were thawed, washed and reconstituted in AIM V serum-free medium (Life Technologies, Oslo, Norway) containing 0.1% human serum albumin. After an overnight rest, cells were pulse-labelled with carboxyfluorescein diacetate succinimidyl ester (CFSE, Life Technologies) at a concentration of 2 μM for 5 minutes. The following blocking antibodies were added to parallel culture wells at a final concentration of 10 μg/mL: anti-IL-10 (R&D Systems, MN, USA; clone 23738), anti-TGF-β (R&D; clone 1D11), anti-PD-L1 (eBioscience, CA, USA; clone MIH1), and anti-HVEM (R&D; clone 94801).
After a 30 minute incubation, cultures were stimulated with either Gag or Env 15-mer overlapping peptide panels (NIH AIDS Research and Reference Reagent Program, MD, USA) at a final concentration of 2 μg/mL/peptide. Staphylococcal Enterotoxin B (SEB, Sigma-Aldrich, MO, USA) at a final concentration of 0.5 μg/mL was used as a positive control.
Cells were cultured at 37°C in 5% CO2 for 5 days, harvested, and stained with the following fluorochrome-conjugated antibodies: CD3 V450, CD8 APC-H7, HLA-DR BV605, CD45RA APC (all BD) and CD25 PE (Biolegend). 7-aminoactinomycin D (7-AAD, BD) was added for dead cell exclusion. Flow cytometry data were acquired on a BD FACS Canto II with BD Diva 6.1 software, and analyzed in FlowJo X (FlowJo LLC, OR, USA).
Gating, readouts and parameter definitions
Antigen-specific, proliferated CD8+ T cells were defined by CFSE fluorescence between the second and sixth generation of CFSEdim cells (Fig 1A). The first generation was omitted based on the assumption that these late proliferating cells more likely represent unspecific bystander proliferation. Activated CD8+ T cells were defined by co-expression of CD25 and HLA-DR. Activated regulatory T cells (aTregs) were defined by the phenotype CD3+CD8-CD25hiCD45RA- [24]. In the absence of discrete cell populations, gating cut-offs were determined by the Fluorescence Minus One (FMO) method. Examples of all gates are given in S1 Fig.
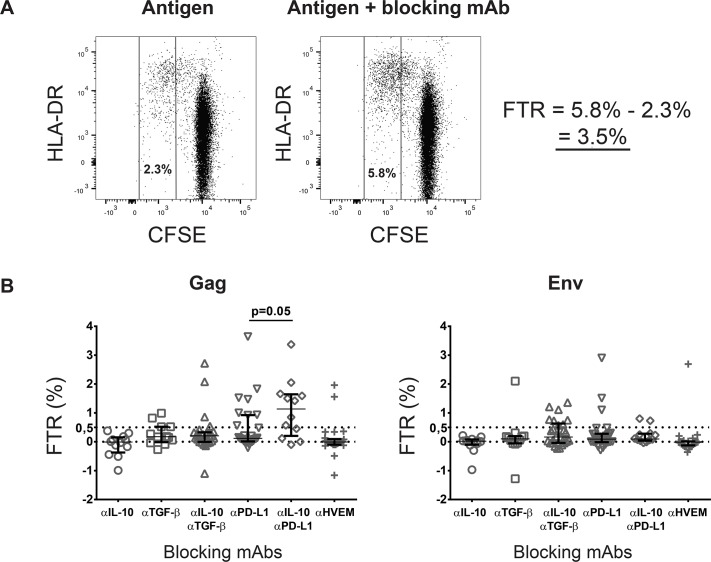
Fig 1. Functional T cell regulation (FTR) of CD8+ T cells from HIV-infected patients.
(A) FTR as the difference between proliferating (CFSEdim) CD8+ T cells, in HIV antigen-stimulated cultures with and without blocking antibodies. Proliferating cells also express the activation marker HLA-DR. (B) Gag and Env FTR, assessed by single or dual blockade of inhibitory pathways. Median and upper/lower quartiles indicated. FTR defined as an increase in proliferating cells above a threshold of 0.5% (indicated by upper dashed line). P-value derived from paired test.
Proliferative responses were defined as the difference in CFSEdim CD8+ T cell fractions between antigen-stimulated and unstimulated cultures. Functional T cell regulation (FTR) was defined as the difference in the percentage of CFSEdim CD8+ T cells between stimulated cultures with and without blocking antibodies, respectively (Fig 1A). In addition, a lower threshold of 0.5% was set to signify FTR beyond potential intra-assay variability, as we observed occasional negative FTR values between 0 and -0.5% (excluding a few outliers). FTR values >0.5% were consistently found on repeated analysis of selected samples.
Statistics
All statistical methods used were non-parametric. Comparisons between groups were assessed by Kruskal-Wallis and Mann-Whitney U tests. Correlations between parameters were assessed by Spearman rank sum tests. Paired data was assessed by Wilcoxon matched pair tests. Analyses were performed in SPSS v. 21 (IBM Corp. Armonk, NY) and Graphpad Prism 6 (Graphpad Software, La Jolla, CA). P-values below 0.05 were considered statistically significant.
Results
Heterogeneity in Gag and Env regulation
Samples from all patients were first assessed for CD8+ FTR mediated by IL-10/TGF-β, the PD-1/PD-L1 and the CD160/HVEM pathways, respectively. Overall, 14 patients (54%) had detectable FTR. In a subgroup of 12 patients who had quantifiable Gag and/or Env FTR detected by IL10/TGF-β dual blockade, we repeated the experiments with single blockade of IL-10 and TGF-β, to quantify the individual regulatory contribution of these two cytokines. In addition, we investigated the FTR effects of PD-L1, IL-10/PD-L1 and HVEM blockade in Gag and Env-stimulated cultures, respectively (Fig 1B).
We observed considerable heterogeneity, not only between Gag and Env FTR, but also between the regulatory effects of the different inhibitory pathways (Fig 1B). For example, we found no Gag or Env FTR mediated by IL-10 alone, while four patients had Gag FTR mediated by TGF-β alone, one of which also had Env FTR mediated by TGF-β. However, Env FTR assessed by IL-10/TGF-β dual blockade was observed in eight patients, suggesting additive or synergistic effects of the two cytokines on Env responses. For Gag FTR, an additive effect was obtained by blocking IL-10 in combination with PD-L1 (p = 0.05), but no such synergy was found for Env FTR (Fig 1B).
Blockade of the CD160 ligand HVEM identified only two patients with Gag FTR and another patient with Env FTR. No clear associations were found between HVEM FTR assessments and FTR obtained by the other inhibitory pathways.
Taken together, substantial inter-individual differences were noted for FTR in relation to both signalling pathways and HIV antigens.
Different blocking conditions best identify patients with Gag and Env FTR
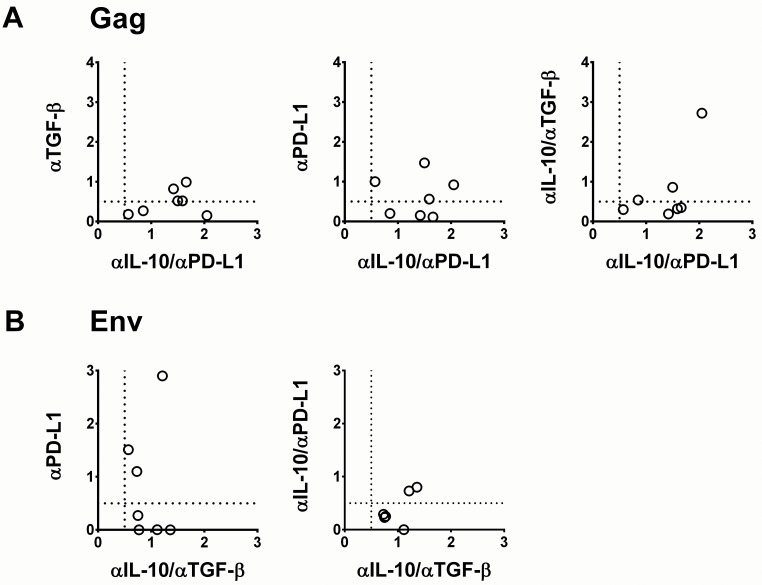
Because we hypothesized that HIV antigen-specific FTR is important to quantify in a clinical immunotherapy setting, we next selected the assay conditions which revealed the highest number of patients with quantifiable Gag and Env FTR, respectively. Notably, dual blockade of IL-10/PD-L1 revealed all patients with Gag FTR, whereas combined blockade of IL-10/TGF-β identified all patients with Env FTR (Fig 2).
Fig 2. Gag and Env FTR is best identified by different combinations of blocking mAbs.
The relationship between FTR mediated by various pathways in (A) Gag- and (B) Env-stimulated cultures. Plots include patients with detectable FTR on one or both axes. Only dual blockade of IL-10/PD-L1 (x-axis) identifies all patients with Gag FTR whereas dual blockade of IL-10/TGF-β (x-axis) identifies all patients with Env FTR. FTR defined as an increase in proliferating cells above a threshold of 0.5% (dashed lines).
The heterogeneity among patients who had detectable FTR was once again exemplified by there being no significant concordance between Gag and Env FTR mediated by the same signalling pathways (data not shown).
Regulation associated with viremia and CD4 counts
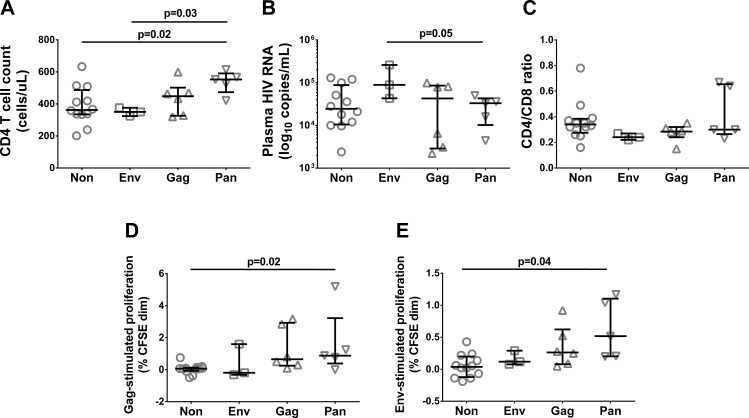
Despite the heterogeneity in FTR by signal pathways and antigens, patients were tentatively grouped on the basis of whether they exhibited FTR associated with Gag only (Gag-regulators, n = 6), Env only (Env-regulators, n = 3), both Gag and Env (Pan-regulators, n = 5), or neither Gag nor Env (Non-regulators, n = 12). There were no differences in age or time since HIV diagnosis between the regulator subgroups. While the paucity of Env-regulators hampered statistical analysis, this group nevertheless had lower CD4 counts (p = 0.03) and higher viral loads (p = 0.05) than Pan-regulators (Fig 3A and 3B). All Env regulators had a low CD4/CD8 ratio, although there were no differences between groups (Fig 3C). Pan-regulators, on the other hand, had higher CD4 counts (p = 0.02) and higher both Gag- and Env-specific proliferative responses than Non-regulators (p = 0.02 and p = 0.04, respectively) (Fig 3D and 3E).
Fig 3. Clinical and immunological variables of regulator subgroups.
(A) CD4 count, (B) Plasma HIV RNA, (C) CD4/CD8 ratio, (D) Gag-stimulated proliferation and (E) Env-stimulated proliferation, by regulator subgroup. Median and upper/lower quartiles indicated. P-values derived from unpaired tests.
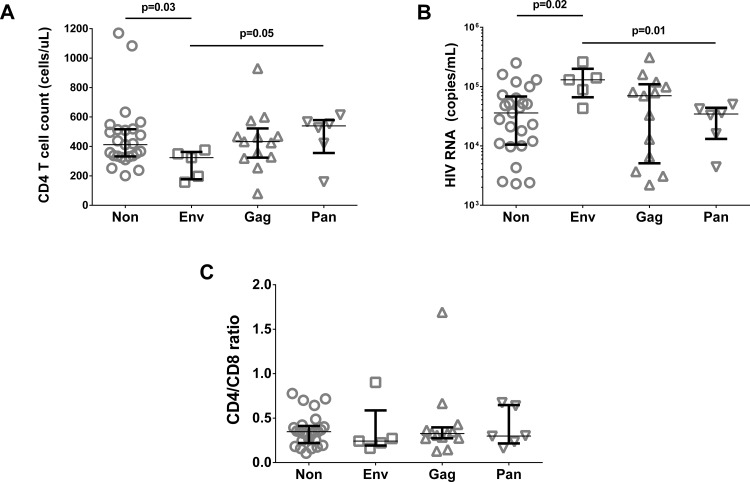
In order to confirm these observations, in particular the characteristics of more rapidly progressive HIV disease exhibited by Env-regulators, we reanalyzed CD8+ T cell Gag and Env FTR in another cross-sectionally sampled HIV cohort of 23 ART-naïve viremic patients. This supplementary cohort had similar clinical characteristics to the study cohort (CD4+ T cell count 425 (255–549) cells/uL; CD8+ T cell count 1222 (771–2019) cells/uL; plasma HIV RNA 52 000 (13 000–130 000) copies/mL; n.s.). When regulator subgroups were compared in the two cohorts combined (n = 49; 13 Gag-, 5 Env-, 6 Pan-, and 25 Non-regulators), we found that Env-regulators indeed had lower CD4 counts and higher HIV RNA than both Non- and Pan-regulators (see Fig 4).
Fig 4. Lower CD4 counts and higher HIV viral loads in Env-regulators.
(A) CD4 count, (B) Plasma HIV RNA and (C) CD4/CD8 ratio by regulator subgroup in the expanded cohort (n = 49). Median and upper/lower quartiles indicated. P-values derived from unpaired tests.
Concordant proliferative responses to HIV Gag and Env
As observed in previous studies, the proliferative responses of CD8+ T cells to Gag stimulation were greater than those induced by Env (p = 0.046, data not shown). In contrast to assessments of FTR, the CD8+ proliferative responses to the two antigens correlated (r = 0.65, p<0.001). The CD8+ T cell activation phenotype, as defined by co-expression of CD25 and HLA-DR, correlated closely with the proliferative responses in all culture conditions (r = 0.76–0.89, p<0.001).
Underscoring the complex relationship between proliferation and regulatory mechanisms, no significant correlations were found between antigen-specific proliferative responses and FTR mediated by any inhibitory pathway.
Frequencies of activated Tregs following HIV antigen stimulation and signal blockade
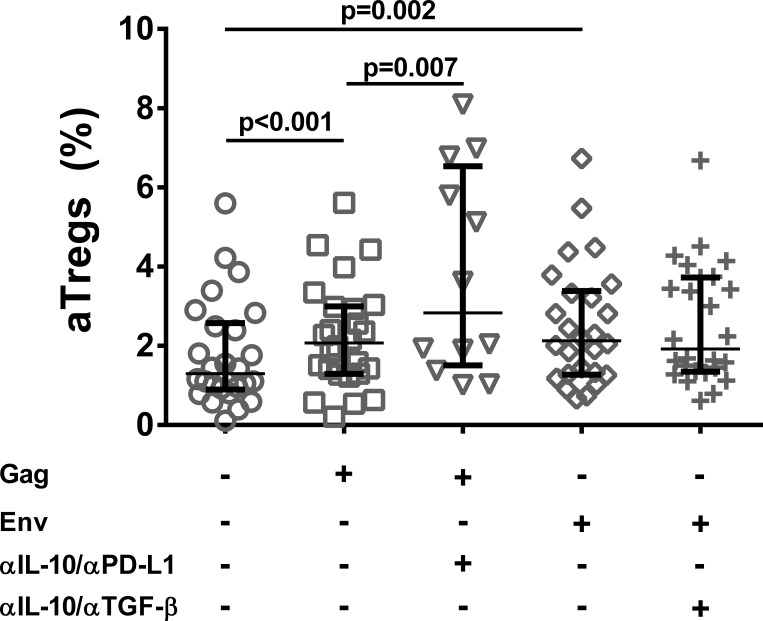
In the study cohort as a whole, the percentage of aTregs increased significantly after both Gag and Env stimulation in vitro (p<0.01, Fig 5). Dual blockade of IL-10/PD-L1 in Gag-stimulated cultures increased the aTreg fraction further (p = 0.007), whereas no such increase were observed in Env-stimulated cultures with dual blockade of IL-10/TGF-β.
Fig 5. Increase in aTreg frequency after IL10/PD-1 dual blockade of Gag-stimulated cultures.
Frequencies of activated regulatory T cells (CD25hiCD45RA-) in unstimulated cultures and in antigen-stimulated cultures with and without blocking antibodies. Median and upper/lower quartiles indicated. P-values derived from paired tests.
Discussion
The primary aim of this study was to explore and quantify antigen-specific functional T cell regulation by blocking defined inhibitory pathways in HIV antigen-stimulated cultures, with an ultimate goal of testing such parameters in the setting of functional HIV cure interventions. Our previous work to assess regulation with combined blockade of IL-10 and TGF-β was here expanded to include the PD-L1/PD-1 and CD160/HVEM pathways. We cross-sectionally tested samples from 26 HIV-infected, ART naïve patients, of who 14 (54%) had detectable FTR. Our main finding was substantial heterogeneity in the prevalence and magnitude of FTR between patients, inhibitory pathways as well as Gag and Env antigens, consistent with our previous observations [17]. Moreover, we have previously reported a similar variability in patients on ART, where this type of assay may help select patients for and/or predict the efficacy of therapeutic HIV vaccines [19].
In preliminary experiments, Antigen-specific, Cytokine-mediated regulation (RAC), as defined in our previous work [17] was also determined, taking into account downregulation of bystander proliferation in control cultures. We found minimal effects on background proliferation of blocking any of the assessed inhibitory pathways and thus a high concordance between RAC and FTR (data not shown).
We chose to use T cell proliferation after 5 days of culture as our primary read-out in assessing FTR. While interferon (IFN) γ secretion detected by ELISPOT or intracellular cytokine staining [25] and polyfunctionality [5,26] are commonly used measures of effector T cell responses to HIV peptides, these assays typically involve 6–18 hours of culture before analysis. We expected antigen-related regulation to develop more slowly, secondary to primary cell activation. In addition, the capacity of HIV-specific effector T cells to proliferate has been linked to delayed or non-progression of HIV infection [27,28], even at an epitope level [29]. Furthermore, proliferation has been linked to perforin expression by CD8+ T cells [28], essential for cytolysis of infected target cells. The high concordance between T cell proliferation and expression of the activation markers CD25 and HLA-DR was here additional evidence of T cell activation processes involving proinflammatory cytokines such as IFN-γ and IL-2 [30,31].
In our previous studies, we have quantified functional regulation by dual blockade of IL-10 and TGF-β [17–19]. This assay condition still proved most effective in revealing Env FTR in the current study cohort. By contrast, Gag FTR was best identified by combined IL-10 and PD-L1 blockade, in keeping with Porichis et al. [23]. Blockade of the PD-1/PD-L1 pathway is known to restore cytokine secretion and proliferative capacity of exhausted CD8+ T cells in HIV infection [32]. We found that the PD-1/PD-L1 pathway was involved in both Gag and Env FTR, but Gag-specific responses were more affected. As Gag-specific T cell responses in particular have been linked to control of HIV viremia [2,33], our data may lend further support to the use of anti-PD-1 antibodies in HIV immunotherapy [22]. Of note, the additive effect of concurrent PD-1 and IL-10 blockade was only apparent upon Gag stimulation.
Somewhat surprisingly, our assay neither detected Gag nor Env regulation by blocking IL-10 alone, as effects of IL-10Ra blockade on Gag p24-specific CD8+ T cell proliferation have been reported [34]. Notably, the monoclonal antibody used in our study (clone 23738) neutralises IL-10 bio-activity [17,35]. On the other hand, TGF-β alone mediated regulation in some patients, in accordance with Garba et al. [36]. The additive effect of blocking IL-10 and TGF-β in the same cultures may therefore suggest a potential recruitment of IL-10 dependent mechanisms during TGF-β blockade.
Regulatory effects of the CD160/HVEM pathway were detected in very few patients; two upon Gag stimulation and another patient upon Env stimulation. This contrasts with the results of Peretz et al. [37], who found significantly increased HIV-specific proliferation and cytokine secretion of CD8+ T cells during HVEM blockade. However, their experiments utilized peptides from HIV antigens other than Gag and Env.
Non-regulator patients with little or no detectable FTR by any of the two HIV peptide pools were clinically heterogeneous, with a wide range of CD4 counts and viral loads. This group was, however, also characterised by weak antigen-specific proliferative responses (i.e. unblocked), an observation which was confirmed in the expanded cohort (data not shown). Whether other regulatory mechanisms may be at play, or whether these HIV-specific T cell clones have in fact been deleted cannot be determined by our experiments. In a recent trial of therapeutic vaccination in another HIV-positive cohort, however, we have observed that patients who would have been denominated Non-regulators at study baseline may still develop FTR against the vaccine antigens [unpublished data].
Regulator patients displayed the same clinical heterogeneity as Non-regulators. While the apparent paucity of Env-regulators (approx. 10% in the expanded cohort) limits the conclusions which can be drawn, one may speculate that this group, exhibiting low HIV-specific proliferative capacity of CD8+ T cells, constitutes a patient phenotype more prone to clinical progression, in keeping with their higher HIV RNA levels compared to Non- and Pan-regulators. These findings are also in accordance with our previous work in another patient cohort, in which Env regulation was associated with accelerated CD4 loss [17]. Pan-regulators, meanwhile, had better T cell responses to both antigens, and induction of FTR may merely be a bystander effect of a strong proliferative drive in this group.
As could be expected, HIV antigen stimulation of PBMC increased the frequency of activated Tregs in culture. This potential for generation of Tregs after antigen exposure of CD4+ T cells is well established [38]. Interestingly, we observed a further increase in aTreg frequencies in Gag-stimulated cultures with dual blockade of IL-10 and PD-L1, but not in Env-stimulated cultures with IL-10/TGF-β blockade. As both TGF-β and the PD-1/PD-L1 axis has been implicated in the induction of Tregs [39–42], the mechanisms contributing to these antigen-dependent differences are unclear, and cannot be elucidated by our straightforward in vitro assay.
The increase in ART coverage and potential shift of focus to functional cure strategies in HIV infection encourage the extension of our study to ART-treated patients with suppressed viremia. The limited number of patients in this study as well as the substantial heterogeneity in T cell proliferation, FTR and HIV antigen dependency restrict the conclusions we can draw. Furthermore, while the regulation profiles of CD8+ T cells as described here may have clinical significance, a cross-sectional approach cannot ascertain whether they represent a cause or consequence of progression.
In conclusion, assuming that enhanced proliferation of CD8+ T cells by blockade of key inhibitory pathways represents functional regulation in vivo, our data confirm that FTR of HIV-specific CTL responses is common in HIV-infected patients. Although our data indicate that Gag and Env antigens are differentially regulated in patients, we nevertheless found combinations of inhibitory pathway blockers that identified all patients with Gag and Env FTR, respectively. Taken together, our data support the notion that interventions to improve HIV-specific immunity should be individually tailored and that assessments of FTR should be further explored in clinical trials.
Supporting Information
(TIF)
Data Availability
To protect participant confidentiality data are available upon request from the Corresponding Author.
Funding Statement
This work was supported by the Norwegian Health Region South-East (Grant #2013033) and The Research Council of Norway (GLOBVAC grant #192514), both recieved by DK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Betts MR, Krowka JF, Kepler TB, Davidian M, Christopherson C, Kwok S, et al. Human immunodeficiency virus type 1-specific cytotoxic T lymphocyte activity is inversely correlated with HIV type 1 viral load in HIV type 1-infected long-term survivors. AIDS Res Hum Retroviruses. 1999;15: 1219–1228. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76: 2298–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68: 4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68: 6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107: 4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104: 6776–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96: 3094–3101. [PubMed] [Google Scholar]
- 8.Kalams SA, Buchbinder SP, Rosenberg ES, Billingsley JM, Colbert DS, Jones NG, et al. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73: 6715–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantaleo G, Soudeyns H, Demarest JF, Vaccarezza M, Graziosi C, Paolucci S, et al. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc Natl Acad Sci U S A. 1997;94: 9848–9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10: 29–37. 10.1038/ni.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migueles SA, Weeks KA, Nou E, Berkley AM, Rood JE, Osborne CM, et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol. 2009;83: 11876–11889. 10.1128/JVI.01153-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lozano JM, De la Rosa O, Garcia-Jurado G, Luque J, Solana R, Kindelan JM, et al. Impaired response of HIV type 1-specific CD8(+) cells from antiretroviral-treated patients. AIDS Res Hum Retroviruses. 2007;23: 1279–1282. [DOI] [PubMed] [Google Scholar]
- 13.Chun TW, Justement JS, Murray D, Hallahan CW, Maenza J, Collier AC, et al. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. Aids. 2010;24: 2803–2808. 10.1097/QAD.0b013e328340a239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International ASSWGoHIVC, Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12: 607–614. 10.1038/nri3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382: 1525–1533. 10.1016/S0140-6736(13)61809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36: 491–501. 10.1016/j.immuni.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lind A, Brekke K, Pettersen FO, Mollnes TE, Troseid M, Kvale D. A Parameter for IL-10 and TGF-beta Mediated Regulation of HIV-1 Specific T Cell Activation Provides Novel Information and Relates to Progression Markers. PLoS One. 2014;9: e85604 10.1371/journal.pone.0085604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brekke K, Lind A, Holm-Hansen C, Haugen IL, Sorensen B, Sommerfelt M, et al. Intranasal administration of a therapeutic HIV vaccine (Vacc-4x) induces dose-dependent systemic and mucosal immune responses in a randomized controlled trial. PLoS One. 2014;9: e112556 10.1371/journal.pone.0112556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind A, Brekke K, Sommerfelt M, Holmberg JO, Aass HC, Baksaas I, et al. Boosters of a therapeutic HIV-1 vaccine induce divergent T cell responses related to regulatory mechanisms. Vaccine. 2013;31: 4611–4618. 10.1016/j.vaccine.2013.07.037 [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182: 5891–5897. 10.4049/jimmunol.0803771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hryniewicz A, Boasso A, Edghill-Smith Y, Vaccari M, Fuchs D, Venzon D, et al. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108: 3834–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velu V, Shetty RD, Larsson M, Shankar EM. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology. 2015;12: 14 10.1186/s12977-015-0144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porichis F, Hart MG, Zupkosky J, Barblu L, Kwon DS, McMullen A, et al. Differential impact of PD-1 and/or interleukin-10 blockade on HIV-1-specific CD4 T cell and antigen-presenting cell functions. J Virol. 2014;88: 2508–2518. 10.1128/JVI.02034-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30: 899–911. 10.1016/j.immuni.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 25.Goulder PJ, Addo MM, Altfeld MA, Rosenberg ES, Tang Y, Govender U, et al. Rapid definition of five novel HLA-A*3002-restricted human immunodeficiency virus-specific cytotoxic T-lymphocyte epitopes by elispot and intracellular cytokine staining assays. J Virol. 2001;75: 1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandt L, Benfield T, Kronborg G, Gerstoft J, Fomsgaard A, Karlsson I. HIV-1-infected individuals in antiretroviral therapy react specifically with polyfunctional T-cell responses to Gag p24. J Acquir Immune Defic Syndr. 2013;63: 418–427. 10.1097/QAI.0b013e31828fa22b [DOI] [PubMed] [Google Scholar]
- 27.McKinnon LR, Kaul R, Kimani J, Nagelkerke NJ, Wachihi C, Fowke KR, et al. HIV-specific CD8+ T-cell proliferation is prospectively associated with delayed disease progression. Immunol Cell Biol. 2012;90: 346–351. 10.1038/icb.2011.44 [DOI] [PubMed] [Google Scholar]
- 28.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 29.Horton H, Frank I, Baydo R, Jalbert E, Penn J, Wilson S, et al. Preservation of T cell proliferation restricted by protective HLA alleles is critical for immune control of HIV-1 infection. J Immunol. 2006;177: 7406–7415. [DOI] [PubMed] [Google Scholar]
- 30.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15: 749–795. [DOI] [PubMed] [Google Scholar]
- 31.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12: 180–190. 10.1038/nri3156 [DOI] [PubMed] [Google Scholar]
- 32.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12: 1198–1202. [DOI] [PubMed] [Google Scholar]
- 33.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13: 46–53. [DOI] [PubMed] [Google Scholar]
- 34.Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114: 346–356. 10.1182/blood-2008-12-191296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166: 4312–4318. [DOI] [PubMed] [Google Scholar]
- 36.Garba ML, Pilcher CD, Bingham AL, Eron J, Frelinger JA. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J Immunol. 2002;168: 2247–2254. [DOI] [PubMed] [Google Scholar]
- 37.Peretz Y, He Z, Shi Y, Yassine-Diab B, Goulet JP, Bordi R, et al. CD160 and PD-1 co-expression on HIV-specific CD8 T cells defines a subset with advanced dysfunction. PLoS Pathog. 2012;8: e1002840 10.1371/journal.ppat.1002840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6: 1219–1227. [DOI] [PubMed] [Google Scholar]
- 39.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172: 5149–5153. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105: 9331–9336. 10.1073/pnas.0710441105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206: 3015–3029. 10.1084/jem.20090847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
To protect participant confidentiality data are available upon request from the Corresponding Author.