Abstract
Cholesterol is essential to the growth and viability of cells. The metabolites of cholesterol include: steroids, oxysterols, and bile acids, all of which play important physiological functions. Cholesterol and its metabolites have been implicated in the pathogenesis of multiple human diseases, including: atherosclerosis, cancer, neurodegenerative diseases, and diabetes. Thus, understanding how cells maintain the homeostasis of cholesterol and its metabolites is an important area of study. Acyl-coenzyme A:cholesterol acyltransferases (ACATs, also abbreviated as SOATs) converts cholesterol to cholesteryl esters and play key roles in the regulation of cellular cholesterol homeostasis. ACATs are most unusual enzymes because (i) they metabolize diverse substrates including both sterols and certain steroids; (ii) they contain two different binding sites for steroidal molecules. In mammals, there are two ACAT genes that encode two different enzymes, ACAT1 and ACAT2. Both are allosteric enzymes that can be activated by a variety of sterols. In addition to cholesterol, other sterols that possess the 3-beta OH at C-3, including PREG, oxysterols (such as 24(S)-hydroxycholesterol and 27-hydroxycholesterol, etc.), and various plant sterols, could all be ACAT substrates. All sterols that possess the iso-octyl side chain including cholesterol, oxysterols, various plant sterols could all be activators of ACAT. PREG can only be an ACAT substrate because it lacks the isooctyl side chain required to be an ACAT activator. The unnatural cholesterol analogs epi-cholesterol (with 3-alpha OH in steroid ring B) and ent-cholesterol (the mirror image of cholesterol) contain the iso-octyl side chain but do not have the 3-beta OH at C-3. Thus, they can only serve as activators and cannot serve as substrates. Thus, within the ACAT holoenzyme, there are site(s) that bind sterol as substrate and site(s) that bind sterol as activator; these sites are distinct from each other. These features form the basis to further pursue ACAT structure-function analysis, and can be explored to develop novel allosteric ACAT inhibitors for therapeutic purposes.
Keywords: Acyl-CoA:cholesterol acyltransferase (ACAT/SOAT), Cholesterol, Pregnenolone, Oxysterols, Plant sterols
ACAT enzymes
Acyl-CoA:cholesterol acyltransferases (ACATs), also known as sterol O-acyltransferases (SOATs), play important roles in cellular cholesterol homeostasis and are drug targets for therapeutic intervention of several diseases including atherosclerosis (reviewed in [1]), Alzheimer’s disease [2], [3], [4], [5] and cancer [6]. In mammals, two genes that encode two different proteins exist: Acat1 and Acat2 [7]. Along with acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1), ACAT1 and ACAT2 are founding members of the membrane-bound O-acyltransferase (MBOAT) enzyme family [8]. MBOATs are multi-span membrane enzymes that use long-chain or medium-chain fatty acyl-CoA as the first substrate, and catalyze the transfer of the fatty acyl group to the 3β –hydroxyl moiety of a certain hydrophobic substance as the second substrate. An MBOAT contains two active sites: a histidine within a long hydrophobic peptide region, and an asparagine located within a long hydrophilic peptide region. In humans, there are 11 MBOAT members, with similar catalytic mechanisms but with diverse biological functions. At present, there are no complete crystal structures for any members of the MBOAT family. For ACAT1 and ACAT2, the major sterol substrate is cholesterol; both enzymes use long chain fatty acyl-coenzyme A as the fatty acyl donor to convert cholesterol to cholesteryl esters. For ACAT1, the preferred fatty acyl-CoA is oleoyl coenzyme A [9]. Cholesterol is a lipid molecule; it partitions well within the phospholipid bilayer of various cell membranes. Unlike free (unesterified) cholesterol, the cholesteryl esters do not partition well within the lipid bilayer; instead, cholesteryl esters coalesce in aqueous medium and form cytoplasmic lipid droplets. The over accumulation of free cholesterol in the membranes can be cytotoxic to cells. Thus, a major function of ACATs is to protect against the unnecessary built up of free cholesterol within the cell membranes. ACAT1 is ubiquitously expressed in essentially all tissues examined; it is a resident enzyme at the endoplasmic reticulum (ER). ACAT2 is mainly expressed in the intestines and hepatocytes. It is also expressed in various other tissues, but at much lower levels than ACAT1. In intestines, ACAT2 provides cholesteryl esters for lipoprotein assemblies. In humans, both ACAT1 and ACAT2 are expressed in hepatocytes; the relative roles of ACAT1 and ACAT2 in human hepatic lipoprotein assembly are yet to be clarified. (Reviewed in [1]). Homologs of ACAT1 and ACAT2 have been identified in yeast saccharomyces cerevisiae [10, 11] and other species.
The Acat1 gene was identified by functional complementation of a Chinese hamster ovary cell mutant lacking ACAT activity [12]. Unlike most other genes, human Acat1 is located in two different chromosomes, chromosomes 1 and 7, with each site containing a distinct promoter: chromosome 1 contains exons 1–16, and chromosome 7 contains the optional long exon Xa [13]. The majority of ACAT1 mRNAs is transcribed from exons 1–16; this mRNA translates into a 50-kDa size protein on SDS-PAGE [14]. In addition, the pre-mRNAs produced from exons 1–16 and the pre-mRNAs produced from the optional exon Xa participate in a trans-splicing event to produce an endogenous, chimeric 4.3 kb ACAT1 mRNA. Remarkably, the endogenous 4.3 kb mRNA then undergoes a second trans-splicing event with an exogenous transcript encoded by the antisense strand of Amp(r) (asAmp), which is present in common Amp(r)-plasmids, to produce a novel mRNA species. This novel mRNA species translates into a 56-kDa size protein [15]. The 56-kDa protein has less ACAT enzyme activity than that of the 50-kDa protein [16]. The chimeric 4.3 kb ACAT1 mRNA found in human cannot be found in mouse. In mouse the Acat1 gene is located solely on chromosome 1, contains 17 exons [17], and produces a single protein of 48-kDa size protein on SDS-PAGE [18]. Human Acat2 is located in chromosome 12 and produces a single 46-kDa size protein on SDS-PAGE [19]. Farese and colleagues [18], [20] generated and characterized the Acat1 and Acat2 knockout mice. These mice have served as valuable tools in lipoprotein metabolism, atherosclerosis and neurodegenerative disease research.
The recombinant 50-kDa human ACAT1 has been purified to homogeneity with full retention in enzymatic activity [21]. When assayed in reconstituted liposomes or in mixed micelles, the enzyme responds to cholesterol as its substrate in a sigmoidal manner. ACAT2 activity also responds to cholesterol in a sigmoidal manner. Additional kinetic analyses show that both ACAT1 and ACAT2 can use a variety of sterols as substrates, and their activities are significantly activated by a variety of sterols. Among the sterols tested (including oxysterols, plant sterols, yeast sterols, and several synthetic sterol analogs), cholesterol is the best substrate and the best activator [22]. Those sterols that act both as ACAT activators and as substrates all contain a 3β hydroxyl group at steroid ring A. The sterols that contain alterations at the iso-octyl side chain, such as the plant sterol sitosterol, are both poor substrates and poor activators of ACAT. In addition, epi-cholesterol, which contains the 3α hydroxyl, is not a substrate and is a very poor activator. Likewise, enantiomeric cholesterol (ent-cholesterol), which is the mirror image of cholesterol and possesses essentially the same biophysical properties of cholesterol [23], is also not a substrate and a very poor activator [24]. These studies implicate that both ACAT1 and ACAT2 are allosteric enzymes, and the structural feature of a given sterol as an ACAT substrate deviates from that as an activator. A shortcoming of these studies was that a sterol-like molecule that is only a substrate but not an activator was not found. In the absence of such molecule, in order to test the structural features of various sterols as activators, sitosterol was chosen as a substrate [24]; however, sitosterol might act as an activator. Thus, by using sitosterol as the substrate in the ACAT activation assay, certain essential structural feature of a given sterol to act as an activator might have been masked.
PREG esterification and ACAT
PREG is the obligatory precursor for all steroid hormones. Biosynthesis of PREG occurs in mitochondria, using cholesterol (CHOL) as the precursor [25],[26]. Once produced, PREG can be converted by enzymes in the mitochondria and in the ER to various steroid hormones. In addition, PREG can be stored as fatty acyl esters. Steroid fatty acyl esters can provide a means to quickly provide a substrate pool in times of need. Lipoidal conjugates of PREG were first identified in the bovine adrenal [27]. Using bovine adrenal homogenates, Mellon and Hochberg [28] demonstrated that PREG could form lipoidal derivatives in vitro. In the adrenals of dog, rat and guinea-pig, PREG esters have been reported to be up to 40% of the total adrenal PREG content, while in human adrenals, PREG esters comprise more than 3 times the amount of free PREG[29].
Lavallée et al. [30] reported that PREG could be esterified by lecithin:cholesterol acyltransferase (LCAT). LCAT uses cholesterol and phosphatidyl choline present in the newly formed high-density lipoprotein (HDL) as substrates and convert them into cholesteryl esters and lysophosphatidyl choline. The LCAT enzyme is found in the blood. At the cellular level, Damon and Chavis [31] demonstrated that by adding labeled PREG to embryonic rat fibroblast they could obtain lipoidal PREG esters. Pahuja and Hochberg [32] used an in vitro assay and demonstrated the conversion of radiolabelled dehydroepiandrosterone (DHEA; a metabolite of PREG), estrogen, or corticosterone to their fatty acyl esters, when rat hepatic microsomes were used as the source of enzyme(s). However, the addition of a specific ACAT inhibitor to this in vitro assay did not reduce esterification of the steroids tested, suggesting that enzyme(s) other than ACAT [33] carried out the steroid esterification. More recently, based on ACAT sterol substrate specificity analysis, Rogers el al. [34] speculated that PREG, which possesses the same classical A, B, C, D steroid ring and 3β-hydroxyl moiety but with a much shorter side chain attached to ring D, might be a substrate for ACAT. They tested this possibility by using recombinant ACAT1 or recombinant ACAT2 as the enzyme source, and showed that both enzymes could catalyze the conversion of PREG to PREG esters. In addition, they added radio-labeled PREG to several mouse and human cell types and showed that it was efficiently converted to PREG esters; this esterification process could be inhibited by 80 to 90% when specific ACAT inhibitors were added at appropriate concentration. These results demonstrate that both ACAT1 and ACAT2 can convert PREG to PREG esters in vitro and in intact cells. DHEA was found to be a much inferior ACAT substrate to PREG. Rogers et al. next employed the Acat1 KO mouse, the Acat2 KO mouse, and the Lcat KO mouse to evaluate the roles of ACAT1, ACAT2, and LCAT in contributing to the total adrenal PREG ester levels in vivo. The result showed that neither Acat1 knockout nor Acat2 knockout caused significant reduction in levels of PREG fatty acyl ester, while the loss of Lcat resulted in a partial reduction of PREG fatty acyl ester in the adrenal. These results suggest that in the adrenal glands, multiple enzymes may be involved in the generation of PREG fatty acyl esters, with LCAT playing a larger role and with ACAT1/ACAT2 playing auxiliary roles. Interestingly, in the human adrenal H295R cell line, Ferraz-de-Souza et al. [35] reported a decreased ACAT1 mRNA expression when steroidogenic factor-1 is overexpressed, and increased ACAT1 mRNA expression when steroidogenic factor-1 is knocked down. Steroidogenic factor-1 is a master regulator of adrenal steroidogenesis and controls the expression of steroid acute regulatory protein (STAR) and CYP11a1 enzyme at the transcriptional level. This result suggests that ACAT1 may be involved in negatively regulating steroidogenesis in human adrenal cells.
Analysis of ACAT activators when PREG is used as the substrate
Rogers et al. [34] showed that, in the absence of cholesterol, PREG is a very poor substrate. However, the addition of cholesterol in the assay mixture increases the rate of PREG esterification by 100-fold. The result of the converse experiment showed that when CHOL was used as the substrate, PREG had minimal effect on CHOL esterification. These results show that PREG is a substrate but it cannot serve as an activator for ACAT1. Similar results were obtained when ACAT2 was used as the enzyme source. The purified ACAT1 is an intrinsically fluorescent protein; using this property, Chang et al. [9] devised a direct binding assay between purified ACAT1 and various ligands. Using this assay, Rogers et al. showed that ACAT1 directly binds to PREG with a Kd= 0.6 μM, which is 58-fold lower than the concentration for half maximal CHOL binding (35 μM). This result showed that PREG binds to ACAT1 with strong affinity. Rogers et al. next used PREG as the substrate to probe the structural specificity of sterols that could serve as the activator. The results showed that essentially all sterols tested, including the unnatural cholesterol analogs epicholesterol, ent-cholesterol; etc. could activate PREG esterification to a certain degree, with cholesterol providing the maximal activation. Based on these data, Rogers et al. proposes that ACAT1 contains two types of binding sites: a substrate site and an activator site (Figure 1). ACAT1 has 9 transmembrane domains [36] and resides at the ER. The ACAT1 enzyme exists as a homotetramer [37], but it may act as a dimer of dimer [38]. Within each dimer, it may contain two identical sterol substrate sites (designated as Site S), and one or two sterol activator site(s) (designated as site A). Site S preferentially binds PREG; it can also bind a variety of sterols including CHOL, plant sterols, and oxysterols. The binding between site S and the steroid is mainly stereospecific and does not involve extensive interaction with phospholipid in membrane. Site S does not bind ent-CHOL, or epi-CHOL. Site A prefers to bind CHOL but cannot bind PREG; it can also bind a variety of other sterols such as plant sterols, oxysterols, yeast sterol, epi-cholesterol, and ent-CHOL; etc. The binding between Site A and the steroid involves the ability of the sterol molecule to interact biophysically with phospholipid in membrane, in addition to the stereospecific structure of the sterol. When only PREG is present, PREG binds Site S, but it fails to trigger appropriate conformational changes, and the enzyme can only catalyze PREG esterification at a very low rate. When PREG and CHOL (or other sterol analogs) are both present, binding of CHOL at Site A causes conformational changes, enabling the enzyme to increase the rate of esterification reaction much more efficiently. It is unlikely that ACATs would be able to recognize steroids with a hydroxyl-moiety located at C-7, or at C11, or at C17beta as substrates; however, these possibilities have not been formally tested.
Fig.1.
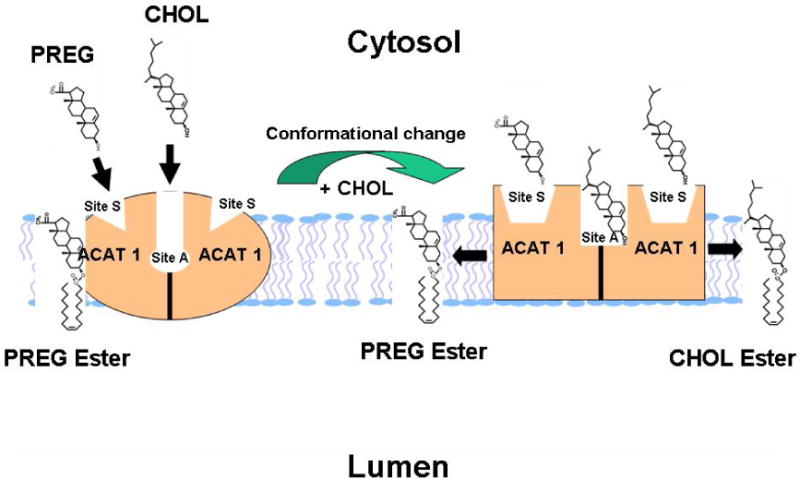
A working model for ACAT1 allosterism [34].
ACAT1 may act as a dimer of dimer [38]. Within each dimer, it may contain two identical sterol substrate sites (designated as Site S), and one or two sterol activator site(s) (designated as Site A). Site S preferentially binds pregnenolone (PREG); it can also bind a variety of sterols that contain 3-beta-hydroxy moieties. Site A prefers to bind CHOL but cannot bind PREG; it can also bind a variety of other sterols such as plant sterols, oxysterols, yeast sterol, epi-cholesterol, and ent-CHOL; etc. When only PREG is present, PREG binds Site S, but it fails to trigger appropriate conformational changes, and the enzyme can only catalyze PREG esterification at a very low rate. When PREG and CHOL (or other sterol analogs) are both present, binding of CHOL at site A causes conformational changes, enabling the enzyme to increase the rate of esterification reaction much more efficiently.
ACAT and neurosteroid esterification?
Neurosteroids including the steroids PREG, DHEA, progesterone and allopregnanolone; etc., are synthesized de novo in the brain by neurons and astrocytes, or are produced in peripheral tissues but accumulate in the central nervous system [39], [40]. Various neurosteroids possess many interesting biological activities. For example, neurosteroids can allosterically regulate gamma-aminobutyric acid receptors [41]. PREG sulfate, which contains a sulfate ester at the 3β-hydroxyl moiety, can potentiate the calcium conductivity of the N-methyl-aspartate receptor channels in rat hippocampal and cortical neurons [42], [43]. In animal studies, administration of neurosteroids can have beneficial effects on learning and memory behavior [44]. More recently, Vallée et al. [45] reported that free, unesterified PREG present in the rodent brain reduces several effects of the main active principle of Cannabis sativa (marijuana), Δ9 tetrahydrocannabinol. This result suggests that PREG can protect the brain from type-1 cannabinoid over-activation, which may have implications for drug intoxication and addiction. In rodent brains, the concentration of free PREG ranges from 2 to 13 ng/g wet brain weight [46]. PREG sulfate levels are almost undetectable, while PREG fatty acyl ester levels are higher [47]. In mouse brain, ACAT1 protein expression and its enzymatic activity has been demonstrated [3]. However, whether ACAT1 is involved in fatty acylation of PREG in the central nervous system remains to be demonstrated.
ACAT and oxysterol esterification
Cholesterol is the precursor for various oxidized sterols, such as: 7α-hydroxycholesterol, 22(R)-hydroxycholesterol, 24(S)-hydroxcholesterol, 25-hydroxycholesterol and 27-hydroxycholesterol [48]. 7α-hydroxycholesterol is the obligatory precursor for bile acid biosynthesis. 22(R)-hydroxycholesterol is the obligatory sterol intermediate in order to form PREG. 24(S)-hydroxycholesterol is the major oxysterol present in the brain, and plays important role in mediating the excretion of cholesterol from the brain [49]. Other oxysterols are involved in regulation of numerous cellular processes [50], [51] including sterol synthesis, DNA synthesis, cell growth, proliferation, cell death, etc.
In the plasma, significant portions of 24(S)-hydroxycholesterol, 25-hydroxycholesterol and 27-hydroxycholesterol can all be converted into fatty acyl esters through the enzyme LCAT [52]. In macrophages, Sinensky and colleagues [53], [54] showed that certain oxysterols added to growth medium led to cell apoptosis; blocking ACAT enzyme activity with ACAT inhibitors, or with genetic ablation of ACAT1, significantly increased macrophage apoptosis. These results show that oxysteryl esters formed by ACAT1 play important role in mediating apoptosis in macrophages in culture. In a different cell system, Lu et al. [55] reported that in human Huh7 and HepG2 cells, adding a selective ACAT2 inhibitor, but not a selective ACAT1 inhibitor, caused significant reduction in the secretion of oxysteryl esters (27-hydroxycholesterol, 24(S)-hydroxycholesterol). ACAT2 inhibition also led to a significant increase in the cellular oxysteryl ester concentration, suggesting that blocking ACAT2 led to increased esterification of oxysterols by ACAT1.
In human neuroblastoma SH-SY5Y cells and in lymphoma Jurkat cells, Yamanaka et al. [56] showed that exogenously added 24(S)-hydroxycholesterol could be efficiently esterified by ACAT1. These results suggest that both ACAT1 and ACAT2 can control the oxysterol levels by directly esterifying them, in a cell-type specific manner. ACAT could also control oxysterol levels by altering the cholesterol pool from which oxysterols are derived. For example, in the triple transgenic mouse model for Alzheimer’s disease, Bryleva et al. [3] reported that genetic ablation of ACAT1 increased the steady state concentration of 24(S)-hydroxycholesterol in the mouse brain. Bryleva et al. speculated that in the absence of cholesterol esterification, an increased substrate pool of cholesterol for biosynthesis of 24(S)-hydroxycholesterol might occur.
In various cell types, sterol/steroid received from lipoproteins, and from endogenous biosynthesis can move to mitochondria where they can be metabolized to produce both oxysterols (OXY), such as 22(R)-hydroxycholesterol and 27-hydroxycholesterol, and steroids including PREG (PREG). The free cholesterol can also move to the ER to produce other cholesterol metabolites like 24(S)-hydroxycholesterol and 25-hydroxycholesterol by resident microsomal enzymes at the ER. These free sterol/steroid pool can be put in a storage form through the enzymatic action of acyl-CoA:cholesterol acyltransferase (ACAT1/SOAT1). ACAT1 has been reported to be enriched in the mitochondria associated membrane (MAM) [57, 58], which forms the transitional zones between the endoplasmic reticulum and the mitochondria. Thus, ACAT1 is in a position to esterify cholesterol and various cholesterol metabolites (i.e., oxysterols, PREG; etc.) traversing through the mitochondria and ER membranes. The exact location of ACAT2 in various cell types is yet to be clarified.
Future perspectives
ACATs are drug targets. Previously, most of the effort has been focused on targeting ACAT to treat atherosclerosis. Recent work demonstrated substantial benefits of inhibiting ACAT1 in mouse models of Alzheimer’s disease [2], [3], [4], [5]. An additional area of interest is the role ACAT in cancer: ACAT inhibition has been used to block carcinogenesis in vitro in a variety of cancer types including breast cancer [59], glioblastoma [60], and lymphocytic leukemia [61]. In addition, ACAT1 has been suggested as a potential prognostic marker of prostate cancer progression [62]. Yue et al. [6] reported that advanced human prostate cancer samples had altered cholesteryl ester accumulation, and that blocking ACAT1 mediated cholesteryl ester storage reduced cancer proliferation, impaired its invasive capabilities, and suppressed tumor growth in a mouse model. The authors proposed that the mechanism of action was to work through increased free cholesterol following ACAT1 inhibition, leading to inhibition of sterol regulatory element-binging protein (SREBP) mediated transcription of the low-density lipoprotein receptor (LDLR). The mechanism as proposed is plausible. In addition, given the connection between ACAT and oxysterol esterification, and the role of oxysterols in regulation of cell growth in liver cancer cells [55], it would be worthwhile to explore whether ACAT inhibition alters oxysterol levels in prostate or other cancer models. The existing ACAT inhibitors have both positive and negative attributes, as reviewed in Chang et al. [1] and in Ohshiro and Tomoda [63]. The identification of PREG as an ACAT substrate but not as an activator may help to identify novel ACAT inhibitors that inhibit the allosteric property of ACAT1 without interfering the enzyme’s active sites. Such inhibitors would provide more specificity and with less toxicity than the traditional active site inhibitors. PREG is the precursor for all neurosteroids. The fact that ACAT1 uses PREG as an excellent substrate suggests that ACAT1 may play a key role in controlling the free PREG content in the central nervous system. These are some of the open areas of investigations in the future.
Highlights.
ACATs esterify both sterols and certain steroids including pregnelonone
ACATs contain two different steroidal binding sites
ACATs are drug targets to treat several human diseases.
The novelty of ACAT enzymology can be exploited to develop allosteric ACAT inhibitors
Acknowledgments
We thank Joseph Granger and Bryan Neumann for careful reading of this manuscript. This work is supported by NIH grants AG37609 and HL 60306 to TYC and CCYC.
ABBREVIATIONS
- ACAT
Acyl-coenzyme A:cholesterol acyltransferase
- DGAT1
Acyl-CoA:diacylglycerol acyltransferase 1
- MBOAT
membrane-bound O-acyltransferase
- LCAT
Lecithin:cholesterol acyltransferase
- OXY
Oxysterol
- CHOL
Cholesterol
- ent-cholesterol
Enantiomeric cholesterol
- HDL
High-density lipoprotein
- MAM
Mitochondria associated membrane
- PREG
Pregnenolone
- SREBP
Sterol regulatory element-binging protein
- SOAT
Sterol-O-acyltransferase
- STAR
Steroid- acute regulatory protein
- LXR
Liver X receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang TY, Li BL, Chang CC, Urano Y. Acyl-coenzyme A:cholesterol acyltransferases. American journal of physiology. Endocrinology and metabolism. 2009;297:E1–9. doi: 10.1152/ajpendo.90926.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutter-Paier B, Huttunen HJ, Puglielli L, Eckman CB, Kim DY, Hofmeister A, Moir RD, Domnitz SB, Frosch MP, Windisch M, Kovacs DM. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer’s disease. Neuron. 2004;44:227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 3.Bryleva EY, Rogers MA, Chang CC, Buen F, Harris BT, Rousselet E, Seidah NG, Oddo S, LaFerla FM, Spencer TA, Hickey WF, Chang TY. ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3081–3086. doi: 10.1073/pnas.0913828107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharyya R, Barren C, Kovacs DM. Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:11169–11183. doi: 10.1523/JNEUROSCI.4704-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy SR, Chang CC, Dogbevia G, Bryleva EY, Bowen Z, Hasan MT, Chang TY. Acat1 knockdown gene therapy decreases amyloid-beta in a mouse model of Alzheimer’s disease. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:1497–1506. doi: 10.1038/mt.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X, Ratliff TL, Cheng JX. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell metabolism. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buhman KF, Accad M, Farese RV. Mammalian acyl-CoA:cholesterol acyltransferases. Biochimica et biophysica acta. 2000;1529:142–154. doi: 10.1016/s1388-1981(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 8.Chang CC, Sun J, Chang TY. Membrane bound O-acyltransferases (MBOAT) Frontiers in Biology. 2011;6:177–182. [Google Scholar]
- 9.Chang CC, Miyazaki A, Dong R, Kheirollah A, Yu C, Geng Y, Higgs HN, Chang TY. Purification of Recombinant Acyl-Coenzyme A:Cholesterol Acyltransferase 1 (ACAT1) from H293 Cells and Binding Studies between the Enzyme and Substrates Using Difference Intrinsic Fluorescence Spectroscopy. Biochemistry. 2010;49:9957–9963. doi: 10.1021/bi1013936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H, Bard M, Bruner DA, Gleeson A, Deckelbaum RJ, Aljinovic G, Pohl TM, Rothstein R, Sturley SL. Sterol esterification in yeast: a two-gene process. Science. 1996;272:1353–1356. doi: 10.1126/science.272.5266.1353. [DOI] [PubMed] [Google Scholar]
- 11.Yu C, Kennedy NJ, Chang CCY, Rothblatt JA. Molecular cloning and characterization of two isoforms of saccharomyces cerevisiae acyl-coA:sterol acyltransferase. J Biol Chem. 1996;271:24157–24163. doi: 10.1074/jbc.271.39.24157. [DOI] [PubMed] [Google Scholar]
- 12.Chang CC, Huh HY, Cadigan KM, Chang TY. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. The Journal of biological chemistry. 1993;268:20747–20755. [PubMed] [Google Scholar]
- 13.Li BL, Li XL, Duan ZJ, Lee O, Lin S, Ma ZM, Chang CC, Yang XY, Park JP, Mohandas TK, Noll W, Chan L, Chang TY. Human acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) gene organization and evidence that the 4.3-kilobase ACAT-1 mRNA is produced from two different chromosomes. The Journal of biological chemistry. 1999;274:11060–11071. doi: 10.1074/jbc.274.16.11060. [DOI] [PubMed] [Google Scholar]
- 14.Chang CC, Chen J, Thomas MA, Cheng D, Del Priore VA, Newton RS, Pape ME, Chang TY. Regulation and immunolocalization of acyl-coenzyme A: cholesterol acyltransferase in mammalian cells as studied with specific antibodies. The Journal of biological chemistry. 1995;270:29532–29540. doi: 10.1074/jbc.270.49.29532. [DOI] [PubMed] [Google Scholar]
- 15.Hu GJ, Chen J, Zhao XN, Xu JJ, Guo DQ, Lu M, Zhu M, Xiong Y, Li Q, Chang CC, Song BL, Chang TY, Li BL. Production of ACAT1 56-kDa isoform in human cells via trans-splicing involving the ampicillin resistance gene. Cell research. 2013;23:1007–1024. doi: 10.1038/cr.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Lee O, Chen J, Chen J, Chang CC, Zhou P, Wang ZZ, Ma HH, Sha HF, Feng JX, Wang Y, Yang XY, Wang L, Dong R, Ornvold K, Li BL, Chang TY. Human acyl-coenzyme A:cholesterol acyltransferase 1 (acat1) sequences located in two different chromosomes (7 and 1) are required to produce a novel ACAT1 isoenzyme with additional sequence at the N terminus. The Journal of biological chemistry. 2004;279:46253–46262. doi: 10.1074/jbc.M408155200. [DOI] [PubMed] [Google Scholar]
- 17.Uelmen PJ, Oka K, Sullivan M, Chang CC, Chang TY, Chan L. Tissue-specific expression and cholesterol regulation of acylcoenzyme A:cholesterol acyltransferase (ACAT) in mice. Molecular cloning of mouse ACAT cDNA, chromosomal localization, and regulation of ACAT in vivo and in vitro. The Journal of biological chemistry. 1995;270:26192–26201. doi: 10.1074/jbc.270.44.26192. [DOI] [PubMed] [Google Scholar]
- 18.Meiner VL, Cases S, Myers HM, Sande ER, Bellosta S, Schambelan M, Pitas RE, McGuire J, Herz J, Farese RV., Jr Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14041–14046. doi: 10.1073/pnas.93.24.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang CC, Sakashita N, Ornvold K, Lee O, Chang ET, Dong R, Lin S, Lee CY, Strom SC, Kashyap R, Fung JJ, Farese RV, Jr, Patoiseau JF, Delhon A, Chang TY. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. The Journal of biological chemistry. 2000;275:28083–28092. doi: 10.1074/jbc.M003927200. [DOI] [PubMed] [Google Scholar]
- 20.Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, Farese RV., Jr Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 21.Chang CC, Lee CY, Chang ET, Cruz JC, Levesque MC, Chang TY. Recombinant acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) purified to essential homogeneity utilizes cholesterol in mixed micelles or in vesicles in a highly cooperative manner. The Journal of biological chemistry. 1998;273:35132–35141. doi: 10.1074/jbc.273.52.35132. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Yu C, Liu J, Spencer TA, Chang CC, Chang TY. Cholesterol is superior to 7-ketocholesterol or 7 alpha-hydroxycholesterol as an allosteric activator for acyl-coenzyme A:cholesterol acyltransferase 1. The Journal of biological chemistry. 2003;278:11642–11647. doi: 10.1074/jbc.M211559200. [DOI] [PubMed] [Google Scholar]
- 23.Mannock DA, McIntosh TJ, Jiang X, Covey DF, McElhaney RN. Effects of natural and enantiomeric cholesterol on the thermotropic phase behavior and structure of egg sphingomyelin bilayer membranes. Biophysical journal. 2003;84:1038–1046. doi: 10.1016/S0006-3495(03)74920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Chang CC, Westover EJ, Covey DF, Chang TY. Investigating the allosterism of Acyl Coenzyme A: cholesterol acyltransferase (ACAT) by using various sterols: In vitro and intact cell studies. Biochem J. 2005;391:389–397. doi: 10.1042/BJ20050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller WL. Steroidogenic enzymes. Endocrine development. 2008;13:1–18. doi: 10.1159/000134751. [DOI] [PubMed] [Google Scholar]
- 26.Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (Lond) 2010;7:47–73. doi: 10.1186/1743-7075-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochberg RB, Bandy L, Ponticorvo L, Lieberman S. Detection in bovine adrenal cortex of a lipoidal substance that yields pregnenolone upon treatment with alkali. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:941–945. doi: 10.1073/pnas.74.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellon-Nussbaum S, Hochberg RB. Biosynthesis of lipoidal derivatives of pregnenolone and dehydroisoandrosterone by the adrenal. The Journal of biological chemistry. 1980;255:5566–5572. [PubMed] [Google Scholar]
- 29.Belanger B, Caron S, Belanger A, Dupont A. Steroid fatty acid esters in adrenals and plasma: effects of ACTH. J Endocrinol. 1990;127:505–511. doi: 10.1677/joe.0.1270505. [DOI] [PubMed] [Google Scholar]
- 30.Lavallee B, Provost PR, Belanger A. Formation of pregnenolone- and dehydroepiandrosterone-fatty acid esters by lecithin-cholesterol acyltransferase in human plasma high density lipoproteins. Biochimica et biophysica acta. 1996;1299:306–312. doi: 10.1016/0005-2760(95)00222-7. [DOI] [PubMed] [Google Scholar]
- 31.Damon M, Chavis C. GLC/MS identification of new pregnenolone metabolites in confluent embryonic rat fibroblast cultures. J Steroid Biochem. 1982;16:771–778. doi: 10.1016/0022-4731(82)90034-6. [DOI] [PubMed] [Google Scholar]
- 32.Pahuja SL, Hochberg RB. A comparison of the esterification of steroids by rat lecithin:cholesterol acyltransferase and acyl coenzyme A:cholesterol acyltransferase. Endocrinology. 1995;136:180–186. doi: 10.1210/endo.136.1.7828529. [DOI] [PubMed] [Google Scholar]
- 33.Hochberg RB. Biological esterification of steroids. Endocr Rev. 1998;19:331–348. doi: 10.1210/edrv.19.3.0330. [DOI] [PubMed] [Google Scholar]
- 34.Rogers MA, Liu J, Kushnir MM, Bryleva E, Rockwood AL, Meikle AW, Shapiro D, Vaisman BL, Remaley AT, Chang CC, Chang TY. Cellular pregnenolone esterification by acyl-CoA:cholesterol acyltransferase. The Journal of biological chemistry. 2012;287:17483–17492. doi: 10.1074/jbc.M111.331306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferraz-de-Souza B, Hudson-Davies RE, Lin L, Parnaik R, Hubank M, Dattani MT, Achermann JC. Sterol O-acyltransferase 1 (SOAT1, ACAT) is a novel target of steroidogenic factor-1 (SF-1, NR5A1, Ad4BP) in the human adrenal. The Journal of clinical endocrinology and metabolism. 2011;96:E663–668. doi: 10.1210/jc.2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo ZY, Lin S, Heinen JA, Chang CC, Chang TY. The active site His-460 of human acyl-coenzyme A:cholesterol acyltransferase 1 resides in a hitherto undisclosed transmembrane domain. The Journal of biological chemistry. 2005;280:37814–37826. doi: 10.1074/jbc.M508384200. [DOI] [PubMed] [Google Scholar]
- 37.Yu C, Chen J, Lin S, Liu J, Chang CC, Chang TY. Human acyl-CoA:cholesterol acyltransferase-1 is a homotetrameric enzyme in intact cells and in vitro. The Journal of biological chemistry. 1999;274:36139–36145. doi: 10.1074/jbc.274.51.36139. [DOI] [PubMed] [Google Scholar]
- 38.Yu C, Zhang Y, Lu X, Chen J, Chang CC, Chang TY. Role of the N-terminal hydrophilic domain of acyl-coenzyme A:cholesterol acyltransferase 1 on the enzyme’s quaternary structure and catalytic efficiency. Biochemistry. 2002;41:3762–3769. doi: 10.1021/bi0120188. [DOI] [PubMed] [Google Scholar]
- 39.Baulieu EE, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- 40.Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- 41.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 42.Bowlby MR. Pregnenolone sulfate potentiation of N-methyl-D-aspartate receptor channels in hippocampal neurons. Molecular pharmacology. 1993;43:813–819. [PubMed] [Google Scholar]
- 43.Takebayashi M, Kagawa A, Uchitomi Y, Yokota N, Horiguchi J, Yamawaki S. Differential regulation by pregnenolone sulfate of intracellular Ca2+ increase by amino acids in primary cultured rat cortical neurons. Neurochem Int. 1998;32:205–211. doi: 10.1016/s0197-0186(97)00070-3. [DOI] [PubMed] [Google Scholar]
- 44.Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, Thompson RF, Brinton RD. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6498–6503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, Kasanetz F, Baillie GL, Panin F, Cathala A, Roullot-Lacarriere V, Fabre S, Hurst DP, Lynch DL, Shore DM, Deroche-Gamonet V, Spampinato U, Revest JM, Maldonado R, Reggio PH, Ross RA, Marsicano G, Piazza PV. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343:94–98. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tagawa N, Sugimoto Y, Yamada J, Kobayashi Y. Strain differences of neurosteroid levels in mouse brain. Steroids. 2006;71:776–784. doi: 10.1016/j.steroids.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Morfin R, Young J, Corpechot C, Egestad B, Sjovall J, Baulieu EE. Neurosteroids: pregnenolone in human sciatic nerves. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6790–6793. doi: 10.1073/pnas.89.15.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell DW. Oxysterol biosynthetic enzymes. Biochim Biophys Acta. 2000;1529:126–135. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 49.Russell DW, Halford RW, Ramirez DM, Shah R, Kotti T. Cholesterol 24-hydroxylase: an enzyme of cholesterol turnover in the brain. Annual review of biochemistry. 2009;78:1017–1040. doi: 10.1146/annurev.biochem.78.072407.103859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 51.Smith LL, Johnson BH. Biological activities of oxysterols. Free radical biology & medicine. 1989;7:285–332. doi: 10.1016/0891-5849(89)90136-6. [DOI] [PubMed] [Google Scholar]
- 52.Szedlacsek SE, Wasowicz E, Hulea SA, Nishida HI, Kummerow FA, Nishida T. Esterification of oxysterols by human plasma lecithin-cholesterol acyltransferase. The Journal of biological chemistry. 1995;270:11812–11819. doi: 10.1074/jbc.270.20.11812. [DOI] [PubMed] [Google Scholar]
- 53.Freeman NE, Rusinol AE, Linton M, Hachey DL, Fazio S, Sinensky MS, Thewke D. Acyl-coenzyme A:cholesterol acyltransferase promotes oxidized LDL/oxysterol-induced apoptosis in macrophages. Journal of lipid research. 2005;46:1933–1943. doi: 10.1194/jlr.M500101-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu M, Hu XH, Li Q, Xiong Y, Hu GJ, Xu JJ, Zhao XN, Wei XX, Chang CC, Liu YK, Nan FJ, Li J, Chang TY, Song BL, Li BL. A specific cholesterol metabolic pathway is established in a subset of HCCs for tumor growth. Journal of molecular cell biology. 2013;5:404–415. doi: 10.1093/jmcb/mjt039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamanaka K, Urano Y, Takabe W, Saito Y, Noguchi N. Induction of apoptosis and necroptosis by 24(S)-hydroxycholesterol is dependent on activity of acyl-CoA:cholesterol acyltransferase 1. Cell death & disease. 2014;5:e990. doi: 10.1038/cddis.2013.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof AJ, Madra M, Ikenouchi J, Umeda M, Bird TD, Sturley SL, Schon EA. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. The EMBO journal. 2012;31:4106–4123. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rusinol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. The Journal of biological chemistry. 1994;269:27494–27502. [PubMed] [Google Scholar]
- 59.Antalis CJ, Arnold T, Rasool T, Lee B, Buhman KK, Siddiqui RA. High ACAT1 expression in estrogen receptor negative basal-like breast cancer cells is associated with LDL-induced proliferation. Breast cancer research and treatment. 2010;122:661–670. doi: 10.1007/s10549-009-0594-8. [DOI] [PubMed] [Google Scholar]
- 60.Bemlih S, Poirier MD, El Andaloussi A. Acyl-coenzyme A: cholesterol acyltransferase inhibitor Avasimibe affect survival and proliferation of glioma tumor cell lines. Cancer biology & therapy. 2010;9:1025–1032. doi: 10.4161/cbt.9.12.11875. [DOI] [PubMed] [Google Scholar]
- 61.Mulas MF, Abete C, Pulisci D, Pani A, Massidda B, Dessi S, Mandas A. Cholesterol esters as growth regulators of lymphocytic leukaemia cells. Cell proliferation. 2011;44:360–371. doi: 10.1111/j.1365-2184.2011.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saraon P, Trudel D, Kron K, Dmitromanolakis A, Trachtenberg J, Bapat B, van der Kwast T, Jarvi KA, Diamandis EP. Evaluation and prognostic significance of ACAT1 as a marker of prostate cancer progression. The Prostate. 2014;74:372–380. doi: 10.1002/pros.22758. [DOI] [PubMed] [Google Scholar]
- 63.Ohshiro T, Tomoda H. Isoform-specific inhibitors of ACATs: recent advances and promising developments. Future medicinal chemistry. 2011;3:2039–2061. doi: 10.4155/fmc.11.158. [DOI] [PubMed] [Google Scholar]


