Abstract
We report a case of Trousseau’s syndrome with cholangiocarcinoma complicated by a fatal pulmonary embolism after liver biopsy. A 69-year-old man who presented with right upper quadrant pain was found to have portal vein thrombosis and nonspecific liver hypodensities after imaging by computerized tomography. Following four days of anticoagulation, heparin was held for percutaneous liver biopsy. After the biopsy, he developed acute hepatic failure, acute kidney injury, lactic acidemia, and expired. Autopsy revealed intrahepatic cholangiocarcinoma and a pulmonary embolism. Trousseau’s syndrome with cholangiocarcinoma is rarely reported and has a poor prognosis. This case highlights a fundamental challenge in the diagnosis and early management of intrahepatic cholangiocarcinoma with hypercoagulability. Diagnostic biopsy creates an imperative to reduce post-operative bleeding risk, but this conflicts with the need to reduce thrombotic risk in a hypercoagulable state. Considering the risk of withholding anticoagulation in patients with proven or suspected cholangiocarcinoma complicated by portal vein thrombosis, physicians should consider biopsy procedures with lesser bleeding risks, such as transjugular liver biopsy or plugged percutaneous liver biopsy, to minimize interruption of anticoagulation.
Keywords: Cholangiocarcinoma, Venous thrombosis, Portal vein, Heparin, Biopsy, Large-Core Needle
In 1865 the French physician Armand Trousseau identified migratory superficial phlebitis as a sign of malignancy, an association known as Trousseau’s syndrome.1 He later described this condition in himself before dying of gastric cancer.2 Sack et al3 expanded this definition in 1977 to include the occurrence of venous or arterial thromboembolism – namely chronic disseminated intravascular coagulopathy, platelet-rich microthrombi, microangiopathic hemolytic anemia, and Libman-Sacks endocarditis – in the setting of an underlying malignancy. Recent definitions restrict Trousseau’s syndrome to unexplained thrombotic events that occur before or concomitantly with the presentation of visceral malignancy.4 While common in some gastrointestinal neoplasms, Trousseau’s syndrome is rarely reported in patients with cholangiocarcinoma. When this occurs, the requirements to both diagnose the malignancy with tissue biopsy and manage the prothrombotic state have contradictory anticoagulation requirements that can create a precarious scenario.
Case Report
A 69-year-old man with a history of hypertension, dyslipidemia, and post-traumatic stress disorder was admitted following 10 days of worsening right upper quadrant abdominal pain, anorexia, and dark urine. His previous surgical history was notable for a colostomy, long since reversed, and an abdominal shrapnel injury. Medications on admission were amlodipine 10 mg daily, hydrochlorothiazide 12.5 mg daily, lisinopril 20 mg daily, aspirin 81 mg daily, pravastatin 80 mg daily, lorazepam 0.5 mg as needed, and ibuprofen 600 mg as needed. He reported an allergy to contrast media. He was a long-time smoker who had quit 7 years prior, and he exercised frequently.
On presentation, the patient was afebrile with blood pressure of 180/80 mm Hg, pulse of 110 bpm, and respiratory rate of 20 bpm; he was not jaundiced. His physical examination demonstrated right upper quadrant tenderness to palpation and no upper or lower extremity tenderness or swelling. Laboratory evaluation revealed cholestasis and transaminitis with normal hepatic synthetic function (Table 1). Hematuria (550 red blood cells per high power field) without bilirubinuria was noted. Ultrasound examination disclosed portal vein thrombosis and a large, coarsely irregular liver with increased echogenicity. Heparin was administered.
Table 1.
Significant laboratory values
| Serum laboratory test | Value |
|---|---|
| ALT | 255 U/L |
| AST | 255 U/L |
| Alkaline phosphatase | 357 U/L |
| Total bilirubin | 4.3 mg/dL |
| Direct bilirubin | 2.9 mg/dL |
| Protein | 7.9 g/dL |
| Albumin | 3.0 g/dL |
| INR | 1.22 |
| GGT | 292 u/L |
| LDH | 571 U/L |
| Haptoglobin | 137 mg/dL |
ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma-glutamyl transferase; INR, international normalized ratio; LDH, lactate dehydrogenase
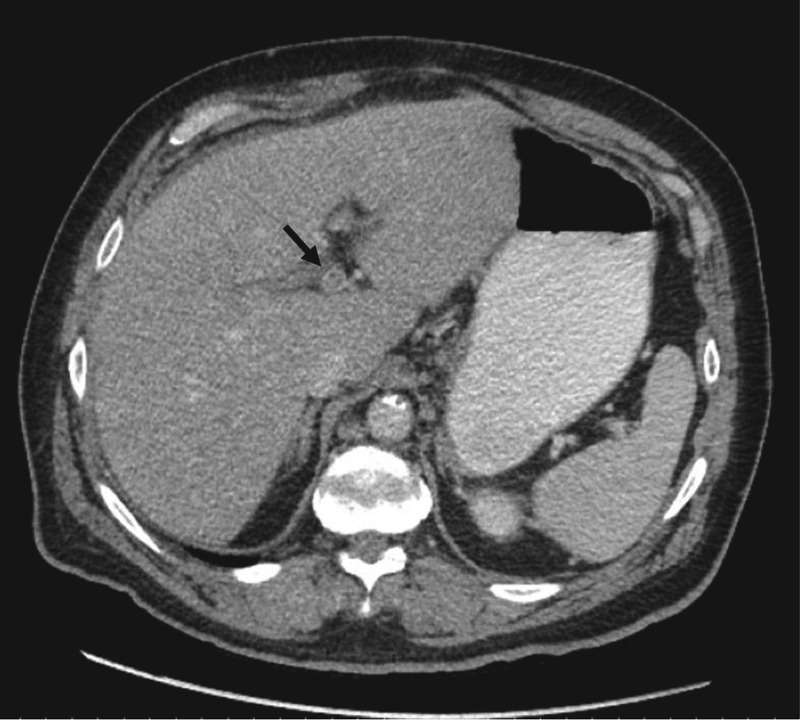
Further work-up showed that alpha-fetoprotein was within normal limits and lupus anticoagulant was not detected. Prothrombin 20210, factor V Leiden, Jak2, CD55, and CD59 mutation assays and hepatitis serologies were negative. Triple phase abdominal computerized tomography (CT) demonstrated a heterogeneous liver with focal, right-lobe hypodensities on arterial phase and persistent left portal vein thrombosis (Figure 1).
Figure 1.
Venous phase abdominal computed tomography with thrombus in left portal vein at bifurcation. Ring of contrast tracking around central thrombus.
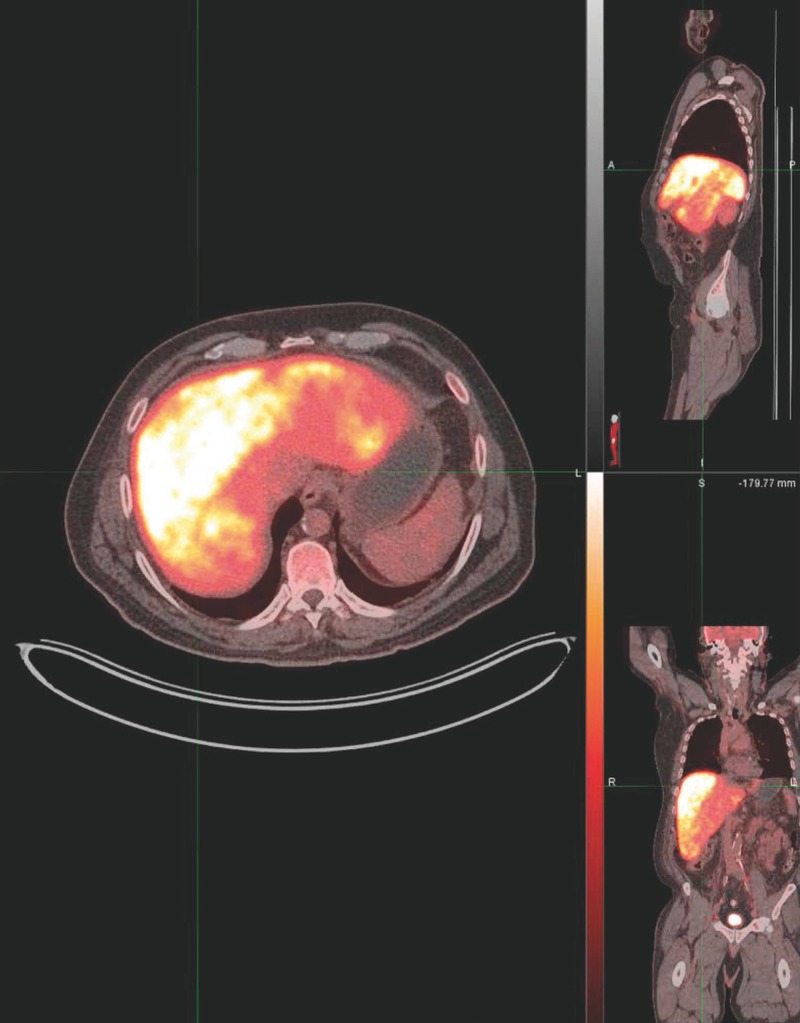
On hospital day 5, heparin was held for an ultrasound-guided percutaneous liver biopsy. Estimated blood loss during the procedure was 0 mL to 10 mL. A positron emission tomography (PET) scan showed diffuse hypermetabolism throughout the right and left hepatic lobes (Figure 2). Sixteen hours after biopsy, before restarting heparin, he developed nausea, diffuse abdominal pain, and delirium. He rapidly decompensated with fulminant hepatic failure, acute kidney injury, and severe lactic acidemia (lactate of 30.1 mg/dL, pH of 6.8, and bicarbonate of 3 mEq/L). He was intubated and maintained on two pressors. Repeat right upper quadrant ultrasound showed no sign of post-biopsy bleeding. Given his poor prognosis, his family opted to discontinue life support and the patient expired.
Figure 2.
Positron emission tomography/computed tomography demonstrating diffuse hypermetabolism throughout right and left hepatic lobes.
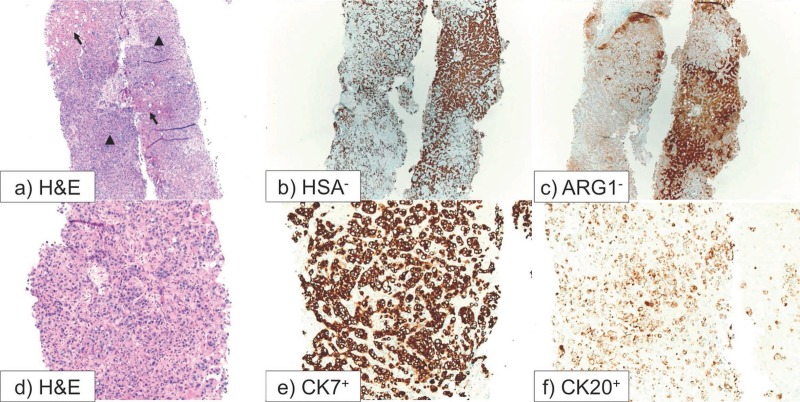
Post-mortem examination of a core needle liver biopsy performed the day before death revealed poorly differentiated adenocarcinoma of the liver. The tumor cells were reactive to immunohistochemical stains for CK7 and CK20, and nonreactive to hepatocyte markers including Hepatocyte Specific Antigen (HSA) and Arginase-1 (ARG-1). Non-tumoral liver demonstrated reverse staining pattern: negative for CK7 and CK20 and positive for HAS and ARG-1 (Figure 3). Tumor cells were also non-reactive to TTF-1, Napsin-A, CDX-2, P63, and mucin. Autopsy demonstrated bilateral lower extremity pitting edema, extensive liver autolysis, portal vein thrombosis, and pulmonary saddle embolus. In the absence of extrahepatic lesions on imaging studies and autopsy, the patient was diagnosed with intrahepatic cholangiocarcinoma.
Figure 3.
Liver, needle biopsy: (a) Hematoxylin and eosin (H&E) stain. Low power picture with arrows indicating uninvolved liver parenchyma and arrow heads pointing to infiltrating tumor cells; (b) Hepatocyte Specific Antigen (HSA) immunohistochemical stain. Uninvolved hepatocytes are staining. The tumor cells show no staining; (c) Arginase-1 (ARG-1) immunohistochemical stain. Uninvolved hepatocytes are staining. The tumor cells show no staining; (d) H&E: High power picture showing infiltrating tumor; (e) Cytokeratin-7 (CK7) immunohistochemical stain showing positive staining by tumor cells. Normal hepatocytes show no staining; (f) Cytokeratin-20 (CK20) immunohistochemical stain showing positive staining by tumor cells. Normal hepatocytes show no staining.
Discussion
Trousseau’s syndrome occurs through a spectrum of complicated and incompletely understood pathways.4 Inappropriate mucin secretion activates microthrombi formation, and tissue factor release triggers the coagulation cascade. Tissue hypoxia and oncogene activation stimulate the production of coagulant factors, and inflammatory cytokines activate endothelial adhesion molecules.4 Herein, we will review the current understanding of this syndrome in cholangiocarcinoma and discuss our patient in the context of these findings.
In a Danish cohort of patients who presented with venous thromboembolism (VTE), 11% were found to have an associated malignancy. Lung, prostate, colorectal, pancreatic, and breast cancers were most commonly diagnosed.5 Ten case reports of cholangiocarcinoma with Trousseau’s syndrome are published in English (Table 2). The first case was reported in 1991 and involved a portal vein thrombosis.6 Of the 11 cases, including this one, the most common presenting thromboembolism was deep vein thrombosis (4 cases) followed by pulmonary embolism (3 cases). Portal vein thrombosis was ultimately uncovered in four of the eleven cases. Splenic thrombosis, stroke, cardiac valve vegetations, and superficial thrombophlebitis were also reported in at least two cases. Only three patients survived for more than 3 months following their initial presentation of thrombosis, which probably reflects both the poor overall prognosis for cholangiocarcinoma (5-year survival, 5–10%)7 and the advanced stage of disease when patients present with thrombosis.
Table 2.
Case Reports of Cholangiocarcinoma with Trousseau’s Syndrome
| Author | Age/Sex | Presenting Thrombosis | Other Thrombosis | Anticoagulation | Outcome & Time After Thrombosis Presentation | Diagnosis | Other |
|---|---|---|---|---|---|---|---|
| Bandyopadhyay et al20 | 38 F | DVT | Spleen, IVC, PVT | Warfarin | Death? (refused therapy), 8 months | ERCP punch biopsy | |
| Blum et al | 69 M | PVT | PE | Heparin | Death, 1 week | U/S-guided liver biopsy | |
| Ching6 | 76 M | PVT | — | — | Death, 5 days | Autopsy | 1st reported case |
| Hernández et al21 | 45 F | PE | DVT, PVT | Heparin | Death, few days | Autopsy | |
| Jang et al22 | 56 M | PE | — | LMWH | Alive, 3 months | U/S-guided liver biopsy | |
| Koskinas et al8 | 30 F | PE | — | Heparin | Death, 6 days | Autopsy | HBsAg+, hepatocellular-cholangiocarcinoma |
| Martins et al (case 1)23 | 67 M | DVT | STP | Warfarin | Death, 8 weeks | CT-guided liver biopsy | PSC with CD |
| Martins et al (case 2)23 | 39 M | DVT | STP | — | Death, 6 weeks | FNA | PSC with UC |
| Muñoz-Ortego et al9 | 51 F | Stroke | PE, kidney, spleen, mitral valve vegetations | Heparin (and immunoglobulin) | Death, timing unknown | Autopsy | Lung adenocarcinoma and intrahepatic cholangiocarcinoma |
| Samadian et al24 | 78 F | DVT | PE | Warfarin | Death, 2 years | Autopsy | DVT & PE with INR of 3.0 |
| Yuri et al10 | 73 F | Stroke | Mitral valve vegetations, spleen, rectum | “Anticoagulant therapy” | Death, 3 months | Autopsy | CA 19-9, CA15-3, CA-125 active in tumor cytoplasm |
CD, Crohn disease; CRP, C-reactive protein; DVT, deep vein thrombosis; ERCP, endoscopic retrograde cholangiopancreatography; F, female; FNA, Fine Needle Aspiration; HBsAg+, Hepatitis B Virus surface antigen positive; IVC, inferior vena cava; LDH, lactate dehydrogenase; LMWH, low-molecular-weight heparin; M, male; PE, pulmonary embolism; PSC, primary sclerosing cholangitis; PVT, portal vein thrombosis; STP, superficial thrombophlebitis; UC, ulcerative colitis; U/S, ultrasound
All but two cases involved an isolated cholangiocarcinoma; the others included a hepatocellular-cholangiocarcinoma8 and a patient with both cholangiocarcinoma and lung adenocarcinoma.9 Antiphospholipid antibodies were identified in three cases. One study identified several markers of mucin expression.10 Diagnosis of cholangiocarcinoma was made during autopsy for six cases and by CT or ultrasound-guided liver biopsy, fine needle aspiration, or endoscopic retrograde cholangiopancreatography punch biopsy in the remaining cases. No biopsy complications were reported.
Jeon et al11 conducted a retrospective cohort analysis of 273 South Korean patients with cholangiocarcinoma spanning a four year period, 40 of whom developed VTE. Ten of these patients presented with VTE at the time of cholangiocarcinoma diagnosis. Elevated C-reactive protein, advanced cancer stage, and use of chemotherapy were associated with development of VTE. Pulmonary thromboembolism and portal vein thrombosis were the most common sites of VTE, and development of VTE was significantly associated with reduced survival compared to non-VTE patients, even among patients with advanced stage disease. This study did not include patients whose VTE preceded cholangiocarcinoma diagnosis. There is a discrepancy between the numerous VTE patients in this cohort and the scant case reports in the literature, which the authors attribute to the two to four-fold higher incidence of cholangiocarcinoma in Korea compared with Western countries. The English-language case reports tend to highlight VTE prior to or with cholangiocarcinoma diagnosis, while the majority of the Korean cohort developed VTE after diagnosis.
The fundamental challenge in this case is balancing the need for anticoagulation in managing portal vein thrombosis with the need for a diagnostic liver biopsy, which requires temporary discontinuation of anticoagulation. Current guidelines for management of acute portal vein thrombosis address the overarching category of splanchnic vein thrombosis and recommend prolonged anticoagulation for symptomatic patients to prevent progression and recurrence of splanchnic and non-splanchnic thrombosis.12 Diagnosis of focal liver lesions often requires biopsy, since definitive diagnosis is not always made on imaging.13 For cholangiocarcinoma in particular, diagnosis cannot be made reliably with imaging alone, and biopsy is recommended if surgery is not already planned.13 Liver biopsy guidelines categorize percutaneous liver biopsy as having a moderate risk of bleeding and recommend discontinuing heparin-products 12 hours to 24 hours before biopsy to reduce the risk of bleeding, though this risk must be weighed against need for anticoagulation.14 Perioperative antithrombotic bridging guidelines recommend resuming therapeutic heparin 24 hours after surgery.15 Two retrospective studies found the risk of significant bleeding following percutaneous liver biopsy to be 0.078% to 0.32%,16,17 and peripheral coagulation parameters do not correlate to bleeding risk.18
Unfortunately, the clinical experience describing the outcome of hypercoagulable patients that undergo liver biopsy is poorly documented. In this patient, we hypothesize that withdrawing heparin in the perioperative period may have removed inhibition of an ongoing procoagulant process. We also consider the possibility that biopsy-associated trauma to tumor parenchyma or an inflammatory response to injury triggered further release of procoagulant factors, in which case the absence of heparin would have indirectly contributed to the development of a pulmonary embolism. No embolic source of patient’s fatal pulmonary embolism was identified during the patient’s hospital course, but it is estimated that 10% to 15% of pulmonary embolisms are without a source.19 Transjugular liver biopsy and plugged percutaneous liver biopsy are alternative methods that may have lower bleeding risks.14 These techniques could allow clinicians to withhold anticoagulants for less time, enabling a safer diagnosis of cholangiocarcinoma in a hypercoagulable state.
Physicians should carefully consider the high risk of withholding anticoagulation in patients with proven or suspected cholangiocarcinoma complicated by portal vein thrombosis. Biopsy techniques that minimize interruption of anticoagulation should be strongly considered.
References
- 1.Trousseau A. Phlegmasia Alba Dolens. Lectures on Clinical Medicine, Delivered at the Hotel-Dieu, Paris. Vol 5 London, England; 1865. [Google Scholar]
- 2.Khorana AA. Malignancy, thrombosis and Trousseau: the case for an eponym. J Thromb Haemost 2003;1:2463–2465. [DOI] [PubMed] [Google Scholar]
- 3.Sack GH, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore) 1977;56:1–37. [PubMed] [Google Scholar]
- 4.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood 2007;110:1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–1850. [DOI] [PubMed] [Google Scholar]
- 6.Ching CK. Trousseau’s syndrome in a patient with cholangiocarcinoma. Am J Gastroenterol 1991;86:928–929. [PubMed] [Google Scholar]
- 7.Anderson CD, Pinson CW, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004;9:43–57. [DOI] [PubMed] [Google Scholar]
- 8.Koskinas J, Betrosian A, Kafiri G, Tsolakidis G, Garaziotou V, Hadziyannis S. Combined hepatocellular-cholangiocarcinoma presented with massive pulmonary embolism. Hepatogastroenterology 2000;47:1125–1128. [PubMed] [Google Scholar]
- 9.Muñoz-Ortego J, Blanco Lopez L, Carbonell Abello J, Monfort Faure J. Multiple thromboemboli associated to two occult tumors: a case mimicking catastrophic antiphospholipid syndrome. Joint Bone Spine 2011;78:405–408. [DOI] [PubMed] [Google Scholar]
- 10.Yuri T, Kato K, Hirohara J, Kinoshita Y, Emoto Y, Yuki M, Yoshizawa K, Tsubura A. Trousseau’s Syndrome Caused by Intrahepatic Cholangiocarcinoma: An Autopsy Case Report and Literature Review. Case Rep Oncol 2014;7:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon HK, Kim DU, Baek DH, Ha DW, Lee BE, Ryu DY, Cheong JH, Kim GH, Song GA, Jang AL. Venous thromboembolism in patients with cholangiocarcinoma: focus on risk factors and impact on survival. Eur J Gastroenterol Hepatol 2012;24:444–449. [DOI] [PubMed] [Google Scholar]
- 12.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e419S–e494S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrero JA, Ahn J, Rajender Reddy K. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol 2014;109:1328–1347; quiz 1348. [DOI] [PubMed] [Google Scholar]
- 14.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology 2009;49:1017–1044. [DOI] [PubMed] [Google Scholar]
- 15.Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, Dunn AS, Kunz R; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S–e350S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Thiel DH, Gavaler JS, Wright H, Tzakis A. Liver biopsy. Its safety and complications as seen at a liver transplant center. Transplantation 1993;55:1087–1090. [DOI] [PubMed] [Google Scholar]
- 17.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol 1986;2:165–173. [DOI] [PubMed] [Google Scholar]
- 18.Ewe K. Bleeding after liver biopsy does not correlate with indices of peripheral coagulation. Dig Dis Sci 1981;26:388–393. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz T, Hingorani A, Ascher E, Marks N, Shiferson A, Jung D, Jimenez R, Jacob T. Pulmonary embolism without deep venous thrombosis. Ann Vasc Surg 2012;26:973–976. [DOI] [PubMed] [Google Scholar]
- 20.Bandyopadhyay SK, Sarkar N, Ghosh S, Dasgupta S. Cholangiocarcinoma presenting with recurrent venous thrombosis. J Assoc Physicians India 2003;51:824–825. [PubMed] [Google Scholar]
- 21.Hernández JL, Riancho JA, González-Macías J. Cholangiocarcinoma presenting as Trousseau’s syndrome. Am J Gastroenterol 1998;93:847–848. [DOI] [PubMed] [Google Scholar]
- 22.Jang JW, Yeo CD, Kim JD, Bae SH, Choi JY, Jung ES, Rha SE, Byun JY, Yoon SK. Trousseau’s syndrome in association with cholangiocarcinoma: positive tests for coagulation factors and anticardiolipin antibody. J Korean Med Sci 2006;21:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martins EB, Fleming KA, Garrido MC, Hine KR, Chapman RW. Superficial thrombophlebitis, dysplasia, and cholangiocarcinoma in primary sclerosing cholangitis. Gastroenterology 1994;107:537–542. [DOI] [PubMed] [Google Scholar]
- 24.Samadian S, Estcourt L. Recurrent thrombo-embolic episodes: the association of cholangiocarcinoma with antiphospholipid syndrome. Postgrad Med J 1999;75:45–46. [DOI] [PMC free article] [PubMed] [Google Scholar]