Abstract
Objectives:
The association of inflammatory reactions with almost all types of cancer supports the concept that inflammation is a critical component of tumor progression. The present study aimed to evaluate the relationship of serum markers of chronic inflammation with the stage of and histopathological size of colorectal carcinoma (CRC).
Methods:
This cross-sectional study included 90 patients of both sexes, mean age 66.2 (range 47-78) years, with clinically and histologically confirmed CRC, who were admitted to the Clinic for abdominal surgery UCCS for surgical treatment of CRC. The patients according to the stage of disease were divided into three groups (stage II–IV). The control group consisted of 30 subjects with no signs of malignancy and acute inflammatory diseases. Staging of CRC was done according to the TNM classification. In each patient, the preoperative blood samples were taken for determination of the parameters of inflammation: the erythrocyte sedimentation rate, white blood cells, C-reactive protein (CRP), fibrinogen and alpha 2 globulins.
Results:
It was confirmed that increasing markers of inflammation followed increasing stages of colorectal cancer, depth of tumor invasion and the occurrence of metastatic disease. CRP is a biomarker that consistently and significantly increases from the second to the fourth stage of colorectal cancer (7.2 (2.3-14.6) mg/L vs. 21.85 (12.3-41) mg/L vs. 38.6 (21.5-79) mg/L; p<0.01) and significantly correlates positively with the stage of CRC (r= 0.783, p<0.001), and the tumor size (r=0.249, p<0.05).
Conclusion:
The study results point to an increase in the degree of chronic inflammation throughout the progression of colorectal cancer. The most consistent marker of chronic inflammation that accompanies the progression of colorectal carcinoma is CRP.
Keywords: colorectal cancer, chronic inflammation
1. INTRODUCTION
Inflammation is part of the innate immunity that takes part in the destruction of infectious agents, as well as cancer cells. On the other hand, chronic inflammation is a pathological condition in which the inflammatory state is constantly supported. Such inflammatory conditions may administer or promote oncogenic transformation of the genetic and epigenetic changes in malignant cells. It is believed that inflammatory microenvironment can support tumor progression (1, 2, 3), stimulating cell growth and creating opportunity to build up a larger number of mutations in the tissue. Monocytes/macrophages are the most common components of the inflammatory infiltrate in the majority of malignant tumors, and are referred to as tumor-associated macrophages (TAM). Phagocytic infiltrates occur early in a non-invasive tumor stage and progressively increase, with gradual conversion of the M1 form of a pro-inflammatory phenotype into cancer-promotional M2 phenotype. This event directly affects the behavior of the tumor cells, fueling tumor remodeling, immunomodulation, angiogenesis and tumor progression (4).
Many cancers grow at sites of infection, chronic irritation, and inflammation. Recent studies point to molecular pathways link between inflammation and cancer, representing the concept that inflammation is a critical component of tumor progression (5, 6). Colorectal carcinoma (CRC) is the third most common malignant tumor in human pathology worldwide and the second leading cause of death due to malignant disease (7). It occurs in the tissues of the colon, in only partially known complex sequence of molecular events. Chronic inflammatory bowel disease, particularly ulcerative colitis, increases the risk of developing colorectal cancer. According to a meta-analysis of 116 studies, the prevalence of colorectal cancer in patients with ulcerative colitis was 3.7%. The cumulative risk of colorectal cancer as a result of the duration of ulcerative colitis is estimated at 2% at 10 years, 8% at 20 years and 18% at 30 years of the disease (8).
2. AIM OF THE STUDY
The aim of this study was to evaluate the relationship of markers of chronic inflammation with the stage of and histopathological size of colorectal cancer and to examine the possible role of inflammation in the promotion, progression and metastasis of colorectal carcinoma.
3. MATERIALS AND METHODS
This cross-sectional study included 90 patients of both sexes, with clinically and histologically confirmed colorectal cancer, who had been admitted to the Clinic for abdominal surgery of the Clinical Center of the University of Sarajevo (CCUS) for surgical treatment of colorectal carcinoma. All 90 cases were pathologically confirmed as adenocarcinoma. Patients who refused surgical treatment, patients with proven neoplasm on another organ that is not linked to colorectal carcinoma, as well as the patients with active infection were not included in the study. The control group consisted of 30 patients of the appropriate age and gender, without any clinical and laboratory signs of malignant or inflammatory disease.
The study protocol was approved by the Ethics Committee of the Clinical Center of the University of Sarajevo. All participants signed informed written consent after the explanation of the study procedure. The study was conducted in accordance with basic principles of the last revision of The Declaration of Helsinki on the Rights of patients involved in biomedical research.
Blood samples of each patient were preoperatively taken by venipuncture from the cubital vein for the purpose of laboratory measurement of inflammatory serum markers: the erythrocyte sedimentation rate, white blood cells number, the value of C-reactive protein (CRP), fibrinogen and alpha 2 globulins. These parameters are determined by standard laboratory analysis at the Institute of Clinical Chemistry and Biochemistry of the CCUS, on the day of blood sampling on an empty stomach, and before surgery.
Surgical treatment of colorectal cancer was performed at the Clinic of Abdominal Surgery, Clinical Center of the University of Sarajevo, as part of the therapeutic process. Ontological Surgery principle “en bloc” resection of organs with associated lymph-vascular arcade was honored within the operative treatment.
Histopathologic analysis of biopsy samples and resected specimen was performed at the Clinic of Pathology of the CCUS. Microscopic analysis included the determination of: a) histological type of malignoma (categorized according to World Health Organization); b) depth of tumor invasion in the intestinal wall (pT), and c) number of pericolic lymph nodes infiltrated with cancerous tissue. The stages of colorectal cancer are determined according to the TNM classification of the American Committee on Cancer (American Join Committee on Cancer -AJCC) in 2010 (9). All patients on the basis of stage of colorectal carcinoma were divided into three groups, with 30 patients: group 1–stage II, group 2–stage III and group 3–stage IV.
Statistical analysis
All statistical calculations were performed with the MedCalc Software for Windows, version 12.6.1.0 (Mariakerke, Belgium). The distribution of variables was tested by Kolmogorov-Smirnov or Shapiro-Wilk test. Values with normal distribution were expressed as mean±standard deviation, while those without normal distribution were expressed as median and interquartile range. Student’s t-test (for variables with normal distribution) and Mann Whitney U test (for variables without normal distribution) were used to compare mean values between two groups. ANOVA and Kruskall-Wallis test was used for statistical evaluation of more than 3 groups. Correlation of monitored variables is determined by Spearman. P-values less than 0.05 were considered statistically significant.
4. RESULTS
Clinical and histopathologic colorectal cancer is more commonly confirmed in older people (mean age was 66.2 (range 47-78) years at the time of surgery) of which 60% were male. Adenocarcinoma was found in 100% of cases, predominantly grade 2. According to the depth of tumor invasion of the intestine, histopathological pT3 is the most common (63.3%), while pT2 is the least common (8.9%).
Analysis of chronic inflammation parameters through the stages of colorectal cancer progression shows an evident increase of monitored parameters with the progression of the disease (Table 1). The values of inflammatory markers C-reactive protein and alpha 2 globulin serum, as well as plasma fibrinogen concentration in the second stage of colorectal carcinoma are significantly different in comparison with the healthy control group (p<0.05).
Table 1.
The values of the parameters of inflammation in control group and in different stages of of colorectal carcinoma (CRC). Data are presented as median and interquartile range. ESR, erythrocyte sedimentation rate; WBC, white blood cells; CRP, C reactive protein; *–Significant difference between the control group and second stages of CRC; †–Significant difference between II and III stages of CRC; ‡–Significant difference between III and IV stages of CRC; I – Significant difference between II and IV stages of CRC.
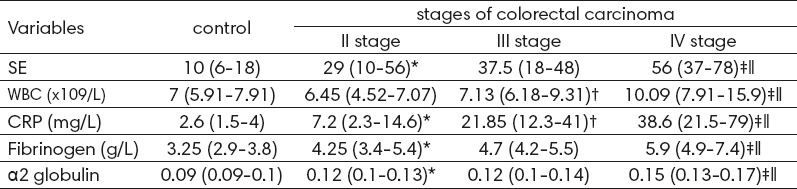
A significant difference in a number of WBC (p <0.05) and the value of CRP in serum (p <0.001) between the second and third stages of malignant diseases of the colon is observed, while between the third and fourth stages, and between the second and fourth stages of colorectal carcinoma there is a significant difference between all markers of inflammation (p <0.01) (Table 1).
From analyzed parameters of inflammation by depth of tumor invasion in the intestinal wall (pT), the values and CRP in serum showed consequently an increase with the depth of tumor invasion of histologically T2 to T4 tumor size (Table 2). Erythrocyte sedimentation rate was significantly higher in pT4 tumor size compared to pT3 (55 (33-75) vs 38 (17-56) mg / L, p = 0.012) as well as in comparison to pT2 tumor size (55 (33-75) vs. 30.5 (12-54.5) mg / L, p = 0.014), while between pT2 and pT3 there was no statistical difference in this parameter (p = 0.478). And the serum concentration of C-reactive protein in patients pT4 group was significantly higher than the concentration of the biomarkers of the pT3 groups (29.7 (14.7-50) vs. 19 (6-44) mg / L, p = 0.029), and pT2 group (29.7 (14.7-50) vs 17.75 (7.3-20.25) mg / L, p = 0.012) (Table 2).
Table 2.
The parameters of inflammation of respondents by the depth of tumor invasion in the intestinal wall (pT). Data are presented as median and interquartile range. ESR, erythrocyte sedimentation rate; WBC, white blood cells; CRP, C reactive protein. pT2, tumour invades muscularis propria; pT3, tumor invades serosis; pT4, tumor invades adjacent organs or breaks visceral peritoneum. *–Significant difference between pT4 and pT3 group; †–Significant difference between pT4 and pT2 group.
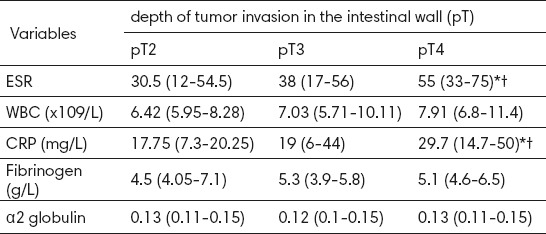
All monitored parameters of inflammation in serum of the patients with metastatic form (M1) of colorectal cancer were significantly higher compared to the patients with colorectal cancer without the existence of metastasis (M0) (Table 3). There was a significant statistical difference in mean erythrocyte sedimentation rate (p = 0.001), WBC count (p <0.001), the concentration of CRP (p <0.001), fibrinogen (p <0.001), and alpha2 globulin (p <0.001).
Table 3.
The differences in the parameters of inflammation in patients without and in patients with metastatic form of colorectal carcinoma. Data are presented as mean±SD or as median and interquartile range; M0, without metastatic disease; M1, with metastatic disease.
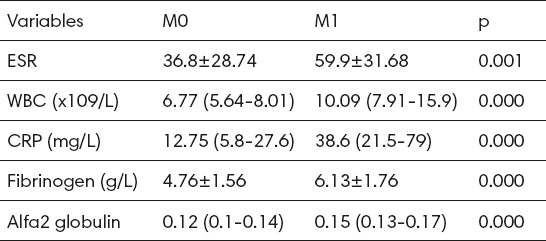
It was found that the stage of colorectal cancer significantly and positively correlated with markers of inflammation, including: erythrocyte sedimentation rate (r = 0.573, p <0.001), WBC (r = 0.400, p <0.001), CPR value (r = 0.783, p <0001), fibrinogen (r = 0.601, p <0.001) and alpha 2 globulins (r = 0.656, p <0.001) (Table 4). The size of the tumor (pT) significantly positively correlated with ESR (r = 0.216, p<0.05), the number of leukocytes (r = 0.207, p <0.05) and CRP (r = 0.249, p <0.05).
Table 4.
Correlation of monitored biomarkers with the stage and the histopathologic size of colorectal carcinoma. r – koeficijent korelacije; *p<0.05; **p<0.001
5. DISCUSSION
Previous studies have shown that the strongest association of chronic inflammation with the malignant disease is expressed in colon carcinogenesis, particularly in patients with inflammatory bowel disease, for example, chronic ulcerative colitis and Crohn’s disease (10). The molecular mechanisms by which the inflammation promotes cancer development are still uncovered and may differ between colitis-associated cancer (CAC) and other forms of colorectal cancer (11).
The concept suggesting that inflammation is a critical component in tumor progression is the result of the fact that many cancers arise from the site of infection, chronic irritation, and inflammation (12). There is evidence suggesting that the tumor microenvironment, which is largely influenced by inflammatory cells, is an indispensable actor in the neoplastic process, in fostering proliferation, and survival and migration of tumor cells. In addition, the tumor cells have some co-opted signaling molecules of the innate immune system, such as selectins, chemokines and their receptors for invasion, migration and metastasis, which has enhanced new therapeutic approaches to cancer development.
This research revealed that the highest number of patients with clinically and histologically confirmed colorectal cancer are male (60%), median age 66.2 years (range 47-78 years of age), which is consistent with the data of the published studies (13, 14). It is believed that chronic inflammation is most strongly associated with the development of colorectal cancer in the elderly population. The risk of occurrence and development of colorectal cancer increases greatly after 50 years of age. Therefore in many countries the recommended screening programs exist for people older than 50 years of age (15, 16).
Our research identified statistically significant differences in the values of markers of inflammation: ESR, CRP, fibrinogen and alpha2 globulin between the control group and the patients with the second stage of colorectal cancer (p <0.01). An increase of all monitored inflammatory markers was observed with the disease progression, including the determined significant differences between third and fourth, and the fourth and second stage of colorectal cancer (p <0.001), indicating the presence and influence of chronic inflammation in the pathogenesis of this malignancy. In addition, serum CRP proved to be the constant biomarker of the disease progression, as it progressively significantly increased with the increase of the disease stage. In evaluating the level of serum CRP during colorectal carcinogenesis, Italian authors Biasi and colleagues also found an increase in levels of CRP in the serum of patients with colorectal cancer, particularly in the second and third stages of the disease (14). In this research, it was also revealed that the markers of inflammation were significantly positively correlated with the stage of colorectal cancer (p <0.001). Erlinger et al. (17) showed a significant association between baseline C-reactive protein levels and the subsequent risk of colon cancer in a case-control study within the CLUE II cohort, and a prospective study was conducted to find clues to prevent cancer and heart disease. Otani et al. (18) in a large Japan Public Health Center-Based Prospective Study also found a significant association of plasma levels of C-reactive protein and the subsequent risk of colon cancer. The results of this study also showed that plasma C-reactive protein was associated with the intramucosal rather than the invasive type of colon cancer. In contrast, the study found that the values of ESR and CRP levels progressively increased with the depth of tumor invasion in the intestinal wall of T2 to T4 tumor size and were significantly highest in patients with T4 tumor size, or in patients with the tumor penetration through the wall of the visceral peritoneum.
It was also found that the patients with metastatic form (M1) of colorectal cancer have a significantly higher value of chronic inflammation markers in the serum (p <0.001), indicating that the increase in the index of inflammation significantly contributes to increasing the size of colorectal cancer and its metastasis. This finding supports the significance of chronic inflammation in occurrence and progression of colorectal cancer. Inflammatory infiltrates occur in the early, non-invasive tumor stage. Stimulated by inflammation, the colon cancer cells may secrete the cytokines involved in the tumorigenesis in the colon, with the increase of angiogenesis (19) and metastatic progression of tumors (20). Cell proliferation, that contributes to DNA damage and / or mutagenic attack, creates a microenvironment rich in inflammatory cells and growth factors that support their growth (21). In this regard, tumors act as wounds that are not healed.
6. CONCLUSION
The results provide evidence that all monitored serum markers of inflammation show an increase along with an increase in the stage of colorectal carcinoma, the depth of tumor invasion of the wall intestine and the metastasis of the disease. The most consistent marker of chronic inflammation that accompanies the progression of colorectal carcinoma is CRP. This biomarker showed significant correlation with the size and stage of colorectal carcinoma, which highlights the importance of the location and CRP in the follow-up of colorectal carcinoma. These data support the hypothesis that inflammation is a risk factor for the development and progression of colon cancer.
Footnotes
• Author’s contribution: all authors were included in all phases of preparing this article, including final proof reading.
• Conflict of interest: none declared.
REFERENCES
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature Reviews Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Web Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431(7007):405–6. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 7.Haggar FA, Boushey RP. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin Colon Rectal Surg. 2009;22(4):191–7. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AJCC Cancer Staging Manual. 7th ed. Springer-Verlage New-York, Inc; 2010. [Google Scholar]
- 10.Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14:3937–47. doi: 10.3748/wjg.14.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35(2):229–44. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–5. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 14.Biasi F, Guina T, Maina M, Nano M, Falcone A, Aroasio E, Saracco GM, Papotti M, Leonarduzzi G, Poli G. Progressive Increase of matrix metalloprotease-9 and interleukin- 8 serum levels during carcinogenic process in human colorectal tract. PLoS ONE. 2012;7(7):e41839. doi: 10.1371/journal.pone.0041839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzpatrick D. Cancer in Northern Ireland 1993-2001 a comprehensive report. Belfast: Northern Ireland Cancer Registry; 2004. [Google Scholar]
- 16.Davies RJ, Miller R, Coleman N. Colorectal cancer screening: prospects for molecular stool analysis. Nat Rev Cancer. 2005 Mar;5(3):199–209. doi: 10.1038/nrc1569. [DOI] [PubMed] [Google Scholar]
- 17.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–90. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 18.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S, et al. Plasma C-Reactive Protein and Risk of Colorectal Cancer in a Nested Case-Control Study: Japan Public Health Center - Based Prospective Study. Cancer Epidemiol Biomarkers Prev. 2006;15(4):690–5. doi: 10.1158/1055-9965.EPI-05-0708. [DOI] [PubMed] [Google Scholar]
- 19.Ning Y, Lenz HJ. Targeting IL-8 in colorectal cancer. Expert Opin Ther Targets. 2012;16(5):491–7. doi: 10.1517/14728222.2012.677440. [DOI] [PubMed] [Google Scholar]
- 20.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–14. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]


