Abstract
Introduction:
Appropriate oral health care is fundamental for any individual’s health. Dental caries is still one of the major public health problems. The most effective way of caries prevention is the use of fluoride.
Aim:
The aim of our research was to review the literature about fluoride toxicity and to inform physicians, dentists and public health specialists whether fluoride use is expedient and safe.
Methods:
Data we used in our review were systematically searched and collected from web pages and documents published from different international institutions.
Results:
Fluoride occurs naturally in our environment but we consume it in small amounts. Exposure can occur through dietary intake, respiration and fluoride supplements. The most important factor for fluoride presence in alimentation is fluoridated water. Methods, which led to greater fluoride exposure and lowered caries prevalence, are considered to be one of the greatest accomplishments in the 20th century`s public dental health. During pregnancy, the placenta acts as a barrier. The fluoride, therefore, crosses the placenta in low concentrations. Fluoride can be transmitted through the plasma into the mother’s milk; however, the concentration is low. The most important action of fluoride is topical, when it is present in the saliva in the appropriate concentration. The most important effect of fluoride on caries incidence is through its role in the process of remineralization and demineralization of tooth enamel. Acute toxicity can occur after ingesting one or more doses of fluoride over a short time period which then leads to poisoning. Today, poisoning is mainly due to unsupervised ingestion of products for dental and oral hygiene and over-fluoridated water.
Conclusion:
Even though fluoride can be toxic in extremely high concentrations, it`s topical use is safe. The European Academy of Paediatric Dentistry (EAPD) recommends a preventive topical use of fluoride supplements because of their cariostatic effect.
Keywords: diet, fluorides, topical administration, mechanism of action, toxicity, standards
1. INTRODUCTION
The most efficient way to prevent caries is by using fluoridated dental products (1, 2). Fluoride enters the body with food, through respiration and products containing fluoride (3). Fluoride is a part of the natural environment and is therefore constantly present in people’s lives. However, concentration of fluoride can vary from one region to another. From a chemical point of view, it is the most electronegative and reactive of all the elements due to its small atomic radius. Since it is highly reactive, it is usually bound as inorganic fluoride and not found in its elementary state (4). It ranks 13th in terrestrial abundance and represents 0.06-0.09% of weight of the Earth’s crust. Fluorine is present in the lithosphere, atmosphere, hydrosphere and biosphere. A large amount of fluorine can be found in rocks of volcanic origin. It enters the environment through volcanic eruptions, rock dissolution and numerous human activities (coal burning, ore processing, production and use of fertilizers, and industrial plants) (4, 5). Fluoride is found in all natural waters. Seawater contains 1.2-1.5 ppm of fluoride. Freshwater concentrations are usually lower ranging from 0.01 to 0.3 ppm. Higher concentrations of fluoride in water can be present near hot springs of volcanic origin (5). Normal accumulation of fluoride from the soil is low. Flora growing in acidic soil tends to accumulate more fluoride. There are some plants which can accumulate a few 100 ppm of fluoride; the best known is the tea plant (Camellia sinensis, syn. Thea sinensis) (6).
2. AIM
The aim of our research was to review the literature about fluoride toxicity and to inform physicians, dentists and public health specialists whether fluoride use is expedient and safe.
3. METHODS
3.1 Sources and search strategy
Data we used in our review were systematically searched from articles published until 2015 and collected from official web pages and documents published from different international institutions. Professional criteria were taken into account. The search was conducted between 16th and 27th March 2015 and revised between 1st and 18th December 2015. At the end of our review we summarized current EAPD guidelines for fluoride use published in 2009 (7).
3.2 Reviewed fields
Topics discussed in this review are:
Dietary intake of fluoride,
Fluoride metabolism,
History of fluoride use,
The mechanism of fluoride action,
Overuse and toxicity of fluoride.
4. RESULTS AND DISCUSSION
4.1 Dietary intake of fluoride and fluoride supplements
Even though fluoride is generally present in our everyday life, we consume it in small amounts. In general, it can be found in meat, fish, and cereals (2, 5). In higher concentrations, it can also be found in canned anchovies, canned fruits, ground chicken meat products (with a higher percentage of ground bones), chocolate milk and some baby dietary supplements (8). Table 1 shows the average concentrations of fluoride in different types of food (8).
Table 1.
Fluoride concentrations for different types of food. Source: United States Agriculture Department (USDA), 2005.
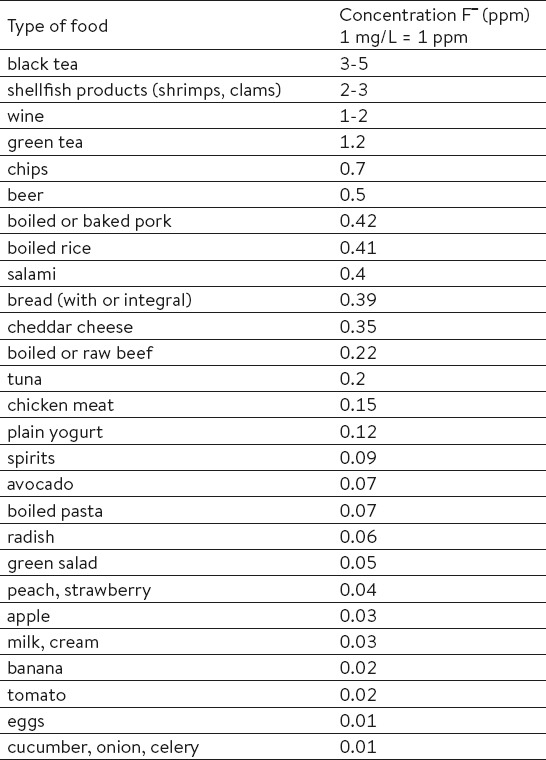
Fluoride content in food can also depend on materials used in food preparation. For instance, Teflon cookware is a great source of fluoride ions (9). The most important factor for fluoride presence in alimentation is fluoridated water (8). The reason behind this are preventive programs for fluoridation of drinking water (in the USA, the Healthy People 2010 objective was to increase proportion of USA population served by community water systems with optimally fluoridate water to 75%) (10). These methods are especially important in regions where other preventive programs are not available (11).
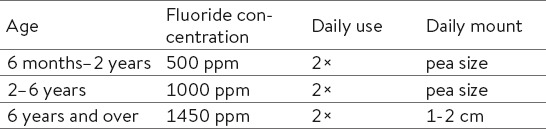
In certain countries, preventive methods also include fluoridated milk and salt (7). Fluoridated salt has been widely used in Germany, France and Switzerland since 1955. Nowadays, 30 to 80% of marketed salt is fluoridated (12). Salt usually contains 250 ppm of fluoride (11), whereas milk contains 2.5 ppm or up to 5 ppm of fluoride (11). However, this method is becoming less suitable, because of modern guidelines for low salt diets (7). According to different evidence based research, there is insufficient evidence that fluoridated milk has caries-protective effects (13). Products used for oral hygiene are also an important means of systemic fluoride intake. A certain amount of toothpaste is consumed during brushing. It is therefore recommended that children use toothpaste with a lower fluoride concentration in small amounts (Table 2) (14).
Table 2.
Recommended use of fluoride toothpaste for children. Source: European Academy of Paediatric Dentistry (EAPD), 2009.
4.2 Fluoride metabolism
About 90% of fluoride is absorbed in the gastrointestinal tract after consumption (up to 25% in the stomach and around 77% in proximal part of the small intestine). The remaining 10% is excreted in feces (15). After absorption, fluoride is transported into the bloodstream and is distributed through the organism (4). The mean time of the peak concentration is 20-60 minutes after consumption (15). In the plasma, fluoride ions are bound to plasma protein (15). Concentration rarely exceeds 0.06 ppm (parts per million). It is usually about 0.01 ppm and is not homeostatically regulated in the blood. Adults retain around 36% of fluoride, whereas children retain approximately 50% of fluoride; 99% of that is contained in mineralized tissues (bone and teeth) and 1% can be found in soft tissue (15). The remaining part of the absorbed fluoride is excreted through the kidneys into the urine; excretion through saliva and sweat is negligible (4). The kidneys are therefore the only human organ that helps maintain the fluoride concentration in our bodies. There are different factors that can influence fluoride metabolism. The most important are: acid base disorders, hematocrit, altitude, physical activity, circadian rhythm, hormones, kidney function, genetic predispositions and diet (15). In pregnant women, uptake of fluorides in the placenta is dependent on the fluoride concentration in the mother`s bloodstream. When the concentration is low, fluoride is transmitted into the placenta (16). On average, the concentration in the placenta is about 60% of the concentration in the mother`s bloodstream (17). If the fluoride concentration increases over 0.4 ppm (17), the placenta works as a barrier, preventing the fluoride from passing through and thus protecting the fetus from a high fluoride concentration (16). Fluoride can also be transmitted through the plasma into the mother’s milk; however, the concentration is low (18).
4.3 Historical overview of fluoride use
The idea of using fluoride as a beneficial agent in caries prevention is not new. The pioneers in the field were Black and McKay. They started observing and describing the effects of fluoride in the late 19th and beginning of the 20th century (19). McKay was studying brown stains on enamel observed in the population from different parts of the USA. A condition which he termed mottled enamel. In 1931, Churchill’s chemical analysis discovered the reason behind those changes – fluoride (20, 21). After the discovery, Dean systematically conducted a series of epidemiological investigations (22). He listed enamel spots based on size and color and measured the degree of tooth impairment. He compared enamel changes to fluoride concentration in drinking water and made the connection between fluoride content and the quantity of enamel spots, which he termed dental fluorosis (23). He documented dental fluorosis prevalence in the USA until 1942 and then compared it to caries prevalence in children. He noticed a strong inverse relationship (24). After concluding his 21-city study (25, 26), he found that drinking water with 1 ppm of fluoride can prevent dental caries, increase tooth strength and does not have a negative impact on enamel. The first studies about positive effects were conducted in 1945 with systemic fluoridation of drinking water in four American cities (Grand Rapids, Evanston, Brantford and Newberg), where 1 mg of fluoride per liter was added to drinking water. The results were convincing. Caries incidence reduction was at least 50%. They came to the conclusion that fluoride in suitable concentrations significantly affects dental caries prevalence (27-30). These observations and discoveries triggered massive drinking water fluoridation, the use of fluoridated salt and milk and an increase in diet supplement production (pills, drops, chewing gum, lozenges). Consequently, caries prevalence was successfully decreased. In 1950, Bibby et al (31) conducted a study comparing the efficacy of fluoride-coated pills intended to be swallowed, with fluoride lozenges intended to be dissolved slowly in the mouth. Research showed a lower number of new caries lesions in the research group which consumed lozenges. This research triggered different studies comparing pre- and post-eruptive effects, which consequently led to a rethinking of the theory of systemic use of fluoride and its incorporation into enamel during teeth development (odontogenesis). In the last 30 years, studies have shown that the maximum anti-caries benefits of fluoride are primarily through topical use and direct contact on the tooth surface. Daily use of topical supplements with suitable fluoride concentration is beneficial (2).
Methods, which led to greater fluoride exposure and lowered caries prevalence, are considered to be one of the greatest accomplishments in the 20th century`s public dental health (32).
4.4 The mechanism of fluoride action
The primary and most important action of fluoride is topical, when the fluoride ion is present in the saliva (33) in the appropriate concentration. Hydroxyapatite is the main mineral responsible for building the permanent tooth enamel after the development of the teeth is finished (34). During tooth growth, the enamel is constantly exposed to numerous demineralization processes, but also important remineralization processes, if the appropriate ions are present in the saliva. These processes can either weaken or strengthen the enamel. The presence of fluoride in an acidic environment reduces the dissolution of calcium hydroxyapatite. The main action is inhibition of demineralization of enamel, which is carried out through different mechanisms. There are different cariogenic bacteria in the plaque fluid the most important being S. mutans. When bacteria metabolize sugars, they produce lactic acid (33) which decreases the pH in saliva. When the pH falls below the critical level of hydroxyapatite (pH 5.5), the process of demineralization of enamel takes place and caries is formed. At the beginning, the process is reversible and it is possible to reduce the formation of new lesions with appropriate preventive measures. If fluoride is present in plaque fluid, it will reduce the demineralization, as it will adsorb into the crystal surface and protect crystals from dissolution. Because the fluoride ion coating is only partial, the uncoated parts of the crystal will undergo dissolution on certain parts of the tooth, if the pH falls below level 5.5. When the pH rises above the critical level of 5.5, the increased level of fluoride ion leads to remineralization, because it absorbs itself into the enamel and forms fluorhydroxyapatite (33). After repeated cycles of demineralization and remineralization, the outer parts of enamel may change and become more resistant to the acidic environment due to a lowered critical pH level of newly formed crystals (pH 4.5) (33). The most important effect of fluoride on caries progression is thus on demineralization and remineralization processes. It has also been proposed, that the fluoride ion can affect the physiology of microbial cells, which can indirectly affect demineralization. Fluoride ions affect bacterial cells through several mechanisms. One of them being a direct inhibition of cellular enzymes – glycolytic enzymes, H+ATPases). It affects cellular membrane permeability and also lowers cytoplasmic pH, resulting in a decrease in acid production from glycolysis (33).
4.5 Overuse and toxicity of fluoride
Dental fluorosis is a developmental disturbance of enamel which occurs during enamel forming. It is caused by systemic overexposure to fluoride during the first six years of life, when the enamel of the crowns of permanent teeth is formed. The enamel contains more protein, is porous, opaque and less transparent. Clinical manifestation vary from (quantitative) narrow, white horizontally running lines, larger patches or yellow to light brown colored areas of porous enamel, to (qualitative) loss of enamel in varying degrees (35). For the optimal effect of fluoride toothpaste, it is important to follow recommended guidelines for the use of products containing fluorides (Table 2) (7). In this way, the probability for fluorosis is decreased and the protective effect of fluoride on the development of caries is significantly important (1).
Just like any other substance we are exposed to in our everyday lives (oxygen, water, table salt), fluoride can be toxic in certain quantities. Acute toxicity can occur after ingesting one or more doses of fluoride over a short time period which then leads to poisoning. The stomach is the first organ that is affected. First signs and symptoms are nausea, abdominal pain, bloody vomiting and diarrhea. This is followed by a collapse with paleness, weakness, shallow breathing, weak heart sounds, wet, cold skin, cyanosis, dilated pupils, hypocalcaemia and hyperkalemia, and in to two to four hours even death. Other possible effects include muscle paralysis, carpopedal spasms and extremity spasms. Based on research papers and some overdose cases, the probable toxic dose (PTD) was defined at 5 mg/kg of body mass. The PTD is the minimal dose that could trigger serious and life-threatening signs and symptoms and requires immediate treatment and hospitalization (36). Example: PTD in a 20 kg child would be achieved at ingesting 100g (75 ml) of toothpaste which contains 1000-1500 ppm of fluorides or 100 pills, that contain fluorides (0,5-1 mg fluoride) (36).
Despite the widespread presence of fluoride in our life and the seriousness of the conditions associated with its toxicity, the number of cases of acute toxicity today, compared to the first half of twenty first century, is very rare. At that time, sodium fluoride was used as a pesticide and rat poison. Because of its appearance it was often mistaken for flour, powdered sugar or any other white powder product, which is used in the kitchen. The most notable example is from the Oregon State Hospital from 1942. During the preparation of scrambled eggs powdered milk was replaced by sodium fluoride which caused 467 cases of acute poisoning of which 47 were fatal. This incident is considered to be the largest mass fluoride poisoning (37).
Today, poisoning is mainly due to unsupervised ingestion of products for dental and oral hygiene and over-fluoridated water (example: Hooper Bay, Alaska, 1992) (37).
4.6 European directive for the use of fluorides
The European Academy of Paediatric Dentistry (EAPD) recommends to everybody, including pregnant women, the preventive use of fluoride toothpaste as a primary preventive measure against caries (7). The most effective way to prevent dental caries is tooth brushing twice a day. Children should only spit out the toothpaste and not rinse with water afterwards.
Parents should start brushing children’s teeth with fluoride toothpaste as soon as the first tooth erupts in the concentration and quantity recommended by the EAPD (Table 2) (7). Parents should use the recommended amount of toothpaste and assist or supervise their children with tooth brushing at least up to the age of 7.
5. CONCLUSION
Fluoride occurs naturally in our environment and is always present in our lives. Exposure can occur through dietary intake, respiration and fluoride supplements. Fluoride can be toxic in extremely high concentrations. Its everyday use in concentrations present in beverages for dental hygiene is safe. The European Academy of Paediatric Dentistry (EAPD) recommends a preventive topical use of fluoride supplements because of their cariostatic effect.
“You don’t have to brush all your teeth, just the ones you want to keep.” (Unknown)
Acknowledgement
The authors acknowledge the support of Lijana Zaletel-Kragelj, MD, PhD, Professor, Chair of Public Health, Faculty of Medicine, University of Ljubljana, Slovenia for helpful comments on earlier drafts of this review.
Footnotes
• Author’s contribution: all authors contributed equally in the preparation of the manuscript.
REFERENCES
- 1.Wong MCM, Clarkson J, Glenny AM, Lo ECM, Marinho VCC, Tsang BWK, et al. Cochrane reviews on the benefits/risks of fluoride toothpastes. J Dent Res. 2011;90(5):573–9. doi: 10.1177/0022034510393346. doi: 10.1177/0022034510393346. [DOI] [PubMed] [Google Scholar]
- 2.Petersen PE, Lennon MA. Effective use of fluorides for the prevention of dental caries in the 21st century: the WHO approach. Community Dent Oral Epidemiol. 2004;32:319–21. doi: 10.1111/j.1600-0528.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 3.Cagetti MG, Campus G, Milia E, Lingström P. A systematic review on fluoridated food in caries prevention. Acta Odontol Scand. 2013;7(3-4):381–7. doi: 10.3109/00016357.2012.690447. doi: 10.3109/00016357.2012.690447. [DOI] [PubMed] [Google Scholar]
- 4.Fawell J, Bailey K, Chilton J, Dahi E, Fewtrell L, Magara Y. Fluoride in drinking-water. London: World Health Organization (WHO); 2006. [Access: March 16 2015]. http://apps.who.int/iris/bitstream/10665/43514/1/9241563192_eng.pdf . [Google Scholar]
- 5.Australian Government. National Health and Medical Research Council. A systematic review of the efficacy and safety of fluoridation. PART A: review of methodology and results. 2007. [Access: March 18 2015]. https://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/eh41_1.pdf .
- 6.Gao HJ, Zhao Q, Zhang XC, Wan XC, Mao JD. Localization of fluoride and aluminum in subcellular fractions of tea leaves and roots. J Agric Food Chem. 2014;62(10):2313–9. doi: 10.1021/jf4038437. doi: 10.1021/jf4038437. [DOI] [PubMed] [Google Scholar]
- 7.European Academy of Paediatric Dentistry. European Archives of Paediatric Dentistry. Guidelines on the use of fluoride in children: an EAPD policy document. 2009. [Access: March 19 2015]. http://www.eapd.eu/dat/82C0BD03/file.pdf . [DOI] [PubMed]
- 8.United States Agriculture Department. USDA National Fluoride Database of Selected Beverages and Foods - Release 2. 2005. [Access: March 19 2015]. http://www.ars.usda.gov/services/docs.htm?docid=6312 .
- 9.Peckham S, Awofeso N. Water fluoridation: a critical review of the physiological effects of ingested fluoride as a public health intervention. The Scientific World Journal. 2014 doi: 10.1155/2014/293019. Article ID 293019: 10 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Healthy People 2010. Final Review. 2010. [Access: March 25 2015]. http://www.cdc.gov/nchs/healthy_people/hp2010/hp2010_final_review.htm.
- 11.World Health Organization. Water Sanitation and Health (WSH) Water fluoridation. [Access: March 25 2015]. http://www.who.int/water_sanitation_health/oralhealth/en/index2.html .
- 12.Marthaler TM, Petersen PE. Salt fluoridation - an alternative in automatic prevention of dental caries. Int Dent J. 2005;55:351–8. doi: 10.1111/j.1875-595x.2005.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 13.Yeung CA, Hitchings JL, Macfarlane TV, Threlfall AG, Tickle M, Glenny AM. Fluoridated milk for preventing dental caries. Cochrane Database Syst Rev. 2005;3:CD003876. doi: 10.1002/14651858.CD003876.pub2. doi: 10.1002/14651858.CD003876. [DOI] [PubMed] [Google Scholar]
- 14.Mascarenhas AK, Burt BA. Fluorosis risk from early exposure to fluoride toothpaste. Community Dent Oral Epidemiol. 1998;26:241–8. doi: 10.1111/j.1600-0528.1998.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 15.Buzalaf MA, Whitford GM. Fluoride metabolism. Monogr Oral Sci. 2011;22:20–36. doi: 10.1159/000325107. doi: 10.1159/000325107. [DOI] [PubMed] [Google Scholar]
- 16.GS M, Mohanty S, Rao P. Role of placenta to combat fluorosis (in fetus) in endemic fluorosis area. NJIRM. 2010;1(4):16–19. [Google Scholar]
- 17.Gupta S, Seth AK, Gupta A, Gavane AG. Transplacental passage of fluorides. J Pediatr. 1993;123:139–41. doi: 10.1016/s0022-3476(05)81558-6. [DOI] [PubMed] [Google Scholar]
- 18.Ekstrand J, Boréus LO, de Chateau P. No evidence of transfer of fluoride from plasma to breast milk. Br Med J. (Clin Res Ed.) 1981;283:761–2. doi: 10.1136/bmj.283.6294.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black GV, McKay FS. Original Communications. Mottled teeth: an endemic developmental imperfection of the enamel of the teeth heretofore unknown in the literature of dentistry. Dental Cosmos. 1916;58:129–56. [Google Scholar]
- 20.Churchill HV to McKay FS. Jan. 20, 1931, in the ALCOA (Aluminum Company of America) papers [Google Scholar]
- 21.Churchill HV. Occurrence of fluorides in some waters of the United States. J Ind Eng Chem. 1931;23:996–8. [Google Scholar]
- 22.Dean HT. Classification of mottled enamel diagnosis. J Am Dent Assoc. 1934;21:1421–6. [Google Scholar]
- 23.Dean HT, Elvove E. Studies on the minimal threshold of the dental sign of chronic endemic fluorosis (mottled enamel) Public Health Rep. 1935;50:1719–29. [Google Scholar]
- 24.Dean HT. On the epidemiology of fluorine and dental caries. In: Gies WJ, editor. Fluorine in dental public health. New York, NY: New York Institute of Clinical Oral Pathology; 1945. pp. 19–30. [Google Scholar]
- 25.Dean HT, Jay P, Arnold FA, Jr, Elvove E. Domestic water and dental caries. II. A study of 2,832 white children, aged 12 to 14 years, of 8 suburban Chicago communities, including Lactobacillus acidophilus studies of 1,761 children. Public Health Rep. 1941;56:761–92. doi: 10.1111/j.1753-4887.1976.tb05724.x. [DOI] [PubMed] [Google Scholar]
- 26.Dean HT, Arnold FA, Jr, Elvove E. Domestic water and dental caries. V. Additional studies of the relation of fluoride domestic waters to dental caries experience in 4,425 white children, aged 12 to 14 years, of 13 cities in 4 states. Public Health Rep. 1942;57:1155–79. [Google Scholar]
- 27.Arnold FA, Jr, Likins RC, Russell AL, Scott DB. Fifteenth year of the Grand Rapids fluoridation study. J Am Dent Assoc. 1962;65:780–85. [Google Scholar]
- 28.Blayney JR, Hill IN. Fluorine and dental caries. J Am Dent Assoc. 1967;74:225–302. [PubMed] [Google Scholar]
- 29.Hutton WL, Linscott BW, Williams DB. Final report of local studies on water fluoridation in Brantford. Can J Public Health. 1956;47:89–92. [PubMed] [Google Scholar]
- 30.Ast DB, Fitzgerald B. Effectiveness of water fluoridation. J Am Dent Assoc. 1962;65:581–7. doi: 10.14219/jada.archive.1962.0311. [DOI] [PubMed] [Google Scholar]
- 31.Bibby BG, Wilkins E, Witol E. A preliminary study of the effects of fluoride lozenges and pills on dental caries. Oral Surg Oral Med Oral Pathol. 1955;8(2):213–6. doi: 10.1016/0030-4220(55)90195-x. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Achievements in public health 1900-1999. Fluoridation of drinking water to prevent dental caries. Morb Mort Wkly Rep. 1999;48:933–40. doi: 10.1001/jama.283.10.1283. [DOI] [PubMed] [Google Scholar]
- 33.Buzalaf MA, Pessan JP, Honório HM, ten Cate JM. Mechanisms of action of fluoride for caries control. Monogr Oral Sci. 2011;22:97–114. doi: 10.1159/000325151. doi: 10.1159/000325151. [DOI] [PubMed] [Google Scholar]
- 34.Fincham AG, Moradian-Oldak J, Simmer JP. The structural biology of the developing dental enamel matrix. Journal of Structural Biology. 1999;126:270–99. doi: 10.1006/jsbi.1999.4130. [DOI] [PubMed] [Google Scholar]
- 35.Denbesten P, Li W. Chronic fluoride toxicity: dental fluorosis. Monogr Oral Sci. 2011;22:81–96. doi: 10.1159/000327028. doi: 10.1159/000327028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitford GM. Acute toxicity of ingested fluoride. Monogr Oral Sci. 2011;22:66–80. doi: 10.1159/000325146. doi: 10.1159/000325146. [DOI] [PubMed] [Google Scholar]
- 37.Salem Public Library. Salem Online History. 467 Poisoned at Oregon State Hospital. [Access: March 26 2015]. http://www.salemhistory.net/brief_history/state_hospital_poisoning.htm .


