Abstract
Background:
Allergic rhinitis (AR) related to local weeds pollen sensitization (Chenopodiaceous family) is the most common cause of respiratory allergy in Kuwait. Local nasal accumulation of different cells typical of allergic inflammation is responsible for clinical symptoms of AR. Although nasal smear for Eosinophils (NSE) is one of the earliest included valuable test in diagnosis of AR, with time is underestimated.
Aim:
Explore possible correlation of natural pollen allergen stimulation with appearance and quantity of Eosinophils in nasal smear.
Methods:
A group of randomly selected patients with clinical history suggestive for seasonal AR (SAR), who came to Al Rashed Allergy Center in period from October 2014 to October 2015, obtain Nasal Smear for Eosinophils as a screening test before further diagnostic evaluation. Nasal samples were collected by passing a sterile swab, from each nasal cavity, along the medial surface of the inferior turbinate 2 to 3 times and the specimen smeared on a clear glass slide. Nasal smears were examined by light microscopy after staining with hematoxylin and eosin stain. Skin prick test is performed in all symptomatic patients with a battery of inhalant allergens that include local pollens. The control group was recruited, with their voluntary consent, from the medical stuff with a negative history of any allergic nasal symptoms. In this group we performed only nasal smear for Eosinophils. Air Biology Laboratory Kuwait provided us with daily pollen count.
Results:
From total 158 study participants, 132 had SAR symptoms and are divided in four groups. Fifth, control, group is non symptomatic. For 38.6% of symptomatic patients NSE were positive, while 45% of these patients have negative SPT. From 62.1% NSE negative patients, 37.8% have negative SPT. Our results showed expected positive correlation of NSE positive patients with pollen season in Kuwait, in SPT positive group. However, presence of Eosinophils in nasal smear was moderate to high also in patients with negative SPT during the highest peak of season, in contrast to control group.
Conclusion:
NES showed moderate sensitivity, relatively high specificity and importance as screening test in SPT negative patients. Evaluation of AR demand wide and improved diagnostic approach due to significant number of SPT negative patients with positive NSE based on natural allergen stimulation. Our results emphasize locale allergic response of nasal mucosa and importance of target organ diagnostic approach.
Keywords: seasonal allergic rhinitis, nasal smear for eosinophils
1. INTRODUCTION
Allergic rhinitis (AR) has become increasingly prevalent in the Middle East Gulf region; estimates indicate that up to 36% of the region’s population may be affected. Rapid development, the oil industry, and modernization have led to pollution and the introduction of non-native species of plants and grass and amenities such as carpeting and air conditioning, contributing to an overall increased sensitivity to allergens (1, 2). Large epidemiologic studies consistently show a significantly higher percentage of the population with rhinitis symptoms than those with rhinitis symptoms and positive allergy tests (3).
The diagnosis of AR is based on clinical manifestations and supported by a positive result for skin prick test (SPT) or serum specific immunoglobulin E (sIgE) antibodies to aeroallergens (4). In contrast, rhinitis is diagnosed as non allergic when an allergic cause has been ruled out by the presence of an inconsistent clinical history, a negative SPT, and the absence of serum sIgE antibodies (5). Local allergic rhinitis (LAR) has recently been suggested to be a distinct rhinitis endotype characterized by symptoms similar to AR associated with Local (nasal) allergen-specific IgE (sIgE), but with no evidence of Systemic sIgE (6).
Indoor allergens seem to play a lesser role in respiratory allergies in Kuwait. Most allergic patients become sensitized to pollens; the strongest and most frequent reaction is from Chenopodiaceous family (Salsola pollen) (7). Seasonal AR occurs during two periods in Kuwait, i.e. September - October and April - May, with September - October being the main season. The rise in AR during late summer in Kuwait is mainly associated with the pollination of Chenopodiaceae species (8). During the pollen season, patients show all the clinical signs of the disease, and nasal cytology is characterized by the presence of neutrophils, lymphocytes, eosinophils and mast cells, mostly degranulating. In patients evaluated outside the pollen season, there are no clinical or cytological signs that may be detected; this aspect is even more evident if the pollen season and the allergen exposure finished more than 30 days before evaluation. In these cases, for an effective diagnosis, it is mandatory to perform a nasal provocation test with the specific allergen or a cytological study during the peak of pollination (9). Eosinophils in the nasal smear have been reported to display the best correlation with all the clinical and immunological parameters in allergic rhinitis (10). It is a cheap, non- invasive test that may be easily repeated on the same patient, with is essential both in the follow-up of the disease and to monitor the efficacy of medical and surgical interventions. Nasal cytology could help the physicians, including general practitioners and allergists, in assessing the biological expression of AR in individual patients (10). Nasal smears for eosinophils are not necessary for routine use in diagnosing allergic rhinitis when the diagnosis is clearly supported by the history, physical examination, and specific IgE diagnostic studies but may be a useful adjunct when the diagnosis of allergic rhinitis is in question (11).
Although nasal smear for Eosinophils (NSE) is one of the earliest included valuable test in diagnosis of AR, with time is underestimated. This study was conducted to evaluate possible correlation of natural pollen allergen stimulation on appearance and quantity of Eosinophils in nasal smear.
2. MATERIALS AND METHODS
A group of randomly selected patients with clinical history suggestive for seasonal AR (SAR) who came to Al Rashed Allergy Center in period from October 2014 to October 2015 obtain Nasal Smear for Eosinophils as a screening test before further diagnostic evaluation. Nasal samples were collected by passing a sterile swab, from each nasal cavity, along the medial surface of the inferior turbinate 2 to 3 times and the specimen smeared on a clear glass slide. Nasal smears were examined by light microscopy after staining with hematoxylin and eosin stain. Skin prick test is performed in all symptomatic patients with a battery of inhalant allergens that include local pollens. The control group was recruited, with their voluntary consent, from the medical stuff with a negative history of any allergic nasal symptoms. In this group we performed only nasal smear for Eosinophils.
Air Biology Laboratory Kuwait provided us with daily pollen count.
Exclusion Criteria for our study were: Pregnant and nursing women, Malignant disease, Chronic autoimmune disease, Acute respiratory tract infection, Patients who had used local or systemic anti-inflammatory drugs (e.g., corticosteroids, antihistamines, or disodium cromoglycate) in the 1 weeks prior to diagnostic test, oral corticosteroids in the last 6 weeks, Antihistamine and Leukotrien receptor antagonist 2 weeks, Polypectomy in the last 6 months.
Scale used to interpret nasal smear eosinophilia is based on data from Immunology laboratory at Al Rashed Allergy Center: 0-3 negative, 5-10 weak positive, 10-30 moderate positive, >30 strong positive result.
Statistical analysis
Non-parametric and parametric methods are used to calculate statistical significance. The distribution value is determined D’Agostino and Pearson omnibus test normality. Student’s t-test, Mann-Whitney test, Fisher’s test and χ2 test were used for calculating the difference between the groups. ANOVA test was used to calculate the relative difference distribution variance between variables. The statistical hypotheses were tested at the level of α = 0.05, and the difference between the groups in the sample was considered significant when p <0.05 or less. Statistical significance is depicted as: p <0.05, p <0.01 and p <0.001. All data were analyzed using GraphPad Prism version 7 (San Diego, California, USA).
3. RESULTS AND DISCUSSION
The sensitivity for nasal smear eosinophilia in the diagnosis of allergic rhinitis varies in different studies from 51.3% to 74% with a specificity of 88.5% to 90% (3, 12). At Immunology laboratory in Al Rashed Allergy Centre, sensitivity of NSE is 58,3% and specificity 82,7%.
Total of 158 patients are divided in V (five) groups based on results of skin prick test (SPT) and nasal smear for eosinophils (NSE), group V is control group (Table 1).
Table 1.
Evaluated groups of patients and volunteers.
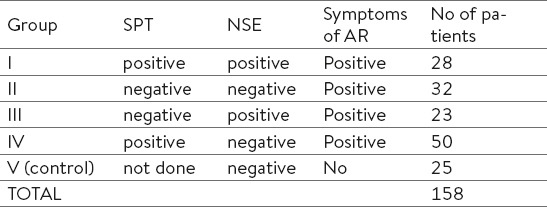
In the entire study group (N=158), the ratio of female : male was 1.29: 1. Female were significantly over-represented compared to male patients in the group III (p <0.05) and IV (p <0.0001), while group I and II have an equal gender distribution (p> 0.05).
The patients in group I are significantly younger than in group II (p <0.01), while other groups had equal mean age distribution (p> 0.05). Females are significantly older compared to males (p<0.05). The youngest patient is 7 years old (male), and the oldest 75 years old (male and female).
The prevalence of AR peaks around the age of 16-24 and decrease in the subsequent years up to the age of 65-70. The subject with non allergic rhinitis (NAR) reported the onset of symptoms at older ages compared to subjects with AR (13). The age related decrease in the AR prevalence may be due to the allergen specific IgE level decrease that occurs with aging in atopic individuals (14). It has also been proposed that AR is less common in subjects over 60 years of age than in younger subjects, possibly because the allergic epidemics started quite recently (15).
Among our patients with symptoms of allergic rhinitis, having negative NSE was more common than positive NSE (p<0.001). Among patients with symptoms of allergic rhinitis and positive NSE, those with negative skin prick test were represented equally to those with positive skin prick (p>0.05).
Behbehani et al. evaluated seasonal variation of AR in Kuwait and concluded that there is a significant seasonal variation with a bimodal increase in the number of patients with AR. The main peak in the number of patients occurred in September - October, and there was a smaller peak in April - May. Similarly, the average daily pollen count varied from 3.7 ± 1.0 pollens per mm3 in January to 124 ±92 in October. There was strong positive correlation between the number of new AR patients and the average total pollen count of Chenopodiaceae (weed) pollens (8).
Skin prick test negative patients in our study, showed no significant correlation in number with the daily pollen count (p>0.05; total Spearman coefficient r=0.3275; 95%CI -0.3210 to 0.7669). On the other side, in skin prick test positive patients there is significant positive correlation between the number of pollen particles and number of patients (p<0.01; total Spearman coefficient r=0.6715; 95%CI 0.1397 to 0.9026) (Graph 1).
Graph 1.
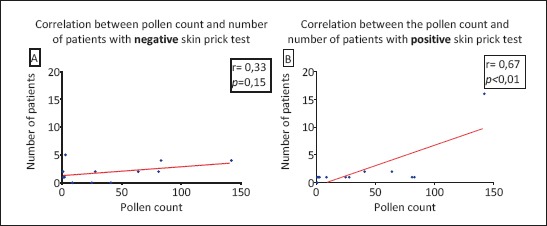
(A and B). Correlation between the pollen count and number of patients with negative (A) and positive (B) skin prick test (symptoms of allergic rhinitis positive and nasal smear for eosinophils positive).
Skin prick test is positive in 79 patients (59.4%). Among skin prick test positive patients with allergic rhinitis (groups I and IV), Salsola was more frequent than other allergens (p<0.0001).
Group III, SPT negative, NSE positive and highly symptomatic patients, is evaluated based on distribution of number of patients per month over a one year period but in correlation with number of patients in group I, who were SPT positive, NSE positive and highly symptomatic and number of pollen particle per month in same period. The average daily pollen count peaked during the months of September and October, when it reached 104 ±45 and 124 ±92 pollens per mm3, respectively, while in January and April, the pollen count was 3.7 ±1.0 and 41.8 ±26.9 pollens per mm3, respectively (Graph 2).
Graph 2.
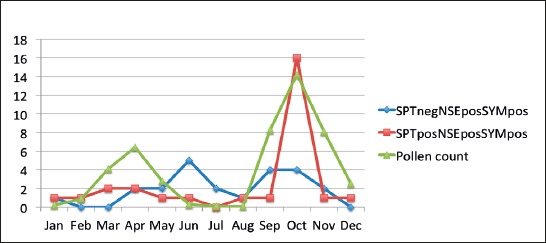
Correlation between number of symptomatic patients in SPT negative, NSE positive and SPT positive and NSE positive groups with monthly pollen count.
There is notable correlation in number of patients and pollen count over the evaluated period of time. Most of the group III patients were moderate to high positive for number of eosinophils in nasal smear at main peaks of pollen season (Graph 3).
Graph 3.
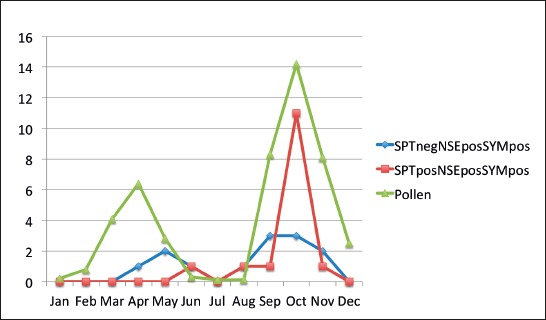
Number of moderate and strongly positive NSE patients during pollen season
4. CONCLUSION
Evaluation of AR demand wide and improved diagnostic approach due to notable number of SPT negative patients with positive NSE based on natural allergen stimulation.
NES showed moderate sensitivity, relatively high specificity and importance as screening test in SPT negative patients.
There is expected positive correlation of NSE positive patients with pollen season in Kuwait in SPT positive group. However, presence of Eosinophils in nasal smear was moderate to high also in patients with negative SPT during the highest peak of season, in contrast to control group.
Our results emphasize locale allergic response of nasal mucosa and importance of target organ diagnostic approach.
Footnotes
• Author’s contribution: Jasmina Nurkic: substantial contribution to conception and design, substantial contribution to acquisition of data, drafting the article. Mona AlAhmed: substantial contribution to conception and design, substantial contribution to acquisition of data, critically revising the article for important intellectual content, final approval of the version to be published. Nermina Arifhodzic: substantial contribution to conception and design, substantial contribution to acquisition of data, critically revising the article for important intellectual content, final approval of the version to be published. Edin Jusufovic: substantial contribution to analysis and interpretation of data.
• Conflict of interest: None declared.
REFERENCES
- 1.Alsowaidi S, Abdulle A, Shehab A, Zuberbier T, Bernsen R. Allergic rhinitis: prevalence and possible risk factors in a Gulf Arab population. Allergy. 2010;65:208–12. doi: 10.1111/j.1398-9995.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- 2.Zeamuzie C, Thomson M, Al-Ali S, Dowaisan A, Khan M, Hijazi Z. Asthma in the desert: spectrum of the sensitizing aeroallergens. Allergy. 2000;55(2):157–62. doi: 10.1034/j.1398-9995.2000.00375.x. [DOI] [PubMed] [Google Scholar]
- 3.Akhshaee M, Fereidouni M, Ferzadnia M, Varasteh R. The Nasal Smear for Eosinophils, It’s Value, and It’s Relation to Nasal Mucosal Eosinophilia in Allergic Rhinitis. The Iranian Journal of Otorhinolaryngology. 2010;22(60):73–8. [Google Scholar]
- 4.Bousquet J, Khaltaev N, Cruz A, Denburg J, et al. ARIA (Allergic Rhinitis and its Impact on Asthma). Update In collaboration with the World Health Organization, GA2LEN and AllerGen. 2008:13–14. [Google Scholar]
- 5.Rondon C, Dona I, Torres MJ, Campo P, Blanca M. Evolution of patients with nonallergic rhinitis supports conversion to allergic rhinitis. J Allergy Clin Immunol. 2009;123:1098–1102. doi: 10.1016/j.jaci.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos N, Bernstein J, Demoly P, Dykewicz M, Fokken W, Hellings P, Peters A, Rondon C, Togias A, Cox L. Phenotypes and endotypes of rhinitis and their impact on management: a PRACTALL report. Allergy. 2015;70(5):474–94. doi: 10.1111/all.12573. [DOI] [PubMed] [Google Scholar]
- 7.Al-Dowaisan A, Fakim N, Arifhodzic N, Fakim N, Khan R, Panicker R, Hanoon A, Khan I. Salsola pollen as a predominant cause of respiratory allergies in Kuwait. Ann Allergy Asthma Immunol. 2004;92(2):262–7. doi: 10.1016/S1081-1206(10)61558-X. [DOI] [PubMed] [Google Scholar]
- 8.Behbehani N, Arifhodzic N, Al-Mousawi M, Marafie S, Ashkanani L, Moussa M, Al Duwaisan A. The Seasonal Variation in Allergic Rhinitis and Its Correlation with Outdoor Allergens in Kuwait. Int Arch Allergy Immunol. 2004;133:164–7. doi: 10.1159/000076622. [DOI] [PubMed] [Google Scholar]
- 9.Gelardi M, Marseglia G, Licari A, Landi M, DellAlbani I, Incorvaia C, Frati F, Quaranta N. Nasal cytology in children: recent advances. Italian journal of paediatrics. 2012;38:51. doi: 10.1186/1824-7288-38-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelardi M, Incorvaria C, Passalacqua G, Quaranta N, Frati F. The classification of allergic rhinitis and its cytological correlate. Allergy. 2011;66(12):1624–5. doi: 10.1111/j.1398-9995.2011.02741.x. [DOI] [PubMed] [Google Scholar]
- 11.Wallace D, Dykewicz M, Bernstein D, Blessing-Moore J, Cox L, et al. The diagnosis and management of rhinitis: An updated practice parameter. JACI. 2008;122(2):S1–S84. doi: 10.1016/j.jaci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadiafshar A, Taghiloo D, Esmailzadeh A, Falakaflaki B. Nasal eosinophilia as a marker for allergic rhinitis: A controlled study of 50 patients. ENT Journal. 2012:2–4. doi: 10.1177/014556131209100309. [DOI] [PubMed] [Google Scholar]
- 13.Cazzoletti L, Ferrari M, Olivieri M, Verlato G, Antonicelli L, Bono R, Casali L, Cerveri I, Marchetti P, et al. The gender, age and risk factor distribution differs in self-reported allergic and non-allergic rhinitis: a cross-sectional population-based study Allergy, Asthma & Clinical Immunology. 2015;11:36. doi: 10.1186/s13223-015-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slavin R. Allergic rhinitis: managing the adult spectrum. Allergy Asthma Proc. 2006;27:9–11. [PubMed] [Google Scholar]
- 15.Bousquet J, Fokkens W, Burney P, Durham R, Bachert C, Akdis A, Canonica G, Dahlen S, Zuberbier T, et al. Important research questions in allergy and related diseases: nonallergic rhinitis: a GA2LEN paper. Allergy. 2008;63:842–53. doi: 10.1111/j.1398-9995.2008.01715.x. [DOI] [PubMed] [Google Scholar]


