Abstract
Introduction:
Hypothyroidism has been reported to affect renal function and structure. However, the association of hypothyroidism with distal renal tubular acidosis (dRTA) is rarely reported in children.
Case Presentation:
We present a 6-year-boy with Down syndrome admitted in our department due to vomiting, weakness, polyuria, polydipsia, irritability and weight loss in the last few weeks. Investigations revealed features of hypokalemia, metabolic acidosis and alkaline urine consistent with dTRA. Abdominal ultrasound found nephrocalcinosis. In addition, Antithyroid peroxidase antibodies were positive, suggesting an autoimmune background for the pathogenesis of the tubular dysfunction. Treatment for dRTA and hypothyroidism was started and symptomatic improve was noticed.
Conclusion:
dRTA should be excluded in children with autoimmune disorders who develop weakness, polyuria, polydipsia or growth failure. Early diagnosis would reduce long-term complications.
Keywords: Hashimoto thyroiditis, distal renal tubular acidosis, nephrocalcinosis, Down syndrome
1. INTRODUCTION
Renal function and structure is affected by the disorders of thyroid gland (1-3). Also, it is reported that renal growth improves after intake of thyroxine supplements in congenital hypothyroidism patients (4). Hypothyroidism of autoimmune as well as non-autoimmune etiology has been associated with distal renal tubular acidosis (dRTA) in adult population (5, 6). However, in pediatric population there is only one case of dTRA associated with Hashimoto thyroiditis reported till date (7). Hereby, we have reported a case of a 6-year-old boy with Down syndrome and Hashimoto thyroiditis who was diagnosed to have dRTA and nephrocalcinosis.
2. CASE PRESENTATION
A 6-year-old boy was admitted to our department due to vomiting, weakness, polyuria, polydipsia, irritability and weight loss in the last few weeks. The patient is the third child of the fourth pregnancy, one of which ended up with abortion. He was born at term, through Cesarean section, weighting 3400 g. Down syndrome was suspected clinically at birth and confirmed by karyotyping (47XX, t21).
The body weight on admission was 14.5 kg and the body height was 93 cm (20th and 5th percentile for sex and age in Down syndrome, respectively) (8).
Typical features of Down syndrome were present, including typical facies and generalized hypotonia. A 3/6 heart murmur was heard on auscultation, and transthoracic echocardiography revealed a low grade aortic regurgitation. The rest of his physical examination was unremarkable.
Laboratory studies revealed red blood cell count 2.14 × 1012/l, Hb 6.1 gr/dL, Htc 17.5%, platelet count 272000/mm3, white blood cell count 7800/mm3, ESR 60 mm/h, CRP 26 mg/l, urea 12.7 mmol/1, creatinine 160 umol/1, Na 136 mEq/L, K 2.7 mEq/L, Cl 108 mEq/L, Ca 9.8 mg/dL, ionised Ca 1.28 mmol/1, Mg 1.0 mmol/1, total serum proteins 59.6 g/l, albumins 32.3 g/l, fasting blood glucose 5.6 mmol/l, cholesterol 6,1 mmol/l, triglycerides 2.3 mmol/l and alkaline phosphatase 49 U/l. Results of the capillary blood gas analysis were as follows: pH 7.25, PCO2 26 mmHg, HCO3 14 mEq/L and serum anion gap 14 mEq/L.
Routine urine analysis showed mild proteinuria (+) and negative glucose. Urine microscopy showed 15 erythrocytes/hpf, 20 leucocytes/hpf and 20-30 bacteria/hpf; specific gravity was 1.005, and urinary pH was 7.0. Urine culture resulted positive for E. Coli.
Urinary uric acid was low at 774.0 umol/24h (normal values 1480-4430 umol/24h) as was citrate 21 mg/24h (normal value >115mg/24h). The urine calcium to creatinine ratio was 1.2 mmol/mmol (normal value < 1.1 mmol/mmol). In the view of normal anion gap hyperchloremic metabolic acidosis, alkaline urine and hypokalemia the diagnosis of dTRA was made.
Additional analysis searching for etiology of dTRA revealed serum 1,25-dihydroxyvitamin D 1.2 ng/ml (normal values 10.8-54 ng/ml), parathyroid hormone 27.2 pg/ml (normal values 6.5-36.8 pg/ml), free serum T4 2.20 pmol/l (normal values 10.3-25.8 pmol/l) and thyroid-stimulating hormone >1000.0 mU/l (normal values 0.54-4.21 mU/l). Prolactin was 163.9 ng/mL (normal values 3.2-20 ng/mL), while other hormonal profiles such as adrenocorticotropic hormone, luteinizing hormone, follicle-stimulating hormone and growth hormone were normal. Anti-thyroid peroxidase (TPO) antibody was >1000.0 IU/ml (normal value <50 IU/ml) and Thyroglobulin (hTg) was 3.41 ng/ml (normal values 0.2-70.0 ng/ml). Hence, Hashimoto thyroiditis as a possible cause was postulated. Moreover, antinuclear antibodies (ANA) test resulted negative.
The renal ultrasound showed hyper-echoic regions in the renal medulla consistent with bilateral nephrocalcinosis grade I (Figure 1), also confirmed by abdominal CT (Figure 2). Brain magnetic resonance imaging revealed pituitary enlargement (Figure 3).
Figure 1.
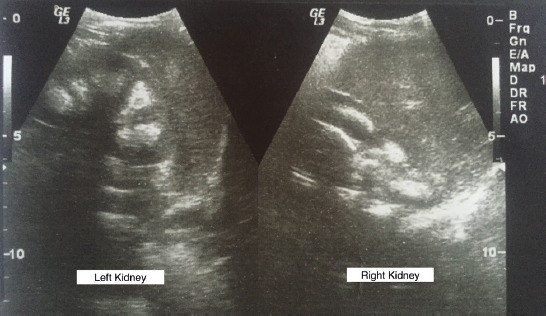
Abdominal ultrasound image showing bilateral nephrocalcinosis
Figure 2.
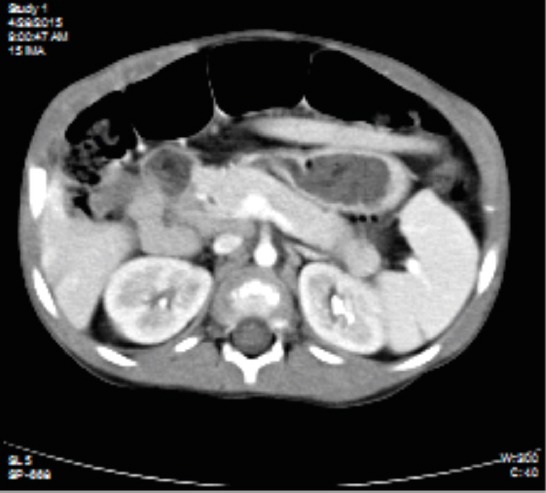
Abdominal CT section showing bilateral nephrocalcinosis
Figure 3.
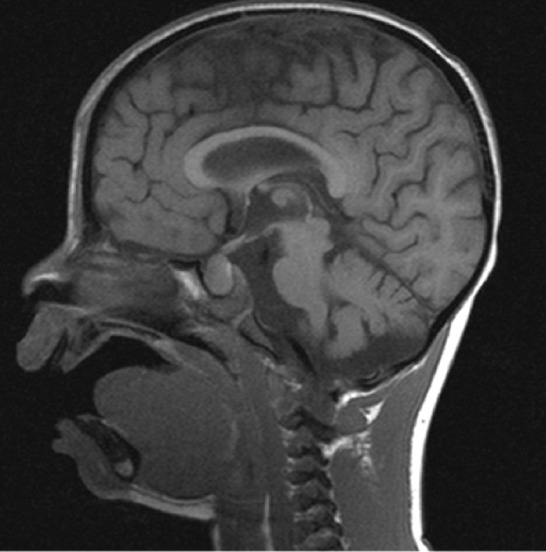
MRI of brain showing pituitary enlargment.
Treatment and follow up
Intravenous rehydration was started. The bicarbonate deficit was calculated, and KCl was added after the patient had his first urinary void. Blood gas analysis after 12 hours revealed increasing pH, HCO3 and K values. Packed red blood cells were then administered. The patient was put on potassium citrate and thyroid hormone substitution therapy. Also, intravenous antibiotherapy and oral ferrous supplements were started to treat urinary tract infection and anemia, respectively. On follow-up after six months, no evidence of metabolic acidosis and a nearly normal serum potassium level (3.4 mEq/L) were found. Urinary random analysis was normal. Patient’s weight was 16 kg and his height 98 cm (25th and 10th percetile, respectively). His thyroid hormone profile was within normal range. Also, prolactin, cholesterol, triglycerides and complete blood count analysis were normalizing. The patient is on regular follow-up.
3. DISCUSSION
The combination of autoimmune hypothyroidism and dRTA has been rarely described in the literature (1, 9). However, dTRA has been reported in other autoimmune disorders including Sjogrens syndrome, systemic lupus erythematosus, etc. (10). Although the mechanism remains unclear, immune-related dRTA as a result of a gradient defect with bicarbonate leak into the tubular lumen has been reported as a possible mechanism (11). On the other hand, dRTA was also reported in non-autoimmune hypothyroidism (12). Likewise, a defect in the renal acidification was observed in hypothyroid rats (13).
The association of congenital hypothyroidism with nephrocalcinosis has been reported previously. The postulated mechanism was that mitochondria accumulate calcium against concentration gradient in proximal or distal renal tubular cells. Hypothyroidism affects this mechanism, leading to high intracytoplasmic calcium concentrations predisposing to nephrocalcinosis (14).
Because nephrocalcinosis itself can cause dRTA and vice versa, distinguishing the initial insult can be difficult. However, calcium levels do not have to be increased for nephrocalcinosis to develop in hypothyroidism (14). Thus, hypothyroidism, as a cause of simultaneous dTRA and nephrocalcinosis development seems the most possible mechanism. Then, vicious circle between dTRA and nephrocalcinosis might have hastened the condition.
Down syndrome was also reported to be associated with nephrocalcinosis. But, in contrast to our case, very high serum calcium levels were found in all reported patients (15, 16) and passive calcium absorption in the gut was suggested as a possible mechanism.
Hyperprolactinemia and pituitary enlargement secondary to pituitary hyperplasia has been previously reported in patients with primary hypothyroidism (17). Decreased prolactin level after thyroxine therapy in our patient adds more evidence.
At the best of our knowledge this is the second case reporting on Hashimoto thyroiditis associated with dTRA. The first reported case presented a 12-year-old girl with a multiple autoimmune disorders. She showed Hashimoto thyroiditis and distal renal tubular acidosis at 5 years of age, pernicious anemia at the age of 9 and encephalopathy at the age of 12. Hence, patients with autoimmune disorders should be carefully followed-up for possible consequences and further multisystem disorders development.
Footnotes
• Author’s contribution: Study concept and design: Lidvana Spahiu, Haki Jashari and Vjosa Mulliqi-Kotori. Analysis and interpretation of data: Lidvana Spahiu, Haki Jashari. Drafting of the manuscript: Lidvana Spahiu. Critical revision of the manuscript for important intellectual content: Haki Jashari, Vjosa Mulliqi-Kotori, Besart Merovci and Blerta Elezi-Rugova
• The authors have no conflict of interest to disclose.
REFERENCES
- 1.Mason AM, Golding PL. Renal tubular acidosis and autoimmune thyroid disease. Lancet (London, England) 1970;2(7683):1104–7. doi: 10.1016/s0140-6736(70)92296-8. [DOI] [PubMed] [Google Scholar]
- 2.Nishimoto A, Tomiyoshi Y, Sakemi T, Kanegae F, Nakamura M, Ikeda Y, et al. Simultaneous occurrence of minimal change glomerular disease, sarcoidosis and Hashimoto's thyroiditis. American journal of nephrology. 2000;20(5):425–8. doi: 10.1159/000013621. [DOI] [PubMed] [Google Scholar]
- 3.Khalil S, Sethi SK, Sankar J, Dubey NK. Unusual renal manifestations in a girl with hyperthyroidism. Indian journal of pediatrics. 2012;79(2):260–1. doi: 10.1007/s12098-011-0482-1. [DOI] [PubMed] [Google Scholar]
- 4.Bulbul M, Cetinkaya S, Eksioglu S, Ozkasap S, Ginis T. Kidney growth in children with congenital hypothyroidism. Pediatric nephrology (Berlin, Germany) 2009;24(2):333–40. doi: 10.1007/s00467-008-0992-x. [DOI] [PubMed] [Google Scholar]
- 5.Basak RC, Sharkawi KM, Rahman MM, Swar MM. Distal renal tubular acidosis, hypokalemic paralysis, nephrocalcinosis, primary hypothyroidism, growth retardation, osteomalacia and osteoporosis leading to pathological fracture:a case report. Oman medical journal. 2011;26(4):271–4. doi: 10.5001/omj.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laway BA, Ali I, Bashir MI, Mir SA, Ganie MA, Wani IA. Distal renal tubular acidosis associated with non-autoimmune hypothyroidism. Saudi journal of kidney diseases and transplantation:an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2012;23(4):846–9. doi: 10.4103/1319-2442.98185. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki N, Mitamura R, Ohmi H, Itoh Y, Yano K, Okuno A, et al. Hashimoto thyroiditis, distal renal tubular acidosis, pernicious anaemia and encephalopathy:a rare combination of auto-immune disorders in a 12-year-old girl. European journal of pediatrics. 1994;153(2):78–9. doi: 10.1007/BF01959211. [DOI] [PubMed] [Google Scholar]
- 8.Cronk C, Crocker AC, Pueschel SM, Shea AM, Zackai E, Pickens G, et al. Growth charts for children with Down syndrome:1 month to 18 years of age. Pediatrics. 1988;81(1):102–10. [PubMed] [Google Scholar]
- 9.Drukker A, Dolberg M, Landau H. Renal tubular acidosis in a patient with hypothyroidism due to autoimmune thyroiditis–improvement with hormone replacement therapy. The International journal of pediatric nephrology. 1982;3(3):205–9. [PubMed] [Google Scholar]
- 10.Pessler F, Emery H, Dai L, Wu YM, Monash B, Cron RQ, et al. The spectrum of renal tubular acidosis in paediatric Sjogren syndrome. Rheumatology (Oxford, England) 2006;45(1):85–91. doi: 10.1093/rheumatology/kei110. [DOI] [PubMed] [Google Scholar]
- 11.Zawadzki J. Permeability defect with bicarbonate leak as a mechanism of immune-related distal renal tubular acidosis. American journal of kidney diseases:the official journal of the National Kidney Foundation. 1998;31(3):527–32. doi: 10.1053/ajkd.1998.v31.pm9506692. [DOI] [PubMed] [Google Scholar]
- 12.Fang JT, Huang CC. Distal renal tubular acidosis associated with non-autoimmune hypothyroidism. Nephrology, dialysis, transplantation:official publication of the European Dialysis and Transplant Association - European Renal Association. 1996;11(6):1146–7. [PubMed] [Google Scholar]
- 13.Michael UF, Chavez R, Cookson SL, Vaamonde CA. Impaired urinary acidification in the hypothyroid rat. Pflugers Archiv :European journal of physiology. 1976;361(3):215–20. doi: 10.1007/BF00587285. [DOI] [PubMed] [Google Scholar]
- 14.Mantan M, Mishra D. Congenital hypothyroidism and nephrocalcinosis. Indian pediatrics. 2010;47(3):281. [PubMed] [Google Scholar]
- 15.Proesmans W, De Cock P, Eyskens B. A toddler with Down syndrome, hypercalcaemia, hypercalciuria, medullary nephrocalcinosis and renal failure. Pediatric nephrology (Berlin, Germany) 1995;9(1):112–4. doi: 10.1007/BF00858987. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 16.Filler G, Kotecha S, Milanska J, Lawson ML. Trisomy 21 with hypercalcemia, hypercalciuria, medullary calcinosis and renal failure - a syndrome? Pediatric nephrology (Berlin, Germany) 2001;16(1):99–100. [PubMed] [Google Scholar]
- 17.Grubb MR, Chakeres D, Malarkey WB. Patients with primary hypothyroidism presenting as prolactinomas. The American journal of medicine. 1987;83(4):765–9. doi: 10.1016/0002-9343(87)90911-9. [DOI] [PubMed] [Google Scholar]


