Abstract
Spinal cord injury (SCI) activates circulating leukocytes that migrate into the injured cord and bystander organs using adhesion molecule-mediated mechanisms. These cells cause oxidative damage, resulting in secondary injury to the spinal cord, as well as injury to bystander organs. This study was designed to examine, over a 6-h to 2-week period, changes in adhesion molecule surface expression on human peripheral leukocytes after SCI (9 subjects), using as controls 10 uninjured subjects and 6 general trauma patients (trauma controls, TC). Both the percentage of cells expressing a given adhesion molecule and the average level of its expression was quantified for both circulating neutrophils and monocytes. The percentage of neutrophils and monocytes expressing the selectin CD62L was unchanged in TC and SCI patients after injury compared to uninjured subjects. Concurrently, the amount of surface CD62L on neutrophils was decreased in SCI and TC subjects, and on monocytes after SCI. The percentage of neutrophils expressing α4 decreased in TC, but not in SCI, subjects. Likewise, the percentage of neutrophils and monocytes expressing CD11d decreased markedly in TC subjects, but not after SCI. In contrast, the mean surface expression of α4 and CD11d by neutrophils and monocytes increased after SCI compared with uninjured and TC subjects. The percentage of cells and surface expression of CD11b were similar in neutrophils of all three groups, whereas CD11b surface expression increased after SCI in monocytes. In summary, unlike changes found after general trauma, the proinflammatory stimulation induced by SCI increases the surface expression of adhesion molecules on circulating neutrophils and monocytes before they infiltrate the injured spinal cord and multiple organs of patients. Integrins may be excellent targets for anti-inflammatory treatment after human SCI.
Keywords: inflammation, integrins, macrophage, neutrophil, selectin
Introduction
Spinal cord injury (SCI) induces immediate damage to neuronal cell bodies and axons as well as glia, which is followed by progressive, secondary damage at and near the injury site (Blight, 1992; Fleming et al., 2006). This secondary damage exacerbates the functional losses of the SCI patient. Inflammation in the injury site after SCI plays an important role in secondary cell death (Blight, 1985; Taoka and Okajima, 1998; Tator and Fehlings, 1991; Young, 1993). The hallmark of the inflammatory reaction to SCI is the large numbers of leukocytes that invade the injured spinal cord (Blight, 1992; Fleming et al., 2006; Saville et al., 2004), and bystander organs such as the lungs and kidneys (Gris et al., 2008). In response to stress or injury, leukocytes are released into the blood from marginal pools and the bone marrow (Dimitrov et al., 2010; Steppich et al., 2000), increasing the number of cells available to participate in inflammation-associated secondary injury in the central nervous system (CNS) lesion, and elsewhere in the body. Upon activation in the circulation, leukocytes can upregulate expression of their oxidative enzymes (Bao et al., 2009). Such activation readies the cells for even greater pro-inflammatory and oxidative activity once they enter an injury site such as a spinal cord lesion (Longbrake et al., 2007; Saiwai et al., 2010). The potential to infiltrate and cause damage to the spinal cord and other organs relates to the maturity of the leukocytes and their expression of various integrins and receptors. Further knowledge of these aspects of circulating human neutrophils and monocytes after SCI is key to devising treatments that limit their role in secondary SCI damage.
Leukocyte migration out of the bloodstream into the sites of inflammation entails a sequence of leukocyte–adhesion molecule interactions with endothelial cell and extracellular matrix ligands (Bevilacqua, 1993). Different groups of adhesion molecules mediate this cell–cell and cell–extracellular matrix adhesion. Migration of neutrophils or monocytes into tissues is regulated by specific combinations of adhesion receptors and chemoattractants. L-selectins (CD62L) are transmembranous glycoproteins that recognize carbohydrate residues on endothelial cells (Springer, 1990). Selectins are the first molecules involved in the extravasation of leukocytes, as they transiently attach leukocytes to inflamed endothelial cells, slowing their travel within the blood vessel and commencing a process known as “rolling” (Bevilacqua et al., 1991). This receptor is known to be shed after ligation (Bevilacqua, 1993), a process that permits rolling at optimal velocities, an important determinant of leukocyte recruitment (Hafezi-Moghadam and Ley, 1999). After selectins initiate the first leukocyte attachment to the vascular endothelium, the next step in leukocyte migration is tethering and firm adhesion, mediated by membrane-bound integrin molecules. Upon recognition of inflammatory signals, neutrophils and monocytes are activated to increase surface expression of integrins (Albelda et al., 1994; Granger and Kubes, 1994; Hogg and Berlin, 1995), enabling the cells to stop rolling, bind to the endothelium, and migrate rapidly between endothelial cells into neighboring tissue (Adams and Shaw, 1994; Kansas, 1996). The leukocyte integrin, α4β1 (CD49d/CD29 or very late antigen-4 [VLA-4]), plays a role in leukocyte rolling and firm adhesion to vascular walls, and also has important roles in leukocyte activation and migration into tissue (Davenpeck et al., 1998; Nandi et al., 2004; Yednock et al., 1995). It also facilitates leukocyte adhesion to the vascular endothelium and to CNS matrix proteins, while activating cell-signaling pathways and upregulating β2 integrin expression. Ligation of α4β1 also induces production of reactive oxygen species by neutrophils (Alon et al., 1995; Poon et al., 2001; van den Berg et al., 2001).
The β2 integrins also have an important role in leukocyte migration into tissues, and are required for the spreading of firmly-attached leukocytes on the endothelial cell surface (Luscinskas et al., 1994). The β2 integrin CD11b/CD18 (Mac-1) is the most abundant and is important for neutrophil and monocyte adhesion to endothelial cells (Carlos and Harlan, 1994). The more recently discovered CD11d/CD18 is expressed on neutrophils and monocyte/macrophages, and binds to vascular cell adhesion molecule-1 (VCAM-1) in rats, and to induced endothelial cell adhesion molecule-3 (ICAM-3) and VCAM-1 in humans, facilitating firm adhesion to extracellular matrix molecules (Grayson et al., 1998; Van der Vieren et al., 1999; Van der Vieren et al., 1995). Leukocyte migration is an important mechanism in the pathogenesis of inflammatory disease. Thus, blocking leukocyte migration may have therapeutic potential against inflammation and associated diseases. Indeed, several studies in animals have shown beneficial effects of such blockade on neurological outcomes after SCI (Fleming et al., 2008; Gris et al., 2004; Oatway et al., 2005; Taoka and Okajima, 1998).
Although experimental animals have been studied in detail, the human inflammatory response to SCI is less well understood, especially in the acute period immediately after injury (Fleming et al., 2006; Yang et al., 2004). Accordingly, we used flow cytometry to characterize the ensuing cellular inflammatory response in the blood caused by human SCI, and examined changes in the expression of key adhesion molecules present on circulating neutrophils and monocytes after SCI.
Methods
These studies were approved by the University of Western Ontario Research Ethics Board for the Review of Health Sciences Research Involving Human Subjects. Venous blood samples were obtained from 25 subjects, of whom 6 were female and the remainder were male (Table 1). The groups included 10 uninjured subjects (aged 30–60 years), 9 cord-injured subjects (aged 20–87 years), and 6 trauma controls (aged 22–54 years). Patients were enrolled after obtaining consent at the Robarts Research Institute (uninjured subjects) or London Health Sciences Centre (trauma controls and cord-injured subjects), London, Ontario. Exclusion criteria for our study included a personal or family history of peripheral neuropathy or autoimmune disease, significant cognitive limitations, a history of malignant cancer within 5 years prior to the study, chronic liver disease, regular medication with anti-inflammatory drugs, diseases of the blood, and pregnancy or lactation. If a subject enrolled in the study required a blood transfusion (typically during surgery, at about 24–48 h after injury), then no further samples were obtained from that patient. One SCI subject died at 60 h after injury. The blood samples were taken at 6, 12, 24, 48, and 72 h after injury, as well as at 1 week and 2 weeks after injury. The SCI involved exclusively cervical segments in three cases, exclusively thoracic segments in three cases, and exclusively lumbar segments in two cases. One subject had injury to both cervical and lumbar segments. The SCI subjects did not have significant other injuries and were not considered to have multi-system trauma. The trauma controls had significant fractures of the vertebrae (n =2), or long bones (n =4), without CNS injury or multi-system trauma. The causes of injury for these patients were motor vehicle accidents or falls. Details about the patients are shown in Table 1.
Table 1.
Description of the Subjects
| Subject group | Case no. | Age | Sex | Nature of accident | Initial assessmenta | Follow-up assessment | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| SCI | Spinal level(s) | AIS | Motor score | AIS | Motor score | Months post-SCI | ||||
| C5 | P#1 | 41 | M | MVA | A | 10 | –b | – | – | |
| T12 | P#2 | 47 | F | Fall | A | 50 | B | 53 | 10 | |
| L1 | P#4 | 47 | M | MVA | A | 52 | D | 75 | 18 | |
| T12 | P#5 | 33 | M | MVA | A | 50 | A | 50 | 18 | |
| L1 | P#6 | 20 | F | MVA | C | 60 | C | 77 | 15 | |
| C5–C7 | P#7 | 20 | M | MVA | C | 40 | D | 80 | 16 | |
| T12 | P#10 | 44 | M | MVA | A | 50 | B | 50 | 12 | |
| C6 | P#13 | 87 | M | Fall | C | 53 | D | 74 | 6 | |
| L1 | P#14 | 57 | M | MVA | A | 60 | A | 71 | 5 | |
|
| ||||||||||
| Trauma control | Fracture location | |||||||||
| T7–T10 | P#3 | 33 | M | MVA | ||||||
| C6–C7 | P#8 | 47 | M | Fall | ||||||
| Multiple | P#9 | 25 | M | MVA | ||||||
| Multiple | P#11 | 54 | F | MVA | ||||||
| Multiple | P#12 | 22 | M | MVA | ||||||
| Multiple | P#15 | 21 | F | MVA | ||||||
| Uninjured | #1 | 60 | F | |||||||
| #2 | 30 | M | ||||||||
| #3 | 35 | M | ||||||||
| #4 | 30 | F | ||||||||
| #5 | 60 | M | ||||||||
| #6 | 55 | M | ||||||||
| #7 | 35 | M | ||||||||
| #8 | 35 | M | ||||||||
| #9 | 35 | M | ||||||||
| #10 | 55 | M | ||||||||
AIS, American Spinal Injury; C, cervical; L, lumbar; MVA, motor vehicle accident; T, thoracic;
at admission;
patient deceased at 60 h after SCI.
Used with permission from Bao et al., 2009.
Flow cytometry study of neutrophils and monocytes
Blood samples from the uninjured, trauma control, and SCI subjects were analyzed by flow cytometry. Heparinized whole blood samples were incubated with 5% normal human IgG for 30 min to block nonspecific antibody binding. Next the samples were incubated with fluorochrome-labeled antibodies, including isotype-matched mAbs for 15 min in the dark at 4°C. Leukocytes were isolated by ammonium chloride lysis of red blood cells. Cell-associated fluorescence was determined following the immunostaining using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). At least 50,000 events were analyzed for each blood sample. Neutrophils and monocytes were initially gated by their characteristic forward- and side-scatter profiles that represent size and granularity of the cells, respectively (see CD62L in Fig. 1A). The total population of cells within the gate was distributed as a scatterplot of fluorescence intensity versus side-scatter to determine the percentage of neutrophils and monocytes expressing a specific antigen (Fig. 1B and C). Gating for this analysis was derived from the fluorescence pattern of the isotype-matched control antibody. In the figures included here, the example of the percentage data and statistical analysis of the percentage data will typically correspond to the sample time at which the first significant changes in mean fluorescence intensity (MFI) occurred. The percentage data within the 12–72 h samples after the injuries were similar.
FIG. 1.
An example of flow cytometry analysis of surface protein (CD62L) expression in neutrophils and monocytes. Expression was detected by fluorescent antibodies to the surface antigens. Neutrophils and monocytes were gated by their characteristic forward- and side-scatter profiles (A), and then cells in each respective gate (B) were analyzed using two methods for mean fluorescence intensity (MFI). First, fluorescence was plotted against side-scatter to obtain the percentage of cells expressing the protein (C and E). Background fluorescence generated by the isotype-matched control antibody (left quadrants) was used to define the gating for detection of cells expressing the protein (right quadrants). Next, fluorescence was plotted against cell number and the area under this histogram was used as MFI (D and F). Again, fluorescence of the isotype-matched antibody was used to determine background fluorescence. Normalized MFI of each surface protein was calculated by subtracting the area of background fluorescence from the area of fluorescence of the specific protein.
Separately, the MFI, a measure of overall intensity of surface expression of the phenotypic markers, was determined for the total population of cells in each respective gate (Fig. 1B and C). For calculation of the MFI, areas under a histogram of cell number versus fluorescence were obtained, and then normalized by subtracting the area of fluorescence for the isotype-matched control antibody. Background fluorescence in the absence of antibody was typically 1–2% of the mean channel fluorescence of each antigen tested, whereas fluorescence in the presence of the control mAb ranged from 5–25%. These analyses were performed using FlowJo software (Tree Star Inc., Ashland, OR).
Antibodies used in the study
To detect adhesion molecules expressed by the human leukocytes, the following mouse monoclonal antibodies (mAbs) and their isotype-matched control mAbs were used: APC-conjugated mouse anti-human CD62L mAbs, and APC-conjugated mouse IgG1, κ monoclonal immunoglobulin isotype control; PE-Cy5 conjugated mouse anti-human α4 (CD49d) mAbs, and PE-Cy5 conjugated mouse IgG1, κ monoclonal immunoglobulin isotype control; Alexa Fluor 488-conjugated mouse anti-human CD11b/Mac-1 mAbs, and Alexa Fluor-488 conjugated mouse IgG1, κ monoclonal immunoglobulin isotype control (all from BD Biosciences, Toronto, ON). The Alexa Fluor 488-conjugated mouse anti-human CD11d mAbs and Alexa Fluor 488-conjugated isotype control 1B7 were gifts from the former ICOS Corp. (mAbs currently owned by Eli Lilly & Co., Indianapolis, IN).
Statistical analysis
Data are expressed as the mean±standard error (SE). Differences among groups were established using one-way analysis of variance (ANOVA), and post-hoc Fisher’s protected t-tests (Sokol and Rohlf, 1981). Sequential data collected during the first 72 h after SCI were compared to those of the uninjured subjects in a separate analysis from the values collected at 1 and 2 weeks after SCI, due to the smaller number of subjects available at the 1- and 2-week time points. Use of a two-way ANOVA was not possible, as time was shown not to be a significant factor in most of the measurements. Statistical significance was set at p <0.05. Details of the ANOVA results are given in the text. Statements of significance assume p ≤0.05 by Fisher’s protected t-tests.
Results
Adhesion molecule expression by neutrophils and monocytes
L-selectin (CD62L)
Examples of scatterplots of CD62L surface expression on neutrophils are shown in Figure 2A. These plots are representative of the CD62L expression by neutrophils of an uninjured subject, and of trauma control and SCI subjects, at 24 h after injury. Background fluorescence generated by the isotype control mAb is also shown. The majority of the neutrophils in the blood of uninjured subjects were CD62L-positive, with 86 ± 10% of the cells expressing this molecule (Fig. 2A). At 24 h after injury, the percentages of neutrophils expressing CD62L in trauma controls (82 ± 11%) and in SCI subjects (83 ± 8%) were similar to those of uninjured subjects. In contrast, the level of CD62L surface expression on positive neutrophils, as shown by the MFI, was uniformly reduced in the trauma control and SCI subjects throughout the time course of the study compared to that in uninjured subjects. The average MFI for all patients in the trauma control and SCI groups (Fig. 2C) was significantly lower than that of uninjured subjects at almost all time points sampled, with the exception of a return to the uninjured range by 2 weeks post-SCI (ANOVA, 6–72 h, F10,41 = 2.02, p =0.037; 1–2 week, F4,13 = 3.87, p =0.028). CD62L expression in trauma controls did not differ from that in SCI subjects.
FIG. 2.
The L-selectin (CD62L) expression on neutrophils and monocytes of uninjured (U), trauma control (TC), and spinal cord injury (SCI) subjects. Panels A and B illustrate examples of fluorescence plotted against side-scatter to obtain percentages of neutrophils (A) and monocytes (B) expressing CD62L. Gating was defined as shown by the fluorescence generated after incubation with the isotype-matched control antibody (Isotype). The larger numerals in one of each of the quadrants indicate the percentage of positive cells in that quadrant. The percentages of neutrophils and monocytes expressing CD62L were similar in these examples from an uninjured subject (Uninjured), and from a TC and a SCI subject, obtained 24 h after injury. Averages and typical examples of mean fluorescence intensity (MFI) in neutrophils and monocytes from the uninjured group, and from the TC and SCI groups, plotted at 6–72 h, 1 week, and 2 weeks after injury, are shown in panels C and D. The left panels illustrate mean values from each subject group, plotted at the indicated sampling times. The right panels show a typical histogram example for each group, including the isotype control. TC and SCI examples are for 24 h post-injury. Neutrophils from TC and SCI subjects had decreased CD62L MFI (mean ± standard error) compared to that of uninjured subjects. Decreases in monocytes were significant at all time points after SCI, but only at 6 h and 2 weeks in the TC subjects (*p ≤0.05 versus the uninjured group, n =5). Animal numbers at 6, 12, 24, 48, and 72 h, and 1 and 2 weeks after injury, respectively: TC: n =4, 5, 5, 5, 4, 3, 3; SCI: n =3, 6, 6, 6, 4, 5, 3.
A high percentage of monocytes (70 ± 12%) in the uninjured subjects were CD62L-positive (Fig. 2B). At 24 h after injury, this percentage was similar in the trauma controls (70 ± 12%) and SCI (61 ± 10%) subjects. Quantitative assessment of the average MFI in the SCI and TC groups revealed decreased CD62L surface expression on CD62L-positive monocytes of TC subjects only at 6 h and 2 weeks after injury (Fig. 2D), compared to the uninjured subjects, whereas decreases in SCI subjects occurred at all sampling times except 2 weeks (ANOVA, 6–72 h, F10,41 = 2.8, p =0.026; 1–2 week, F4,13 = 4.37, p =0.019). The SCI and TC groups did not differ from each other.
α4 Subunit of the α4β1 integrin
In uninjured subjects, 36 ± 7% of neutrophils expressed the α4 subunit of the α4β1 integrin (Fig. 3A). At 24 h after injury, only 13 ± 4% of neutrophils of trauma controls expressed α4, whereas after SCI 32 ± 9% of neutrophils expressed this integrin subunit. Although this decrease in TC subjects did not achieve significance when all three groups were compared with an ANOVA (F2,16 = 2.31, p =0.131), a comparison between the two groups (uninjured and trauma controls) demonstrated a significant difference (F1,9 = 8.61, p =0.017). When the average MFI on positive neutrophils was examined, α4 surface expression (Fig. 3C) decreased significantly in trauma controls throughout the early sampling periods (6–72 h) and at 1 week after injury, compared to that seen in uninjured subjects (ANOVA, 6–72 h, F10,56 = 7.313, p <0.001; 1–2 weeks, F4,19 = 4.085, p =0.015). Thus the number of α4-positive neutrophils was reduced in trauma controls, and those neutrophils that were positive expressed lower levels of α4 on their surface. In contrast, surface expression of α4 on positive neutrophils was increased after SCI compared to levels in uninjured subjects, at 12 h, 24 h, and 1 week after injury. This expression was also significantly different from that seen in trauma controls from 12–72 h and at 1 week after injury.
FIG. 3.
The α4 subunit expression on neutrophils and monocytes of uninjured (U), trauma controls (TC), and spinal cord injury (SCI) subjects. Panels A and B illustrate examples of fluorescence plotted against side-scatter to obtain percentages of neutrophils (A) and monocytes (B) expressing α4. The format of this figure is identical to that of Figure 2. The percentages of neutrophils expressing α4 in this example at 24 h after injury were lower in the TC than in the uninjured subjects, whereas the SCI subjects were similar to the uninjured subjects. The percentages of monocytes expressing α4 in the uninjured, TC, and SCI subjects were similar. Mean fluorescence intensity (MFI) (±standard error) for α4 on neutrophils and monocytes was decreased in TC subjects compared to uninjured and SCI subjects, at both early and late sampling times (C and D). In contrast, after SCI, α4 MFI on neutrophils was increased compared to uninjured and TC subjects. α4 MFI on monocytes was greater in SCI than TC subjects (*p ≤0.05 versus uninjured subjects; #p ≤0.05 versus TC subjects; U: n =6 at all time points). Animal numbers at 6, 12, 24, 48, and 72 h, and 1 and 2 weeks after injury, respectively: TC: n =5, 6, 6, 6, 5, 4, 4; SCI: n =4, 8, 8, 8, 5, 6, 4.
Most monocytes (95 ± 2%) showed surface expression of α4 with a frequency that was not significantly different between trauma control and SCI subjects (Fig. 3B). At 24 h after injury 85 ± 8% of monocytes in trauma controls and 84 ± 6% of monocytes in SCI subjects expressed this integrin subunit. Despite the similar percentages of monocytes expressing the α4 integrin in the three groups, the average MFI for α4 on the surface of monocytes of trauma controls was significantly less than that of uninjured subjects from 6 h to 1 week after injury (Fig. 3D; ANOVA, 6–72 h, F10,56 = 4.377, p <0.001; 1–2 weeks, F4,19 = 4.972, p =0.006). In contrast, based on MFI, the surface expression on α4-positive monocytes of SCI patients was no different from that of uninjured subjects, and furthermore, from 24 h to 1 week after injury, the monocyte α4 surface expression in SCI subjects was significantly greater than in trauma controls.
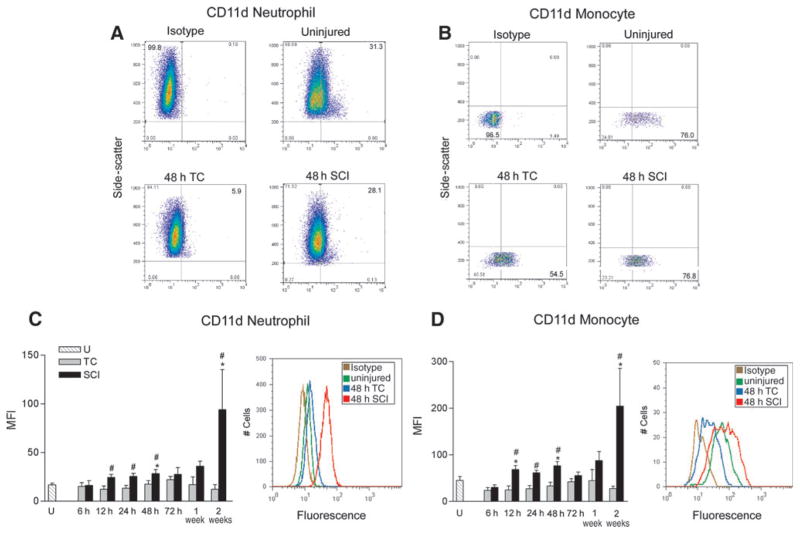
CD11d subunit of the β2 integrin CD11d/CD18
Uninjured subjects and subjects at 48 h after SCI exhibited very similar percentages of CD11d-positive neutrophils (32 ± 9% and 35 ± 11%, respectively), but the percentage of CD11d-positive neutrophils was greatly reduced (7 ± 2%) in trauma controls (ANOVA, F2,15 = 3.53, p =0.05; Fig. 4A) at 24 h post-injury. The MFI for surface expression on CD11d-positive neutrophils of uninjured, TC, and SCI subjects, was generally low at all time points except at 2 weeks after SCI, when it increased substantially (Fig. 4C). Surface expression of CD11d on positive neutrophils from trauma controls was approximately constant throughout the time course of this study. Despite the lowered number of neutrophils expressing CD11d in the trauma controls, the MFI for those cells was similar to that of uninjured subjects. In contrast, neutrophil CD11d surface expression after SCI generally tended to be increased compared to uninjured controls, reaching a significant 42% increase by 48 h after SCI, and a substantial (~6-fold) increase at 2 weeks (ANOVA, 6–72 h, F10,55 = 2.50, p =0.015; 1 and 2 weeks, F4,18 = 3.83, p <0.02). At 12–48 h after injury the MFI of CD11d surface expression in SCI subjects was significantly greater (~2-fold) than in trauma controls, and at 2 weeks this difference was even more substantial (~7-fold).
FIG. 4.
CD11d subunit expression on neutrophils and monocytes of uninjured (U), trauma controls (TC), and spinal cord injury (SCI) subjects. Panels A and B illustrate examples of fluorescence plotted against side-scatter to obtain percentages of neutrophils (A) and monocytes (B) expressing CD11d. The format of this figure is identical to that of Figure 2. The percentages of neutrophils expressing CD11d in this example at 48 h after injury were lower in the TC than in the uninjured subjects, and were higher in the SCI subjects. The percentages of monocytes expressing CD11d in the TC subjects was lower than that in the uninjured subjects, but in the SCI subjects this percentage was similar to that of the uninjured subjects. Mean fluorescence intensity (MFI) (± standard error) is shown in panels C and D. The histogram examples were taken at 48 h after injury. CD11d MFI was not changed in neutrophils or monocytes of TC subjects compared to uninjured subjects. After SCI, the CD11d MFI of neutrophils and monocytes was significantly greater than that of uninjured subjects and TC both at early and late sampling times (*p ≤0.05 versus uninjured subjects; #p ≤0.05 versus TC subjects; U: n =5). Animal numbers at 6, 12, 24, 48, and 72 h, and 1 and 2 weeks after injury: TC: n =5, 6, 6, 6, 5, 4, 4; SCI: n =4, 8, 8, 8, 5, 6, 4.
The majority of monocytes (77 ± 2%) in uninjured subjects expressed CD11d, and again the percentage was decreased in the TC subjects (Fig. 4B). For example, at 48 h after injury in TC subjects, 41 ± 10% of monocytes expressed CD11d, and this percentage was significantly lower than in uninjured (77 ± 2%) and SCI (77 ± 3%) subjects (ANOVA, F2,15 = 10.82, p =0.0012). Despite the decreased numbers of monocytes expressing CD11d in trauma controls, mean fluorescence did not differ from that in uninjured subjects, and remained relatively constant throughout the time course of the study (Fig. 4D). In SCI subjects, CD11d surface expression on positive cells was greater than in uninjured controls, by 34% and 42% at 12 and 48 h, and by sevenfold at 2 weeks after injury (ANOVA, 6–72 h, F10,55 = 6.014, p <0.001; 1 and 2 weeks, F4,18 = 3.891, p <0.019). At 12–48 h after SCI, the surface expression of CD11d on positive monocytes was about twice that of trauma controls (significantly greater) and, similarly to neutrophil expression, this difference was even larger at 2 weeks (Fig. 4D).
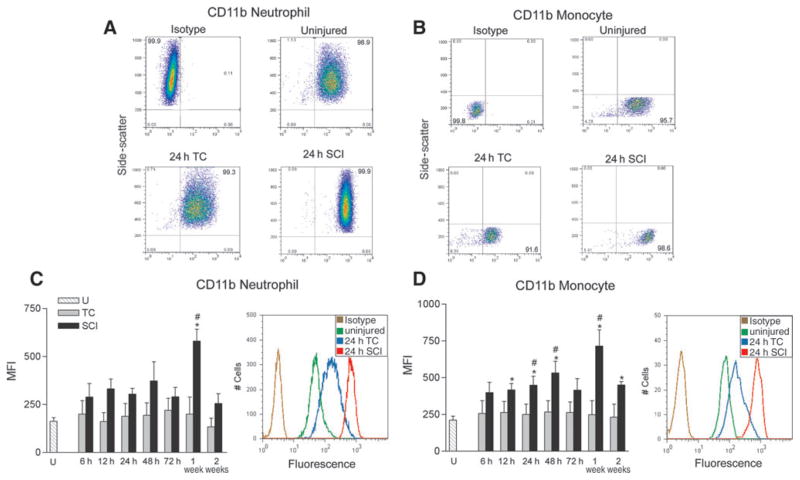
The CD11b subunit of the β2 integrin CD11b/CD18
The CD11b subunit of the β2 integrin CD11b/CD18 is expressed on the surface of nearly all neutrophils and monocytes (Fig. 5A and B). Most neutrophils (Fig. 5A) in the blood of uninjured subjects surface expressed CD11b (97 ± 1%). This prevalence was very similar in trauma controls (99 ± 0%), and SCI subjects (100 ± 0%), at 24 h after injury. When the MFI was analyzed, the average expression of CD11b on positive neutrophils of trauma controls and SCI subjects at 6–72 h after injury (Fig. 5C) was no different from that of uninjured subjects, although a tendency toward increased expression was noted after SCI (ANOVA, F10,51 = 1.44, p =0.19). The CD11b surface expression on positive neutrophils at 1 week after SCI was significantly greater, by fourfold, than on neutrophils of uninjured and trauma control subjects (ANOVA, F4,17 = 11.682, p <0.001). When CD11b MFI from all seven sampling times were averaged, the CD11b expression on neutrophils of SCI subjects was significantly greater than that of uninjured and trauma controls (ANOVA, F2,16 = 11.77, p <0.001), but no differences were found between trauma controls and uninjured subjects.
FIG. 5.
CD11b subunit expression on neutrophils and monocytes of uninjured (U), trauma controls (TC), and spinal cord injury (SCI) subjects. Panels A and B illustrate examples of fluorescence plotted against side-scatter to obtain percentages of neutrophils (A) and monocytes (B) expressing CD11b. The format of this figure is identical to that of Figure 2. The percentages of neutrophils and monocytes expressing CD11b in these TC and SCI examples at 24 h after injury were no different from those in the uninjured subjects. Mean fluorescence intensity (MFI) (±standard error) for CD11b on neutrophils and monocytes was unchanged in TC subjects compared to uninjured subjects (C and D). CD11b MFI of neutrophils in SCI subjects was greater than that in uninjured and TC subjects only at 1 week after injury. In monocytes, the CD11b MFI was greater than that of uninjured and TC subjects at several early and later sampling times (*p ≤ 0.05 versus uninjured subjects; #p ≤ 0.05 versus TC subjects; U: n =5). Animal numbers at 6, 12, 24, 48, and 72 h, and 1 and 2 weeks after injury, respectively: TC: n =5, 6, 6, 6, 5, 4, 4; SCI: n =4, 7, 7, 7, 4, 5, 4.
Most monocytes (Fig. 5B) in uninjured subjects also exhibited CD11b surface expression (95 ± 2%). Again, this prevalence was similar in trauma controls (97 ± 1%) and SCI subjects (98 ± 1%) at 24 h after injury. The monocyte CD11b MFI was significantly greater, by one- to threefold, in SCI subjects than in uninjured subjects at 12–48 h, and at 1 and 2 weeks after injury (ANOVA, 6–72 h, F10,51 = 2.459, p =0.017; 1 and 2 weeks, F4,17 = 7.941, p <0.001; Fig. 5D). CD11b surface expression on monocytes from trauma controls did not change. At 24 h, 48 h, and 1 week post-injury, the surface expression of CD11b was significantly greater in SCI subjects than in trauma controls.
Summary of results
As described in detail above, after either general trauma or SCI, changes in adhesion molecule expression generally occurred within 24–48 h after injury. Surface expression of CD62L usually decreased in TC and SCI subjects. Expression of the integrins often decreased after general trauma and increased after SCI. The observed changes were more or less maintained for the 72 h after injury, often lasting up to 2 weeks. Sharp increases in expression of the two β integrins occurred in the 1- to 2-week period relative to both the uninjured and TC subjects. To facilitate comparisons between the adhesion molecules, a summary of these data for the 24–48 h period is presented in Table 2.
Table 2.
Summary of Adhesion Molecule Expression in Uninjured Subjects and at 24–48 Hours after Injury in Trauma Control and Spinal Cord Injury Subjects
| Neutrophils
|
Monocytes
|
|||||
|---|---|---|---|---|---|---|
| Uninjured | TC | SCI | Uninjured | TC | SCI | |
| Percentage of cells expressing molecule | Percentage of cells expressing molecule | |||||
| CD62L | 86 ± 10 | 82 ± 11 | 83 ± 8 | 70 ± 12 | 70 ± 12 | 61 ± 10 |
| α4 | 36 ± 7 | 13 ± 4 | 32 ± 9 | 95 ± 2 | 85 ± 8 | 84 ± 6 |
| CD11d | 32 ± 9 | 7 ± 2 | 35 ± 11 | 77 ± 2 | 41 ± 10 | 77 ± 3 |
| CD11b | 97 ± 1 | 99 ± 0 | 100 ± 0 | 95 ± 2 | 97 ± 1 | 98 ± 1 |
| MFI change from uninjured | MFI change from uninjured | |||||
| CD62L | 58% ↓ | 47% ↓ | 12% | 42% ↓ | ||
| α4 | 45% ↓ | 35% ↑ | 49% ↓ | 1% | ||
| CD11d | 6% | 65% ↑ | 27% ↓ | 69% ↑ | ||
| CD11b | 17% ↑ | 88% ↑ | 18% ↑ | 111% ↑ | ||
All values with the exception of CD11d are illustrated at 24 h after injury. CD11d samples are shown at 48 h.
TC, trauma control; SCI, spinal cord injury; MFI, mean fluorescence intensity.
Discussion
Our study shows, for the first time in human subjects, that the surface expression of adhesion molecules on circulating neutrophils and monocytes is changed after SCI. The expression of the selectin CD62L decreased to a similar degree after general trauma and SCI, suggesting that trauma is the common cause of this response. In contrast, the responses of the integrins α4β1, CD11d/CD18, and CD11b/CD18 differed between general trauma and SCI subjects, indicating a specific role of the cord injury in the response. Surface expression on neutrophils and monocytes of the integrins studied almost always increased after SCI, whereas it decreased (α4 and CD11d), or remained essentially unchanged (CD11b), after general trauma.
The results of this study show that the high percentage of neutrophils and monocytes expressing CD62L did not change with injury, but the extent of this expression decreased markedly on the neutrophils of TC and SCI subjects, and on monocytes of SCI subjects. Presumably, this decrease reflects shedding of the selectin CD62L that is known to follow leukocyte activation and transient binding of CD62L to one of its glycoprotein counter-receptors (Bevilacqua et al., 1991). As CD62L is the first adhesion molecule to begin the process of leukocyte diapedesis, and it responds quickly to the first signs of inflammation, the similar responses of neutrophils to SCI and general trauma are understandable. Although monocytes also decreased their surface expression of CD62L after SCI, the reduction seen after general trauma was inconsistent, suggesting that this molecule is not essential for their diapedesis after an injury outside the CNS.
The pattern of the changes in β1 and β2 integrin expression on neutrophils and monocytes differed between SCI and TC subjects. The mechanism underlying these disparate responses in SCI and TC subjects is unclear, but likely relates to qualitative and quantitative differences in the inflammatory responses to the two conditions. Our previous study has shown, in these same two groups of subjects and at the same sampling times, that SCI causes more intense activation of the leukocytes than does general trauma (Bao et al., 2009), impacting both neutrophils and monocytes. An additional factor contributing to the leukocyte profiles seen after SCI is that activated neutrophils are more long-lived (Gris et al., 2008). The sustained and even increased α4 surface expression by neutrophils after SCI may reflect this greater leukocyte activation, with greater recruitment of the receptor to the cell surface, and perhaps greater expression of the integrin proteins. The greater degree of leukocyte activation seen after SCI may also explain the increased CD11d and CD11b surface expression. CD11d requires a much stronger proinflammatory signal for activation than other β2-integrin family members (Noti et al., 2000). Accordingly, only the enhanced inflammatory response after SCI may be adequate to trigger increased CD11d expression on neutrophils and on the marginal pool and circulating monocyte populations. We cannot explain the greatly increased CD11d surface expression seen at 2 weeks after SCI by any comorbid condition. The patients in this group did not suffer any clinical evidence of infection or show any obvious cause for an additional inflammatory response at this time point.
The decreased surface α4 seen in the leukocytes of TC subjects may be attributable to internalization of this integrin in the circulation after a ligation to its endothelial receptor that was insufficient to cause diapedesis. Ligation of α4β1 by antibody binding causes internalization of this receptor (Leone et al., 2003). Our previous study of these TC subjects (Bao et al., 2009) demonstrated rather weak activation of neutrophils and monocytes, a stimulus less likely to increase CD11d expression as discussed above. The dramatic drop in the percentage of CD11d-positive neutrophils seen after general trauma may also have been due to the release from bone marrow into the circulation of relatively immature, unactivated neutrophils that lack CD11d expression, since most types of trauma initiate a transient neutrophilia (Gris et al., 2008).
The pattern of CD11d surface expression on monocytes may also relate to their heterogeneity. Although most peripheral monocytes are “conventional” CD14+ cells, a smaller population of proinflammatory CD14lowCD16+ monocytes also exists (Tacke and Randolph, 2006; Ziegler-Heitbrock, 2007). CD11d is proposed to be expressed primarily on the CD14lowCD16+ subpopulation, but this contention has not been confirmed (Steppich et al., 2000). CD11d-expressing proinflammatory monocytes may exit the circulation after general trauma, are attracted to sites of inflammation, or they may adhere to blood vessel walls. Indeed, the number of CD11d+ monocytes decreased by ~35% in the TC group, suggesting that as the CD11d+ cells leave the circulation, the relative proportion of CD11d− monocytes increases. The apparently conflicting increases in proinflammatory CD11d+ monocytes that have been noted in the circulation occur within minutes of stress or trauma and last less than 20 min (Dimitrov et al., 2010; Steppich et al., 2000). Therefore the effects of this mobilization probably would not have been observed in our study. A direct analysis using polychromatic flow cytometry is required to examine CD11d in the two different monocyte populations after general trauma and SCI.
We can offer some speculation regarding the cause of the different effects of SCI and general trauma on surface integrin expression. The enhanced leukocyte response to SCI may occur because SCI damages crucial CNS feedback systems that control inflammation, whereas general trauma would likely leave them intact. Recent studies in animal models of SCI show that trauma to the CNS can lead to inhibition of a cholinergic anti-inflammatory pathway, resulting in heightened systemic inflammatory responses, especially after cervical or high-to-mid-thoracic SCI (Rosas-Ballina and Tracey, 2009). This system limits proinflammatory cytokine production. Increases in proinflammatory chemokine and cytokine concentrations that have been observed in the circulation after SCI (Ankeny et al., 2006; Campbell et al., 2005) would impact greatly on the properties of leukocytes. For example, the unregulated release of proinflammatory cytokines immediately after injury causes activation and priming of neutrophils (Kobayashi et al., 2003; Ogura et al., 1999). Such priming was found as early as 2 h after SCI in one of our studies (Gris et al., 2008). Stimulation by chemokines and cytokines upregulates neutrophil adhesion molecules, enhancing their ability to migrate (Sunil et al., 2002; Walzog et al., 1999; Wittmann et al., 2004). The proinflammatory cytokines and an inflammation-induced release of epinephrine also activate monocytes, inducing them to migrate from marginal pools, and to adopt a macrophage phenotype when they reach sites of inflammation (Dimitrov et al., 2010). General trauma would not disrupt this anti-inflammatory pathway, permitting the TC subjects more capacity to limit the systemic inflammatory response.
The presence of the α4β1 and CD11d/CD18 integrins on the surface of human neutrophils and monocytes strengthens the idea that blocking the actions of these adhesion molecules would be a good strategy to limit early inflammation after SCI. Our previous studies in rats have shown that antibodies to the α4 subunit of α4β1, and to the CD11d subunit of CD11d/CD8 (αDβ2), when administered within 2–6 h after SCI can block migration of neutrophils and monocytes into the injured cord (Ditor et al., 2006; Fleming et al., 2008; Gris et al., 2004; Oatway et al., 2005; Saville et al., 2004). The present study shows that surface expression of each of these key integrins is well maintained or increased on the circulating leukocytes after SCI, a response consistent with the view that they participate in cell migration after this CNS injury.
Our data show that the onset of increased surface expression was about 12 h after SCI, providing a good window of opportunity for clinical treatment. Their basal expression on leukocytes at 6 h post-SCI would also render them available to an earlier course of treatment. Maintained surface expression throughout the acute period (72 h) after injury suggests that they could remain an important target during this time. Although we did observe increases in integrin surface expression after SCI, this finding does not permit the conclusion that such an increase is necessary for them to have a functional role in leukocyte migration after SCI. They may have functioned well without such an increase. Indeed, a key response of the α4β1 integrin to activation is a conformational change that increases adhesivity to ligands, suggesting that increased amounts of expression of this integrin may not be necessary for its function (Yednock et al., 1995). Moreover, the functional implications of the amount of increase cannot be extrapolated from our data. We found increases ranging from about 20–100%, and a more detailed study ex vivo would be required to understand the functional implications of these changes. In contrast, the decreases in integrin expression that occurred exclusively in the TC subjects might indicate a lessening of capacity to facilitate leukocyte migration, but that too would require further analysis.
In a rat SCI model, we have studied the effectiveness of different onset times of intravenous treatment with an anti-CD11d blocking mAb, finding excellent efficacy with a 6-h delay in treatment, and even good efficacy with a delay of 12 h (Ditor et al., 2006). Although the time course of the rat inflammatory response cannot be precisely extrapolated to the human condition, the robust expression of the human integrins in the acute hours after SCI suggests that they would be available targets for therapeutic interventions. In contrast to the promising role of an anti-integrin strategy, the loss of the surface CD62L selectin by human neutrophils and monocytes, presumably due to shedding by the activated cells, makes the selectins a less ideal target for therapy.
Conclusion
In this study we demonstrated that circulating neutrophils and monocytes not only maintain their surface expression of adhesion molecules after SCI, but increase it. This response is significant and is often opposite the response seen after general trauma. The increases sometimes lasted up to weeks after SCI, at time points when other measures of the immune system show depression (Campagnolo et al., 1997; Cruse et al., 1996). An examination in a larger patient population of the inflammatory responses that we found may reveal a means by which secondary complications can be predicted or detected more readily after SCI. Moreover, this aspect of the systemic inflammatory response may also provide a target for the development of acute treatments that provide neuroprotection after SCI.
Acknowledgments
We thank all of the volunteers and patients who contributed to this study. We also thank all of the fellows, nurses, and staff at the University Health Sciences Centre Victoria Hospital Campus and Robarts Research Institute, who facilitated our work by coordinating the timely acquisition and transport of samples to our laboratory. We are indebted to Dr. Canio Polosa for his critical evaluation of this manuscript. This research was supported by the Canadian Institutes of Health Research (CIHR).
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- Adams DH, Shaw S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet. 1994;343:831–836. doi: 10.1016/s0140-6736(94)92029-x. [DOI] [PubMed] [Google Scholar]
- Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504–512. [PubMed] [Google Scholar]
- Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- Bao F, Bailey CS, Gurr KR, Bailey SI, Rosas-Arellano MP, Dekaban GA, Weaver LC. Increased oxidative activity in human blood neutrophils and monocytes after spinal cord injury. Exp Neurol. 2009;215:308–316. doi: 10.1016/j.expneurol.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M, Butcher E, Furie B, Furie B, Gallatin M, Gimbrone M, Harlan J, Kishimoto K, Lasky L, McEver R. Selectins: a family of adhesion receptors. Cell. 1991;67:233. doi: 10.1016/0092-8674(91)90174-w. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Ann Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Blight AR. Delayed demyelination and macrophage invasion: A candidate for “secondary” cell damage in spinal cord injury. CNS Trauma. 1985;2:299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- Blight AR. Macrophages and inflammatory damage in spinal cord injury. J Neurotrauma. 1992;9 (Suppl 1):S83–S91. [PubMed] [Google Scholar]
- Campagnolo DI, Bartlett JA, Keller SE, Sanchez W, Oza R. Impaired phagocytosis of Staphylococcus aureus in complete tetraplegics. Am J Phys Med Rehabil. 1997;76:276–280. doi: 10.1097/00002060-199707000-00005. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Perry VH, Pitossi FJ, Butchart AG, Chertoff M, Waters S, Dempster R, Anthony DC. Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol. 2005;166:1487–1497. doi: 10.1016/S0002-9440(10)62365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- Cruse JM, Keith JC, Bryant ML, Jr, Lewis RE., Jr Immune system-neuroendocrine dysregulation in spinal cord injury. Immunol Res. 1996;15:306–314. doi: 10.1007/BF02935314. [DOI] [PubMed] [Google Scholar]
- Davenpeck KL, Sterbinsky SA, Bochner BS. Rat neutrophils express alpha4 and beta1 integrins and bind to vascular cell adhesion molecule-1 (VCAM-1) and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) Blood. 1998;91:2341–2346. [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184:503–511. doi: 10.4049/jimmunol.0902189. [DOI] [PubMed] [Google Scholar]
- Ditor DS, Bao F, Chen Y, Dekaban GA, Weaver LC. A therapeutic time window for anti-CD11d monoclonal antibody treatment yielding reduced secondary tissue damage and enhanced behavioral recovery following severe spinal cord injury. J Neurosurg Spine. 2006;5:343–352. doi: 10.3171/spi.2006.5.4.343. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Bao F, Chen Y, Hamilton EF, Relton JK, Weaver LC. a4b1 integrin blockade after spinal cord injury decreases damage and improves neurological function. Exp Neurol. 2008;214:147–159. doi: 10.1016/j.expneurol.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquales-Style M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- Grayson MH, Van der Vieren M, Sterbinsky SA, Gallatin WM, Hoffman PA, Staunton DE, Bochner BS. adb2 integrin is expressed on human eosinophils and functions as an alternative ligand for vascular cell adhesion molecule 1 (VCAM-1) J Exp Med. 1998;188:2187–2191. doi: 10.1084/jem.188.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gris D, Hamilton EF, Weaver LC. The systemic inflammatory response after spinal cord injury damages lungs and kidneys. Exp Neurol. 2008;211:259–270. doi: 10.1016/j.expneurol.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J Exp Med. 1999;189:939–948. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N, Berlin C. Structure and function of adhesion receptors in leukocyte trafficking. Immunol Today. 1995;16:327–330. doi: 10.1016/0167-5699(95)80147-2. [DOI] [PubMed] [Google Scholar]
- Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- Kobayashi SD, Voyich JM, DeLeo FR. Regulation of the neutrophil-mediated inflammatory response to infection. Microbes Infect. 2003;5:1337–1344. doi: 10.1016/j.micinf.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Leone DR, Giza K, Gill A, Dolinski BM, Yang W, Perper S, Scott DM, Lee WC, Cornebise M, Wortham K, Nickerson-Nutter C, Chen LL, LePage D, Spell JC, Whalley ET, Petter RC, Adams SP, Lobb RR, Pepinsky RB. An assessment of the mechanistic differences between two integrin alpha 4 beta 1 inhibitors, the monoclonal antibody TA-2 and the small molecule BIO5192, in rat experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther. 2003;305:1150–1162. doi: 10.1124/jpet.102.047332. [DOI] [PubMed] [Google Scholar]
- Longbrake EE, Lai W, Ankeny DP, Popovich PG. Characterization and modeling of monocyte-derived macrophages after spinal cord injury. J Neurochem. 2007;102:1083–1094. doi: 10.1111/j.1471-4159.2007.04617.x. [DOI] [PubMed] [Google Scholar]
- Luscinskas FW, Kansas GS, Ding H, Pizcueta P, Schleiffenbaum BE, Tedder TF, Gimbrone MA., Jr Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, beta 1-integrins, and beta 2-integrins. J Cell Biol. 1994;125:1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T cell extravasation. Immunity. 2004;20:455–465. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- Noti JD, Johnson AK, Dillon JD. Structural and functional characterization of the leukocyte integrin gene CD11d. Essential role of Sp1 and Sp3. J Biol Chem. 2000;275:8959–8969. doi: 10.1074/jbc.275.12.8959. [DOI] [PubMed] [Google Scholar]
- Oatway MA, Chen Y, Bruce JC, Dekaban GA, Weaver LC. An anti-CD11d integrin antibody treatment restores normal serotonergic projections to the dorsal, intermediate and ventral horns of the injured spinal cord. J Neurosci. 2005;25:637–647. doi: 10.1523/JNEUROSCI.3960-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura H, Tanaka H, Koh T, Hashiguchi N, Kuwagata Y, Hosotsubo H, Shimazu T, Sugimoto H. Priming, second-hit priming, and apoptosis in leukocytes from trauma patients. J Trauma. 1999;46:774–781. doi: 10.1097/00005373-199905000-00004. [DOI] [PubMed] [Google Scholar]
- Poon BY, Ward CA, Cooper CB, Giles WR, Burns AR, Kubes P. alpha(4)-integrin mediates neutrophil-induced free radical injury to cardiac myocytes. J Cell Biol. 2001;152:857–866. doi: 10.1083/jcb.152.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265:663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiwai H, Ohkawa Y, Yamada H, Kumamaru H, Harada A, Okano H, Yokomizo T, Iwamoto Y, Okada S. The LTB4-BLT1 axis mediates neutrophil infiltration and secondary injury in experimental spinal cord injury. Am J Pathol. 2010;176:2352–2366. doi: 10.2353/ajpath.2010.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville LR, Pospisil CH, Mawhinney LA, Bao F, Simedria FC, Peters AA, O’Connell PJ, Weaver LC, Dekaban GA. A monoclonal antibody to CD11d reduces the inflammatory infiltrate into the injured spinal cord: A potential neuroprotective treatment. J Neuroimmunol. 2004;156:42–57. doi: 10.1016/j.jneuroim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Sokol RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. W.H. Freeman; San Francisco: 1981. [Google Scholar]
- Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Steppich B, Dayyani F, Gruber R, Lorenz R, Mack M, Ziegler-Heitbrock HW. Selective mobilization of CD14(+)CD16(+) monocytes by exercise. Am J Physiol Cell Physiol. 2000;279:C578–C586. doi: 10.1152/ajpcell.2000.279.3.C578. [DOI] [PubMed] [Google Scholar]
- Sunil VR, Connor AJ, Zhou P, Gordon MK, Laskin JD, Laskin DL. Activation of adherent vascular neutrophils in the lung during acute endotoxemia. Respir Res. 2002;3:21. doi: 10.1186/rr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Taoka Y, Okajima K. Spinal cord injury in the rat. Prog Neurobiol. 1998;56:341–358. doi: 10.1016/s0301-0082(98)00049-5. [DOI] [PubMed] [Google Scholar]
- Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- van den Berg JM, Mul FP, Schippers E, Weening JJ, Roos D, Kuijpers TW. Beta1 integrin activation on human neutrophils promotes beta2 integrin-mediated adhesion to fibronectin. Eur J Immunol. 2001;31:276–284. doi: 10.1002/1521-4141(200101)31:1<276::AID-IMMU276>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Van der Vieren M, Crowe DT, Hoekstra D, Vazeux R, Hoffman PA, Grayson MH, Bochner BS, Gallatin WM, Staunton DE. The leukocyte integrin aDb2 binds VCAM-1: Evidence for a binding interface between I domain and VCAM-1. J Immunol. 1999;163:1984–1990. [PubMed] [Google Scholar]
- Van der Vieren M, Trong HL, Wood CL, Moore PF, St John T, Staunton DE, Gallatin WM. A novel leukointegrin, adb2, binds preferentially to ICAM-3. Immunity. 1995;3:683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Walzog B, Weinmann P, Jeblonski F, Scharffetter-Kochanek K, Bommert K, Gaehtgens P. A role for beta(2) integrins (CD11/CD18) in the regulation of cytokine gene expression of polymorphonuclear neutrophils during the inflammatory response. FASEB J. 1999;13:1855–1865. doi: 10.1096/fasebj.13.13.1855. [DOI] [PubMed] [Google Scholar]
- Wittmann S, Rothe G, Schmitz G, Frohlich D. Cytokine upregulation of surface antigens correlates to the priming of the neutrophil oxidative burst response. Cytometry A. 2004;57:53–62. doi: 10.1002/cyto.a.10108. [DOI] [PubMed] [Google Scholar]
- Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN. Early expression and cellular localization of proinflammatory cytokines interleukin-1Beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine. 2004;29:966–971. doi: 10.1097/00007632-200405010-00004. [DOI] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Vandevert C, Goldbach EG, Shaw G, Ellis DK, Liaw C, Fritz LC, Tanner LI. Alpha 4 beta 1 integrin-dependent cell adhesion is regulated by a low affinity receptor pool that is conformationally responsive to ligand. J Biol Chem. 1995;270:28740–28750. doi: 10.1074/jbc.270.48.28740. [DOI] [PubMed] [Google Scholar]
- Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med. 1993;11:13–22. [PubMed] [Google Scholar]
- Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]