Abstract
OBJECTIVE
The aim of this study was to assess whether computer-assisted detection–processed MRI kinetics data can provide further information on the biologic aggressiveness of breast tumors.
MATERIALS AND METHODS
We identified 194 newly diagnosed invasive breast cancers presenting as masses on contrast-enhanced MRI by a HIPAA-compliant pathology database search. Computer-assisted detection–derived data for the mean and median peak signal intensity percentage increase, most suspicious kinetic curve patterns, and volumetric analysis of the different kinetic patterns by mean percentage tumor volume were compared against the different hormonal receptor (estrogen-receptor [ER], progesterone-receptor [PR], ERRB2 (HER2/neu), and triple-receptor expressivity) and histologic grade subgroups, which were used as indicators of tumor aggressiveness.
RESULTS
The means and medians of the peak signal intensity percentage increase were higher in ER-negative, PR-negative, and triple-negative (all p ≤ 0.001), and grade 3 tumors (p = 0.011). Volumetric analysis showed higher mean percentage volume of rapid initial enhancement in biologically more aggressive ER-negative, PR-negative, and triple-negative tumors compared with ER-positive (64% vs 53.6%, p = 0.013), PR-positive (65.4% vs 52.5%, p = 0.001), and nontriple-negative tumors (65.3% vs 54.6%, p = 0.028), respectively. A higher mean percentage volume of rapid washout component was seen in ERRB2-positive tumors compared with ERRB2-negative tumors (27.5% vs 17.9%, p = 0.020).
CONCLUSION
Peak signal intensity percentage increase and volume analysis of the different kinetic patterns of breast tumors showed correlation with hormonal receptor and histologic grade indicators of cancer aggressiveness. Computer-assisted detection–derived MRI kinetics data have the potential to further characterize the aggressiveness of an invasive cancer.
Keywords: breast neoplasms, computer-assisted detection, kinetics, MRI, volumetric analysis
The use of contrast-enhanced MRI for breast cancer provides information about the vascular properties of these tumors on the basis of their contrast-enhancement properties [1–5]. This may help to provide useful information on tumor aggressiveness, disease prognostication, choice and timing of therapy, and therapy response of breast cancer. In recent years, computer-assisted detection systems with postprocessing software have been used for semiautomatic time curve analysis of enhancement kinetics of breast lesions. Parameters obtained from computer-aided detection include the peak contrast-enhanced percentage signal intensity increase, the different kinetic curve patterns found within the tumor, and the volumetric assessment of each enhancement kinetic pattern within the tumor. The objective of this study was to investigate whether any of these computer-assisted detection processed data on tumor enhancement kinetics could provide information on tumor biologic behavior by investigating any correlation with pathologic indicators for tumor aggressiveness, such as the hormonal receptor expression status and histologic grading.
Materials and Methods
Study Cohort
Institutional review board approval was obtained for this HIPAA-compliant study. A pathology database search revealed 357 patients who had newly diagnosed breast cancers on core biopsies and underwent breast MRI from March 2005 to March 2009. Forty-seven cases had incomplete data and were excluded. Of the remaining cases, pure ductal carcinoma in situ cancers (n = 9) and rare types of breast cancers, such as adenoid cystic carcinoma, metaplastic carcinoma, and phyllodes tumor as well as nonepithelial malignancies, such as angiosarcoma, osteosarcoma, and others (n = 3) were excluded. Other exclusions included cancers that had no appreciable residual mass after biopsy or underwent prior surgical excision (n= 40), lesions that presented as nonmasslike enhancement or masses containing contiguous areas of nonmasslike enhancement (n = 47), and cases with prior neoadjuvant chemotherapy (n = 20). Multifocal, multicentric, or contralateral tumors were evaluated separately as individual cancers. Thus, 194 invasive breast cancers from 191 patients were evaluated.
MRI Technique
Five different MRI scanners from two manufacturers were used for imaging: Signa 1.5-T or Signa HDx 3-T (both GE Healthcare) or Trio 3-T (Siemens Healthcare). Scheduling availability determined which magnet would be used. Dedicated surface breast coils with either a seven-channel coil (InVivo, InVivo Research) or an eight-channel coil (NORAS, GE Healthcare) were used.
The unenhanced and contrast-enhanced dynamic sequences were performed in the axial or sagittal plane on the Signa 1.5-T and Signa HDx 3-T scanners and in the axial plane on the Trio 3-T scanners. The parameters acquired in the sagittal plane for the T1-weighted volume imaging for breast assessment VIBRANT (GE Healthcare) fat-suppressed dynamic sequences in the 1.5-T and 3-T Signa MRI scanners were TR/TE, 4.3/1.7; flip angle, 8°; slice thickness, 2.0 mm with no gap; measurements, 1; matrix, 256 × 256; and FOV, 18 × 18 cm. The parameters acquired in the axial plane for the T1-weighted VIBRANT fat-suppressed dynamic sequences in the 1.5-T and 3-T Signa scanners were TR/TE, 4.3/1.7; flip angle, 8°; slice thickness, 2.0 mm with no gap; measurements, 1; matrix, 512 × 512; and FOV, 30 × 30 cm. The parameters acquired in the axial plane for the T1-weighted volume interpolated breath-hold examination VIBE (Siemens Healthcare) fat-suppressed dynamic sequences on the 3-T Trio scanners were TR/TE, 4.6/1.7, flip angle, 8°; slice thickness, 2.0 mm with no gap; measurements, 1; matrix, 448 × 448; and FOV, 280 × 280 cm. The dynamic sequences were performed before and at approximately 90, 180, 270, and 360 seconds IV administration of 0.1 mmol/kg of gadopentetate dimeglumine (Magnevist, Berlex). Postprocessed subtraction images and computer-assisted diagnosis were routinely used.
MRI and Computer-Assisted Detection Image Interpretation
The contrast-enhanced enhancement kinetics data of the breast tumors were analyzed prospectively by a full-time breast imaging radiologist with 14 years of experience in breast imaging. The radiologist was blinded to the clinical and pathologic data of the breast tumors.
The different kinetic curve patterns were described according to the BI-RADS lexicon for MR breast imaging. In this study, the patterns between the early and delayed phases of the curves were analyzed separately. In the early phase, the kinetic pattern depended on the percentage signal intensity increase from the unenhanced baseline to the signal intensity detected at the first contrast-enhanced dynamic sequence at 90 seconds after contrast administration. The patterns were grouped into rapid enhancement type (more than 100% increase in signal intensity from the unenhanced baseline), medium type (60–100% signal increase), or slow type (less than 60% signal increase). The delayed phase of enhancement, which was evaluated between the first and fourth dynamic sequences after contrast administration, would be categorized into persistent type curve if there was continuing enhancement that rose by more than 10% in signal intensity, rapid washout type curve if the enhancement showed more than 10% decrease, or a plateau type curve if the continuing enhancement was within a 10% range of the signal intensity from the first contrast-enhanced dynamic sequence at 90 seconds.
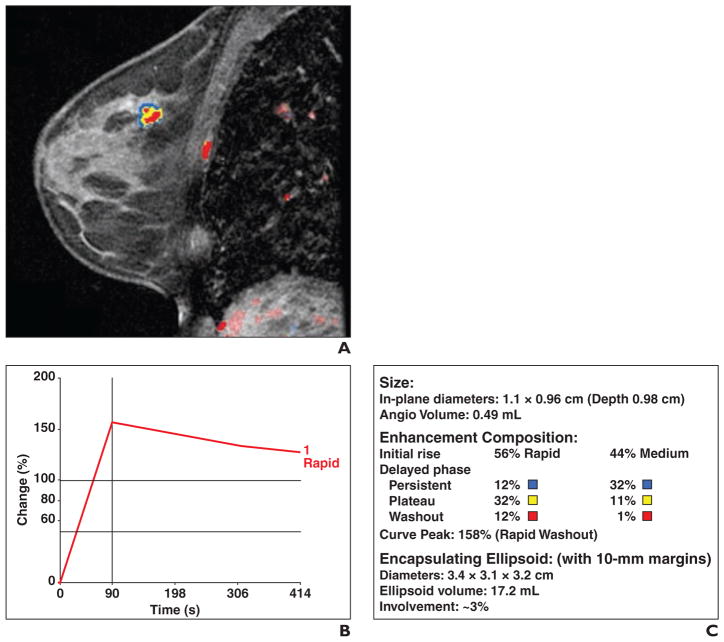
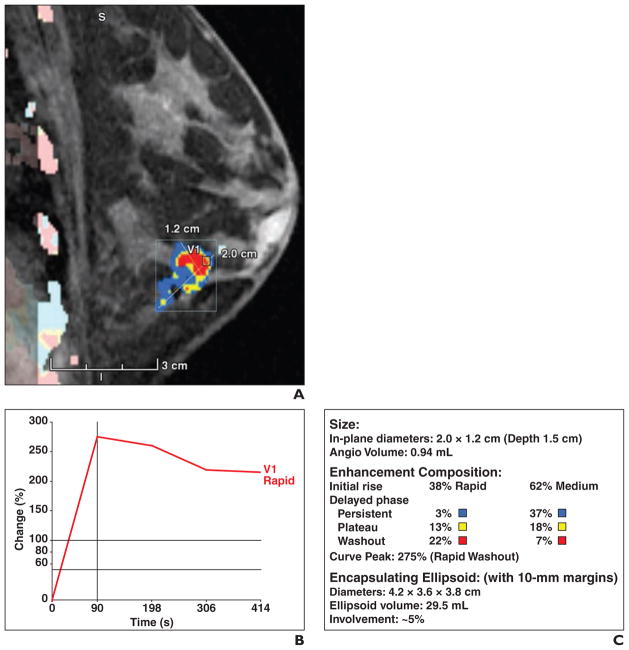
The dynamic MRI data were processed by a computer-assisted detection system (CADstream, version 4.1.3.963, Confirma) that analyzed the signal intensities within each voxel of the FOV obtained during the dynamic sequences, forming the patterns of the kinetic curves. After analysis of the kinetic curves, the computer-assisted detection system could then identify the abnormally enhancing areas. The computer-assisted detection system that was used in the study had a function that could automatically identify and outline the margins of a selected tumor mass and provide information on its size and internal MRI kinetics (Figs. 1 and 2).
Fig. 1. 51-year-old woman with grade 2 estrogen receptor–positive, progesterone receptor–positive, and ERRB2-negative invasive ductal carcinoma.
A, Sagittal contrast-enhanced, T1-weighted volume imaging for breast assessment (VIBRANT, GE Healthcare) fat-suppressed sequence shows computer-assisted detection color overlay over breast mass. Areas in red indicate rapid washout type delayed enhancement pattern, areas in yellow indicate plateau type delayed enhancement pattern, and areas in blue indicate persistent type delayed enhancement pattern. B, Graph of worst kinetics pattern within tumor shows rapid initial enhancement and rapid washout type curve. C, Volumetric assessment by computer-assisted detection calculates volume of tumor and enhancement composition of tumor mass. With respect to initial enhancement phase, 56% of tumor shows rapid wash-in and 44% shows medium rate of wash-in. For delayed enhancement component, only 13% (12% + 1%) of mass showed rapid washout type pattern; 44% (12% + 32%) of mass contained delayed persistent type curve, and 43% (32% + 11%) contained plateau type curve.
Fig. 2. 57-year-old woman with MRI computer-assisted detection assessment of grade 2 estrogen receptor–positive, progesterone receptor–positive, and ERRB2-negative invasive lobular carcinoma.
A, Sagittal contrast-enhanced, T1-weighted volume imaging for breast assessment (VIBRANT, GE Healthcare) fat-suppressed sequence shows computer-assisted detection color overlay over breast mass. Areas in red indicate rapid washout type delayed enhancement pattern, areas in yellow indicate plateau type delayed enhancement pattern, and areas in blue indicate persistent type delayed enhancement pattern. B, Graph of worst kinetic curve shows rapid initial enhancement and rapid washout type curve. C, Volumetric assessment shows 38% of mass had rapid initial enhancement, 29% of mass showed rapid washout, 40% of mass contained delayed persistent type curve, and 31% contained plateau type curve.
The following computer-assisted detection–processed data were used for analysis. Tumor size: Tumor volume calculated by the computer-assisted detection software was used for analysis. Peak signal intensity percentage increase: The peak signal intensity percentage increase refers to the percentage signal intensity change seen at the initial enhancement phase from the unenhanced sequence to the first contrast-enhanced sequence at 90 seconds after contrast administration. Only the highest percentage signal intensity change found within the tumor was observed and documented. Most suspicious kinetic pattern: Analysis was performed on only the most suspicious early and delayed types of curve patterns. The more suspicious curve in the initial phase of enhancement was taken to be the rapid type, followed by medium type enhancement. Computer-assisted detection would not highlight the slow uptake type of early enhancement and this component was not assessed. For the delayed enhancement phase, rapid washout was taken to be the most suspicious, followed by plateau and persistent type patterns. Volumetric assessment of the different kinetic curve components refers to the percentage volume of each different kinetic curve component found within the tumors. A set of percentage tumor volumes would be shown separately for the early enhancement phase (showing the volume distribution between the rapid and medium type curves) and the delayed enhancement phase (for persistent, plateau, and rapid washout type curves).
Histologic Grade Assessment
On the basis of the modified Bloom-Richardson-Elston grading system, histologic grading was stratified into grades 1, 2, and 3, which relate to low, intermediate, and high tumor grades, respectively.
Assessment of Hormonal Receptor Subtypes
The estrogen (ER), progesterone (PR), and ERRB2/neu (HER2/neu, ERRB2) hormonal receptor subtypes were evaluated. Hormone receptors were considered positive when their concentration was above 10%. ERRB2 overexpression was considered positive when complete, and intense membrane staining was seen in more than 10% of the tumor cells. The receptors were also evaluated collectively as a group for triple-receptor expressivity. A triple-negative tumor refers to a tumor with negative expression of all three receptor subtypes. Non-triple-negative tumors were defined as cancers with positive expression of at least one of the hormonal receptors.
Data and Statistical Analysis
The mean age of the patients was compared against the histopathologic cancer types, hormonal receptor subtypes, and histologic grade. The histologic grade and median tumor volume were compared against the histopathologic cancer types and hormonal receptor subtypes. The mean and median peak signal intensity percentage increase, most suspicious kinetic curve patterns, and volumetric analysis of the different kinetic patterns by mean percentage volume were compared against the different hormonal receptor and histologic grade subgroups. MRI kinetics characteristics between the tumors that were scanned in the 1.5-T and 3-T MRI magnet strengths were also analyzed.
Comparison of continuous data between two groups was performed using Student t tests and ANOVA for comparison of three groups. Comparison of median tumor size among groups was performed with the Mann-Whitney U test for comparison of two groups and the Kruskal-Wallis test for three groups because of skewed distribution of the data. Comparison of categoric variables among groups was performed using either the chi-square test or Fisher exact test. Adjustment of post-hoc comparison of all pairs of two groups was done by the Bonferroni method whenever three comparison groups were present. Statistical analysis was performed with SPSS, version 17, statistical software.
Results
We evaluated 194 breast cancer masses from 191 women. One patient had two multi-focal cancers 0.8 cm apart, and two patients had contralateral cancers.
Age
The mean age (± SD) of the women was 50.1 ± 10.6 years. Patients who had mixed invasive ductal and lobular carcinoma were significantly older than the patients with invasive ductal carcinoma (mean age, 56.8 ± 10.5 vs 48.8 ± 10.5, p = 0.003). There were no differences in mean age seen within the different hormonal receptor and histologic grade subgroups (ER negative, 50.7 ± 12.7 years vs ER positive, 49.7 ± 9.6, p = 0.588; PR negative, 49.6 ± 12.7 vs PR positive, 50.2 ± 9.5, p = 0.705; ERRB2 negative, 50.3 ± 0.8 vs ERRB2 positive, 48.9 ± 2.1, p = 0.479; triple negative, 51.6 ± 12.2 vs nontriple negative, 49.6 ± 10.2, p = 0.316; and grade 1, 51.0 ± 9.1 vs grade 2, 50.6 ± 10.2 vs grade 3, 49.4 ± 11.1, p = 0.688).
Histopathologic Characteristics
Invasive ductal carcinoma formed the largest histopathologic group (79.9%), followed by mixed invasive ductal and lobular carcinoma (10.8%) and invasive lobular carcinoma (9.3%). For hormonal receptor subtypes, most were ER positive (72.2%), PR positive (68.6%), or ERRB2 negative (79.4%); 18.6% were triple negative. Most of the invasive ductal carcinomas (60.0%) were of histologic grade 3, compared with 27.8% for invasive lobular carcinomas and 14.3% for mixed invasive ductal and lobular carcinomas (p < 0.001). A high number of ER-negative (94.4%), PR-negative (88.5%), ERRB2-positive (80.0%), and triple-negative (97.2%) cancers were of histologic grade 3 compared with ER-positive (35.7%), PR-positive (35.3%), ERRB2-negative (44.8%), and non-triple-negative (41.8%) cancers, respectively (p < 0.001 for all subgroups) (Table 1). For the patient with two multifocal tumors, both cancers were grade 3 invasive ductal carcinoma with ER-negative, PR-positive, and ER-RB2-positive receptor subtypes. Of the two patients with contralateral cancers, one had grade 2 invasive ductal carcinoma with ER-positive, PR-positive, and ERRB2-negative receptor subtypes, and the other had a grade 1 invasive ductal carcinoma with ER-positive, PR-positive, and ERRB2-negative receptor subtypes and a contralateral grade 1 invasive ductal carcinoma with ER-positive, PR-negative, and ERRB2-negative receptor subtypes.
TABLE 1.
Distribution of Histologic Grades and Sizes Within Different Histopathologic Diagnoses and Hormonal Receptor Subtypes
| Characteristic | Histologic Grade
|
p | Median Tumor Volume (cm3) | p | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
|
| ||||||
| Histopathologic diagnosis | < 0.001 | 0.283 | ||||
| Invasive ductal carcinoma (n = 155) | 13 (8.4) | 49 (31.6) | 93 (60.0) | 4.2 (1.5–13.4) | ||
| Invasive lobular carcinoma (n = 18) | 5 (27.8) | 8 (44.4) | 5 (27.8) | 2.3 (1.4–24.7) | ||
| Mixed invasive ductal and lobular carcinoma (n = 21) | 4 (19.0) | 14 (66.7) | 3 (14.3) | 2.5 (0.7–6.6) | ||
| Hormonal receptor subtypes | ||||||
| ER negative (n = 54) | 0 (0) | 3 (5.6) | 51 (94.4) | < 0.001 | 10.1 (2.6–23.0) | 0.001 |
| ER positive (n = 140) | 22 (15.7) | 68 (48.6) | 50 (35.7) | 3.3 ± 2.9 (1.1–10.1) | ||
| PR negative (n = 61) | 2 (3.3) | 5 (8.2) | 54 (88.5) | < 0.001 | 11.2 (2.5–17.9) | 0.001 |
| PR positive (n = 133) | 20 (15.0) | 66 (49.6) | 47 (35.3) | 3.2 (1.0–9.5) | ||
| ERRB2 negative (n = 154) | 20 (13.0) | 65 (42.2) | 69 (44.8) | < 0.001 | 3.1 (1.0–11.7) | 0.002 |
| ERRB2 positive (n = 40) | 2 (5.0) | 6 (15.0) | 32 (80.0) | 10.6 (3.3–24.1) | ||
| Triple negative (n = 36) | 0 (0) | 1 (2.8) | 35 (97.2) | < 0.001 | 7.7 (1.9–20.0) | 0.054 |
| Nontriple negative (n = 158) | 22 (13.9) | 70 (44.3) | 66 (41.8) | 3.4 (1.2–11.8) | ||
Note—Data in parentheses for histologic grade are percentages. Data in parentheses for tumor volume are interquartile range. ER = estrogen receptor, PR = progesterone receptor.
Size
There were no differences in median volume size among the different histopathologic groups of invasive ductal, invasive lobular, and mixed cancers (p = 0.283). ER-negative, PR-negative, and ERRB2-positive cancers were all found to be larger in size compared with their respective receptor counterparts (p ≤ 0.002 for all subgroups). Triple-negative cancers were also larger in size compared with nontriple-negative cancers, which was almost statistically significant (p = 0.054) (Table 1). Grade 3 cancers (9.5 cm3) were larger than grade 2 (2.5 cm3) and grade 1 (1.3 cm3) cancers (p < 0.001).
Peak Signal Intensity Percentage Increase
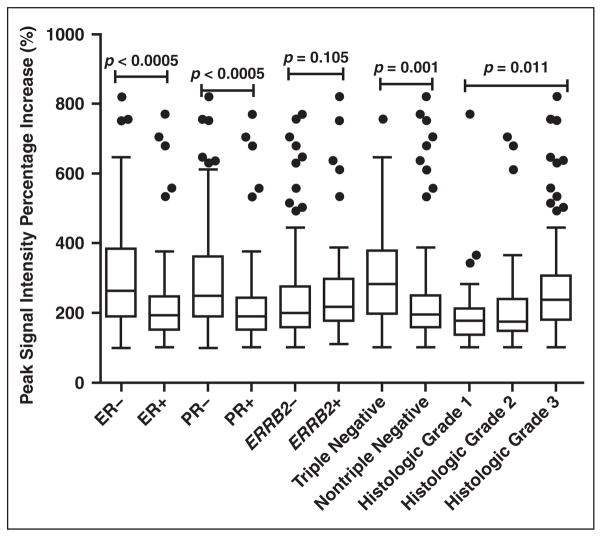
The means and medians of the peak signal intensity percentage increase obtained for all the tumors in the different subgroups are summarized in the box plots in Figure 3. The means of the percentage increase were higher with statistical significance in ER-negative (317 ± 178%), PR-negative (306 ± 172%), and triple-negative tumors (312 ± 155%) compared with the ER-positive (215 ± 106%, p < 0.001), PR-positive (215 ± 107%, p < 0.001), and nontriple-negative tumors (228 ± 128%, p = 0.001), respectively. There was no statistical significance seen between the means of the ERRB2-negative and ER-RB2-positive cancers (235 ± 128% vs 275 ± 166%, p = 0.105). The mean percentage increase for grade 3 tumors was also higher (272 ± 146%) with statistical significance compared with that of grade 1 (213 ± 142%) and grade 2 (213 ± 114%) tumors (p = 0.011).
Fig. 3.
Box and whisker plots show distribution of peak signal intensity percentage increase after contrast administration within various cancer receptor subgroups and histologic grades. Ends of boxes represent 25th and 75th percentiles and horizontal lines within boxes represent median. Whiskers below and above boxes represent minimum and maximum values that are not outliers. Small black circles represent outliers. Grade 2 tumors had significantly lower peak enhancement signal intensity compared with grade 3 by Bonferroni pairwise comparison test. ER = estrogen receptor, PR = progesterone receptor
Because the distribution curves for all the values were found to be skewed and not of normal distribution, the median percentage increase was also calculated for each subgroup and shown in Figure 3. The medians of every subgroup were found to arrive at similar statistical conclusions as the means.
Analysis Using the Most Suspicious MRI Kinetic Curve
Analysis using the most suspicious MRI kinetic curve revealed that 98.5% (191/194) of the tumors showed rapid enhancement as the most suspicious initial phase kinetic, whereas 93.3% (181/194) showed rapid washout as the most suspicious delayed phase kinetic. Of the tumors within the different hormonal receptor subgroups, 97.2–100% had a component of rapid initial enhancement, whereas 91.7–97.2% had a component of rapid washout; 99.0% of grade 3, 97.2% of grade 2, and 100% of grade 1 tumors showed rapid initial enhancement. Rapid washout was the most suspicious delayed phase kinetic in 97.0% of grade 3 cancers, 90.1% of grade 2 cancers, and 86.4% of grade 1 cancers. No statistical significance was seen for all the subgroups when compared against the most suspicious kinetic curves (Table 2).
TABLE 2.
Assessment on Basis of Most Suspicious Kinetic Pattern With Correlation to Hormonal Receptor Subtypes and Histologic Tumor Grades
| Characteristic | Number of Cases With Most Suspicious Rapid Enhancement Pattern (%)
|
p | Number of Cases With Most Suspicious Delayed Enhancement Pattern (%)
|
p | |||
|---|---|---|---|---|---|---|---|
| Rapid | Medium | Persistent | Plateau | Rapid | |||
|
| |||||||
| Hormonal receptor expression | |||||||
| ER negative (n = 54) | 53 (98.1) | 1 (1.9) | 0.83 | 1 (1.9) | 1 (1.9) | 52 (96.3) | 0.43 |
| ER positive (n = 140) | 138 (98.6) | 2 (1.4) | 2 (1.4) | 9 (6.4) | 129 (92.1) | ||
| PR negative (n = 61) | 60 (98.4) | 1 (1.6) | 0.94 | 1 (1.6) | 1 (1.6) | 59 (96.7) | 0.33 |
| PR positive (n = 133) | 131 (98.5) | 2 (1.5) | 2 (1.5) | 9 (6.8) | 122 (91.7) | ||
| ERRB2 negative (n = 154) | 151 (98.1) | 3 (1.9) | 0.37 | 3 (1.9) | 7 (4.5) | 144 (93.5) | 0.52 |
| ERRB2 positive (n = 40) | 40 (100.0) | 0 (0) | 0 (0) | 3 (7.5) | 37 (92.5) | ||
| Triple negative (n = 36) | 35 (97.2) | 1 (2.8) | 0.51 | 1 (2.8) | 0 (0) | 35 (97.2) | 0.25 |
| Nontriple negative (n = 158) | 156 (98.7) | 2 (1.3) | 2 (1.3) | 10 (6.3) | 146 (92.4) | ||
| Histologic grade | |||||||
| 1 (n = 22) | 22 (100.0) | 0 (0) | 0.52 | 1 (4.5) | 2 (9.1) | 19 (86.4) | 0.20 |
| 2 (n = 71) | 69 (97.2) | 2 (2.8) | 1 (1.4) | 6 (8.5) | 64 (90.1) | ||
| 3 (n = 101) | 100 (99.0) | 1 (1.0) | 1 (1.0) | 2 (2.0) | 98 (97.0) | ||
Note—ER = estrogen receptor, PR = progesterone receptor.
Analysis Using Volumetric Analysis of MRI Kinetic Curves
Overall, the tumors were mostly made up of tissue containing rapid initial enhancement and persistent delayed enhancement by percentage tumor volume. The mean percentage volumes of the early enhancement components of all tumors were 56.5% for rapid enhancement and 43.4% for medium enhancement (p < 0.001). The mean percentage tumor volumes for the different delayed enhancement components were 51.6% for persistent curve, 28.8% for plateau curve, and 19.9% for rapid washout (p < 0.001).
With regard to the initial enhancement curves seen within the different hormonal receptor subgroups, there was a higher mean percentage tumor volume containing the rapid early enhancement over the medium enhancement component seen in for ER-negative (64.1%), PR-negative (65.4%), and triple-negative tumors (65.3%) compared with ER-positive (53.6%, p = 0.013), PR-positive (52.5%, p = 0.001), and triple-negative (54.6%, p = 0.028) tumors. With higher histologic grades, there was a progressive trend for greater percentage tumor volumes of rapid early enhancement, but this did not reach statistical significance (p = 0.293).
For the delayed enhancement curve, there was a higher mean percentage volume of rapid washout component seen in the ERRB2-positive subgroup compared with ERRB2-negative (mean, 27.5% vs 17.9%, p = 0.020). There was otherwise no statistical significance seen in the mean percentage volumes of the persistent, plateau, and rapid type curves within the rest of the hormonal receptor and histologic grade subgroups (Table 3).
TABLE 3.
Comparison of Enhancement Patterns Measured by Percentage Tumor Volume Observed in Tumors of Different Receptors and Histologic Grades Between Categories of Each Receptor and Histologic Grade
| Characteristic | Early Enhancement Phase | Delayed Enhancement Phase | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Rapid Type | Medium Type | p | Persistent Type | p | Plateau Type | p | Rapid Washout | p | |
|
| |||||||||
| Hormonal receptor expression | |||||||||
| ER negative (n = 54) | 64.1 ± 27.2 (68.5) | 35.9 ± 27.2 (31.5) | 0.013 | 50.3 ± 22.2 (52.0) | 0.629 | 29.1 ± 11.1 (28.5) | 0.852 | 21.4 ± 17.3 (17.2) | 0.523 |
| ER positive (n = 140) | 53.6 ± 25.6 (57.0) | 46.4 ± 25.6 (43.0) | 52.2 ± 25.5 (52.5) | 28.7 ± 14.1 (29.0) | 19.3 ± 21.1 (11.5) | ||||
| PR negative (n = 61) | 65.4 ± 26.8 (70.0) | 34.5 ± 26.8 (30.0) | 0.001 | 49.4 ± 24.2 (52.0) | 0.393 | 28.0 ± 10.8 (27.0) | 0.580 | 28.0 ± 10.8 (27.0) | 0.539 |
| PR positive (n = 133) | 52.5 ± 25.3 (54.0) | 47.5 ± 25.3 (46.0) | 52.7 ± 24.8 (53.0) | 29.2 ± 14.3 (29.0) | 29.2 ± 14.3 (29.0) | ||||
| ERRB2 negative (n = 154) | 55.4 ± 26.3 (58.0) | 44.6 ± 26.3 (42.0) | 0.243 | 52.7 ± 24.4 (53.5) | 0.259 | 29.6 ± 14.0 (29.0) | 0.065 | 17.9 ± 18.8 (12.0) | 0.020 |
| ERRB2 positive (n = 40) | 60.9 ± 26.7 (66.5) | 39.1 ± 26.7 (33.5) | 47.7 ± 25.1 (48.5) | 26.0 ± 9.5 (25.9) | 27.5 ± 23.2 (21.6) | ||||
| Triple negative (n = 36) | 65.3 ± 27.0 (70.0) | 34.6 ± 27.0 (30.0) | 0.028 | 50.3 ± 22.2 (52.0) | 0.711 | 30.0 ± 11.6 (29.6) | 0.842 | 20.1 ± 16.7 (17.1) | 0.955 |
| Nontriple negative (n = 158) | 54.6 ± 25.9 (58.0) | 45.4 ± 26.0 (42.0) | 52.0 ± 25.2 (52.5) | 28.6 ± 13.7 (28.0) | 19.8 ± 20.8 (12.5) | ||||
| Histologic grade | |||||||||
| 1 (n = 22) | 51.6 ± 22.9 (55.0) | 48.4 ± 22.9 (45.0) | 0.293 (0.13)a | 54.7 ± 27.8 (56.5) | 0.331 | 27.7 ± 13.6 (29.5) | 0.793 | 18.4 ± 24.2 (5.0) | 0.296 |
| 2 (n = 71) | 54.1 ± 45.9 (53.0) | 45.9 ± 52.2 (47.0) | 54.3 ± 25.9 (55.0) | 28.3 ± 14.6 (28.0) | 17.3 ± 20.0 (8.1) | ||||
| 3 (n = 101) | 59.3 ± 27.8 (64.0) | 40.7 ± 27.9 (35.0) | 49.1 ± 22.9 (49.0) | 29.4 ± 12.3 (29.0) | 22.0 ± 19.1 (17.2) | ||||
Note—Except for p values, data are mean ± SD. ER = estrogen receptor, PR = progesterone receptor.
Test for correlation trend.
MRI Kinetics of Tumors Scanned at Different MRI Magnet Strengths
Of the 194 masses, 150 cases underwent imaging using 1.5 -T MRI magnets, and 44 cases were imaged using 3-T magnets. None were scanned in both field strengths.
The mean peak percentage signal intensity increase was 238.6 ± 123.8% (median, 200.0%) for tumors scanned at 1.5 T and 259.8 ± 176.9% (median, 210.0%) (p = 0.369) for tumors scanned at 3 T. The mean percentage volumes of the early enhancement components were 55.7 ± 25.0% rapid enhancement and 44.3 ± 24.0% medium enhancement for tumors scanned at 1.5 T and 59.3 ± 31.0% rapid enhancement and 40.6 ± 31.0% medium enhancement for tumors scanned at 3 T (p = 0.425). The mean percentage tumor volumes for the different delayed enhancement components were 52.4 ± 24.4% for persistent curve, 28.0 ± 12.7% for plateau curve, and 20.1 ± 19.6% for rapid curve for tumors scanned at 1.5 T versus 48.9% ± 25.2% (p = 0.403) for persistent curve, 31.8 ± 14.9% (p = 0.092) for plateau curve, and 19.2 ± 21.8% for rapid curve (p = 0.793) for tumors scanned at 3 T.
Discussion
The tumoral expressions of the ER, PR, and ERRB2 receptors, together with determination of the histologic grade, are now commonly performed in breast cancer tissue specimens for therapy planning and prognostication. Patients with positive expression of the hormonal receptors can benefit from targeted adjuvant or palliative hormonal or immunomodulating therapy. Although triple-negative cancers have the disadvantage of having fewer treatment options, they are also more aggressive, larger in size [6], and have a worse prognosis [7–10]. With regard to individual hormonal receptor subtypes, ER-negative, PR-negative, and ERRB2-positive cancers are known to be more aggressive and were shown to be of higher grade and larger size in this study. In the case of ERRB2 receptor expression, although ERRB2-negative cancers are less aggressive and have a more favorable prognosis than ERRB2-positive cancers, they do not have the benefit of available targeted immunomodulating therapies. The reported incidence of ER-negative breast tumors is approximately 25–30%, whereas triple-negative breast cancers account for approximately 10–16% of all breast cancers [11]. The incidence rates are similar to those found in our study. We used this available data on receptor expression and histologic grading as pathologic indicators for tumor aggressiveness.
The internal enhancement characteristics of the breast masses can now be automatically analyzed by computer-assisted detection software, providing information, such as size, peak percentage signal intensity increase, and kinetic curve patterns within the tumors. In addition, computer-assisted detection software is able to measure the percentage volume of each different kinetic curve pattern component within breast tumors. Some studies have found no correlation between the MRI enhancement kinetic curve patterns and the expression of the different hormonal receptors [12–14], but they mostly evaluated MRI kinetics in terms of the most suspicious kinetics pattern and included cases of ductal carcinoma in situ and tumors presenting as nonmasslike enhancement on MRI, for which evaluation of MRI kinetics would be unreliable [15, 16]. Unlike these studies, our study included analysis of the different kinetic components by volume and examined the enhancement characteristics of only invasive cancers presenting as masses and excluding ductal carcinoma in situ and cancers presenting with nonmasslike enhancement.
In the assessment of the peak signal intensity percentage increase, the higher overall peak signal intensity seen in ER-negative, PR-negative, triple-negative, and grade three tumors could be due to a combination of factors involving higher perfusion flow, higher microvessel density [17, 18], and greater vascular permeability [19]. In addition, ER-negative and triple-negative tumors are also known to be associated with high microvessel density [20] and higher vascular endothelium growth factor (VEGF) production [21]. VEGF production is important in the development of abnormal neovasculature and loss of blood flow regulation [22, 23] that cause increased vascular permeability in tumors. All these factors are associated with tumors of higher nuclear grade [24]. Thus, it was not surprising that our findings showed most of the ER-negative, PR-negative, and triple-negative cancers were also of high histologic grade.
In assessment of the kinetic patterns within the tumors, solely examining on the basis of the most suspicious kinetic pattern as opposed to volumetric analysis of the different kinetic components did not show any correlation with the biologic tumor indicators. As expected, most of the masses had at least some component of rapid enhancement as the most suspicious initial kinetic pattern and rapid washout as the most suspicious delayed enhancement curve. However, this would not indicate how much of the other kinetic patterns were present in the tumors, and the most suspicious kinetic pattern present might not necessarily be the most dominant type of kinetic pattern by volume. The different vascular properties and heterogeneous internal enhancement in different parts of the tumors would not be reflected in this assessment method, and the actual biologic and aggressive behavior of the tumors might not be fully appreciated.
In contrast, volumetric analysis of the different enhancement kinetic components yielded positive correlation with the tumor indicators. With regard to the early enhancement phase of the kinetic curve, ER-negative, PR-negative, and triple-negative tumors contained dominant volumes of rapid initial enhancement pattern. The rapid type of initial enhancement could be related to high vascular perfusion, increased arteriovenous shunting, and higher microvessel density [25]. It also appears to correlate with a higher histologic grade [26]. There was a progressive trend for greater tumor volume containing rapid early enhancement in tumors of higher histologic grades. It did not achieve statistical significance, but this trend could be related to a small sample size. Overall, our findings appear to show that the greater the tumoral composition of rapid initial enhancement and the greater the tumor neovascularization, the more biologically aggressive the cancers are.
Volumetric analysis of the delayed phase of the kinetic curve showed that ERRB2-positive tumors had a relatively higher rapid washout component than ERRB2-negative cancers. This would suggest that ERRB2-positive cancers had larger vessels that would give rise to a faster clearance of contrast material from its relatively smaller extravascular interstitial space. A larger vascular space would be in keeping with the cancer being more biologically aggressive [27]. However, apart from the ERRB2 receptors, the volumetric composition of the delayed phase kinetics did not show any correlation with the other hormonal receptor subtypes or the histologic grading. However, it was interesting to note that most of the cancers had a large dominant component of persistent type enhancement, and the reason for this is not immediately clear. These areas with a persistent type delayed enhancement may have cells that are less closely packed and have a larger extravascular interstitial volume, such as those found in intra-tumoral desmoplasia and fibrosis [28, 29]. Histopathologic correlation in future studies would be useful to determine whether a large part of an enhancing tumoral mass seen on MRI could be due to desmoplasia.
Clinical Implications
Computer-assisted detection postprocessing of contrast-enhanced dynamic MRI has the advantage of being easy to use as it has the ability to automatically generate kinetic information and volumetric analysis of tumor masses. The findings of this study show that computer-assisted detection–derived information may have clinical relevance in a few areas. Because the peak percentage signal intensity increase and amount of tumor volume with rapid early enhancement appear to correlate with the tumor biologic markers, such as the hormonal receptor status and histologic tumor grading, these different MRI parameters may be used to assess for tumor aggressiveness, metastatic risk [24, 30, 31], risk of disease recurrence, and survival. Heldahl et al. [31], for example, performed MRI volume analysis on breast cancer patients receiving neoadjuvant chemotherapy and found that patients with larger breast tumors or breast tumors with a greater volume of rapid washout type on the delayed enhancement curve on pretreatment breast MRI had significantly shorter survival. Thus, an MRI scoring system may be developed in a way similar to component scoring of the histologic categories of nuclear pleomorphism; the degree of tubule formation and mitotic rate of tumors are combined to give an overall histologic grade. Further research is required but volumetric analysis appears to have the potential to provide information on the biologic behavior of tumors.
The information obtained can also be used to determine the most suitable treatment of targeted therapy. For example, it can be used to predict which patients will better benefit from antiangiogenic agents, such as bevacizumab [32]. Another possible application is to provide a quantifiable method to assess early tumor response to therapy, both in the clinical and drug research setting. The response may be evaluated by measuring the change in the enhancement kinetic components. Traditional assessment with tumor size measurement alone may no longer be adequate because tumor size measurement is not sensitive to early therapy changes and assessment of residual recurrence and meta-static potential after therapy [33].
Study Limitations
The tumors were imaged on different MRI scanners with different magnet strengths. Hence, there were different scanning parameters, and even the signal intensity could be different for the same lesion scanned in a different scanner. The kinetic curves also may not be totally consistent across MRI systems [34]. However, there were no large differences with the components of the analyzed MRI kinetics of the tumors scanned between the 1.5-T and 3-T MRI magnet strengths, although the relatively small number of tumors in the 3-T group would limit any meaningful statistical comparison. The MRI parameters studied in this article were also based on relative ratios rather than absolute measurements. For example, the peak percentage of contrast-enhanced signal intensity increase was calculated relative to the baseline signal intensity, and the volumetric analysis of the MRI kinetics was based on the percentage tumor volume. It is thought that these relative ratios would be less affected by differences in MRI scanning parameters, especially because other factors, such as the rate of infusion of contrast material and volume of infused contrast material adjusted for patient weight were equal in the study.
This study also lacked histopathologic correlation for various prognostic markers, such as microvessel density, VEGF, and p53 and Ki-67 antibodies, and the tumors were not correlated for tumor histopathologic findings of necrosis and desmoplasia that may help explain the computer-assisted detection volumetric findings. There was also no follow-up and correlation with metastatic disease, tumor recurrence, and patient survival. Future studies will require clinical follow-up and histologic correlation to account for the MRI findings and substantiate the value of MRI in prognostication.
Finally, the use of computer-assisted detection software for volumetric assessment has to be validated and the interpretation standardized across all software vendors. Further studies are required to test the robustness of such software applications.
Conclusion
ER-negative, PR-negative, and triple-negative breast tumors showed a higher peak percentage signal intensity increase. Volumetric analysis of the different enhancement kinetic components also showed that tumors containing a higher volume of rapid type initial enhancement and rapid washout type delayed enhancement patterns were associated with cancers of more aggressive hormonal receptor subtypes. Because the overwhelming majority of tumors showed rapid initial enhancement and rapid washout as the most suspicious kinetic pattern, assessment based on the most suspicious kinetic pattern did not reveal any correlation with the tumor indicators examined. We conclude that computer-assisted detection–derived MRI kinetics data of peak percentage signal intensity increase and volumetric analysis of the different enhancement kinetic components have the potential to provide additional information on the biologic aggressiveness of an invasive cancer.
Footnotes
WEB
This is a web exclusive article.
This article is available for credit.
References
- 1.Matsubayashi R, Matsuo Y, Edakuni G, Satoh T, Tokunaga O, Kudo S. Breast masses with peripheral rim enhancement on dynamic contrast-enhanced MR images: correlation of MR findings with histologic features and expression of growth factors. Radiology. 2000;217:841–848. doi: 10.1148/radiology.217.3.r00dc07841. [DOI] [PubMed] [Google Scholar]
- 2.Leach MO. Application of magnetic resonance imaging to angiogenesis in breast cancer. Breast Cancer Res. 2001;3:22–27. doi: 10.1186/bcr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett T, Kobayashi H, Brechbiel M, Choyke PL. Macromolecular MRI contrast agents for imaging tumor angiogenesis. Eur J Radiol. 2006;60:353–366. doi: 10.1016/j.ejrad.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Gaustad JV, Brurberg KG, Simonsen TG, Mollatt CS, Rofstad EK. Tumor vascularity assessed by magnetic resonance imaging and intravital microscopy imaging. Neoplasia. 2008;10:354–362. doi: 10.1593/neo.08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-Guinea O, Andicoechea A, González LO, et al. Relationship between morphological features and kinetic patterns of enhancement of the dynamic breast magnetic resonance imaging and clinicopathological and biological factors in invasive breast cancer. BMC Cancer. 2010;10:8. doi: 10.1186/1471-2407-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JH, Agrawal G, Feig B, et al. Triple-negative breast cancer: MRI features in 29 patients. Ann Oncol. 2007;18:2042–2043. doi: 10.1093/annonc/mdm504. [DOI] [PubMed] [Google Scholar]
- 7.Yaghan R, Stanton PD, Robertson KW, Going JJ, Murray GD, McArdle CS. Oestrogen receptor status predicts local recurrence following breast conservation surgery for early breast cancer. Eur J Surg Oncol. 1998;24:424–426. doi: 10.1016/s0748-7983(98)92341-1. [DOI] [PubMed] [Google Scholar]
- 8.Chang J, Clark GM, Allred DC, Mohsin S, Chamness G, Elledge RM. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer. 2003;97:545–553. doi: 10.1002/cncr.11083. [DOI] [PubMed] [Google Scholar]
- 9.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2/NEU-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 10.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 11.Reis-Filho JS, Tutt AN. Triple negative tumors: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Cho N, Kim SJ, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol. 2008;9:10–18. doi: 10.3348/kjr.2008.9.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JH, Baek HM, Nalcioglu O, Su MY. Estrogen receptor and breast MR imaging features: a correlation study. J Magn Reson Imaging. 2008;27:825–833. doi: 10.1002/jmri.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montemurro F, Martincich L, Sarotto I. Relationship between DCE-MRI morphological and functional features and histopathological characteristics of breast cancer. Eur Radiol. 2007;17:1490–1497. doi: 10.1007/s00330-006-0505-x. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz AC, Smits ML, Veldhuis W, et al. Breast MR-imaging of ductal carcinoma in situ: a systematic review. Imaging Decisions MRI. 2009;13:112–121. [Google Scholar]
- 16.Viehweg P, Lampe D, Buchmann J, Heywang-Köbrunner SH. In situ and minimally invasive breast cancer: morphologic and kinetic features on contrast-enhanced MR imaging. MAGMA. 2000;11:129–137. doi: 10.1007/BF02678476. [DOI] [PubMed] [Google Scholar]
- 17.Stomper PC, Winston JS, Herman S, Klippenstein DL, Arredondo MA, Blumenson LE. Angiogenesis and dynamic MR imaging gadolinium enhancement of malignant and benign breast lesions. Breast Cancer Res Treat. 1997;45:39–46. doi: 10.1023/a:1005897227030. [DOI] [PubMed] [Google Scholar]
- 18.Tuncbilek N, Unlu E, Karakas HM, Cakir B, Ozyilmaz F. Evaluation of tumor angiogenesis with contrast-enhanced dynamic magnetic resonance mammography. Breast J. 2003;9:403–408. doi: 10.1046/j.1524-4741.2003.09508.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuhl C. Dynamic breast magnetic resonance imaging. In: Morris EA, Liberman L, editors. Breast MRI. New York, NY: Springer; 2005. pp. 79–139. [Google Scholar]
- 20.Koukourakis MI, Manolas C, Minopoulos G, Giatromanolaki A, Sivridis E. Angiogenesis relates to estrogen receptor negativity, c-erbB-2 overexpression, and early relapse in node-negative ductal carcinoma of the breast. Int J Surg Pathol. 2003;11:29–34. doi: 10.1177/106689690301100107. [DOI] [PubMed] [Google Scholar]
- 21.Fuckar D, Dekanić A, Stifter S, et al. VEGF expression is associated with negative estrogen receptor status in patients with breast cancer. Int J Surg Pathol. 2006;14:49–55. doi: 10.1177/106689690601400109. [DOI] [PubMed] [Google Scholar]
- 22.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 23.Raghunand N, Gatenby RA, Gillies RJ. Microenvironmental and cellular consequences of altered blood flow in tumors. Br J Radiol. 2003;76(spec no 1):S11–S22. doi: 10.1259/bjr/12913493. [DOI] [PubMed] [Google Scholar]
- 24.Choi WW, Lewis MM, Lawson D, et al. Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF-family gene expression. Mod Pathol. 2005;18:143–152. doi: 10.1038/modpathol.3800253. [DOI] [PubMed] [Google Scholar]
- 25.Buadu LD, Murakami J, Murayama S. Breast lesions: correlation of contrast medium enhancement patterns on MR images with histopathologic findings and tumor angiogenesis. Radiology. 1996;200:639–649. doi: 10.1148/radiology.200.3.8756909. [DOI] [PubMed] [Google Scholar]
- 26.Furuta A, Ishibashi T, Takahashi S, et al. Magnetic resonance imaging of breast cancer: correlation between contrast enhancement and tumor angiogenesis [in Japanese] Nippon Igaku Hoshasen Gakkai Zasshi. 1999;59:682–688. [PubMed] [Google Scholar]
- 27.Koh TS, Thng CH, Hartono S, et al. Dynamic contrast-enhanced MRI of neuroendocrine hepatic metastases: a feasibility study using a dual-input two-compartment model. Magn Reson Med. 2011;65:250–260. doi: 10.1002/mrm.22596. [DOI] [PubMed] [Google Scholar]
- 28.Gokalp G, Topal U, Yildirim N, Tolunay S. Malignant spiculated breast masses: dynamic contrast enhanced MR (DCE-MR) imaging enhancement characteristics and histopathological correlation. Eur J Radiol. 2012;81:203–208. doi: 10.1016/j.ejrad.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Onishi M, Furukawa A, Takahashi M, Murata K. A wide variety of dynamic contrast-enhanced MR appearances of breast cancer: pathologic correlation study. Eur J Radiol. 2008;65:286–292. doi: 10.1016/j.ejrad.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 31.Heldahl MG, Bathen TF, Rydland J, et al. Prognostic value of pretreatment dynamic contrast-enhanced MR imaging in breast cancer patients receiving neoadjuvant chemotherapy: overall survival predicted from combined time course and volume analysis. Acta Radiol. 2010;51:604–612. doi: 10.3109/02841851003782059. [DOI] [PubMed] [Google Scholar]
- 32.Mehta S, Hughes NP, Buffa FM, et al. Assessing early therapeutic response to bevacizumab in primary breast cancer using magnetic resonance imaging and gene expression profiles. J Natl Cancer Inst Monogr. 2011;2011:71–74. doi: 10.1093/jncimonographs/lgr027. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor JP, Jackson A, Parker GJ, Jayson GC. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer. 2007;96:189–195. doi: 10.1038/sj.bjc.6603515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jansen SA, Shimauchi A, Zak L, et al. Kinetic curves of malignant lesions are not consistent across MRI systems: need for improved standardization of breast dynamic contrast-enhanced MRI acquisition. AJR. 2009;193:832–839. doi: 10.2214/AJR.08.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]