Abstract
BACKGROUND
It remains unknown whether care of high-risk vascular patients with both diabetes and peripheral arterial disease is improving. We examined national trends in care of patients with both peripheral arterial disease and diabetes.
METHODS
A cohort of patients diagnosed with peripheral arterial disease and diabetes between 2007–2011 undergoing open or endovascular diagnostic or revascularization procedures was analyzed using Medicare claims data. Main outcome measure was amputation-free survival measured from time of initial revascularization procedure to 24 months, stratified by race and hospital referral region (HRR).
RESULTS
2.3 per 1,000 patients underwent a major amputation from 2007–2011, with the higher rate among black patients (5.5 per 1,000 versus 1.9 per 1,000, p<0.001) compared to non-black. The rate varied widely by HRR (1.2 per 1,000 to 6.2 per 1,000), with higher variation in amputation rates in black patients (2.1 to 16.1 per 1,000). Overall, amputation-free survival was approximately 74.6% at 2 years, 68.4% among black patients and 75.4% among non-black patients, with the disparity between the 2 groups increasing over time.
CONCLUSIONS
Prevalence of concurrent peripheral arterial disease and diabetes is increasing, but amputation rates and amputation-free survival vary significantly by both race and hospital referral region. Prevention and care coordination effort should aim to limit racial disparities in the treatment and outcomes of these high-risk patients.
INTRODUCTION
Multidisciplinary treatment of diabetes in recent years has been associated with a decline in the rates of several diabetes-related complications, including lower extremity amputation.1 Despite this trend, amputation remains common among diabetics, especially those with peripheral arterial disease (PAD).2,3 Along with increased amputation rates, diabetics with PAD have increased rates of re-amputation and death, as well as lower rates of functional independence.4–6 Vascular interventions are frequently used to treat and prevent limb loss in these patients. Several studies suggest that more procedural care, when combined with preventative measures is associated with lower amputation risk.7
However, vascular interventions and their success vary greatly across patients.8 Patient characteristics - such as diabetes, age, race, co-morbid conditions, and functional status -- affect both interventions and their outcome.9 Other non-medical factors - primarily socio-economic and geographic factors, such as race and where patients live – can have a dramatic impact as well on amputation rates,10–13 and that black patients with diabetes are at the highest risk for amputation.14 Within this context, we hypothesized that better care of diabetes may have exhibited differential effects, by race and region, for patients at risk for amputation from diabetes and PAD.
Therefore, using Medicare claims, we investigated the outcomes of vascular interventions in these high risk patients. We examined how these rates differed across race and varied geographically, as well as how the primary outcome of interest (amputation-free survival) varied along these parameters.
METHODS
We used the Medicare Physician and Supplier file and the Medicare Denominator file of the CMS 2007–2011 to identify all patients admitted with diagnoses of PAD and Diabetes during that time. The physician and supplier file contains all claims submitted by physicians for performance of procedures under the Medicare Part B program, including Current Procedural Terminology (CPT) codes, International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes, date of procedure, and age, sex, and race of the beneficiary.
The denominator file contains information about eligibility, including age, gender and race. We excluded patients with missing age, sex, or race variables, who were aged less than 65 or greater than 99 years of age, and who were not enrolled in fee-for-service (FFS) Medicare.
Establishing a Cohort of Patients with Peripheral Arterial Disease and Diabetes
We used the Medicare Physician/supplier file and the Medicare denominator file from the years 2007–2010 to identify patients with ICD-9 codes for diabetes with peripheral arterial disease (See Appendix 1a). We identified the index event using CPT codes for any endovascular or open revascularization procedure. Further information was obtained using the denominator file, which contains information about eligibility by year for part B and information about age, sex, and race of Medicare beneficiaries. We used patients zip code of residence to determine hospital referral region (HRR), as described by the Dartmouth Atlas of Health care.
APPENDIX 1.
Diagnostic and procedural codes used in the analysis
ICD-9 and CPT codes used in the analysis
| Diagnosis | Codes Used |
|---|---|
| Peripheral Arterial Disease | 429xx, 440–448xx, 451–454xx, 585, 709.8, 719.47, 727xx, 728xx, 730xx, 731xx, 733xx, 736xx, 821xx, 823xx, 824xx |
| Diabetes | ICD-9 diagnosis codes: 250xx, 357.2x, 362.0x, 366.41, 648.0x: DRG codes: 294, 295; for Q4, MS-DRG codes 637, 638, 639. |
| Amputation | CPT codes: 27590, 27591, 27592, 27880, 27881, 2788; ICD-9 procedure codes 84.15 to 84.17 |
| Vascular Intervention | 35521, 35302 , 35351, 35303, 35355 35304, 35361, 35305, 35363, 35306, 35537, 35371, 35538, 35372 35539 35533 35540 35556 35541 35558 35546, 35566, 35539, 35571, 35548 35583 35549 35585 35551, 35587 35563, 35656 35565 35666 35621 35671, 35623 35681, 35637 35682 35638 35683 35646 35879 35647 35881 35651 35883 35654 35884 35661 35663 35665 Outflow: 35452 35456 35454 35459 35472 35470 35473 35474 35481 35483 35482 35485 35491 35493 35492 35495 37205 37206 37207 37208 36200 36245 36246 36247 36248 |
Calculating amputation rates
First, we generated variables indicative of major amputation, at the patient level. We studied only the first amputation per patient in any individual year. In our report, we describe only above-ankle amputations, as these have the greatest effect on functional status in patients with diabetes and PAD.
Population-based, HRR-level amputation rates were calculated. We used the patients with diabetes and peripheral arterial disease undergoing amputation as the numerator and the total number of patients with diabetes and PAD within the hospital referral region during that year as the denominator.
Calculating Amputation-free survival
In patients with peripheral arterial disease and diabetes who underwent a vascular intervention from 2007–2010, we determined amputation-free survival time. For this analysis, we excluded patients in 2011 to allow at least one year of follow-up study. Amputation-free survival was defined as time from the index procedure to the first major amputation. Patients were followed for a maximum of 24 months following their index procedure. Groups were stratified into endovascular and open procedures and amputation-free survival at 2 years was calculated using Kaplan-Meier methodology.
RESULTS
Annual prevalence of diabetes and PAD among Medicare patients, by race
In each year between 2007–2011, approximately 3 million Medicare patients have had a hospitalization related to diabetes and PAD, and this number has increased over time (Table 1). While in 2007 there were 2.9 million Medicare patients with both diabetes and PAD, the number of patients increased by 15% by 2011, reaching nearly 3.5 million patients in 2011. The increase in patients with diabetes and PAD occurred in both black and white patients, although the diagnosis grew at a faster rate in black patients than non-black patients (19% versus 15%, p<0.001). The mean age of patients in the cohort was 76 years of age. The proportion of patients on Medicaid during the study period was 20%, and rates of dementia (5%), congestive heart failure (17%), cerebrovascular disease (9%) and chronic renal failure (15%) were all stable during the study period. We did not adjust for changes in Medicare enrollment over this time period, which has remained stable among our population of interest. The total number of older patients enrolled in fee-for-service Medicare increased 1.6% during this time period (29.2 to 29.6 million).15
TABLE 1.
Race characteristics of Medicare beneficiaries with diabetes and peripheral arterial disease: 2007–2011
| Year | 2007 | 2008 | 2009 | 2010 | 2011 | Total |
|---|---|---|---|---|---|---|
| Total Number | 2970448 | 3058067 | 3185860 | 3308308 | 3415080 | 15937763 |
| Race | ||||||
| Non-black (n) | 2629669 | 2708518 | 2814562 | 2917395 | 3006558 | 14076702 |
| 88.5% | 88.6% | 88.3% | 88.2% | 88.0% | ||
| Black (n) | 340779 | 349549 | 371298 | 390913 | 408522 | 1861061 |
| 11.5% | 11.4% | 11.7% | 11.8% | 12.0% | ||
Annual prevalence of vascular procedures among patients with diabetes and PAD, by race
Each year, approximately 2.5% of the nearly 3 million Medicare patients with diabetes and PAD underwent a vascular procedure (Table 2). For example, in 2007, 77,994 patients had a vascular procedure – either a diagnostic endovascular procedure such as an angiogram, a therapeutic endovascular procedure such as angioplasty or stenting, or surgical bypass. While the absolute number of patients treated with an invasive vascular procedure increased each year through 2011 for both nonblack and black patients, the relative proportion of patients treated with invasive treatments actually declined in both populations (15% decline in nonblack patients, 5% decline in black patients). Among black patients, the use of therapeutic endovascular procedures increased 13% during the study period, while those among nonblack patients declined 5%. , a difference that was significant (13% vs −5%, p<0.0001).
TABLE 2.
Open and endovascular procedures among diabetics with peripheral arterial disease, by race 2007–2011
| Procedure | 2007 | 2008 | 2009 | 2010 | 2011 | Total | Change over time (relative rate) | Absolute Change (%) |
|---|---|---|---|---|---|---|---|---|
| Any procedure (n) | 77994 | 75914 | 76495 | 76865 | 77154 | 384422 | −14.1% | |
| Non-Black (%) | 2.54 | 2.39 | 2.3 | 2.21 | 2.14 | 2.31 | 0.84 | (0.81–0.88) |
| Black (%) | 3.28 | 3.23 | 3.19 | 3.18 | 3.14 | 3.20 | 0.96 | (0.95–0.97) |
| Open surgery (n) | 14351 | 13438 | 12891 | 12564 | 12355 | 65599 | −25% | |
| Non-Black (%) | 0.47 | 0.42 | 0.39 | 0.37 | 0.35 | 0 | 0.75(0.69–0.80) | |
| Black (%) | 0.60 | 0.58 | 0.51 | 0.47 | 0.44 | 0.52 | 0.73 | (0.67–0.80) |
| Endovascular therapeutic (n) | 42879 | 42770 | 44535 | 46125 | 47747 | 224056 | −2.8% | |
| Non-Black (%) | 1.40 | 1.34 | 1.32 | 1.30 | 1.31 | 1.33 | 0.94 | (0.92–0.95) |
| Black (%) | 1.81 | 1.89 | 1.99 | 2.07 | 2.05 | 1.97 | 1.13 | (1.10–1.17) |
| Diagnostic endovascular (n) | 68485 | 66365 | 66425 | 66282 | 45217 | 312774 | −42.8% | |
| Non-Black (%) | 2.25 | 2.11 | 2.02 | 1.93 | 1.27 | 1.90 | 0.56 | (0.46–0.66) |
| Black (%) | 2.74 | 2.64 | 2.56 | 2.52 | 1.70 | 2.41 | 0.62 | (0.53–0.71) |
Amputation-free survival after revascularization: effect of procedure type, race, and region
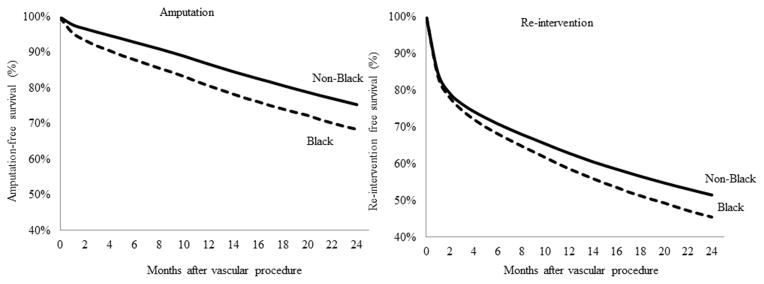
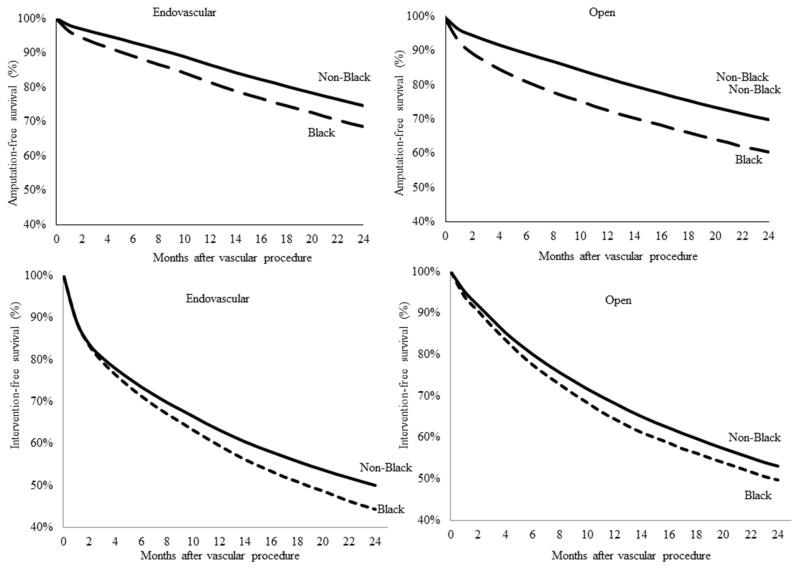
For patients with the most severe vascular disease – those who required vascular procedures – we examined differences in amputation –free survival by race, procedure type, and hospital referral region. Overall, amputation-free survival was approximately 74.6% at 2 years, but results were significantly poorer in black patients when compared non-black patients. Figure 2 demonstrates that 31.6% of black patients had undergone a major amputation, compared to 24.6% of nonblack patients at 2 years. Though the amputation-free survival for black patients was always worse than that for non-black patients, the gap between black and non-black patients who underwent amputation was larger at the end of the follow-up period that at the beginning. When we stratified these results by the type of vascular procedure – open or endovascular – our results were similar, in demonstrating poorer outcomes in black patients.
FIGURE 2.
Amputation and re-intervention-free survival after vascular procedure, by race
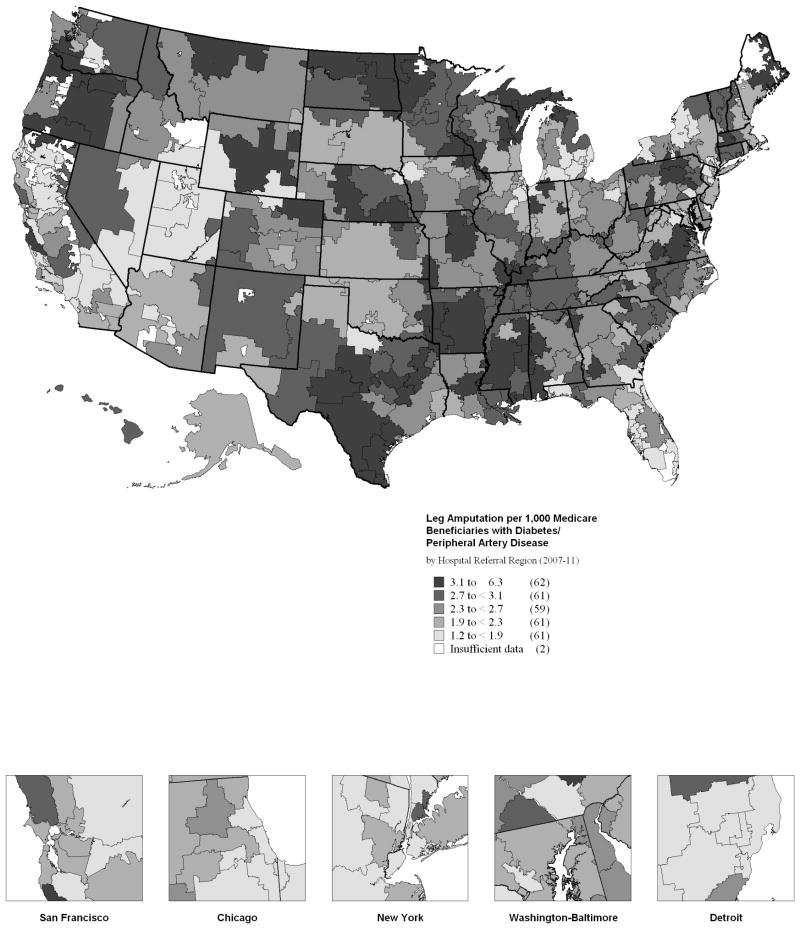
Next, we examined differences in amputation-free survival across the different hospital referral regions as described in the Dartmouth Atlas of Healthcare. Average amputation rate was 2.4/1000, but this rate varied by HRR from a nadir of 1.3 (Royal Oaks, MI) to a maximum of 6.2 (Tupelo, MS). This map revealed some clustering of amputation in the southeastern United States but rates varied even within geographic regions, with surprisingly high rates of amputation in the northern Midwest and Oregon. We examined outcomes by race herein as well, and found disparities even within HRR among the races. For example, among black patients, amputation-free survival at 2 years varies from 53.7 % in Savannah, GA to 76.7% in Gary, IN. Among nonblack patients, amputation-free survival varied from 64.9% in Appleton, WI to 83% (Yakima, WA). The list of highest and lowest amputation rates at 2 years was notably different for the two races, suggesting gross racial disparity in outcomes even within high risk regions.
Finally, amputation free survival was not the only outcome to demonstrate these significant racially-based disparities in care. Re-intervention-free survival also demonstrated significantly poorer results by race, both overall and by procedure type (Figure 3). Given our use of claims data, we are unable to distinguish laterality, and the term re-intervention to mean a repeat intervention on the same patient, not the same limb. Portions of these figures were reproduced from the Dartmouth Atlas of Healthcare.16
FIGURE 3.
Amputation and re-intervention-free survival after vascular procedure, by procedure type and race
DISCUSSION
Our study found that patients with diabetes and peripheral arterial disease have poor amputation free survival overall, but that their survival varies across both race and region. While overall prevalence of peripheral arterial disease and diabetes increased over this time period, so too did the differences in amputation free survival between black and non-black patients, even after attempts at vascular interventions. By studying differences across regions, we were able to demonstrate that variation in vascular care is not always driven by, or a result of, differences in amputation risk.
In the context of improved diabetes care overall, this study suggests that many areas of the country have been left out of this improvement in care. For example, in recent analyses as well as a report published by the Dartmouth Atlas of Healthcare, we have shown that only 30% of patients at high risk of amputation receive Hemoglobin A1C testing, and fewer than 5% receive all three preventative measures recommended by HEDIS and American Diabetes Association (ADA) guidelines.7,12 This echoes earlier work that has shown similar levels of variation in regional diabetes care. 10,12,17 In this current effort, when looking at national rates of open and endovascular interventions, we further describe differences between black and non-black patients, even within these geographic regions. These disparities appear to be independent of treatment type. Black patients are less likely to receive revascularization than non-black patients, but even those patients who receive revascularization have worse amputation-free survival than non-black patients. Our findings echo recent work by Holman et al, who found that black and white Medicare patients with PAD receive significantly fewer interventions in the years leading up to amputation.18
If changing the type of vascular intervention is not likely to dramatically impact racial disparities in vascular care, then what should be the next step forward in trying to limit amputation for high-risk racial subgroups? We hypothesize that the solution may rest in care coordination—integrating proven prevention strategies (eg smoking cessation), comprehensive diabetes management (targeting hemoglobin A1c levels), and targeted vascular care. Certainly the extent of variation seen in both amputation and amputation-free survival in our current work implies that many opportunities to improve care exist, and starting with the least expensive, most evidence-based approaches may be the best, “first” pathway forward.
This study is limited by several factors, most notably its observational and retrospective nature. We did not compare these patients directly to the population with diabetes alone in the course of the analysis, as there have already been a great deal of studies on amputation rates among diabetics. Nonetheless even without direct statistical comparison, amputation-free survival among higher risk patients remains notably worse among this population. As only patients who underwent vascular interventions were studied in the survival analysis, we do not know what steps—if any—are associated with avoiding surgical intervention entirely. Our use of claims data precludes our ability to distinguish laterality of interventions or amputations, clinical details (such as the severity of either PAD or Diabetes) and our ability to definitely tie vascular intervention to amputation prevention. However, regular vascular care should reduce any amputation risk within a high risk patient. Furthermore, while we included Hispanic patients within our analysis, we did not specifically study outcomes among patients of Hispanic ethnicity. Future work on racial and ethnic disparities should distinguish Hispanic patients, who have been shown to have worse outcomes of vascular disease.19
Our study suggests that patients with both diabetes and peripheral arterial disease are at risk for amputation nationwide, and the rate of these amputations has not notably changed over the past 5 years. Furthermore, we identified several areas of variation in surgical interventions, and amputation rates on a regional level that contribute to notable disparities in amputation-free survival. Future efforts to limit racial disparities in the treatment and outcomes of patients with diabetes and peripheral arterial disease have the potential to improve care, and low-cost, evidence-based interventions focusing on care coordination may be a logical and effective pathway forward.
FIGURE 1. Regional variation in amputation rate among patients with diabetes and peripheral artery disease.
Leg amputation by Medicare beneficiaries with Diabetes and Peripheral Arterial Disease (per 1,000 beneficiaries), by Hospital Referral Region
Acknowledgments
This research was funded by the Department of Surgery at Dartmouth Hitchcock Medical Center, The Robert Wood Johnson Foundation, and K08 funding (NHLBI K08HL05676, Philip Goodney, PI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Karina Newhall, Email: Karina.A.Newhall@Hitchcock.org, Department of General Surgery, Dartmouth Hitchcock Medical Center, One Medical Center Drive, Lebanon, NH 03756., Tel 603-650-7692 Fax: 603-650-8030. VA Outcomes Group, White River Junction, VT.
Emily Spangler, Section of Vascular Surgery, Dartmouth Hitchcock Medical Center, Lebanon, NH.
Nino Dzebisashvili, Department of General Surgery, Dartmouth Hitchcock Medical Center, Lebanon, NH. Current: Carolinas Health System, Durham, NC.
David C. Goodman, The Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH. Geisel School of Medicine, Hanover, NH.
Philip Goodney, Section of Vascular Surgery, Dartmouth-Hitchcock Medical Center, Lebanon, NH, VA. Outcomes Group, White River Junction, VT. The Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH.
References
- 1.Gregg EW, Li YF, Wang J, et al. Changes in Diabetes-Related Complications in the United States, 1990–2010. New England Journal of Medicine. 2014;370:1514–23. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 2.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. Journal of the American College of Cardiology. 2006;47:921–9. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 3.Brownrigg JR, Apelqvist J, Bakker K, Schaper NC, Hinchliffe RJ. Evidence-based management of PAD & the diabetic foot. Eur J Vasc Endovasc Surg. 2013;45:673–81. doi: 10.1016/j.ejvs.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Kono Y, Muder RR. Identifying the incidence of and risk factors for reamputation among patients who underwent foot amputation. Ann Vasc Surg. 2012;26:1120–6. doi: 10.1016/j.avsg.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Mueller T, Hinterreiter F, Luft C, Poelz W, Haltmayer M, Dieplinger B. Mortality rates and mortality predictors in patients with symptomatic peripheral artery disease stratified according to age and diabetes. J Vasc Surg. 2014;59:1291–9. doi: 10.1016/j.jvs.2013.11.063. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SM, Kalbaugh CA, Blackhurst DW, et al. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg. 2006;44:747–55. doi: 10.1016/j.jvs.2006.06.015. discussion 55–6. [DOI] [PubMed] [Google Scholar]
- 7.Goodney PP, Holman K, Henke PK, et al. Regional intensity of vascular care and lower extremity amputation rates. J Vasc Surg. 2013;57:1471–79. 80.e1–3. doi: 10.1016/j.jvs.2012.11.068. discussion 9–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodney PP, Travis LL, Nallamothu BK, et al. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes. 2012;5:94–102. doi: 10.1161/CIRCOUTCOMES.111.962233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karam J, Shepard A, Rubinfeld I. Predictors of operative mortality following major lower extremity amputations using the National Surgical Quality Improvement Program public use data. Journal of Vascular Surgery. 2013;58:1276–82. doi: 10.1016/j.jvs.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Jones WS, Patel MR, Dai D, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. Journal of the American College of Cardiology. 2012;60:2230–6. doi: 10.1016/j.jacc.2012.08.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols GA, Schroeder EB, Karter AJ, et al. Trends in Diabetes Incidence Among 7 Million Insured Adults, 2006–2011: The SUPREME-DM Project. American journal of epidemiology. 2015;181:32–9. doi: 10.1093/aje/kwu255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arday DR, Fleming BB, Keller DK, et al. Variation in diabetes care among states: do patient characteristics matter? Diabetes care. 2002;25:2230–7. doi: 10.2337/diacare.25.12.2230. [DOI] [PubMed] [Google Scholar]
- 13.Tunis SR, Bass EB, Klag MJ, Steinberg EP. Variation in utilization of procedures for treatment of peripheral arterial disease. A look at patient characteristics. Archives of internal medicine. 1993;153:991–8. [PubMed] [Google Scholar]
- 14.Jones WS, Patel MR, Dai D, et al. Temporal Trends and Geographic Variation of Lower Extremity Amputation in Patients with Peripheral Artery Disease: Results from U.S. Medicare 2000–2008. Journal of the American College of Cardiology. 2012;60:2230–6. doi: 10.1016/j.jacc.2012.08.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2008–2012 at http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareMedicaidStatSupp/index.html.
- 16.Dartmouth Atlas of Health care. 2014. Variation in the Care of Surgical Conditions: Diabetes and Peripheral Arterial Disease. [PubMed] [Google Scholar]
- 17.Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower-extremity major amputation in individuals with and without diabetes in the Medicare population. Diabetes care. 2001;24:860–4. doi: 10.2337/diacare.24.5.860. [DOI] [PubMed] [Google Scholar]
- 18.Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. Journal of vascular surgery. 2011;54:420–6. 6.e1. doi: 10.1016/j.jvs.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrissey NJ, Giacovelli J, Egorova N, et al. Disparities in the treatment and outcomes of vascular disease in Hispanic patients. Journal of vascular surgery. 2007;46:971–8. doi: 10.1016/j.jvs.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]