Abstract
Objective
Perceived stress; emotional eating; anhedonia; depression and dietary restraint, hunger, and disinhibition have been studied as risk factors for obesity. However, the majority of studies have been cross-sectional and the directionality of these relationships remains unclear. In this longitudinal study, we assess their impact on future weight change.
Methods
Psychological predictors of weight change in short- (6 month) and long-term (>1 year) periods were studied in 65 lean and obese individuals in two cohorts. Subjects participated in studies of food intake and metabolism that did not include any type of medication or weight loss interventions. They completed psychological questionnaires at baseline and weight change was monitored at follow-up visits.
Results
At six months, perceived stress predicted weight gain (r2 = 0.23, P = 0.02). There was a significant interaction (r2 = .38, P=0.009) between perceived stress and positive emotional eating, such that higher scores in both predicted greater weight gain, while those with low stress but high emotional eating scores lost weight. For long-term, higher anhedonia scores predicted weight gain (r2 = 0.24, P=0.04). Depression moderated these effects such that higher scores in both predicted weight gain but higher depression and lower anhedonia scores predicted weight loss.
Conclusion
There are different behavioral determinants for short- and long-term weight change. Targeting perceived stress may help with short-term weight loss while depression and anhedonia may be better targets for long-term weight regulation.
Keywords: depression, mood, emotional eating, longitudinal, weight gain
1.1 Introduction
Research into the causes of and risk factors for obesity has been pursued broadly, including genetic, environmental, lifestyle and psychosocial causes (1). Emotional states can influence eating behavior, which in turn, can result in weight change over time (2). Some of the most commonly studied themes are the cross sectional relationships between stress (5–6), anhedonia (7–8), emotional eating (9–10), depression (11–12), restrained eating (13) and adiposity, while only a few have examined these as predictors of weight change. Several studies report a positive correlation between these emotions and unhealthy behaviors, such as quitting a weight loss program or decreased cardiorespiratory fitness (6–7, 9). A recent meta-analysis of longitudinal studies reported a weak relationship between perceived stress and adiposity during long-term follow up (14), yet other studies found higher levels of perceived stress were associated with lower levels of eating awareness and physical activity, as well as higher consumption of fast foods (18). In other studies, elevated levels of anhedonia were associated with an increased risk of quitting a weight loss program and lower fitness levels in obese participants (7–8). Additionally, studies have shown that emotional eating in response to both positive and negative moods predicted overeating and weight gain (9–10).
Only a small number of studies have extended the relationship between these factors and adiposity to examine mediating effects these factors may have on one another in relation to weight gain in a real-world setting. For instance, restrained and emotional eating have been shown to mediate the effects of stress, such that restrained or emotional eaters may become more hyperphagic in response to stress (19–20). In a recent study of college freshman, this main effect was qualified by an interaction between stress and BMI: students who entered university with high levels of stress gained weight if they also had high BMIs; if they had lower BMIs then they lost weight (21). Anhedonia has been shown to increase in response to chronic stress in rat models (22), although food intake response is not as well studied, particularly in humans.
Behavioral attitudes toward food intake are known contributors to successful weight maintenance. Validated across gender, age, and BMI (23), the Three Factor Eating Questionnaire (TFEQ) is an effective indicator of dietary behaviors. The three factors (restraint, disinhibition, and hunger) of the TFEQ have all been positively correlated with BMI (23). Cognitive restraint has been validated as an indicator of dietary restraint (40). A longitudinal weight loss study demonstrated that those with high restraint are most successful at maintaining weight loss if they also have low disinhibition (24). However, if restraint is disrupted by stress, exposure to palatable foods, or the perception of failure to maintain dietary restrictions, disinhibition and subsequent overeating may occur (25–29). Individuals who maintain weight loss not only score higher on measures of dietary restraint but also demonstrate increased neural activity in regions responsible for executive function (30). In support of this notion, we recently found an interaction between perseveration and restraint was observed on 24 h food intake such that subjects with high perseveration and high restraint ate the least, whereas unrestrained subjects with high perseveration ate the most.
Studies have shown that weight is gradually regained 6 months after weight loss efforts, owing to decreases in time and effort spent on weight control, perceived inadequate long-term rewards for weight control behaviors, as well as differences in eating habit behaviors in short- and long-term time periods (31–33). In addition, other efforts have been made to assess differences that lead to weight loss versus long-term weight maintenance (34). Therefore, it is plausible that there may also be different behavioral contributors to short-term versus long-term weight change but this hypothesis has not yet been fully explored. We hypothesized that psychological constructs including perceived stress; positive and negative emotional eating; anhedonia; depression; and dietary restraint, hunger and disinhibition would be related to either short-term (6 month) or long-term (greater than 1 year) weight changes and that interactions between constructs may exist. We further hypothesized that associated constructs would likely differ between short-term and long-term weight changes.
1.2 Methods
1.2.1 Study Design
Sixty-five non-diabetic, healthy volunteers were recruited from the Phoenix area by means of newspaper advertisements and flyers to participate in one of two inpatient studies (NCT00523627; NCT00342732). Both were observational studies of the effects of overconsumption and different diets on energy expenditure, as well as exploring food intake preference as risk factors for obesity. Neither study included any type of medication or weight loss intervention. Baseline measures were collected on the Clinical Research Unit of the National Institute of Diabetes and Digestive and Kidney Diseases – Phoenix (NIDDK). Inclusion criteria for all studies consisted of healthy adults, between the ages of 18–55, with no evidence of illness by history, physical or basic laboratory measures. No subjects were taking medication. Exclusion criteria included evidence of substance abuse (positive urine test), nicotine use, or reported excess alcohol use (>3 drinks/day). Prior to participation, all subjects were informed of the nature, purpose and risks of the study they participated in and written informed consent was obtained. The experimental protocols were approved by the Institutional Review Board of the NIDDK.
The first week of both studies was identical. The decision to combine data from these studies to assess the impact of psychosocial measures on body weight was pre-planned as these studies were all relatively small. Upon admission, subjects were given a standard weight maintaining diet (20%, 30%, and 50% of daily calories provided as protein, fat and carbohydrate, respectively) for the first 3 days. Weight maintaining energy needs were calculated for each subject based on weight, gender and BMI as previously described (35). Body composition was determined by dual-energy x-ray absorptiometry (LUNAR Prodigy, GE). Within the first 2 days after admission, subjects completed a variety of self-report psychological questionnaires that were subsequently scored by a trained staff member. After 3 days of the weight maintaining diet, a 75g oral glucose tolerance test was done to exclude individuals with diabetes mellitus (36).
1.2.2 Psychological Questionnaires
Participants completed 5 questionnaires:
Physical Anhedonia Scale (PAS) (37): assesses the capability to experience pleasure from typically pleasurable physical stimuli and the extent individuals are motivated to engage in these stimuli. This questionnaire consists of 61 True or False statements, with each anhedonic response given a score of 1. Higher scores indicate an increasing presence of anhedonic symptoms. The Cronbach α for this measure is 0.82 for males and 0.78 for females.
Perceived Stress Scale (PSS) (38): assesses different facets related to stress such as unpredictability, lack of control, burden overload, and stressful life circumstances in the last month. This survey consists of 14 questions with responses scored on a 0–5 Likert scale (“never” to “very often”) and scores range from 0–56, with higher scores indicating a higher degree of perceived stress. The Cronbach α for this measure is 0.85.
Emotional Appetite Questionnaire (EMAQ) (39): measures eating responses to various positive and negative emotions and situations. Subjects answer their eating response to 22 emotions or situations on a Likert scale from 1–9. Example emotions include boredom, anxiety, frustration, happiness; examples of situations include after an argument and after receiving good news. Choices 1–4 coincide with “eating much less”, 5 with “the same” and 6–9 with “eating much more.” Scores can then be separated into eating in response to positive emotions/situations versus eating in response to negative emotions/situations. The Cronbach α for positive emotions/situations is 0.78 and 0.75 for negative emotions/situations.
Inventory for Depressive Symptomatology (IDS) (40): assesses signs and symptoms of depression. It consists of 30 questions that measure the degree of depression on a scaled score from 0–3, where 0 indicates least severe and 3 as most severe. Scores range from 0–90 and scores below 14 indicate no evidence of depression. The Cronbach α for this measure is 0.85.
Three-Factor Eating Questionnaire (TFEQ) (41): has 3 subscales assessing cognitive restraint, disinhibition and hunger. Restraint refers to control of eating behavior, disinhibition measures the degree to which individuals have an uncontrolled response to food and hunger measures an individual’s inclination to eat in response to subjective feelings of hunger. Each subscale has a number of “true/false” or multiple-choice questions. Higher scores indicate greater disruptions in eating behavior. The Cronbach α for this measure ranged from 0.82–0.90 for each subscale.
1.2.3 Follow-up Visits
Subjects in these studies returned for reassessment of body weight. This was done either as scheduled visits requested at six months and one year, and annually up to 5 years in one study. Participants were not prescribed a weight loss intervention during this time nor were they monitored between follow-up visits. Data from return visits to re-enroll into other studies on our research unit with assessment of body composition measures were also used (n = 27). Short-term follow-up was defined as a return visit within 6 to 9 months of baseline (n = 49) whereas long-term follow-up was considered a return visit of at least 1 year after the baseline measures (n = 49).
The sample size for both the short- and long-term analyses was 49 subjects each: 33 subjects had follow-up visits for both short- and long-term analysis; 16 only had short-term follow-up data and 16 had only long-term follow-up data (total n = 65). In a separate analysis of those individuals who had short- and long-term follow-up, analysis was done both on the whole group (n = 49 per group) and only on those who had both short- and long-term follow-up (n = 33) and results were similar. Therefore we chose to present analyses for all those with short-term (n = 49) and all those with long-term (n = 49) follow-up data.
1.2.4 Statistical Analysis
Alpha was set to 0.05. Because our study was exploratory in nature, many of the analyses addressed related questions, and each analysis was of independent interest, we did not adjust for multiple comparisons on a study-wide level. Variables are expressed as mean ± standard deviation as all were normally distributed. Two-tailed t-tests were utilized to assess differences in baseline characteristics between males and females and the short- and long-term groups. Pearson’s correlations were used to describe relationships between each psychological measure and weight change. Significant correlations were investigated using multivariate linear regression, controlling for baseline weight, age, gender and race. Interactions between psychological factors were tested in all models. Because anhedonia is often a component of depression, we assessed for multi-collinearity in our model using variance inflation factors (VIF = 1.03), which confirmed that the PAS was addressing a different psychological construct than the IDS. If an interaction was significant and improved the fit of the model, it was included in the final model. In order to better understand and describe significant interactions, each variable involved was divided into upper and lower halves (separated at the median) to determine categorical differences in weight change. All statistical analyses were done using SAS (Version 9.3) and SAS Enterprise guide (Version 5.1).
1.3 Results
Baseline characteristics of the short- and long-term cohorts are shown in Table 1. Based on established BMI criteria, 19 subjects (29%) were lean and the remainder categorized as overweight/obese. For the short-term analysis, mean follow-up time was 7 ± 1 months (range = 5 to 9 months; n = 49) and the change in weight at follow-up was 1 ± 5 kg (range = −9 kg to 11 kg). For the long-term analysis, the mean follow-up time was 2 ± 2 years (range = 1 year to 5 years; n = 49) and the change in weight at follow-up was 1 ± 5 kg (range = −9 kg to 14 kg). Twenty-two subjects had follow-ups at 1 year, 13 subjects had follow-up at 2 years, 8 subjects had follow-ups at 3 years, 5 subjects had follow-up at 4 years and 1 subject had a 5 year follow-up. Consistent with a physically and psychologically healthy cohort, the scores of psychological measures were not within clinical range. There were no differences in baseline characteristics or psychological measures between our short-term and long-term cohorts. Expected gender differences in body fat percentage were present within each group. BMI was positively correlated with anhedonia (r = 0.30, P = 0.03) and perceived stress (r= 0.29, P = 0.05)
Table 1.
Mean Baseline Characteristics and Psychological Measures
| Variable | Short-Term Follow-Up | Long-Term Follow-Up | ||||
|---|---|---|---|---|---|---|
| (n=49) | (n = 49) | |||||
| All Subjects (n=49) |
Male (n=32) | Female (n=17) |
All Subjects (n=49) |
Male (n=38) |
Female (n=11) |
|
| Race | ||||||
| African American | 9 (18%) | 5 (15%) | 4 (24%) | 7 (14%) | 5 (13%) | 2 (18%) |
| Caucasian | 18 (37%) | 12 (38%) | 6 (35%) | 16 (33%) | 13 (34%) | 3 (27%) |
| Hispanic | 7 (14%) | 6 (19%) | 1 (6%) | 7 (14%) | 7 (19%) | 0 (0%) |
| Native American | 15 (31%) | 9 (28%) | 6 (35%) | 19 (39%) | 13 (34%) | 6 (55%) |
| Age, years | 37±10 | 37 ± 9 | 36±11 | 38±9 | 38±8 | 37±12 |
| Baseline Weight, kg | 83±17 | 85±15 | 80±19 | 86±16 | 86±15 | 84±19 |
| BMI (kg/m2) | 29±6 | 28±5 | 30±7 | 27±5 | 27±4 | 31±5 |
| Percent Body Fat (%) | 32±11 | 27±9* | 42±8 | 31±11 | 27±8* | 45±7 |
| Change in Weight, kg (Range) | 0.7±5 | 1.2±5 (−9, 11) | −0.1 ±4 (−5, 8) | 0.7±5 (−9,14) | 0.7±5 (−9, 14) | 0.7±6 (−9,12) |
| Psychological Questionnaires | ||||||
| Perceived Stress | 21±7 | 21±7 | 21±8 | 21±9 | 21±9 | 22±8 |
| Physical Anhedonia | 14±8 | 15±8 | 12±8 | 15±9 | 15±9 | 13±9 |
| Emotional Appetite, Positive | 5.1±1 | 5.3±1 | 4.9±1 | 5.2±1.4 | 5.4±1 | 4.5±1 |
| Emotional Appetite, Negative | 4±1 | 4.2±1* | 3.4±1 | 4±1.2 | 4.0±1 | 3.7±1 |
| Inventory for Depressive Symptomatology | 12±9 | 12±9 | 11±10 | 13±9 | 12±9 | 15±12 |
| Dietary Restraint | 8±5 | 7±5 | 9±4 | 8±5 | 8±5 | 9±4 |
| Dietary Hunger | 4±3 | 4±2 | 3±4 | 4±3 | 4±3 | 4±4 |
| Dietary Disinhibition | 4±3 | 4±2 | 5±4 | 4±3 | 4±3 | 6±4 |
1.3.1 Short-Term Analysis
Perceived stress scores were positively correlated with short-term weight change (r = 0.35, p = 0.01). After controlling for age, race, gender, and baseline weight, perceived stress was still a predictor of weight change in the short-term (overall model: F= 4.19, r2 = 0.23, p = 0.02) such that a 5 point increase on the perceived stress scale was associated with a 0.95 kg weight gain at follow-up (partial r2 =0.07, p=0.04). Depression, anhedonia, positive and negative emotional eating, and dietary restraint, hunger and disinhibition were not independently correlated with weight change (all p values >0.05). However, in a model of short-term weight change (F = 8.41, r2 = .38, p < 0.01) including both perceived stress (p=0.03) and positive emotional eating (p=0.02) as well as age, race, gender, and baseline weight (all P >0.10), a significant interaction was found between perceived stress and positive emotional eating (p=0.009). There were no other interactions found between psychological variables and weight change at short-term.
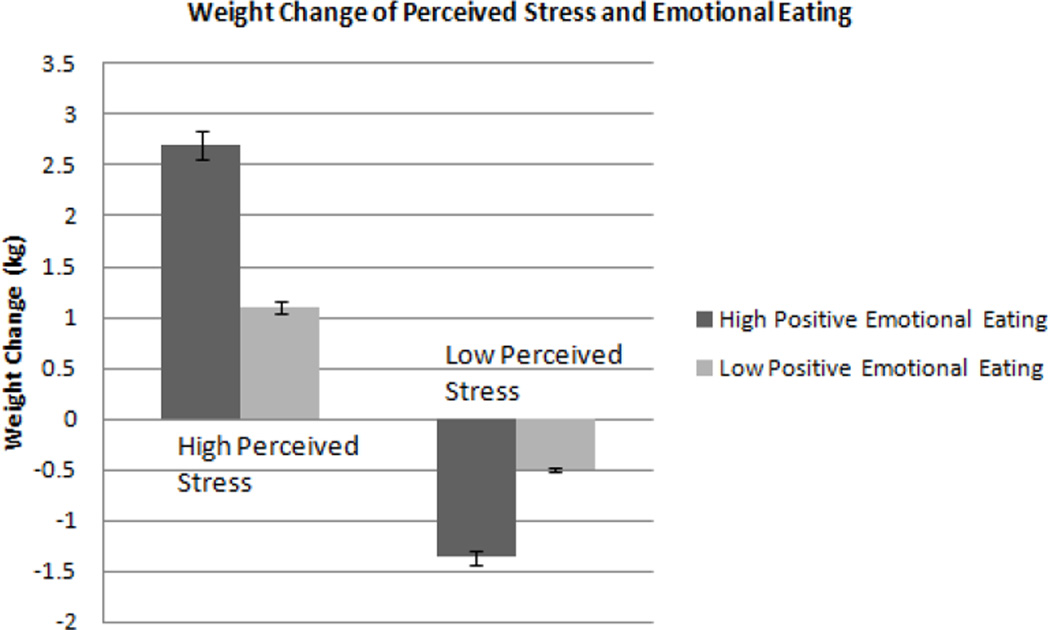
To better understand the observed interaction, perceived stress scores and positive emotional eating scores were divided into lower and upper categories based on the median (21 and 5.05, respectively). Adjusted values of weight change from the model are presented by lower and higher scores of each measure in Figure 1. Graphical representation of the interaction using adjusted data is depicted in Figure 2. Those who scored high on the perceived stress scale gained weight; however, those high scorers who also had high positive emotional eating scores gained the most weight (2.8±5 kg) compared to those with lower positive emotional eating scores (1.1±3kg). Those with lower perceived stress scores did not gain weight in the short-term and those with high positive emotional eating scores actually lost weight over the short-term time period (−1.4±4 kg) while those who scored low on both measures maintained their weight (−0.5±4 kg).
Figure 1.
Weight change of individuals with high versus low perceived stress and positive emotional eating
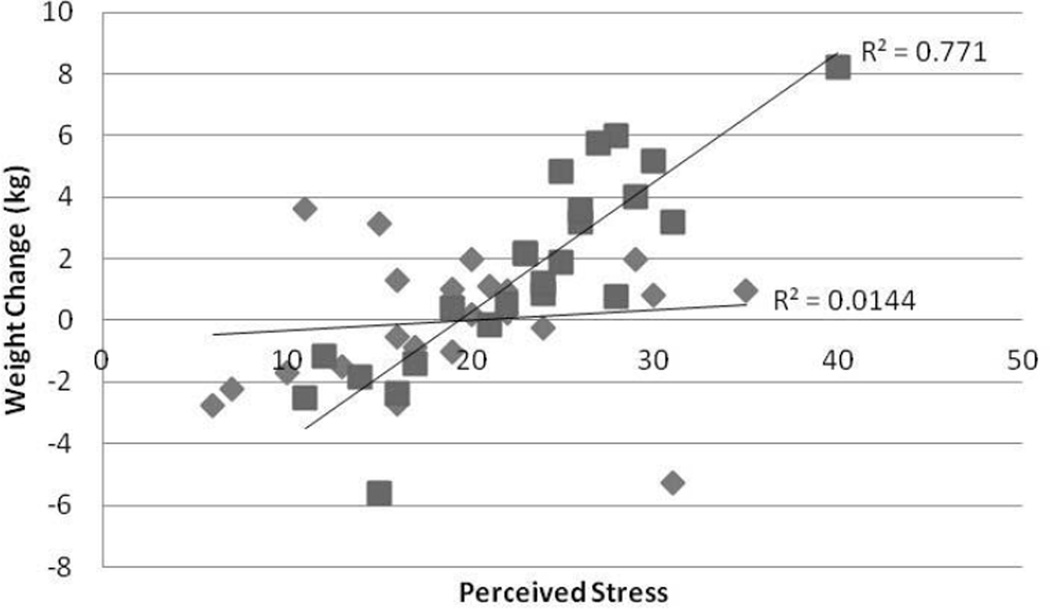
Figure 2.
Adjusted Values of Weight Change versus Perceived Stress categorized by degree of eating in response to Positive Emotions (squares: High positive emotional eaters; Diamonds: low positive emotional eaters). Multiple linear regression was used and adjusted for age, gender, race, and baseline weight. R2 High Positive emotional eaters = 0.77; R2 Low positive emotional eaters = 0.01; p=0.009. Similar results were obtained if the right uppermost outlier was removed.
1.3.2 Long-Term Analysis
Anhedonia was positively correlated with long term weight change (r= 0.32, p = 0.02). After adjusting for age, race, gender, and baseline weight, a 5 point increase in anhedonia score predicted a 0.85 kg weight gain at follow-up (partial r2 = 0.08, p = 0.04), although the overall model was not significant (F = 1.60, r2 = 0.21, p = 0.12). When the least significant covariate was excluded from the model (baseline weight; p=0.81), the model did reach significance with a similar parameter estimate for PAS. In an analysis with the rate of weight change (weight change per year) as the dependent variable, anhedonia still predicted weight change (r2 = 0.24; partial r2 = 0.08; P = 0.04). Depression, perceived stress, positive and negative emotional eating, and dietary restraint, hunger and disinhibition were not independently correlated with weight change (all p values >0.05). However, in a model of weight change (F = 2.21, r2 = .36, p = 0.04) including depression and anhedonia as well as age, race, gender, and baseline weight, there was a significant interaction between depression and anhedonia (p=0.01), but no other interactions between psychological variables and weight change. In a similar analysis where rate of weight change (weight change per year) was the dependent variable, the interaction between anhedonia and depression was still significant (p = 0.005).
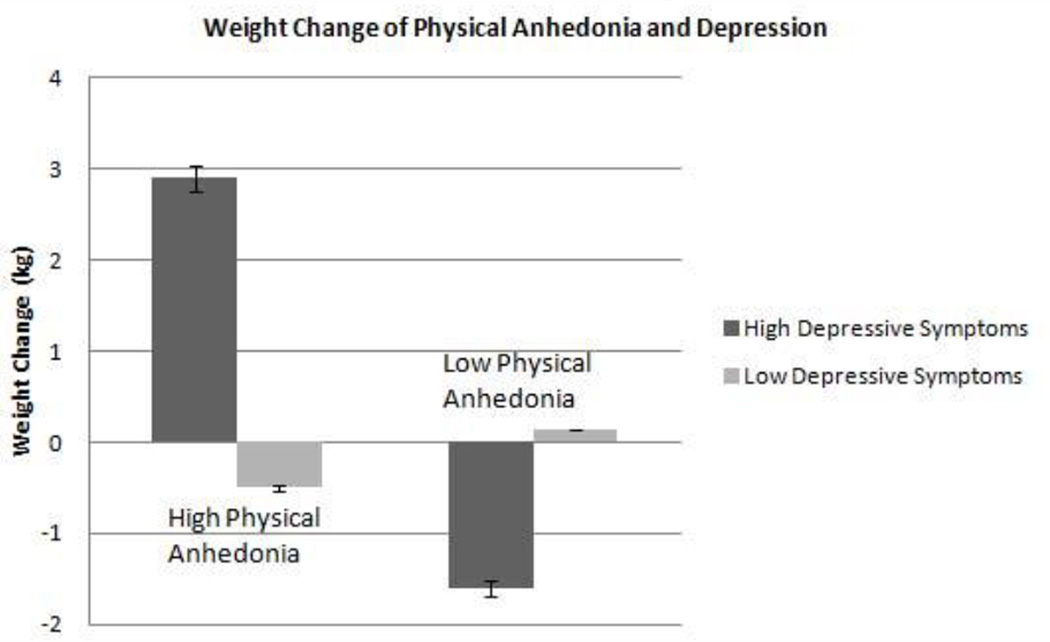
Again, in order to better understand the observed interaction, scores for anhedonia and depression were divided into lower and upper halves based on the median (13 and 10, respectively). Weight change adjusted for covariates is presented by lower and higher scores of each measure in Figure 3. The adjusted continuous data is shown in Figure 4. Those individuals with lower depression scores essentially maintained their weight over time. However, those with both high depression and high anhedonia gained weight (2.90±5 kg) over time while those with high depression but low anhedonia scores lost weight (−1.60±5 kg). 32 of the 49 (65%) individuals assessed in the long-term analysis had no evidence of depression, as defined by the criteria of the IDS.
Figure 3.
Weight change of individuals with high versus low depressive symptoms and anhedonia
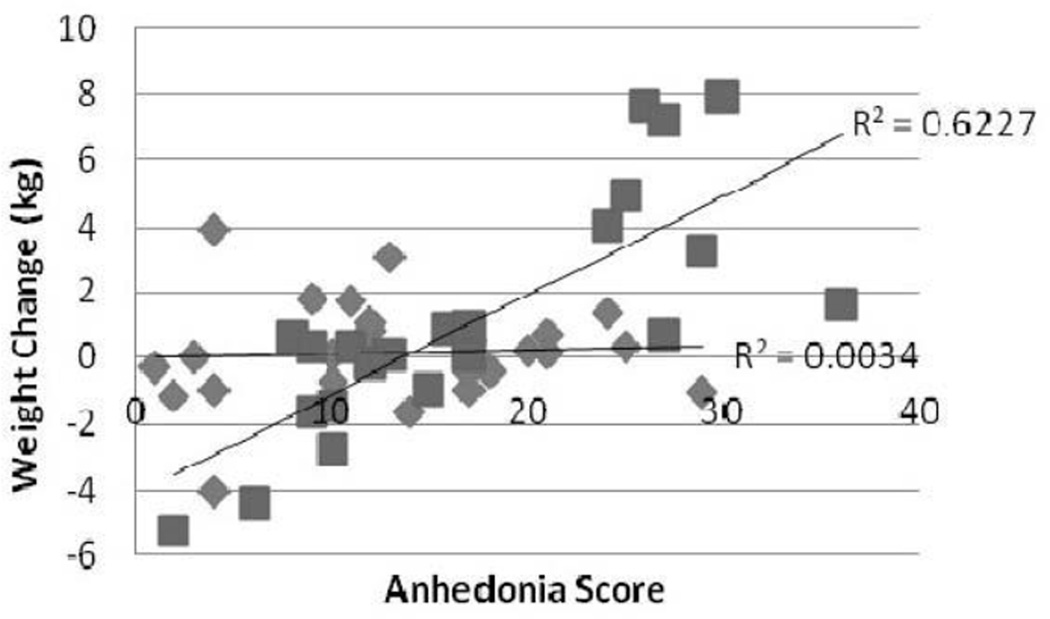
Figure 4.
Adjusted Values of Weight Change versus Anhedonia categorized by degree of depression (squares: High depressive symptoms; Diamonds: Low depressive symptoms). Multiple linear regression was used and adjusted for age, gender, race, and baseline weight. R2 High depressive symptoms = 0.62; R2 Low depressive symptoms = 0.003; p<0.01.
1.4 Discussion
In this longitudinal study of weight change in adults, we found that differing psychological measures predicted short- versus long-term weight change. Higher levels of perceived stress predicted short-term weight gain and this effect was amplified in those with high positive emotional eating scores. Conversely, scoring higher on the positive emotional eating scale in those with lower perceived stress was associated with weight loss during the short-term time period. Anhedonia was associated with long-term weight gain and this effect was observed mainly in individuals with higher scores on the depression scale. On the other hand, individuals with higher depression scores but without anhedonia lost more weight during the long-term time period. Thus, there may be different mechanisms contributing to short-versus long-term weight gain such that perceived stress may be a target to prevent short-term weight gain while attention to anhedonia may be a better target for longer term weight maintenance.
At the short-term follow-up visit, those who perceived themselves as having more stress at baseline gained weight, consistent with previous short-term studies (42–43). It is known that cortisol, the hormone released in response to physiologic stress, can lead to weight gain at supraphysiologic concentrations, in part through appetite stimulation (44–45). However, several human studies have shown that acute psychological stress continually activates the hypothalamic-pituitary-adrenocortical (HPA) axis, which stimulates the secretion of cortisol (46) and one study has shown that in response to a psychological stress task, high cortisol responders ate more compared to low cortisol responders (44). In addition, it is possible that chronic activation of the adrenergic system and down-regulation of adrenergic receptors that is evident in psychological stress (54) may suppress lipolysis (55), thus leading to weight gain in stressed individuals. Unfortunately, we did not have actual measures of 24 hour urinary cortisol.
The effect of perceived stress on future weight gain was dependent on a person’s eating response to positive emotional stimuli. Previous studies have found an inverse relationship between positive emotional eating and BMI (47) but we found that this relationship only occurred in those with low levels of perceived stress. One might expect that individuals susceptible to eating in response to negative emotions would be more likely to gain weight when they perceived a high level of stress but we did not find such a relationship. Instead, we found that individuals who perceive their lives as more stressful may be more likely to gain weight when susceptible to eating in response to positive emotions. This may be due to increased food intake in response to pleasurable feelings and situations in which food might be considered as a reward. Because of their association between food and positive feelings, these individuals may attempt to overcome the effects of stress through increased food intake. When individuals perceive a high level of stress, they may overcompensate to recapture a previous stimuli perceived as both rare and positive by eating to excess in order to reward themselves. Studies have shown that highly palatable foods are preferred in times of increased stress (48) as highly palatable foods stimulate the brain reward system (49). It is possible an increased desire for palatable foods due to a perceived increase in stress coupled with susceptibility to overeat in response to positive moods explain the interaction and increased weight gain in this group. Those who perceived themselves to be less stressed but still reported eating in response to positive emotions likely possess other coping mechanisms and so have a paradoxical decreased need to overeat in response to these stimuli. This may explain both the weight loss we observed in this subset of individuals and the results of prior studies that found an association between lower BMI and positive emotional eating (47). The majority of studies examining emotional eating have focused on negative moods and situations (50), but our findings emphasize the importance of emotional eating in response to positive moods.
Anhedonia, described as joylessness or a lack of motivation to engage in enjoyable activities, was the strongest predictor of long-term weight gain. The Physical Anhedonia Scale used in our study was designed to evaluate anhedonia as a trait rather than a transient state (37). A persistent presence of anhedonic symptoms might contribute to long-term weight gain if it causes a lack of motivation and incentive to compensate for short-term changes or to maintain a healthy lifestyle. Alternatively, the change in brain reward circuitry observed in individuals with anhedonia (51) may be a factor for increased weight gain. Possibly, individuals with anhedonia seek reward and pleasure through food, without recompense, which in turn promotes increased food intake and weight gain. In fact, higher scores on a measure of reward-based eating was associated with increases in BMI over eight years (56).
Previous studies have shown that depressed individuals can either gain or lose weight over time (52), but few have determined factors that may explain why depression-related weight change may differ between individuals. Our study population was not clinically depressed but even within a normative range, scores on the IDS modified the effect of anhedonia on future weight change. In the current study, those individuals with the lowest depression scores were most likely to maintain their weight over a longer time period. However, in those with IDS scores in the upper half of normal in our sample, the level of anhedonia was a major factor in predicting whether they gained or lost weight. Those with higher depression indices but low levels of anhedonia lost weight over time whereas high levels of anhedonia combined with high depression scores resulted in weight gain over time. Studies have demonstrated an inverse relationship between anhedonia and levels of physical activity, possibly due to diminished motivation to engage in physical activity or decreased perception of the rewarding effects of exercise (57). Coupled with higher levels of depression, this may explain a possible mechanism for the increased weight gain seen in our sample.
Previous studies (6–7, 11–12) examining the relationship between psychological measures and obesity have been primarily cross-sectional and focused on only one behavior or symptom. Because it is likely that a complex network of emotions guides human behaviors (49), the examination of multiple psychological measures and their interactions to predict longitudinal outcome variables is a strength of this study. In addition, our assessment of perceived stress rather than actual stress is a strength as it is possible that a subjective measure of stress can more accurately assess emotional response to a situation, rather than an objective measure of the stressor itself (34). Moreover, the baseline psychological assessments were done prior to the change in weight, indicating a potential cause-and effect relationship.
However, the psychological factors were only assessed at baseline, and follow-up assessments might strengthen our observations by examining psychological changes over time. Our small sample size is another limitation. Because of the small number of lean subjects in each cohort, we did not have enough power to examine the observed differences by weight status (lean vs. obese), though we did control for baseline weight in our models. Future studies should survey larger samples to replicate our results and evaluate correlations between other psychological measures that did not reach significance in the current study. In addition, the median split performed in our analyses may possibly skew clinical meaningfulness, given that the majority of our sample was within the nonclinical range. Secondly, we acknowledge that this split may also lead to biased interpretation of the results, as scores clustered around the median may be interpreted as more different than they truly are. However, associations between these variables were present before performing the median split, thus justifying further exploration of their interactive relationships and possible extrapolation to clinical samples. Finally, our sample consisted of individuals who stayed on the Clinical Research Unit for 10–30 days and therefore may not be typical of the general population.
In conclusion, we have demonstrated that different psychological factors predict short- versus long-term weight change. In addition, we demonstrated that these factors interact with one another and therefore we were able to provide a more thorough explanation of the psychological predictors of both short- and long-term weight gain. Our findings indicate that perceived stress, eating in response to positive emotions, depression and anhedonia are important factors when constructing a patient profile assessment for weight loss or weight maintenance strategies.
Highlights.
There may be different mechanisms that predict short- versus long- term weight gain.
We examine psychological predictors and their interactions on weight gain.
Perceived stress and emotional eating interact to predict short-term weight gain.
Depression and anhedonia interact to predict long-term weight gain.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH/NIDDK
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
MI and MST collected and analyzed data. All authors had equal involvement in writing the manuscript and reviewing submitted version.
References
- 1.Hebebrand J, Hinney A. Environmental and Genetic Risk Factors in Obesity. Child and Adolescent Psychiatric Clinics of North America. 2009 Jan;18(1):83–94. doi: 10.1016/j.chc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Levitan RD, Davis C. Emotions and eating behavior: Implications for the current obesity epidemic. University of Toronto Quarterly. 2010;79(2):783–799. [Google Scholar]
- 3.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008 Mar 12;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 4.Ernersson A, Nystrom FH, Lindström T. Long-term increase of fat mass after a four week intervention with fast food based hyper-alimentation and limitation of physical activity. Nutr Metab (Lond) 2010;7:68. doi: 10.1186/1743-7075-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buss J. Associations between obesity and stress and shift work among nurses. Workplace Health Saf. 2012;60(10):453–458. doi: 10.1177/216507991206001007. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Qian L. Association between lifetime stress and obesity in Canadians. Prev Med. 2012;55(5):464–467. doi: 10.1016/j.ypmed.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Komulainen T, et al. Quitting a weight loss program is associated with anhedonia: preliminary findings of the Lifestyle Intervention Treatment Evaluation Study in northern Finland. Int J Circumpolar Health. 2011;70(1):72–78. doi: 10.3402/ijch.v70i1.17795. [DOI] [PubMed] [Google Scholar]
- 8.Shomaker LB, Tanofsky-Kraff M, Zocca JM, Field SE, Drinkard B, Yanovski JA. Depressive symptoms and cardiorespiratory fitness in obese adolescents. Journal of Adolescent Health. 2012;50:87–92. doi: 10.1016/j.jadohealth.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geliebter A, Aversa A. Emotional eating in overweight, normal weight, and underweight individuals. Eating Behaviors. 2003 Jan;3(4):341–347. doi: 10.1016/s1471-0153(02)00100-9. [DOI] [PubMed] [Google Scholar]
- 10.Bongers P, Jansen A, Havermans R, Roefs A, Nederkoorn C. Happy eating: the underestimated role of overeating in a positive mood. Appetite. 2013 Aug;67:74–80. doi: 10.1016/j.appet.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Atlantis E, Baker M. Obesity effects on depression: systematic review of epidemiological studies. Int J Obes (Lond) 2008;32(6):881–891. doi: 10.1038/ijo.2008.54. [DOI] [PubMed] [Google Scholar]
- 12.Luppino FS, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 13.De Lauzon-Guillain B, Basdevant A, Romon M, Karlsson J, Borys J-M, Charles MA, et al. Is restrained eating a risk factor for weight gain in a general population? Am J Clin Nutr. 2006 Jan;83(1):132–138. doi: 10.1093/ajcn/83.1.132. [DOI] [PubMed] [Google Scholar]
- 14.Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A. Stress and Adiposity: A Meta-Analysis of Longitudinal Studies. Obesity. 2011;19(4):771–778. doi: 10.1038/oby.2010.241. [DOI] [PubMed] [Google Scholar]
- 15.De Vriendt T, Clays E, Huybrechts I, De Bourdeaudhuij I, Moreno LA, Patterson E, Molnár D, Mesana MI, Beghin L, Widhalm K, Manios Y, De Henauw S HELENA Study Group. European adolescents' level of perceived stress is inversely related to their diet quality: the Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br J Nutr. 2012 Jul;108(2):371–380. doi: 10.1017/S0007114511005708. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Qian L. Association between lifetime stress and obesity in Canadians. Prev Med. 2012 Nov;55(5):464–467. doi: 10.1016/j.ypmed.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Lahiri K, Rettig-Ewen V, Böhm M, Laufs U. Perceived psychosocial stress and cardiovascular risk factors in obese and non-obese patients. Clin Res Cardiol. 2007 Jun;96(6):365–374. doi: 10.1007/s00392-007-0512-1. [DOI] [PubMed] [Google Scholar]
- 18.Barrington WE, Ceballos RM, Bishop SK, McGregor BA, Beresford SA. Perceived stress, behavior, and body mass index among adults participating in a worksite obesity prevention program, Seattle, 2005–2007. Prev Chronic Dis. 2012 doi: 10.5888/pcd9.120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosom Res. 2000 Feb;48(2):195–202. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- 20.Wallis DJ, Hetherington MM. Stress and eating: the effects of ego-threat and cognitive demand on food intake in restrained and emotional eaters. Appetite. 2004 Aug;43(1):39–46. doi: 10.1016/j.appet.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Boyce JA1, Kuijer RG. Perceived stress and freshman weight change: The moderating role of baseline body mass index. Physiol Behav. 2015 Feb;139:491–496. doi: 10.1016/j.physbeh.2014.12.011. Epub 2014 Dec 4. [DOI] [PubMed] [Google Scholar]
- 22.Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS. Chronic mild stress-induced anhedonia: Greater effect in a genetic rat model of depression. Physiology & Behavior. 1993 Dec;54(6):1215–1220. doi: 10.1016/0031-9384(93)90351-f. [DOI] [PubMed] [Google Scholar]
- 23.Aurélie L, Gilles F, Jean-Jacques D, Agathe A, Sophie V, Daniel T, et al. Characterization of the Three-Factor Eating Questionnaire scores of a young French cohort. Appetite. 2012 Oct;59(2):385–390. doi: 10.1016/j.appet.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Bryant EJ, King NA, Blundell JE. Disinhibition: its effects on appetite and weight regulation. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2008 Sep;9(5):409–419. doi: 10.1111/j.1467-789X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 25.Polivy J, Peter C. Dieting and binging: A causal analysis. Am. Psychol. 1985;40(2):193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- 26.Cools J, Schotte DE, McNally RJ. Emotional arousal and overeating in restrained eaters. J. Abnorm. Psychol. 1992 May;101(2):348–351. doi: 10.1037//0021-843x.101.2.348. [DOI] [PubMed] [Google Scholar]
- 27.Herman CP, Mack D. Restrained and unrestrained eating. J. Pers. 1975 Dec;43(4):647–660. doi: 10.1111/j.1467-6494.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 28.Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J. Psychosom. Res. 2000 Feb;48(2):195–202. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- 29.Johnson F, Pratt M, Wardle J. Dietary restraint and self-regulation in eating behavior. Int. J. Obes. 2005. 2012 May;36(5):665–674. doi: 10.1038/ijo.2011.156. [DOI] [PubMed] [Google Scholar]
- 30.DelParigi A1, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007 Mar;31(3):440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 31.Gibbs BB, Kinzel LS, Gabriel KP, Chang Y, Kuller LH. Short- and long-term eating habit modification predict weight change in overweight, post-menopausal women: results from the WOMAN Study. J Acad Nutr Diet. 2012 Sep;112(9):1347.e2–1355.e2. doi: 10.1016/j.jand.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeffery RW, Kelly KM, Rothman AJ, Sherwood NE, Boutelle KN. The weight loss experience: a descriptive analysis. Ann Behav Med. 2004 Apr;27(2):100–106. doi: 10.1207/s15324796abm2702_4. [DOI] [PubMed] [Google Scholar]
- 33.Kruger J, Blanck HM, Gillespie C. Dietary practices, dining out behavior, and physical activity correlates of weight loss maintenance. Prev Chronic Dis. 2008 Jan;5(1):A11. [PMC free article] [PubMed] [Google Scholar]
- 34.Sciamanna CN, Kiernan M, Rolls BJ, Boan J, Stuckey H, Kephart D, et al. Practices associated with weight loss versus weight-loss maintenance results of a national survey. Am J Prev Med. 2011 Aug;41(2):159–166. doi: 10.1016/j.amepre.2011.04.009. 31. Ferraro R, Boyce VL, Swinburn B, De Gregorio M. [DOI] [PubMed] [Google Scholar]
- 35.Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr. 1991;53/6:1368–1371. doi: 10.1093/ajcn/53.6.1368. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association. Standards of Medical Care in Diabetes-2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapman Loren J, Chapman Jean P, Raulin ML. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976 Aug;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- 38.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983 Dec;(4):385–396. [PubMed] [Google Scholar]
- 39.Geliebter A, Aversa A. Emotional eating in overweight, normal weight, and underweight individuals. Eat Behav. 2003 Jan;(4):341–347. doi: 10.1016/s1471-0153(02)00100-9. [DOI] [PubMed] [Google Scholar]
- 40.Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986 May;18(1):65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 41.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 42.Roberts C, Troop N, Connan F, Treasure J, Campbell IC. The effects of stress on body weight: biological and psychological predictors of change in BMI. Obesity (Silver Spring) 2007 Dec;15(12):3045–3055. doi: 10.1038/oby.2007.363. [DOI] [PubMed] [Google Scholar]
- 43.Serlachius A, Hamer M, Wardle J. Stress and weight change in university students in the United Kingdom. Physiology & Behavior. 2007 Nov 23;92(4):548–553. doi: 10.1016/j.physbeh.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 44.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001 Jan;26(1):37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 45.Arie G. Nieuwenhuizen, Femke Rutters, The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiology & Behavior. 2008 May 23;94(2):169–177. doi: 10.1016/j.physbeh.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007 Oct 10;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 47.Nolan LJ, Halperin LB, Geliebter A. Emotional Appetite Questionnaire. Construct validity and relationship with BMI. Appetite. 2010 Apr;54(2):314–319. doi: 10.1016/j.appet.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosom Med. 2000;62:853–865. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 49.Adam TC, Epel ES. Stress, eating and the reward system. Physiology & Behavior. 2007 Jul 24;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Lowe MR, Maycock B. Restraint, disinhibition, hunger and negative affect eating. Addict Behav. 1988;13(4):369–377. doi: 10.1016/0306-4603(88)90043-3. [DOI] [PubMed] [Google Scholar]
- 51.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends in Neurosciences. 2012 Jan;35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010 Mar;67(3):220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 53.Dolan RJ. Emotion, Cognition, and Behavior. Science. 2002 Nov 8;298(5596):1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- 54.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 55.Robidoux J, Martin TL, Collins S. Beta-adrenergic receptors and regulation of energy expenditure: a family affair. Annu Rev Pharmacol Toxicol. 2004;44:297–323. doi: 10.1146/annurev.pharmtox.44.101802.121659. [DOI] [PubMed] [Google Scholar]
- 56.Epel ES, Tomiyama AJ, Mason AE, Laraia BA, Hartman W, Ready K, et al. The Reward-Based Eating Drive Scale: A Self-Report Index of Reward-Based Eating. PLoS ONE. 2014 Jun 30;9(6):e101350. doi: 10.1371/journal.pone.0101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leventhal AM. Relations Between Anhedonia and Physical Activity. Am J Health Behav. 2012 Nov;36(6):860–872. doi: 10.5993/AJHB.36.6.12. [DOI] [PMC free article] [PubMed] [Google Scholar]