Abstract
Mechanical rigidity in the tumor microenvironment is associated with a high risk of tumor formation and aggressiveness. Adhesion-based signaling driven by a rigid microenvironment is thought to facilitate invasion and migration of cancer cells away from primary tumors. Proteolytic degradation of extracellular matrix (ECM) is a key component of this process and is mediated by subcellular actin-rich structures known as invadopodia. Both ECM rigidity and cellular traction stresses promote invadopodia formation and activity, suggesting a role for these structures in mechanosensing. The presence and activity of mechanosensitive adhesive and signaling components at invadopodia further indicates the potential for these structures to utilize myosin-dependent forces to probe and remodel their ECM environments. Here, we provide a brief review of the role of adhesion-based mechanical signaling in controlling invadopodia and invasive cancer behavior.
Keywords: invadopodia, mechanotransduction, signaling, adhesion, contractility, actin, invasion, extracellular matrix, proteinases, secretion
Introduction
Cells sense the biomechanical properties of the ECM through interactions facilitated by matrix adhesions [1]. Intracellular adhesion proteins link ECM receptors to downstream force-sensing pathways, including non-muscle myosin II (NM II)-dependent contractility of adhesion-associated actin [2] and conformational changes of mechanosensitive proteins [3]. Changes in mechanical signaling pathways can alter cellular phenotypes and contribute to a number of diseases, including deafness, cardiac hypertrophy, and muscular dystrophy [4]. In breast cancer, increased ECM rigidity during tumorigenesis has been shown to drive a malignant phenotype through biomechanical adhesion signaling [5-8], including enhanced invasion and metastasis [8-11]. ECM rigidity changes in breast cancer are thought to occur as a result of a number of factors, including tumor cell packing, ECM deposition and crosslinking, and higher fluid pressures [12]. These factors are common features of many types of cancers [7, 12-14], and several other tumor types have also been quantitatively shown to have greater mechanical properties than neighboring normal tissues [15-17]. Recent studies have shown that mechanical factors alter the invasive properties of diverse cancer cell types in vitro [18-21] suggesting common rigidity-dependent regulatory pathways.
Proteolytic degradation of ECM promotes cancer cell invasion by allowing migration through dense cross-linked tissues such as the basement membranes that surround carcinomas and underlie blood vessels [22]. In addition, proteolytic remodeling of stromal collagen may allow collective migration of cancer cells through tissues [23]. In order to degrade ECM, cancer cells form actin-rich adhesive protrusions called invadopodia (Fig. 1) [24]. Invadopodia are cellular hotspots for secretion of matrix-degrading proteinases [25-27]; thus, formation of invadopodia greatly accelerates matrix remodeling. The ability of cancer cells to form invadopodia correlates well with their in vitro and in vivo invasive behavior [28-35]. In addition, upregulation in tumors of key invadopodia molecules, such as the matrix metalloproteinase MT1-MMP, and the actin assembly protein cortactin, are associated with poor patient prognosis [36, 37]. Similar structures called podosomes are formed in a variety of other cell types that need to remodel tissue or cross tissue barriers, including osteoclasts, endothelial cells, and macrophages [38].
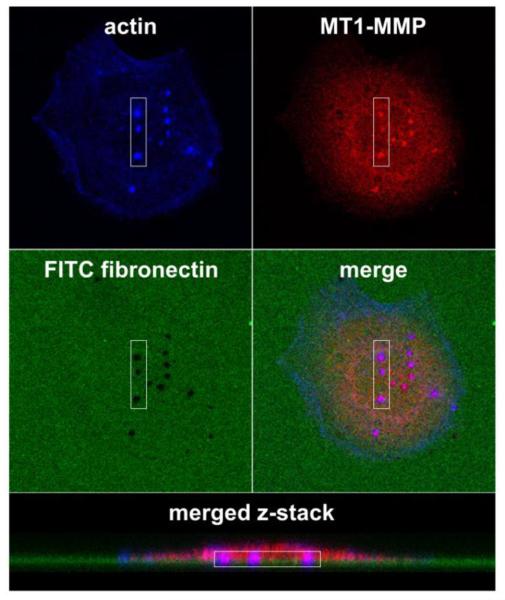
Figure 1.
Invadopodia are actin-rich proteolytic protrusions that are often identified through colocalization of markers with ECM degradation. The in vitro invadopodia assay typically consists of invasive cancer cells cultured on fluoroscently-labeled ECM, in this case FITC-fibronectin-coated crosslinked gelatin. After 6-48 h, the cells are fixed and stained for molecular markers of invadopodia including actin filaments, cortactin, Arp2/3 complex, Tks5, and/or MT1-MMP [44, 45, 76, 99, 108, 109, 119]. In this case, invadopodia are identified by colocalization (purple) of actin filaments (blue) and MT1-MMP (red) using confocal microscopy imaging. Mature invadopodia are further recognized by colocalization of invadopodia markers with areas of ECM degradation (black holes in the green FITC-labeled fibronectin).
In addition to invadopodia and podosomes, invadopodia-independent proteolytic degradation mechanisms have been described in normal and cancer-associated fibroblasts (CAFs) [39, 40]. Matrix degradation by fibroblasts at focal adhesions was regulated by signaling mechanisms that also control invadopodia (e.g. Src, FAK, p130Cas) [40]. However, invadopodia-independent plasma membrane sites were identified that do not depend on the critical invadopodia regulators Cdc42 or Src [39]. These data suggest some flexibility in the mechanisms controlling proteinase expression on the plasma membrane. In contrast, pancreatic CAFs expressing high levels of palladin have been shown to enhance invasion and metastasis of tumor cells through invadopodia-dependent ECM degradation [41]. While invadopodia appear to be the dominant mechanism used by invasive cancer cells to degrade ECM, further investigation is required to elucidate the role and regulation of proteolytic structures in tumor-associated stromal fibroblasts.
Invadopodia formation and structure
Invadopodia are formed in response to signaling events that lead to dynamic branched actin assembly at membrane sites [25, 32, 42]. Shortly thereafter, proteinases are secreted and promote ECM degradation. MT1-MMP has been the most studied proteinase in invadopodia and is essential for degradation of in vitro crosslinked gelatin substrates [43-45] (Fig 1). However, many proteinases are secreted at invadopodia and could collaborate to promote degradation of ECM in tissues. These proteinases include MT1-MMP, MMP-2, MMP-9, seprase, cathepsin B, ADAM12, and uPAR [27, 28, 44, 46-50]. Some of these proteinases are also likely to activate latent ECM- and cell-associated growth factors [51-56].
By electron microscopy (EM), invadopodia are long, slender protrusions that are typically 50 nm in diameter and ~0.5-2 μm in length [30, 32, 57, 58]. While dynamic branched actin is found at the cortex and is an essential part of the formation process, the resemblance to filopodia by EM suggests that the actin found within the invadopodial protrusion is likely to be unbranched. Indeed, key filopodia proteins including fascin, Myosin X, mDia1, and fimbrin have been shown to be essential for invadopodia stabilization and elongation [32, 59, 60]. Thus, both the branched and unbranched actin nucleation machineries collaborate to form stable, active invadopodial protrusions.
Many signaling proteins localize to and regulate invadopodia formation and stability, including tyrosine kinases such as Src, EGFR, and Arg, adhesion proteins such as integrins, focal adhesion kinase (FAK), p130Cas, and integrin-linked kinase (ILK), and scaffold proteins such as Tks5 [24, 25, 61]. Many of these molecules also control podosome and focal adhesion formation and activity [62] (reviewed elsewhere in this issue). Src kinase is a particularly important regulator, as exemplified by the spontaneous formation of invadopodia-like structures in cells engineered to exogenously express constitutively active Src [63-65]. Given their similarities, invadopodia, podosomes, and Src-induced invadopodia-like structures are often referred to collectively as invadosomes [66].
ECM rigidity and cellular contractility control invadopodia formation and activity
One of the first indications that invadopodia might be involved in mechanical signaling came from our work demonstrating that ECM rigidity increases invadopodia numbers and activity [67]. At the same time, podosomes were found to exert shear stresses on flexible substrates and to participate in mechanosensing [68], suggesting general regulation of invadosome structures by substrate rigidity. Interestingly, we found that phosphorylated forms of the mechanosensing proteins p130Cas and FAK localize at actively degrading invadopodia, and their levels are reduced with inhibitors of nonmuscle myosin II (NM II) and myosin light chain kinase (MLCK) [67]. Overexpression of FAK and p130Cas also enhanced invadopodia activity on rigid but not soft substrates. While we did not find significant localization of phosphorylated MLC at invadopodia, in 40% of cells NM IIA was present in a ring-like structure around invadopodia [67]. Combined with the dependence of invadopodia activity on NM II activity, the localization data suggest regulation through the contractile machinery [67, 69]. Using tunable rigidity substrates as well as tissue-derived scaffolds, we further found that substrate rigidity controls invadopodia numbers and activity across a wide elastic modulus range [70]. The peak modulus for invadopodia-associated ECM degradation of our breast cancer model cell line was ~30 kPa which is in the rigidity range of tumor stroma [70]. In contrast, formation of MT1-MMP-positive membrane protrusions by several types of malignant cancer and fibrosarcoma cell lines has been shown to be inversely proportional to ECM rigidity [71]. The impact of these structures on ECM degradation was not quantitated; thus, further study is required to determine how they relate to invadopodia and invasion.
Cellular contractility plays an important role in mechanosensing by distinguishing the levels of ECM rigidity in the microenvironment and adjusting the amount of exerted cellular tension [72, 73]. Actomyosin-generated contractile forces [74] that are transmitted to the ECM can be measured as traction forces or stresses [75, 76]. Using traction force microscopy on substrates of different rigidities, the Parekh group found that the magnitude of overall cellular traction stress within a cell line is predictive of ECM degradation by invadopodia [77]. Across cell lines, the Reinhardt-King group found that the average cell traction stress produced on a substrate of a given rigidity correlates with the metastatic capacity inherent to a given cancer cell line [19]. These data suggest that cellular contractility is an important regulator of invasive behavior.
While the mechanical properties of the ECM may be one significant factor that influences cancer cell invasiveness, cells may exhibit varying responses to the same rigidity level. Thus, invadopodia dynamics may ultimately be dictated by the level of cellular force generation. The Varghese laboratory has recently shown that compressive traction stresses must reach a threshold level to induce invadopodia-like structures capable of ECM degradation [78]. These compressive stresses occurred at the protruding plasma membrane into the underlying substrate and were accompanied by shear and tensile stresses that together triggered proteolytic activity in response to mechanical resistance. These results complement our findings that NM II activity and augmented cellular traction stresses increase invadopodia numbers and induce more ECM degradation [67, 77]. They also suggest that ultimately it is the cellular response that matters, which could differ between cancer cells depending on the baseline signaling state and resultant contractility.
Adhesion components control invadopodia activity
Traction stresses generated by the actomyosin cytoskeleton are transduced to and from the ECM through adhesion complexes [75]. Multiple adhesion and contractility molecules localize to and around invadopodia, suggesting that some of the ECM rigidity response may occur directly at invadopodia [26, 62, 67, 70, 79]. While CD44 and β3 integrins have been found at invadopodia [80, 81], β1 integrins appear to be the predominant adhesion molecules found at or around their actin cores [49, 79, 82-85]. Although this localization could be due to the predominant use of fibronectin matrix for in vitro invadopodia assays, β1 integrins are frequently associated with cancer progression [49, 79, 82-85]. β1 integrins have been found to regulate invadopodia by forming signaling complexes with Src, EGFR, and/or FAK [82, 85]. In addition, β1 integrins have been shown to promote invadopodia maturation to actively degrading structures by mechanisms that include signal complex formation with ezrin at lipid rafts, regulation of actin dynamics by Arg, docking of the gelatinolytic enzyme seprase, and promotion of MMP secretion via integrin-linked kinase-IQGAP interactions (Fig. 2) [49, 79, 83, 84]. While these studies did not directly investigate mechanical signaling, many of these processes may be enhanced by ECM rigidity similar to the maturation of focal adhesions and podosomes that occurs in response to stiff matrices [68, 86]. For example, β1 integrins are known to regulate strength of cellular adhesions while β3 integrins reinforce force-dependent responses through talin-dependent mechanical signaling [87]. Similar mechanisms might operate at invadopodia, through the same molecules.
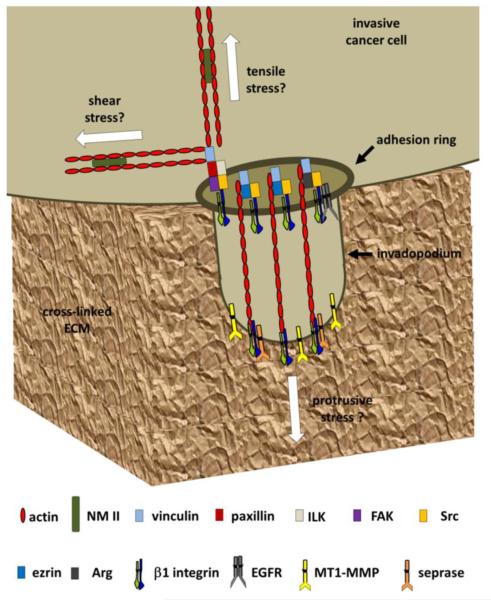
Figure 2.
Adhesion-based signaling in invadopodia. Signaling through β1 integrins at and/or around the actin cores of invadopodia regulates their maturation and ability to degrade ECM with proteases such as MT1-MMP and seprase [49, 79]. Adhesion rings are strongly correlated with invadopodia activity and are dependent on adhesion and signaling components such as vinculin, paxillin, ILK, and FAK [67, 79]. In addition, invadopodia activity is also regulated by the interactions of β1 integrins with other proteins and/or complexes that include ezrin, Arg, and EGFR [83-85]. Each of these pathways is regulated by Src kinase, which is thought to control early signaling cascades necessary for invadopodia formation [25, 61]. Adhesion rings may anchor and thus stabilize nascent invadopodia and generate NM-II generated shear and tensile stresses. Actin polymerization in the core may generate protrusive stresses, similar to those identified in podosomes [78, 91-93]. Periodic fluctuations in the stresses perpendicular to the ECM surface induce oscillations of invadopodia core and ring proteins [79, 91-93]. Together, these processes may be coordinated to constantly sense and respond to local ECM rigidity. Overall, adhesion based signaling in response to ECM rigidity is an important control point for invadopodia.
Similar to podosomes, invadopodia can organize adhesion proteins in rings around the actin cores [49, 67, 79, 88-90]. Using live cell imaging, Branch et al. showed that adhesion rings form shortly after formation of an invadopodial actin core [79]. Furthermore, β1 integrins found in invadopodial adhesion rings specifically enhanced the rate of MT1-MMP secretion [79]. Live cell imaging also revealed oscillations of actin at invadopodia that were paralleled by oscillations of GFP-paxillin-labeled adhesion rings [79]. Oscillatory behavior has been shown to be an important part of podosome mechanosensing and force generation [68, 91] and is regulated by actin polymerization and NM II activity [92, 93]. These data are consistent with a key role for adhesion-induced mechanical signaling in invadopodia maturation [49, 79, 83, 84].
Future Directions
Potential Methods for Studying Mechanical Signaling at Invadopodia
While multiple lines of evidence support the model that local mechanical signaling takes place at invadopodia [30, 49, 67, 70, 77, 79, 82-85, 94], further investigation is required to understand the molecular and biophysical mechanisms that regulate and respond to invadopodia mechanosensing. While studies with tunable rigidity substrates have been useful, more sophisticated methods to measure and manipulate force production at the subcellular level would allow closer investigation of local mechanical control of invadopodia. The standard method for measuring two-dimensional cellular forces is traction force microscopy in which stresses are calculated based on substrate deformations of elastic surfaces or pillars [75, 95, 96]. This method has been used to study podosomes; however, these structures can form large groups or rosettes which allows for easier measurements over a bigger area [68]. In contrast, invadopodia are often found isolated from each other making force generation more difficult to detect in such a small area [69]. However, traction force methods in which forces have been fitted to fluorescently-labeled focal adhesions [97] could be applied to invadopodia puncta in a similar manner. In addition, a novel method using the deformations in Matrigel networks has recently been developed to calculate the three-dimensional stresses generated by cells [78]. Such a method could potentially be further utilized to understand the role of adhesion-based mechanisms in regulating force-dependent proteolysis at invadopodia. In addition, other techniques adapted to study the mechanical nature of podosomes could be applied to invadopodia. For example, atomic force microscopy has been utilized to study the dynamics and stiffness of individual podosomes [92]. This technique was further developed into protrusion force microscopy in which protrusive forces exerted by individual podosomes during mechanosensing were measured based on deformations in polymeric films [91]. Such techniques could be complemented with advanced microscopy methods such as spinning disk confocal, FLIM, FRAP, and STORM with fluorescently tagged mechanosensitive proteins and/or tension biosensors [32, 93, 98, 99]. These techniques could be used to enhance our understanding of the molecular mechanisms that govern or respond to mechanical forces at invadopodia.
Understanding the Role of Local Forces at Invadopodia
One area that remains unclear is the role of different stress components during invadopodia mechanosensing and their part in invadopodia formation and/or maturation (Fig. 2). Focal adhesions generate traction forces via myosin contraction of actin stress fibers running parallel to the ECM surface that promotes their maturation [86, 100]. Similarly, invadopodia have been hypothesized to generate shear stresses through radial arrays of actin that surround their individual cores [62], similar to podosome rosettes [68]. Individual podosomes have recently been shown to exert protrusive or “pushing” stresses at their cores as a result of actin polymerization [91, 93], similar to what occurs at the leading edge of cells [101, 102]. These pushing stresses were accompanied by tensile or “pulling” stresses generated by actomyosin contractility surrounding the podosome cores [91, 93] suggesting that they occurred at the adhesion-based rings. This combination of pushing and pulling lead to oscillatory force generation at podosomes indicating a dynamic mechanism by which these structures are constantly mechanosensing their local microenvironment [91-93]. Similar 3D traction stresses may also regulate mechanosensing at invadopodia since they also exhibit oscillatory behavior (Fig. 2) [79]. However, the consequence of these forces is unclear. For example, the relationship between oscillatory forces, mechanotransduction signaling, and proteinase secretion is unknown.
Determining the Roles of Contractility Regulators in Invadopodia Dynamics
Another area of interest is how actomyosin contractility interfaces with other signals to control invadopodia. For example, molecules that regulate actin dynamics and cellular contractility such as the Rho GTPases act in a multitude of additional signaling pathways that may affect invadopodia independent of NM II-based force generation [26, 61, 103, 104]. For example, Rho-associated kinase (ROCK) is a classic activator of NM II via phosphorylation of MLC [4-6, 105]; however, ROCK has other downstream targets that regulate invadopodia such as LIM kinase, ezrin, and moesin [84, 89, 94, 106, 107]. While these are all actin regulators, they control other aspects including actin polymerization and linking actin to the plasma membrane. Therefore, it will be important to determine how contractile forces in cancer cells synergize with other signals to control invadopodia.
Developing New Models to Study Invadopodia
Validation of new findings regarding mechanical signaling by invadopodia requires models that capture the properties of the tissues encountered by invading cancer cells. The classic model for studying invadopodia has been the in vitro invadopodia assay, which relies on cancer cells plated on fluorescently labeled and cross-linked ECM for detecting degradation (Fig. 1) [108]. While this assay can be modified, e.g. by altering substrate rigidity using tunable synthetic substrates [76, 109], such systems do not fully recapitulate the tumor microenvironment. However, studying invadopodia in more complex environments has presented significant technical challenges given their size, dynamics, and lack of markers for ECM degradation in physiologic systems that can capably be monitored and imaged. Over the last several years, significant progress has been made to overcome some of these issues [110]. For example, Gligorijevic et al. recently identified cellular protrusions as invadopodia in vivo based on the presence and functionality of markers such as cortactin, Tks5, and MMP activity. Using high-resolution multiphoton microscopy of human breast carcinoma xenografts in SCID mice, they found that a variety of microenvironmental factors, including the density of collagen fibers, regulated the locomotion speed of invading cancer cells and resulting disease progression. In particular, slower moving cells required invadopodia in spatially distinct regions of the tumor microenvironment such as near collagen fibers and blood vessels [111]. Using photoconversion of cells expressing invadopodia and an MMP-activated fluorescent substrate, they demonstrated that invadopodia formation and ECM degradation directly correlated with subsequent intravasation and metastasis.
While clinical evidence and animal studies provide strong correlations between tumor ECM density and metastasis [8-11], other microenvironmental factors may significantly contribute to the phenotype of invading cancer cells and their expression of invadopodia [111]. Thus, invasion is influenced by matrix organization and pore size, hypoxia, extracellular vesicles and growth factors, and other cells in the microenvironment [27, 50, 54, 111-115]. In some cases, paradoxical relationships between ECM density and tumor aggressiveness exist. For example, unlike breast cancer, fibrosis appears to play an inhibitory role for pancreatic cancer [116-118]. In one study, depletion of myofibroblasts in a transgenic mouse model of pancreatic cancer resulted in increased invasion and decreased survival despite decreased tumor rigidity [116]. Alteration in immune cell phenotypes was found to account for the aggressive phenotype of pancreatic cancers lacking myofibroblasts, suggesting complex relationships in vivo. Therefore, further studies are required to elucidate how tissue rigidity impacts the aggressiveness of diverse cancer types and the role of invadopodia in that process.
Conclusion
Adhesions and mechanotransduction pathways control a variety of cellular phenotypes. The identification of invadopodia as mechanosensitive organelles suggests that the interface between adhesion signaling and additional molecular pathways can greatly enhance invasive behavior. Understanding that unique interface may help identify novel targets for therapeutic intervention. Future studies should combine a variety of state-or-the-art techniques and pre-clinical models to overcome previous biological and technological limitations and further elucidate molecular pathways. These models will be critical for providing the additional support necessary to link invadopodia to metastasis and explore the relevance of purported molecular mechanisms for therapeutic intervention.
Acknowledgments
Funding was provided by National Institutes of Health grants K25CA143412 and R03AR066875 to A.P. and R01CA163592 and U01CA143069 to A.M.W. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding was provided to A.P. by the Department of Otolaryngology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nature reviews. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends in cell biology. 2007;17:178–186. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- [3].Roca-Cusachs P, Iskratsch T, Sheetz MP. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. Journal of cell science. 2012;125:3025–3038. doi: 10.1242/jcs.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nature reviews. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. Journal of mammary gland biology and neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- [6].Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- [7].Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression, Nature reviews. Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mierke CT. The fundamental role of mechanical properties in the progression of cancer disease and inflammation. Reports on progress in physics. Physical Society. 2014;77:076602. doi: 10.1088/0034-4885/77/7/076602. [DOI] [PubMed] [Google Scholar]
- [10].Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC medicine. 6(2008):11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. The Journal of cell biology. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy, Nature reviews. Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- [13].Ariztia EV, Lee CJ, Gogoi R, Fishman DA. The tumor microenvironment: key to early detection. Critical reviews in clinical laboratory sciences. 2006;43:393–425. doi: 10.1080/10408360600778836. [DOI] [PubMed] [Google Scholar]
- [14].Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- [15].Ophir J, Alam SK, Garra B, Kallel F, Konofagou E, Krouskop T, Varghese T. Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. P I Mech Eng H. 1999;213:203–233. doi: 10.1243/0954411991534933. [DOI] [PubMed] [Google Scholar]
- [16].Sarvazyan A, Hall TJ, Urban MW, Fatemi M, Aglyamov SR, Garra BS. An Overview of Elastography - an Emerging Branch of Medical Imaging. Current medical imaging reviews. 2011;7:255–282. doi: 10.2174/157340511798038684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bhatia KS, Lee YY, Yuen EH, Ahuja AT. Ultrasound elastography in the head and neck. Part II. Accuracy for malignancy. Cancer imaging : the official publication of the International Cancer Imaging Society. 2013;13:260–276. doi: 10.1102/1470-7330.2013.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kraning-Rush CM, Califano JP, Reinhart-King CA. Cellular traction stresses increase with increasing metastatic potential. PLoS ONE. 2012;7:e32572. doi: 10.1371/journal.pone.0032572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Haage A, Nam DH, Ge X, Schneider IC. Matrix metalloproteinase-14 is a mechanically regulated activator of secreted MMPs and invasion. Biochemical and biophysical research communications. 2014 doi: 10.1016/j.bbrc.2014.05.086. [DOI] [PubMed] [Google Scholar]
- [21].Haage A, Schneider IC. Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells. FASEB J. 2014 doi: 10.1096/fj.13-245613. [DOI] [PubMed] [Google Scholar]
- [22].Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Current opinion in cell biology. 2012;24:277–283. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nature cell biology. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- [24].Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clinical & experimental metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- [25].Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nature reviews. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hoshino D, Branch KM, Weaver AM. Signaling inputs to invadopodia and podosomes. Journal of cell science. 2013;126:2979–2989. doi: 10.1242/jcs.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell reports. 2013;5:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Thompson EW, Paik S, Brunner N, Sommers CL, Zugmaier G, Clarke R, Shima TB, Torri J, Donahue S, Lippman ME, et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. Journal of cellular physiology. 1992;150:534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- [29].Coopman PJ, Do MT, Thompson EW, Mueller SC. Phagocytosis of cross-linked gelatin matrix by human breast carcinoma cells correlates with their invasive capacity. Clin Cancer Res. 1998;4:507–515. [PubMed] [Google Scholar]
- [30].Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- [31].Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. The Journal of cell biology. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. The Journal of cell biology. 2010;189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Takkunen M, Hukkanen M, Liljestrom M, Grenman R, Virtanen I. Podosome-like structures of non-invasive carcinoma cells are replaced in epithelial-mesenchymal transition by actin comet-embedded invadopodia. Journal of cellular and molecular medicine. 2010;14:1569–1593. doi: 10.1111/j.1582-4934.2009.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yamaguchi H, Yoshida S, Muroi E, Yoshida N, Kawamura M, Kouchi Z, Nakamura Y, Sakai R, Fukami K. Phosphoinositide 3-kinase signaling pathway mediated by p110alpha regulates invadopodia formation. The Journal of cell biology. 2011;193:1275–1288. doi: 10.1083/jcb.201009126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yamamoto H, Sutoh M, Hatakeyama S, Hashimoto Y, Yoneyama T, Koie T, Saitoh H, Yamaya K, Funyu T, Nakamura T, Ohyama C, Tsuboi S. Requirement for FBP17 in invadopodia formation by invasive bladder tumor cells. The Journal of urology. 2011;185:1930–1938. doi: 10.1016/j.juro.2010.12.027. [DOI] [PubMed] [Google Scholar]
- [36].Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression, Nature reviews. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- [37].Weaver AM. Cortactin in tumor invasiveness. Cancer letters. 2008;265:157–166. doi: 10.1016/j.canlet.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Veillat V, Spuul P, Daubon T, Egana I, Kramer I, Genot E. Podosomes: Multipurpose organelles? The international journal of biochemistry & cell biology. 2015;65:52–60. doi: 10.1016/j.biocel.2015.05.020. [DOI] [PubMed] [Google Scholar]
- [39].Cao H, Eppinga RD, Razidlo GL, Krueger EW, Chen J, Qiang L, McNiven MA. Stromal fibroblasts facilitate cancer cell invasion by a novel invadopodia-independent matrix degradation process. Oncogene. 2015 doi: 10.1038/onc.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. The Journal of cell biology. 2012;196:375–385. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Goicoechea SM, Garcia-Mata R, Staub J, Valdivia A, Sharek L, McCulloch CG, Hwang RF, Urrutia R, Yeh JJ, Kim HJ, Otey CA. Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene. 2014;33:1265–1273. doi: 10.1038/onc.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends in cell biology. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- [43].Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer research. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- [44].Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer research. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- [45].Clark ES, Weaver AM. A new role for cortactin in invadopodia: regulation of protease secretion. European journal of cell biology. 2008;87:581–590. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Greco MR, Antelmi E, Busco G, Guerra L, Rubino R, Casavola V, Reshkin SJ, Cardone RA. Protease activity at invadopodial focal digestive areas is dependent on NHE1-driven acidic pHe. Oncology reports. 2014;31:940–946. doi: 10.3892/or.2013.2923. [DOI] [PubMed] [Google Scholar]
- [47].Artym VV, Kindzelskii AL, Chen WT, Petty HR. Molecular proximity of seprase and the urokinase-type plasminogen activator receptor on malignant melanoma cell membranes: dependence on beta1 integrins and the cytoskeleton. Carcinogenesis. 2002;23:1593–1601. doi: 10.1093/carcin/23.10.1593. [DOI] [PubMed] [Google Scholar]
- [48].Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, Chen WT. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7959–7964. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mueller SC, Ghersi G, Akiyama SK, Sang QX, Howard L, Pineiro-Sanchez M, Nakahara H, Yeh Y, Chen WT. A novel protease-docking function of integrin at invadopodia. The Journal of biological chemistry. 1999;274:24947–24952. doi: 10.1074/jbc.274.35.24947. [DOI] [PubMed] [Google Scholar]
- [50].Diaz B, Yuen A, Iizuka S, Higashiyama S, Courtneidge SA. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. The Journal of cell biology. 2013;201:279–292. doi: 10.1083/jcb.201209151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- [52].Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Becherer JD, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- [53].Izumi Y, Hirata M, Hasuwa H, Iwamoto R, Umata T, Miyado K, Tamai Y, Kurisaki T, Sehara-Fujisawa A, Ohno S, Mekada E. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. The EMBO journal. 1998;17:7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Koshikawa N, Mizushima H, Minegishi T, Iwamoto R, Mekada E, Seiki M. Membrane type 1-matrix metalloproteinase cleaves off the NH2-terminal portion of heparin-binding epidermal growth factor and converts it into a heparin-independent growth factor. Cancer research. 2010;70:6093–6103. doi: 10.1158/0008-5472.CAN-10-0346. [DOI] [PubMed] [Google Scholar]
- [56].Chan KM, Wong HL, Jin G, Liu B, Cao R, Cao Y, Lehti K, Tryggvason K, Zhou Z. MT1-MMP inactivates ADAM9 to regulate FGFR2 signaling and calvarial osteogenesis. Developmental cell. 2012;22:1176–1190. doi: 10.1016/j.devcel.2012.04.014. [DOI] [PubMed] [Google Scholar]
- [57].Enderling H, Alexander NR, Clark ES, Branch KM, Estrada L, Crooke C, Jourquin J, Lobdell N, Zaman MH, Guelcher SA, Anderson AR, Weaver AM. Dependence of invadopodia function on collagen fiber spacing and cross-linking: computational modeling and experimental evidence. Biophysical journal. 2008;95:2203–2218. doi: 10.1529/biophysj.108.133199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Weaver AM. Invadopodia. Curr Biol. 2008;18:R362–364. doi: 10.1016/j.cub.2008.02.028. [DOI] [PubMed] [Google Scholar]
- [59].Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nature reviews. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- [60].Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, Konig I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–345. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Beaty BT, Condeelis J. Digging a little deeper: The stages of invadopodium formation and maturation. European journal of cell biology. 2014 doi: 10.1016/j.ejcb.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. Journal of cell science. 2009;122:3037–3049. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Destaing O, Block MR, Planus E, Albiges-Rizo C. Invadosome regulation by adhesion signaling. Current opinion in cell biology. 2011;23:597–606. doi: 10.1016/j.ceb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- [64].Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annual review of cell and developmental biology. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- [65].Saltel F, Daubon T, Juin A, Ganuza IE, Veillat V, Genot E. Invadosomes: intriguing structures with promise. European journal of cell biology. 2011;90:100–107. doi: 10.1016/j.ejcb.2010.05.011. [DOI] [PubMed] [Google Scholar]
- [66].Linder S. Invadosomes at a glance. Journal of cell science. 2009;122:3009–3013. doi: 10.1242/jcs.032631. [DOI] [PubMed] [Google Scholar]
- [67].Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N. Self-organized podosomes are dynamic mechanosensors. Curr Biol. 2008;18:1288–1294. doi: 10.1016/j.cub.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Parekh A, Weaver AM. Regulation of cancer invasiveness by the physical extracellular matrix environment. Cell adhesion & migration. 2009;3:288–292. doi: 10.4161/cam.3.3.8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Parekh A, Ruppender NS, Branch KM, Sewell-Loftin MK, Lin J, Boyer PD, Candiello JE, Merryman WD, Guelcher SA, Weaver AM. Sensing and modulation of invadopodia across a wide range of rigidities. Biophysical journal. 2011;100:573–582. doi: 10.1016/j.bpj.2010.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gu Z, Liu F, Tonkova EA, Lee SY, Tschumperlin DJ, Brenner MB. Soft matrix is a natural stimulator for cellular invasiveness. Molecular biology of the cell. 2014;25:457–469. doi: 10.1091/mbc.E13-05-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nature reviews. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- [73].Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nature cell biology. 2012;14:777–783. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- [75].Beningo KA, Wang YL. Flexible substrata for the detection of cellular traction forces. Trends in cell biology. 2002;12:79–84. doi: 10.1016/s0962-8924(01)02205-x. [DOI] [PubMed] [Google Scholar]
- [76].Jerrell RJ, Parekh A. Polyacrylamide gels for invadopodia and traction force assays on cancer cells. J Vis Exp. 2015 doi: 10.3791/52343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Jerrell RJ, Parekh A. Cellular traction stresses mediate extracellular matrix degradation by invadopodia. Acta biomaterialia. 2014;10:1886–1896. doi: 10.1016/j.actbio.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Aung A, Seo YN, Lu S, Wang Y, Jamora C, del Alamo JC, Varghese S. 3D traction stresses activate protease-dependent invasion of cancer cells. Biophysical journal. 2014;107:2528–2537. doi: 10.1016/j.bpj.2014.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Branch KM, Hoshino D, Weaver AM. Adhesion rings surround invadopodia and promote maturation. Biol Open. 2012;1:711–722. doi: 10.1242/bio.20121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bourguignon LY, Zhu H, Shao L, Chen YW. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. The Journal of biological chemistry. 2001;276:7327–7336. doi: 10.1074/jbc.M006498200. [DOI] [PubMed] [Google Scholar]
- [81].Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Experimental cell research. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- [82].Hauck CR, Hsia DA, Ilic D, Schlaepfer DD. v-Src SH3-enhanced interaction with focal adhesion kinase at beta 1 integrin-containing invadopodia promotes cell invasion. The Journal of biological chemistry. 2002;277:12487–12490. doi: 10.1074/jbc.C100760200. [DOI] [PubMed] [Google Scholar]
- [83].Beaty BT, Sharma VP, Bravo-Cordero JJ, Simpson MA, Eddy RJ, Koleske AJ, Condeelis J. beta1 integrin regulates Arg to promote invadopodial maturation and matrix degradation. Molecular biology of the cell. 2013;24:1661–1675. doi: 10.1091/mbc.E12-12-0908. S1661-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Antelmi E, Cardone RA, Greco MR, Rubino R, Di Sole F, Martino NA, Casavola V, Carcangiu M, Moro L, Reshkin SJ. ss1 integrin binding phosphorylates ezrin at T567 to activate a lipid raft signalsome driving invadopodia activity and invasion. PLoS ONE. 2013;8:e75113. doi: 10.1371/journal.pone.0075113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Williams KC, Coppolino MG. SNARE-dependent interaction of Src, EGFR and beta1 integrin regulates invadopodia formation and tumor cell invasion. Journal of cell science. 2014;127:1712–1725. doi: 10.1242/jcs.134734. [DOI] [PubMed] [Google Scholar]
- [86].Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. The Journal of cell biology. 2006;172:259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Spinardi L, Rietdorf J, Nitsch L, Bono M, Tacchetti C, Way M, Marchisio PC. A dynamic podosome-like structure of epithelial cells. Experimental cell research. 2004;295:360–374. doi: 10.1016/j.yexcr.2004.01.007. [DOI] [PubMed] [Google Scholar]
- [89].Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21:635–644. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Revach OY, Weiner A, Rechav K, Sabanay I, Livne A, Geiger B. Mechanical interplay between invadopodia and the nucleus in cultured cancer cells. Scientific reports. 2015;5:9466. doi: 10.1038/srep09466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Labernadie A, Bouissou A, Delobelle P, Balor S, Voituriez R, Proag A, Fourquaux I, Thibault C, Vieu C, Poincloux R, Charriere GM, Maridonneau-Parini I. Protrusion force microscopy reveals oscillatory force generation and mechanosensing activity of human macrophage podosomes. Nature communications. 2014;5:5343. doi: 10.1038/ncomms6343. [DOI] [PubMed] [Google Scholar]
- [92].Labernadie A, Thibault C, Vieu C, Maridonneau-Parini I, Charriere GM. Dynamics of podosome stiffness revealed by atomic force microscopy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21016–21021. doi: 10.1073/pnas.1007835107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].van den Dries K, Meddens MB, de Keijzer S, Shekhar S, Subramaniam V, Figdor CG, Cambi A. Interplay between myosin IIA-mediated contractility and actin network integrity orchestrates podosome composition and oscillations. Nature communications. 2013;4:1412. doi: 10.1038/ncomms2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Beaty BT, Wang Y, Bravo-Cordero JJ, Sharma VP, Miskolci V, Hodgson L, Condeelis J. Talin regulates moesin-NHE-1 recruitment to invadopodia and promotes mammary tumor metastasis. The Journal of cell biology. 2014;205:737–751. doi: 10.1083/jcb.201312046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wang JH, Lin JS. Cell traction force and measurement methods. Biomechanics and modeling in mechanobiology. 2007;6:361–371. doi: 10.1007/s10237-006-0068-4. [DOI] [PubMed] [Google Scholar]
- [96].Ribeiro AJ, Denisin AK, Wilson RE, Pruitt BL. For whom the cells pull: Hydrogel and micropost devices for measuring traction forces. Methods. 2015 doi: 10.1016/j.ymeth.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature cell biology. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- [98].van den Dries K, Schwartz SL, Byars J, Meddens MB, Bolomini-Vittori M, Lidke DS, Figdor CG, Lidke KA, Cambi A. Dual-color superresolution microscopy reveals nanoscale organization of mechanosensory podosomes. Molecular biology of the cell. 2013;24:2112–2123. doi: 10.1091/mbc.E12-12-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sharma VP, Eddy R, Entenberg D, Kai M, Gertler FB, Condeelis J. Tks5 and SHIP2 regulate invadopodium maturation, but not initiation, in breast carcinoma cells. Curr Biol. 2013;23:2079–2089. doi: 10.1016/j.cub.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Oakes PW, Beckham Y, Stricker J, Gardel ML. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. The Journal of cell biology. 2012;196:363–374. doi: 10.1083/jcb.201107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochimica et biophysica acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kirkbride KC, Sung BH, Sinha S, Weaver AM. Cortactin: a multifunctional regulator of cellular invasiveness. Cell adhesion & migration. 2011;5:187–198. doi: 10.4161/cam.5.2.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bravo-Cordero JJ, Hodgson L, Condeelis JS. Spatial regulation of tumor cell protrusions by RhoC. Cell adhesion & migration. 2014;8:263–267. doi: 10.4161/cam.28405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer metastasis reviews. 2009;28:137–149. doi: 10.1007/s10555-008-9176-1. [DOI] [PubMed] [Google Scholar]
- [105].Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) The Journal of biological chemistry. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- [106].Hoskin V, Szeto A, Ghaffari A, Greer PA, Cote GP, Elliott BE. Ezrin regulates focal adhesion and invadopodia dynamics by altering calpain activity to promote breast cancer cell invasion. Molecular biology of the cell. 2015 doi: 10.1091/mbc.E14-12-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Scott RW, Hooper S, Crighton D, Li A, Konig I, Munro J, Trivier E, Wickman G, Morin P, Croft DR, Dawson J, Machesky L, Anderson KI, Sahai EA, Olson MF. LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. The Journal of cell biology. 2010;191:169–185. doi: 10.1083/jcb.201002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chen W-T, Yeh Y, Nakahara H. An in vitro cell invasion assay: Determination of cell surface proteolytic activity that degrades extracellular matrix. Journal of Tissue Culture Methods. 1994;16:177–181. [Google Scholar]
- [109].Weaver AM, Page JM, Guelcher SA, Parekh A. Synthetic and tissue-derived models for studying rigidity effects on invadopodia activity. Methods in molecular biology (Clifton, N.J. 2013;1046:171–189. doi: 10.1007/978-1-62703-538-5_10. [DOI] [PubMed] [Google Scholar]
- [110].Genot E, Gligorijevic B. Invadosomes in their natural habitat. European journal of cell biology. 2014;93:367–379. doi: 10.1016/j.ejcb.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Gligorijevic B, Bergman A, Condeelis J. Multiparametric classification links tumor microenvironments with tumor cell phenotype. PLoS biology. 2014;12:e1001995. doi: 10.1371/journal.pbio.1001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. The Journal of cell biology. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer research. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- [114].Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- [115].Arsenault D, Brochu-Gaudreau K, Charbonneau M, Dubois CM. HDAC6 deacetylase activity is required for hypoxia-induced invadopodia formation and cell invasion. PLoS ONE. 2013;8:e55529. doi: 10.1371/journal.pone.0055529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, Stanger BZ. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gore J, Korc M. Pancreatic cancer stroma: friend or foe? Cancer cell. 2014;25:711–712. doi: 10.1016/j.ccr.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Artym VV, Yamada KM, Mueller SC. ECM degradation assays for analyzing local cell invasion. Methods in molecular biology (Clifton, N.J. 2009;522:211–219. doi: 10.1007/978-1-59745-413-1_15. [DOI] [PubMed] [Google Scholar]