Abstract
OBJECTIVE
To test the relationship of anxiety to caloric intake and food cue perception in women and men.
METHODS
Fifty-five twins (26 complete, 3 incomplete pairs; 51% women) underwent 2 functional magnetic resonance imaging (fMRI) scans (before and after a standardized meal) and then ate at an ad libitum buffet to objectively assess food intake. State and trait anxiety were assessed using the State-Trait Anxiety Inventory. During the fMRI scans, participants viewed blocks of fattening and non-fattening food images, and non-food objects.
RESULTS
In women, higher trait anxiety was associated with a higher body mass index (BMI) (r=0.40, P=0.010). Trait anxiety was positively associated with kilocalories consumed at the buffet (r=0.53, P=0.005) and percent kilocalories consumed from fat (r=0.30, P=0.006), adjusted for BMI. In within-pair models, which control for shared familial and genetic factors, higher trait anxiety remained associated with kilocalories consumed at the buffet (ρ=0.66, P=0.014), but not with BMI. In men, higher state anxiety was related to macronutrient choices, but not to total caloric intake or BMI. FMRI results revealed that women with high trait anxiety did not suppress activation by fattening food cues across brain regions associated with satiety perception after eating a standardized meal (mean difference low anxiety: −15.4, P<0.001; high anxiety: −1.53, P=0.82, adjusted for BMI).
CONCLUSIONS
In women, trait anxiety may promote excess caloric consumption through altered perception of high-calorie environmental food cues, placing women with genetic predispositions toward weight gain at risk of obesity.
Keywords: anxiety, obesity, twins, BMI, fMRI, caloric intake
Introduction
By report, anxiety is the most common mental illness in the United States, affecting 18% of the population (1). Cross-sectional studies have consistently found positive associations between obesity and anxiety (2). Much of this work has focused on clinical anxiety disorders (3–6) and is limited to epidemiologic data that cannot discern mechanisms that might account for these associations. However, another line of research suggests that anxiety itself could promote excess food intake. For example, participants with high trait anxiety increase energy intake when exposed to experimental situations that induce stress and anxiety (7, 8). Such findings raise the question of whether anxiety could predispose individuals to weight gain, perhaps via alterations in eating behaviors and/or neural mechanisms. We therefore sought to better understand both the relationship of anxiety to body mass index (BMI) in a genetically-informative community-based population sample of twins and the neural responsiveness to food cues among participants with high levels of anxiety.
Prior functional magnetic resonance imaging (fMRI) studies provide insight into the specific brain regions in which altered responsiveness to food cues could link anxiety to overeating and obesity. Brain regions that are differentially activated in response to high-calorie visual food cues include the orbital frontal cortex, amygdala, insula, dorsal striatum and nucleus accumbens (9–12). Responses in these regions are greater when subjects are hungry compared to when they are satiated (12–14), and, critically, amygdalar, nucleus accumbens, and medial orbital frontal cortex (mOFC) responses predict subsequent choice of high-fat food (12). Moreover, obese women, regardless of their hunger state, exhibit greater activation than lean controls in response to high-calorie food cues in regions associated with food motivation, such as the dorsal striatum (15, 16). Finally, men and women differ in their reactivity to food cues such that women were more responsive to food cues in regions related to food-processing, such as prefrontal and parietal regions, irrespective of hunger state or satiety, compared to men (17–19). In sum, fMRI using visual food cues has documented potentially sex-specific alterations in brain regulation of appetite in obesity, but no studies have used these techniques to examine the effect of anxiety on these responses.
Given this background, we sought to understand relationships between anxiety, eating behavior and neural responses to visual food cues in men and women. Using the State-Trait Anxiety Inventory (STAI), we determined state (current anxiety induced by a situation) and trait (global and enduring) anxiety scores of all participants. Trait anxiety has been shown to affect eating behavior in both lean and obese subjects as well as influence ratings of sweet and bitter flavors, but other studies indicate that state anxiety predicts increased food intake (7, 8, 20, 21). We therefore studied each component of anxiety separately, stratifying our analyses by sex. Next, we used fMRI to understand the potential neural mechanisms linking anxiety to disrupted eating behavior, hypothesizing that high levels of anxiety would be associated with greater food intake and greater activation in response to “fattening” food cues in brain regions implicated in satiety, food choice, and food reward (12, 16). Finally, both anxiety (22) and obesity (23) have strong heritability components. Since our study recruited same-sex monozygotic (MZ) and dizygotic (DZ) twin pairs, we were able to test for confounding by inherited factors in the relationship between anxiety and obesity.
Methods
Participants
MZ and DZ twin pairs were recruited from the community-based University of Washington Twin Registry. Registry composition and procedures are described in an earlier publication (24). MZ and DZ same-sex twin pairs that met study criteria (aged 18–50, BMI 18.5–45kg/m2 and raised together until at least age 15) were selected using a blocked randomized technique to acquire equal numbers of female and male twin pairs. The exclusion criteria included behaviors or conditions that could alter appetite or neural responses to food cues and included: major medical problems (e.g., diabetes mellitus); allergies to foods used in the study; lactose intolerance or inability to taste; vegetarian or vegan diet; daily smoking or heavy alcohol consumption (≥3 drinks per day for males; ≥2 drinks per day for females); medications that affect appetite (e.g., atypical anti-psychotics); a lifetime history of weight loss surgery or eating disorders; excessive exercise (≥8hrs/day); night work; pregnancy or breastfeeding; current participation in weight loss program or use of weight loss medications; and contraindication to MRI. Both individuals within a twin pair had to be eligible in order for the pair to participate. Data were collected over 17 months between 2012 and 2013 and all participants provided informed consent in accordance with procedures approved by the University of Washington Human Subjects Committee.
Twenty-nine twin pairs (MZ and DZ), 58 total individuals, completed the study visit day. Self-reported race was 80% white, 4% Asian, 4% black, and 12% as other or more than one race. Three female participants were excluded from analyses due to illness unrelated to the study, resulting in our final sample of 55 subjects. In addition, 2 participants (1 male, 1 female) were excluded from fMRI analyses due to poor attention to the task (e.g. falling asleep).
Study Procedures
Twins began fasting at 9:30 PM the night before the study visit. At 8:00 A.M., Twin 1 was given a standardized breakfast meal of egg and cheese on an English muffin served with orange juice (15% protein, 35% fat, 50% carbohydrate). The breakfast was titrated to represent 10% of estimated daily caloric requirements (calculated by Mifflin-St. Jeor equation and an activity factor) (25). Twins completed eating behavior questionnaires (see below) and their height and weight were measured to calculate BMI. At 11:30 A.M., Twin 1 completed the first fMRI then had 15 minutes to consume a standardized meal of macaroni and cheese (20% of their daily caloric need; 10% protein, 50% fat, 40% carbohydrate). At 12:30 PM, Twin 1 completed a second fMRI and was then taken to a private room, usually consisting of only a table and chair, where they had 30 minutes to eat at an ad libitum buffet. Participants were not informed the buffet was part of study procedures nor that their food consumption was monitored until a debriefing session that concluded the study. Twin 2 followed the same study procedures as Twin 1 but began at 8:30 A.M. Twin order was counterbalanced by BMI and was assigned prior to the visit day by a statistician who did not interact with study participants. Twins were separated during all meals to prevent any appetite stimulation from food cues or social influences.
Questionnaires
Visual analog scale (VAS) ratings of appetite and nervousness were administered every 30 minutes beginning at 8:00 AM. Twins rated their current nervousness, hunger, fullness and prospective food intake on a 1–100 millimeter scale (26). VAS scores for nervousness were averaged over the whole study day.
Eating behavior was assessed with the Three-Factor Eating Questionnaire-R18 (TFEQ-R18), an 18-item questionnaire with 3 subscales that measure different aspects of eating behavior (unrestrained eating, emotional eating, and cognitive restraint) (27).
Anxiety was evaluated with the State-Trait Anxiety Inventory (STAI) questionnaire. Trait anxiety was measured at the start of the study day and state anxiety was assessed prior to each fMRI scan. The STAI consists of 40-items with 2 subscales, both made of 20-items. Trait anxiety items include: “I worry too much over something that really doesn’t matter” and “I am content; I am a steady person”; whereas state anxiety items include: “I am tense; I am worried” and “I feel calm; I feel secure”. All items are rated on a 4-point scale (e.g., from “Almost Never” to “Almost Always”). Higher scores indicate greater anxiety and a score of 39–40 and above in either section constitutes a clinically significant score of anxiety (28, 29). State anxiety scores obtained prior to the second MRI were used in analyses to reflect the anxiety state during the ad libitum buffet.
Ad libitum caloric intake
Total caloric and macronutrient intake were measured using an ad libitum buffet presented to each twin privately and containing a variety of foods appropriate for a midday meal. The buffet lunch was presented as a thank you for study participation and subjects were not informed food intake was monitored until the debrief session at the conclusion of the study. Food was presented in amounts that widely exceeded each participant’s estimated energy needs (~5000 kilocalories provided) and differed in caloric and macronutrient content as well as hedonic appeal (e.g. bagels, turkey, fruit, pastries). All uneaten food was weighed to determine kilocalories and macronutrient percentages consumed (ProNutra, Viocare Technologies, Princeton, NJ).
Food cue images
Selection and validation of study images were completed in an independent study and have been described previously (12, 30). In sum, “fattening” foods include those rated as unacceptable to eat while dieting to lose weight and were universally characterized by high caloric, fat, and/or sugar content (e.g. candy, desserts, pizzas). “Non-fattening” foods were rated as acceptable to eat while dieting and were lower in kilocalories (e.g. fruits, vegetables, chicken breast).
Imaging paradigm
The imaging paradigm used in the current study has been described elsewhere (12). FMRI sessions consisted of 13 blocks of 10 photographs (7 non-food, alternating with 3 fattening and 3 non-fattening food blocks). Each image was viewed for 2.4 s. Block order was counterbalanced between pairs, but was matched between twins so that each twin pair viewed the blocks in the same order. Non-food images were easily recognizable objects (e.g. pencils, chairs, books). After each scan, twins were asked to distinguish images they had viewed in the scanner from distractor images.
Image acquisition and processing
Scans were acquired with a 32-channel SENSE head coil on a 3-Tesla Philips Achieva MR System (Philips Medical Systems, Best, The Netherlands) with dual Quasar gradients (80mT/m at a slew rate of 110 mT/m/s or 40 mT/m at a slew rate of 220 mT/m/s). In both sessions, a 133 volume, T2*-weighted single-shot echo-planar imaging (EPI) timeseries (44 ascending axial slices, 2.75 × 2.75 × 3.00 mm voxels, repetition time (TR)=2400 ms, echo time (TE)=30 ms, SENSE factor=2) was acquired during passive picture viewing. A B0 field map (TR=10ms; minimum TE=2.8 ms; delta TE 1.0 ms; flip angle=10°) with the same geometry was acquired for distortion correction of the EPI data. A 3D Magnetization-Prepared Rapid Gradient-Echo (MPRAGE) image with 176 sagittal slices (TR=7.5, TE=3.5 ms, flip angle=7°, SENSE factor=2, matrix=256 × 256, 1 mm isotropic voxels) was also acquired in one session for registration of the functional data to standard space.
Timeseries data were processed using tools from FSL (Functional MRI of the Brain (FMRIB) Software Library, www.fmrib.ox.ac.uk/fsl), FreeSurfer (http://surfer.nmr.mgh.harvard.edu/), and AFNI (Analysis of Functional NeuroImages, http://afni.nimh.nih.gov/afni). The following preprocessing steps were applied to the functional data: simultaneous application of fieldmap-based EPI unwarping (FUGUE) (31, 32) and motion correction (MCFLIRT) (33); bias-field correction (FAST) (34); removal of spike artifacts with 3dDespike; correction for slice timing differences using Fourier space-time shifting implemented in slicetimer; mask-based removal of non-brain tissue; spatial smoothing with SUSAN with FWHM=5mm; grand-mean intensity normalization by a single multiplicative factor; high-pass temporal filtering of 90 seconds. The time series statistical analysis was performed with FMRIB’s Improved Linear Model with local autocorrelation correction (35). The regression model included covariates for the fattening and non-fattening stimulus conditions, as well as mean-centered nuisance covariates (average signal time courses in white matter and lateral ventricles defined by FreeSurfer segmentation, motion parameter estimates, and the first derivative of each motion estimate). Each block of fattening and non-fattening visual stimuli was modeled using a boxcar convolved with a gamma function and its temporal derivative. Condition effects were estimated from the average response across blocks for our contrast of interest (fattening vs. non-fattening).
FMRI data for each session were registered to the participant’s high-resolution structural scan using a boundary-based registration procedure (36). The high-resolution structural scans were then registered to the Montreal Neurological Institute template space (ICBM152) with FMRIB’s linear image registration tool (33). For each participant, the derived transformations were concatenated and applied to the statistical images to allow for group-level analyses.
A region of interest (ROI) approach was applied utilizing masks established in an independent study (12). Anatomical areas were chosen based on their known responsiveness to food cues (10–12) or physiologic satiety signals (37). ROIs were established in an independent sample of 23 healthy fasted participants and were functionally defined as voxels exhibiting a greater BOLD response to fattening food cues vs. non-food objects. These functionally-defined areas were combined with anatomical areas defined by the Harvard-Oxford probabilistic atlas (38) and included the bilateral ventral striatum (nucleus accumbens), bilateral amygdala, bilateral dorsal striatum (caudate and putamen), bilateral insula, and mOFC. An overall average of brain activation was calculated for each participant from our a priori ROIs for Pre- and Post-standardized meal.
Nested case-control analysis of anxiety and brain activation
Cases and controls were defined based on STAI trait anxiety scores. A STAI trait anxiety score ≥40 identifies clinically significant anxiety (28) and was used to define high anxiety in this study. There is no established low anxiety category for the STAI, therefore we set the cutoff for low anxiety scores at one standard deviation away from the sex-specific group mean trait anxiety score. This resulted in a low anxiety cutoff of 30 for both men and women. Of those participants with usable fMRI data, 12 women and 12 men qualified as high anxiety, while 8 women and 8 men had STAI trait anxiety scores <30, and were classified as low anxiety participants.
Statistical Analysis
Unless otherwise noted, analyses were performed with generalized estimating equations to account for relatedness within twins. For adjusted values, scatter plots were generated by mean centering the dependent and independent variables, given the mean of the covariate of interest (via regression model). The residuals were determined for each mean-centered variable, and the corresponding mean was added back to each individual’s value for graphing purposes only. Within-pair analyses (N=13 complete pairs) were performed to observe the effects of anxiety while controlling for genetics and other factors including age and shared environment. R values presented were derived from Pearson’s correlations. Spearman’s rank correlations were performed due to the smaller sample size when pairs were the unit of analysis. Effect size was calculated for all significant fMRI outcomes with Cohen’s d for repeated measures. Statistics and graphing were completed using STATA (13.1, College Station, TX) and GraphPad Prism (Version 6.00 for Windows, La Jolla, CA).
Results
Participant characteristics
The final study sample included 55 participants (27 males, 28 females) of which 41 were identified as an MZ twin and 14 as a DZ twin. No significant differences in age, BMI, trait and state anxiety scores, or VAS scores were found between men and women (Table 1), but, as expected, men ate more at the ad libitum buffet (P=0.005).
Table 1.
Subject characteristics by sex.
| Women | Men | Total | |
|---|---|---|---|
| Total number of individuals, N | 28 | 27 | 55 |
| MZ pairs (individuals), N | 8 (18) | 11 (23) | 19 (41) |
| DZ pairs (individuals), N | 5 (10) | 2 (4) | 7 (14) |
| Age, years | 27 ± 7.7 | 27 ± 7.5 | 27 ± 7.6 |
| BMI, kg/m2 | 30 ± 7.3 | 28 ± 5.8 | 28.9 ± 6.6 |
| Trait Anxiety score | 37 ± 9.0 | 39 ± 10 | 37.8 ± 9.4 |
| State Anxiety score | 29 ± 6.6 | 32 ± 8.2 | 30.5 ± 7.5 |
| Emotional Eating score | 7.3 ± 2.4 | 6.0 ± 1.9 | 6.6 ± 2.2 |
| Average Nervousness rating, mm | 10.5 ± 10.6 | 12 ± 13 | 11 ± 12 |
| Hunger, mm | 42 ± 21 | 54 ± 21 | 48 ± 22 |
| Fullness, mm | 39 ± 20 | 30 ± 19 | 34 ± 20 |
| Willingness to eat, mm | 56 ± 18 | 65 ± 20 | 61 ± 19 |
| Kilocalories consumed at the buffet, kcals | 986 ± 382 | 1304 ± 394* | 1142 ± 417 |
Values are means ± standard deviations. Visual analog scale ratings of hunger, fullness and willingness to eat were obtained immediately prior to the ad libitum buffet and are measured in millimeters (mm). All DZ twin pairs were complete. MZ: monozygotic; DZ: dizygotic; BMI: body mass index; kcals: kilocalories. P-values derived from generalized estimating equations.
P=0.005 vs. Women.
Relationship of anxiety to nervousness and subjective appetite in women and men
In women, higher trait and state anxiety scores were significantly associated with greater average nervousness ratings (r=0.46, P=0.003 and r=0.65, P<0.001, respectively), indicating convergent validity. These relationships remained significant when BMI was included in the model (Figure 1A–B). No relationships were found in women between trait or state anxiety and self-reported fullness (r=0.30, P=0.056; r=−0.08, P=0.67), willingness to eat (r=−0.08, P=0.65; r=0.07, P=0.65) or hunger (r=−0.12, P=0.49; r=0.24, P=0.065) prior to the ad libitum buffet.
Figure 1. Sex-specific associations between trait and state anxiety and average nervousness rating (mm).
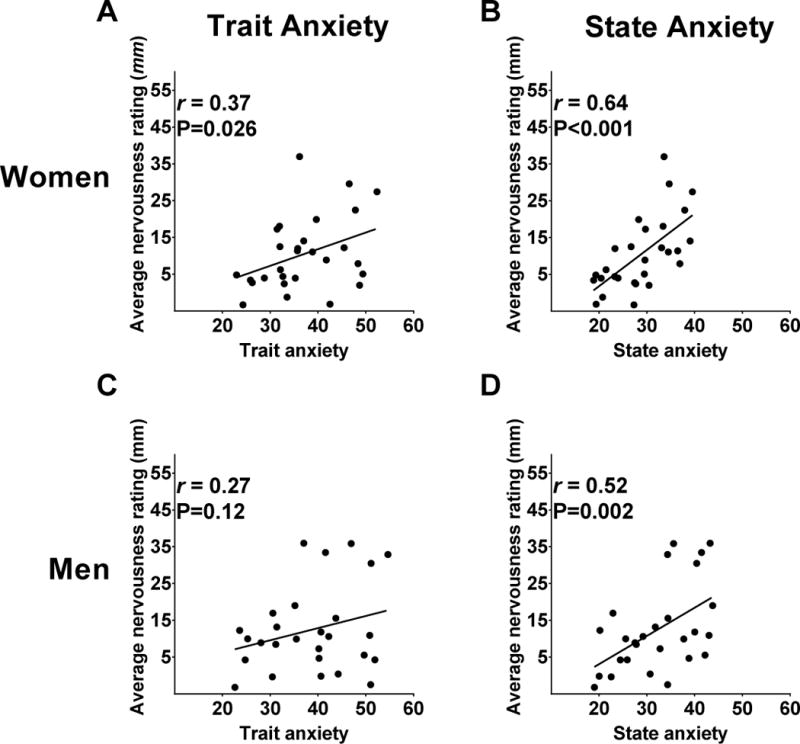
In women, average visual analog scale nervousness ratings were associated with A) trait anxiety, and B) state anxiety (from State-Trait Anxiety Inventory). In men, average nervousness ratings were associated with D) state anxiety only. P-values derived from generalized estimating equations and r-values from Pearson’s Product-moment correlation. Data are adjusted for BMI.
In men, higher average scores of nervousness were also correlated to higher trait (r=0.34, P=0.039) and state (r=0.56, P=0.005) anxiety, but only the association between state anxiety and average scores of nervousness was independent of BMI (Figure 1C–D). No relationships were found in men between trait or state anxiety and self-reported fullness (r=0.33, P=0.13; r=0.13, P=0.63), willingness to eat (r=0.02, P=0.93; r=0.02, P=0.93) or hunger (r=−0.04, P=0.83; r=0.14, P=0.50) prior to the ad libitum buffet.
Relationships between anxiety, BMI and eating behaviors in men and women
In women, trait anxiety scores were positively associated with BMI (Figure 2A), emotional eating scores (r=0.52, P=0.001) and kilocalories consumed at the buffet (r=0.53, P=0.001), but state anxiety scores were not (r=0.20, P=0.28; r=0.29, P=0.13 and r=0.28, P=0.095, respectively). The association between trait anxiety and caloric intake at the buffet, but not emotional eating, persisted after controlling for BMI (Figure 2B–C).
Figure 2. Sex-specific relationships between trait anxiety, BMI, emotional eating and ad libitum buffet intake.
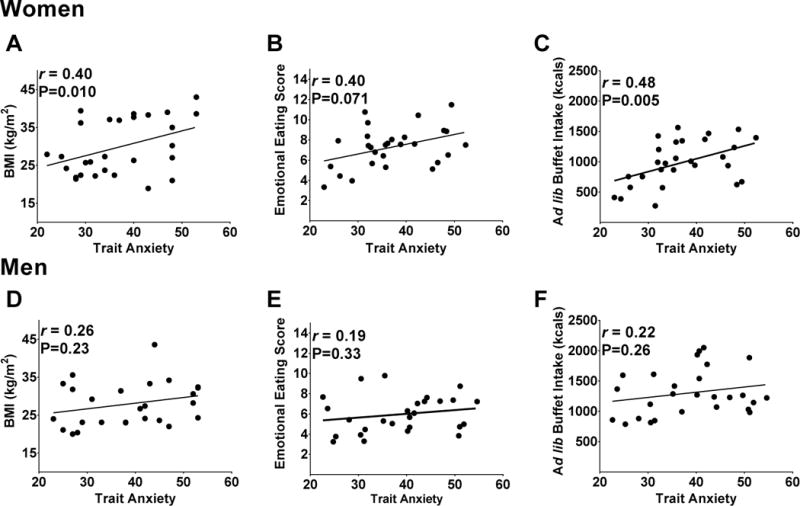
Associations in women are shown between trait anxiety and A) BMI (kg/m2), B) TFEQ-R18 emotional eating subscale scores and C) kilocalories consumed during the ad libitum buffet. In men, associations were not present between trait anxiety and D) BMI (kg/m2), E) TFEQ-R18 emotional eating subscale scores, or F) kilocalories consumed during the ad libitum buffet. P-values derived from generalized estimating equations and r-values from Pearson’s Product-moment correlation. TFEQ-R18 =Three-Factor Eating Questionnaire-Revised 18 item version; BMI=body mass index. Data are adjusted for BMI.
Additionally, trait anxiety scores in women were positively correlated with kilocalories consumed from fat (r=0.30, P=0.033) and negatively correlated with kilocalories consumed from carbohydrates (r=−0.28, P=0.028), but not associated with protein (r=0.06, P=0.76) consumed at the ad libitum buffet. These relationships remained after adjusting for BMI (Figure 3A–C).
Figure 3. Sex-specific relationships between trait anxiety (women) or state anxiety (men) and macronutrient choice.
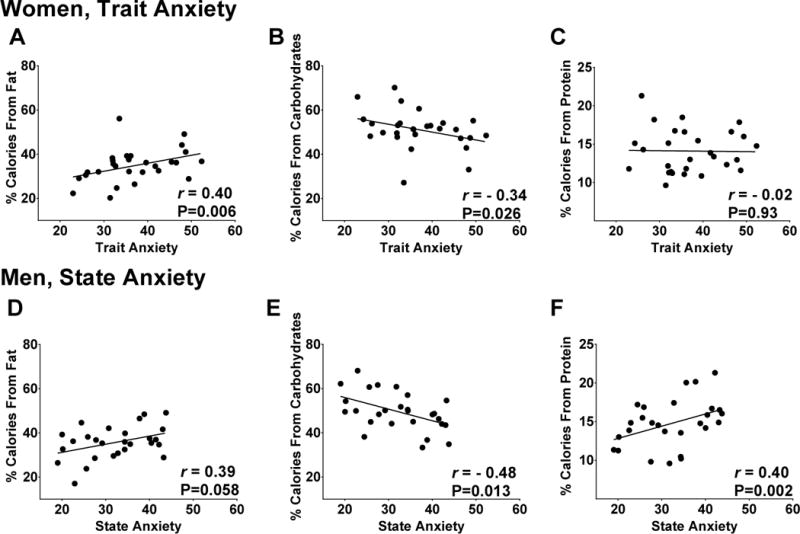
In women, associations were present between trait anxiety and percentage of kilocalories consumed at the ad libitum buffet from A) fat and B) carbohydrates but not C) protein. In men, associations were present between state anxiety and percentage of kilocalories consumed from B) carbohydrates and C) protein; a trend was also present with A) fat. P-values derived from generalized estimating equations and r-values from Pearson’s Product-moment correlation. Trait and state anxiety scored derived from the State-Trait Anxiety Inventory (STAI); BMI=body mass index. Data are adjusted for BMI.
State anxiety scores in women were unrelated to macronutrient intake at the buffet (fat: r=0.12, P=0.50; carbohydrate: r=−0.04, P=0.81; protein: r=−0.19, P=0.34).
Contrary to the findings in women, men’s trait anxiety scores were unrelated to BMI (Figure 2D), emotional eating (r=0.23, P=0.29) or kilocalories consumed during the buffet (r=0.27, P=0.22) (Figure 2E–F). Similar to women, men’s state anxiety scores were unrelated to these measures (BMI: r=0.26, P=0.32; emotional eating: r=0.14, P=0.51; buffet kilocalorie intake: r=0.18, P=0.39).
Trait anxiety in men tended to be positively correlated with the percent of fat consumed at the ad libitum buffet (r=0.36, P=0.075) and negatively correlated with carbohydrate intake (r=−0.39, P=0.071), but unrelated to protein consumption (r=0.25, P=0.24). However, state anxiety was strongly and positively correlated with the percent of kilocalories consumed at the buffet from fat (r=0.45, P=0.032) and protein (r=0.44, P<0.001) and negatively associated with percent carbohydrate intake (r=−0.53, P=0.005). After controlling for BMI, the relationships between state anxiety and percentage of protein and carbohydrates remained significant, and a trend was present with percentage of fat consumed (Figure 3D–F).
Within twin pair analyses of relationships between anxiety and food intake
Within-twin pair models allow analyses to control for age, sex, genetics (100% among MZ; 50% among DZ pairs) and shared familial factors. In women, within-twin pair models revealed that trait anxiety scores were positively correlated with caloric intake during the buffet (Figure 4A). However, there was no relationship between trait anxiety and BMI (Figure 4B). In other words, the twin with higher trait anxiety was not necessarily the heavier twin, despite evidence that those women ate more at the buffet. Within-twin pair models between trait anxiety and fat intake revealed a persistent trend toward an association (ρ=0.54, P=0.058), but relationships were weakened for protein (ρ=0.20, P=0.52) and carbohydrate intake (ρ=−0.36, P=0.22) after controlling for inherited and familial factors. There were no significant within-pair associations in men (data not shown).
Figure 4. Associations of within-twin-pair differences in trait anxiety with total kilocalories consumed at the ad libitum buffet and BMI in women.
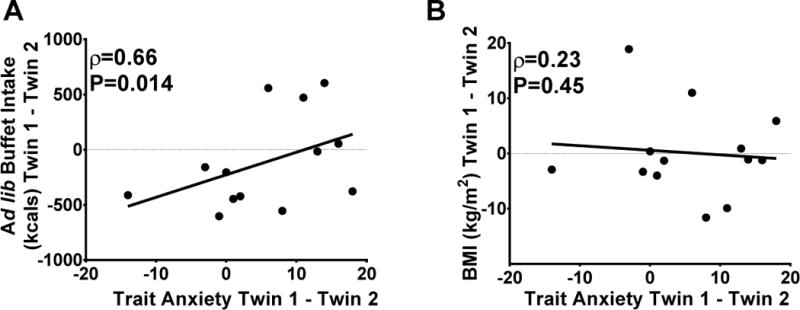
After controlling for age, family background, and genetics (100% in MZ, 50% in DZ), trait anxiety was positively related to A) kilocalories (kcals) consumed during the buffet meal but not to B) BMI. Spearman’s rho and P-values derived from Spearman’s Rank Correlation Coefficient. BMI= body mass index.
Relationships between anxiety and brain response to visual food cues
FMRI analyses were performed as a nested case-control study that included cases with clinically significant trait anxiety scores and controls with low trait anxiety scores, considering men and women separately. Participant characteristics are shown in Table 2. BMI did not differ between high and low anxiety women (Table 2). High-anxiety women had higher state anxiety scores, consumed more kilocalories during the ad libitum buffet and reported higher levels of VAS nervousness and fullness compared to low anxiety controls (Table 2). High anxiety men also had higher state anxiety scores and showed trends for higher VAS nervousness scores and consuming more kilocalories at the buffet compared to low anxiety men (Table 2).
Table 2.
Subject characteristics in the nested case-control study of anxiety.
| High Anxiety Women | Low Anxiety Women | High Anxiety Men | Low Anxiety Men | |
|---|---|---|---|---|
| Total number of individuals, N | 12 | 8 | 12 | 8 |
| Member of MZ pair, N | 6 | 7 | 9 | 8 |
| Member of DZ pair, N | 6 | 1 | 3 | 0 |
| Age, years | 25.8 ± 5.8 | 30.0 ± 10.3 | 29.4 ± 9.6 | 24.5 ± 5.4 |
| BMI, kg/m2 | 32.8 ± 7.9 | 28.1 ± 6.4 | 28.7 ± 4.5 | 26.2 ± 6.4 |
| Kilocalories consumed at the buffet, kcals | 1204 ± 369* | 747 ± 302 | 1454 ± 410# | 1063 ± 387 |
| Trait Anxiety score | 45.9 ± 4.7** | 27.3 ± 2.7 | 46.8 ± 4.8## | 26.4 ± 2.0 |
| State Anxiety score | 33.5 ± 5.1** | 24.8 ± 4.5 | 35.8 ± 6.4## | 24.3 ± 3.1 |
| Average Nervousness rating, mm | 15.7 ± 12.9* | 5.0 ± 5.0 | 16.0 ± 12.8# | 7.3 ± 6.4 |
| Hunger, mm | 42.0 ± 20.1 | 48.6 ± 12.26 | 54.8 ± 20.1 | 49.7 ± 17.8 |
| Fullness, mm | 41.1 ± 14.0* | 23.2 ± 17.7 | 33.3 ± 16.5 | 29.3 ± 19.3 |
| Willingness to eat, mm | 59.3 ± 13.2 | 65.0 ± 17.6 | 65.1 ± 17.9 | 61.0 ± 18.3 |
Values are means ± standard deviation. High anxiety was defined as a STAI trait anxiety score ≥40, a clinically significant anxiety score; low anxiety was defined as a STAI trait anxiety score ≤30, Visual analog scale appetite ratings of hunger, fullness and willingness to eat were obtained immediately prior to the ad libitum buffet. BMI: body mass index; kcals: kilocalories. P-values derived from generalized estimating equations.
P<0.050,
P<0.001 vs. low anxiety women.
P<0.060,
P<0.001 vs. low anxiety men.
FMRI findings averaged across all ROIs showed that, among low anxiety women, a meal induced expected reductions in brain activation by fattening (vs. non-fattening) food cues (mean parameter estimates±SEM, pre-meal 12.4±1.65 vs. post-meal −3.03±5.34; P<0.001, adjusted for BMI; Cohen’s d=1.64). The high anxiety group failed to demonstrate reductions in activation by fattening food cues (8.27±5.11 vs. 6.75±2.97, P=0.82). Subsequent region-by-region analyses revealed that the reductions (pre vs. post meal) in mean activation by fattening food cues were present in all examined regions (amygdala, insula, dorsal striatum, nucleus accumbens, and mOFC) in the low (P=0.001–0.036; Cohen’s d=0.66–1.35), but not the high (P=0.44–0.92), anxiety group. These results were confirmed with an omnibus test indicating a significant interaction of time x region (P=0.009) and remained after adjusting for BMI (Figure 5).
Figure 5. Brain activation by visual food cues pre- and post-standardized meal in low and high anxiety women.
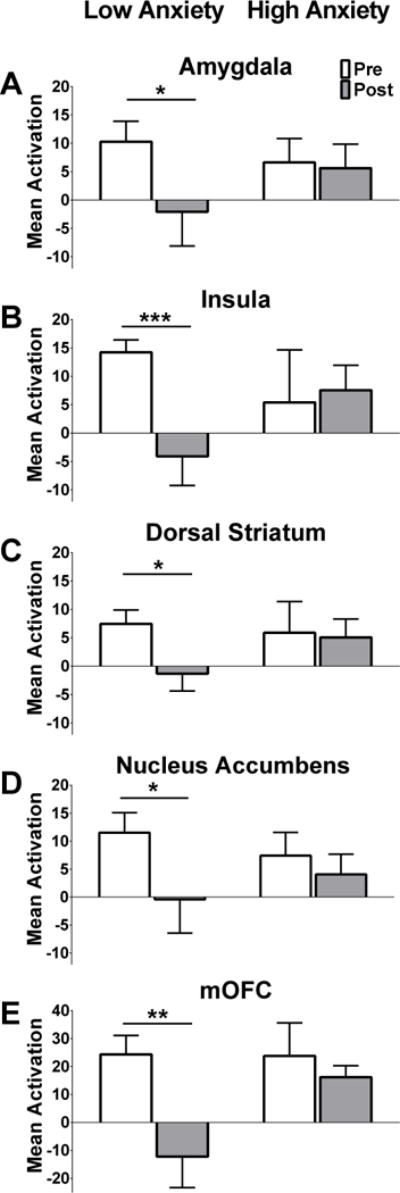
Among low anxiety women, brain activation by fattening vs. non-fattening food cues was significantly suppressed by a standardized meal in each of the five ROIs (A–E bars on left, Nucleus Accumbens, Amygdala, Insula, Dorsal Striatum, and mOFC). Women with high anxiety did not show meal-induced changes in brain activation in any of the ROIs (A–E bars on right). Data are mean parameter estimates for each ROI ± SEM pre- (white bars) and post- (gray bars) standardized meal between low and high anxiety women (left bars and right bars, respectively). Bilateral regions (A–D) were averaged. P-values derived from generalized estimating equations. *P<0.050, **P<0.010, ***P<0.001 pre-meal vs. post-meal in low anxiety women. Data are adjusted for BMI.
In men, average brain activation across all ROIs by fattening (vs. non-fattening) food cues was not significantly reduced by a meal in the low (0.33±2.66 vs. −4.14±4.60, P=0.52) or in the high anxiety group (7.91±2.44 vs. 3.36±6.05, P=0.53).
Discussion
Consistent with our hypotheses, our findings suggest strong relationships between anxiety and eating behavior in women and potential neural mechanisms whereby anxiety could contribute to overeating and obesity. In women, higher trait anxiety was associated with a higher BMI, higher caloric intake, greater consumption of high-fat foods and persistent activation by fattening food cues after a meal. Specifically, low-anxiety women had reduced activation after eating in brain regions that regulate satiety, including the mOFC, amygdala, dorsal striatum, nucleus accumbens and insular cortex, but highly anxious women did not have reduced activation in these same regions. These findings were independent of BMI, and could implicate altered satiety processing as a risk factor for weight gain in highly anxious women. Importantly, even though anxious women tended to overeat during the experiment, their risk of excess weight gain over time appeared to be modified by their genetic background.
As in previous studies (2, 3), anxiety was strongly related to having a higher BMI, but in our study this was specific to trait anxiety and only among women. We provide new information that genetic and familial factors modulate the relationship between anxiety and BMI. When female twins were analyzed as unique individuals by statistically accounting for twin relatedness, higher scores of trait anxiety were significantly associated with a higher BMI. However, when shared environmental and genetic traits between twins were controlled for by using within-pair comparisons, the relationships between trait anxiety and BMI disappeared. This finding is understandable in light of prior twin studies showing that 80% or more of the variance in BMI is linked to genetic factors (39, 40).
We also found relationships between anxiety and specific eating behaviors including total caloric and macronutrient intake that could support the hypothesis that anxiety contributes to weight gain and risk of obesity in genetically susceptible individuals. Women with high trait anxiety consumed more total kilocalories during the ad libitum buffet as well as a higher percentage of kilocalories from fat vs. carbohydrates, independent of BMI. Moreover, within-pair comparisons revealed that the positive relationship between trait anxiety and total kilocalories consumed was independent of genetic (50% for DZ and 100% for MZ twin pairs) and shared familial factors. Put another way, female twins who scored higher in trait anxiety ate more than their twin. These data provide strong evidence that trait anxiety independently increases food consumption. These findings suggest that anxious women increase food intake, but the extent that these extra kilocalories promote weight gain is influenced by genetic and familial factors. The relationship of anxiety to food intake has been a source of debate (41–43) particularly whether eating substantially relieves anxiety and thus serves a functional purpose. Experimentally-induced anxiety has been shown to increase food intake among individuals with a history of loss of control eating (44) or with high emotional eating scores (45), but our data suggest that anxiety is related to increased caloric intake among generally healthy women as well.
Findings from our nested case-control fMRI study provide evidence that women with high anxiety have altered brain response to food cues that could explain their greater caloric intake during the ad libitum buffet. The five regions of interest we examined were selected based on prior research establishing them as markers of satiety (12); in a state of hunger, these regions typically show robust activation by food images which decreases during post-meal satiety. High anxiety women did not reduce activation in these regions, providing evidence of a disruption in satiety perception. Importantly, these women, independent of BMI, also consumed more kilocalories at the post-scan buffet meal that objectively assessed satiety. The extra food consumption occurred without differences in self-reported appetite, further suggesting decoupling of eating behavior from hunger. These findings support theories that neural circuits regulating appetite are disrupted by chronic anxiety or stress in a manner that could promote obesity or eating disorders (46). Alternately, heightened attentional functioning (47) or even improved visual detection among anxious women (48) might explain persistent post-meal responses to visual food cues, a question that deserves further study.
The current findings emphasize potential differences between trait and state anxiety in relation to obesity risk. We show that the effects of state and trait anxiety on food consumption and macronutrient choice varied, and were significantly different by sex. In women, trait (global) anxiety was related to total caloric intake and food choice, whereas in men state (current) anxiety more strongly affected food choice. Moreover, as state anxiety is more closely related to stress, these results suggest that stress might also affect eating behaviors in men. These findings highlight the importance of investigating sex differences.
A strength of our study is the use of a sample of twins, which allowed for examination of confounding of relationships between anxiety, food intake, and BMI by genetic and familial factors. However, due to the cross-sectional study design we cannot discern whether chronic anxiety contributed to the increased BMI among women or whether the altered eating and emotional regulation exhibited was a consequence of weight gain. Although obesity did not appear to explain the relationships seen in our study, previous studies have shown similar findings of persistent brain activation in response to high-calorie food cues in regions associated with food motivation and reward in obese individuals (49, 50). Our findings open the possibility that obesity and anxiety disrupt food cue perception and sensitivity to satiety cues in a similar fashion, either because they are causally related or as a result of shared mechanisms. This study cannot distinguish between these two possibilities. Another limitation was that we selected for relatively healthy individuals and also enrolled a high percentage of Caucasian subjects, therefore our results may not be generalizable to all populations. Additionally, we did not include an instrument capable of identifying clinical anxiety disorders among our sample, therefore, we cannot comment on the clinical severity of symptoms amongst the high anxiety women. We used BMI to relate anxiety to obesity; however, we recognize that measures of body composition or even waist circumference would have been more informative as to cardiometabolic risk.
Future studies examining the role of anxiety in body fat distribution could be informative. Limited sample size (20 women and 20 men), though not uncommon in fMRI studies, could account for the high variability in our measures. We were unable to study the relation of state anxiety to fMRI findings in men due to limited numbers of men scoring high enough to reach clinical cutoffs in state anxiety. Clearly, sex and acute (i.e., experimental) vs. chronic anxiety each influence the ultimate effect of anxiety on eating behavior and should be taken into account in future studies.
In conclusion, the current findings suggest that anxiety promotes caloric consumption and consumption of high-fat foods in women. We also provide evidence that anxiety alters brain responses to satiety such that the normal reduction in activation by high-calorie food cues induced by a meal (12) does not occur in highly anxious women, suggesting a disruption in neural circuitry that could promote overeating. Anxiety may be a risk factor for obesity, but we show this risk is likely limited to people with a genetic susceptibility to weight gain. Future longitudinal studies should examine a larger population to firmly establish whether anxiety and overeating are a risk factor for obesity in women. Interventions to promote a healthy weight in highly anxious women should test the utility of directly addressing anxiety, satiety and emotional eating habits as components of treatment.
Acknowledgments
Source of Funding: This work was supported by the NIH award number R01DK089036 (EAS), R01DK098466 (EAS), P30DK035816, and the National Center for Advancing Translational Sciences of the NIH under award number UL1TR000423.
Glossary
- MZ
Monozygotic
- DZ
Dizygotic
- MRI
Magnetic Resonance Imaging
- fMRI
functional Magnetic Resonance Imaging
- VAS
Visual Analog Scale
- STAI
State-Trait Anxiety Inventory
- BMI
Body Mass Index
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
Clinical Trials Registration: #NCT02483663, https://clinicaltrials.gov
References
- 1.Marniemi J, Kronholm E, Aunola S, Toikka T, Mattlar CE, Koskenvuo M, Ronnemaa T. Visceral fat and psychosocial stress in identical twins discordant for obesity. J Intern Med. 2002;251(1):35–43. doi: 10.1046/j.1365-2796.2002.00921.x. [DOI] [PubMed] [Google Scholar]
- 2.Gariepy G, Nitka D, Schmitz N. The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34(3):407–19. doi: 10.1038/ijo.2009.252. [DOI] [PubMed] [Google Scholar]
- 3.Zhao G, Ford ES, Dhingra S, Li C, Strine TW, Mokdad AH. Depression and anxiety among US adults: associations with body mass index. Int J Obes (Lond) 2009;33(2):257–66. doi: 10.1038/ijo.2008.268. [DOI] [PubMed] [Google Scholar]
- 4.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824–30. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott KM, Bruffaerts R, Simon GE, Alonso J, Angermeyer M, de Girolamo G, Demyttenaere K, Gasquet I, Haro JM, Karam E, Kessler RC, Levinson D, Medina Mora ME, Oakley Browne MA, Ormel J, Villa JP, Uda H, Von Korff M. Obesity and mental disorders in the general population: results from the world mental health surveys. Int J Obes (Lond) 2008;32(1):192–200. doi: 10.1038/sj.ijo.0803701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson SE, Cohen P, Naumova EN, Must A. Association of depression and anxiety disorders with weight change in a prospective community-based study of children followed up into adulthood. Arch Pediatr Adolesc Med. 2006;160(3):285–91. doi: 10.1001/archpedi.160.3.285. [DOI] [PubMed] [Google Scholar]
- 7.Schneider KL, Appelhans BM, Whited MC, Oleski J, Pagoto SL. Trait anxiety, but not trait anger, predisposes obese individuals to emotional eating. Appetite. 2010;55(3):701–6. doi: 10.1016/j.appet.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Acute stress-related changes in eating in the absence of hunger. Obesity (Silver Spring) 2009;17(1):72–7. doi: 10.1038/oby.2008.493. [DOI] [PubMed] [Google Scholar]
- 9.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19(4):1381–94. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 10.van der Laan LN, de Ridder DT, Viergever MA, Smeets PA. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage. 2011;55(1):296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 11.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33(6):653–61. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, Schwartz MW, Schur EA. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 2012;96(5):989–99. doi: 10.3945/ajcn.112.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 14.Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, Durighel G, Hughes E, Waldman AD, Frost G, Bell JD. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30(8):1625–35. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 15.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–21. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169(1):111–9. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99(4):538–43. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr. 2006;83(6):1297–305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- 20.Lau JY, Eley TC, Stevenson J. Examining the state-trait anxiety relationship: a behavioural genetic approach. J Abnorm Child Psychol. 2006;34(1):19–27. doi: 10.1007/s10802-005-9006-7. [DOI] [PubMed] [Google Scholar]
- 21.Platte P, Herbert C, Pauli P, Breslin PA. Oral perceptions of fat and taste stimuli are modulated by affect and mood induction. PLoS One. 2013;8(6):e65006. doi: 10.1371/journal.pone.0065006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158(10):1568–78. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 23.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med. 1990;322(21):1483–7. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 24.Strachan E, Hunt C, Afari N, Duncan G, Noonan C, Schur E, Watson N, Goldberg J, Buchwald D. University of Washington Twin Registry: poised for the next generation of twin research. Twin Res Hum Genet. 2013;16(1):455–62. doi: 10.1017/thg.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–7. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 26.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 27.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 28.Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S467–72. doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 30.Schur EA, Kleinhans NM, Goldberg J, Buchwald DS, Polivy J, Del Parigi A, Maravilla KR. Acquired differences in brain responses among monozygotic twins discordant for restrained eating. Physiol Behav. 2012;105(2):560–7. doi: 10.1016/j.physbeh.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49(1):193–7. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- 32.Jenkinson M, Wilson JL, Jezzard P. Perturbation method for magnetic field calculations of nonconductive objects. Magn Reson Med. 2004;52(3):471–7. doi: 10.1002/mrm.20194. [DOI] [PubMed] [Google Scholar]
- 33.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 35.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 36.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106–9. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 38.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 39.Bergin JE, Neale MC, Eaves LJ, Martin NG, Heath AC, Maes HH. Genetic and environmental transmission of body mass index fluctuation. Behav Genet. 2012;42(6):867–74. doi: 10.1007/s10519-012-9567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJ, Ong KK. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol (Lausanne) 2012;3:29. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herman CP, Polivy J. Anxiety, restraint, and eating behavior. J Abnorm Psychol. 1975;84(6):66–72. [PubMed] [Google Scholar]
- 42.Polivy J, Herman CP, McFarlane T. Effects of anxiety on eating: does palatability moderate distress-induced overeating in dieters? J Abnorm Psychol. 1994;103(3):505–10. doi: 10.1037//0021-843x.103.3.505. [DOI] [PubMed] [Google Scholar]
- 43.Lowe MR, Kral TV. Stress-induced eating in restrained eaters may not be caused by stress or restraint. Appetite. 2006;46(1):16–21. doi: 10.1016/j.appet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Jarcho JM, Tanofsky-Kraff M, Nelson EE, Engel SG, Vannucci A, Field SE, Romer AL, Hannallah L, Brady SM, Demidowich AP, Shomaker LB, Courville AB, Pine DS, Yanovski JA. Neural activation during anticipated peer evaluation and laboratory meal intake in overweight girls with and without loss of control eating. Neuroimage. 2015;108:343–53. doi: 10.1016/j.neuroimage.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Strien T, Herman CP, Anschutz DJ, Engels RC, de Weerth C. Moderation of distress-induced eating by emotional eating scores. Appetite. 2012;58(1):277–84. doi: 10.1016/j.appet.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Hardaway JA, Crowley NA, Bulik CM, Kash TL. Integrated circuits and molecular components for stress and feeding: implications for eating disorders. Genes Brain Behav. 2015;14(1):85–97. doi: 10.1111/gbb.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35(9):527–35. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berggren N, Blonievsky T, Derakshan N. Enhanced visual detection in trait anxiety. Emotion. 2015;15(4):477–83. doi: 10.1037/a0039449. [DOI] [PubMed] [Google Scholar]
- 49.Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes (Lond) 2010;34(10):1494–500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite. 2012;58(1):303–312. doi: 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


