Abstract
As do all things in biology, cell mechanosensation, adhesion and migration begin at the scale of the molecule. Collections of molecules assemble to comprise microscale objects such as adhesions, organelles and cells. And collections of cells in turn assemble to comprise macroscale tissues. From the points of view of mechanism and causality, events at the molecular scale are seen most often as being the most upstream and, therefore, the most fundamental and the most important. In certain collective systems, by contrast, events at many scales of length conspire to make contributions of equal importance, and even interact directly and strongly across disparate scales. Here we highlight recent examples in cellular mechanosensing and collective cellular migration, where physics at some scale bigger than the cell but smaller than the tissue –the mesoscale– becomes the missing link that is required to tie together findings that might otherwise seem counterintuitive or even unpredictable. These examples, taken together, establish that the phenotypes and the underlying physics of collective cellular migration are far richer than previously anticipated.
Understanding cell adhesion and associated mechanisms of mechanosensing remain critical challenges in explaining multiple facets of health and disease, including but not limited to development, wound healing, asthma, cardiovascular disease, and cancer. One finds in the literature two main contrasting approaches. Recent literature emphasizes the microscopic, bottom-up, granular approach in the context of specific molecules, their roles and their interdependencies1–3. This can range from identifying mechanically sensitive actin-linkers4, to characterizing signaling pathways,5 to finding downstream effectors of mechanotransduction such as YAP/TAZ6. The older literature, by contrast, emphasizes the macroscopic, coarsegrained, top-down approach in the context of mechanical forces, fields, and integrative physiological function. In addition to the classical work of D’Arcy Wentworth Thompson7, specific examples of the latter are Wolff’s law8 for adaptation of bone structure to the load that the bone supports, Murray’s Law9 for adaptation of blood vessel diameter to the flow that the vessel carries, and McMahon’s10–12 principle of elastic similarity to explain allometric variations of energy metabolism, muscle mass, and bone size with body mass.
If each were carried to its logical conclusions, one might expect that microscopic and macroscopic approaches, when taken together, would dovetail seamlessly to create a satisfying and comprehensive physical picture. The expectation, then, would be one of complementary parts combining, ultimately and inevitably, to form a complete logical framework that spans all pertinent scales of length. While such cases clearly exist, as when a single mutation affecting mechanosensing can be tied directly to macroscopic effects and disease13, there is reason to believe that such an expectation might be illusory more generally in biology. At the intermediate scale –the mesoscale– pivotal phenomenon can and do emerge that are at once hidden at the macroscale but are not anticipated by or predictable from the microscale14. Nevertheless, at the level of integrated system behavior they become crucial. In the particular context of collective cellular migration, we provide here several such examples15–20. Because of its importance in wound healing, development, and cancer, collective cellular migration has been of interest for more than 100 years21, but, as regards mechanosensing, it has been only in recent years that the mechanical stresses exerted between each cell and its substrate22,23, and between each cell and its immediate neighbors24–26, have been measured and mapped.
Plithotaxis and kenotaxis
We begin with the example of collective cellular migration and the recently discovered mesoscopic phenomenon called plithotaxis. Epithelial cells comprising a confluent layer are known to move in cooperative streaks, strands, packs and clusters27, but the intercellular mechanical stresses that drive these local cell motions were for a long time a matter of pure speculation. Tambe et al.24,25,28 first measured these stresses within the confluent cell layer and showed that these stresses can fluctuate dramatically from cell to cell and from moment to moment; that is to say, intercellular stresses are typified by a dynamic heterogeneity29 wherein fluctuations in space and time dominate. Moreover, Tambe et al. established that each individual cell within the layer can exhibit a strongly preferred stress orientation; that is to say, in addition to dynamic heterogeneity, the field of intercellular stress tends to be strongly anisotropic. When they examined the relationship between motions and stresses, they found that each cell within a cellular collective tends to move along a local orientation corresponding to that in which it pulls hardest upon its immediate neighbors; that stress is called the maximum principal stress, and that orientation is called the maximum principal orientation. Plithotaxis therefore implies the seemingly simple notion that the orientations of local cellular motions and local cellular stresses tend to coincide. However, in proximity to an island in which cells cannot adhere to the substrate, and therefore the monolayer has a cell-free boundary, plithotaxis breaks down altogether; the orientations of local cellular motions versus local cellular stresses tend to depart from one another dramatically and systematically30. Even as the cell migrates parallel to the boundary of the island, cellular tractions polarize so as to pull perpendicular to that boundary; this mesoscopic phenomenon of cells pulling toward a cell-free void is called kenotaxis.31
But whether near such a boundary or far from it, through what molecular processes does a cell within the cell cluster sense mechanical stresses exerted between itself and its immediate neighbors, and then use that information to coordinate its motion with that of the integrated cell cluster? In the mesoscopic process of plithotaxis, Das et al.32 showed that the tumor suppressor merlin plays a key role. As intracellular stresses build up locally within a constituent cell of the layer, merlin disassociates from cortical cell-cell junctions and enters the cytoplasm. Merlin dissociation then leads to Rac1 activation and polarization, and, ultimately, to lamellipodium formation aligned along the direction of the maximal principal stress. Indeed, the orientation of Rac1 polarization matches stress alignment in the presence of merlin, whereas cells lacking merlin do not show alignment of Rac1 polarity and direction of maximal principal stress. Merlin is not required for cellular motion nor does it affect the development intercellular stress. Nevertheless, through this mechanism, merlin is shown to account for the long range cooperativity and alignment of cellular motions and intercellular stresses. Clearly, without measuring mechanical stress our understanding of merlin polarization would likely seem a perplexing process and, conversely, understanding cell alignment without merlin polarization would seem equally perplexing. But in the example of plithotaxis we now see how polarizations of local cellular motions, mechanical stresses, merlin and Rac1 link together across scales to provide an integrated physical picture32.
The intercellular adhesome
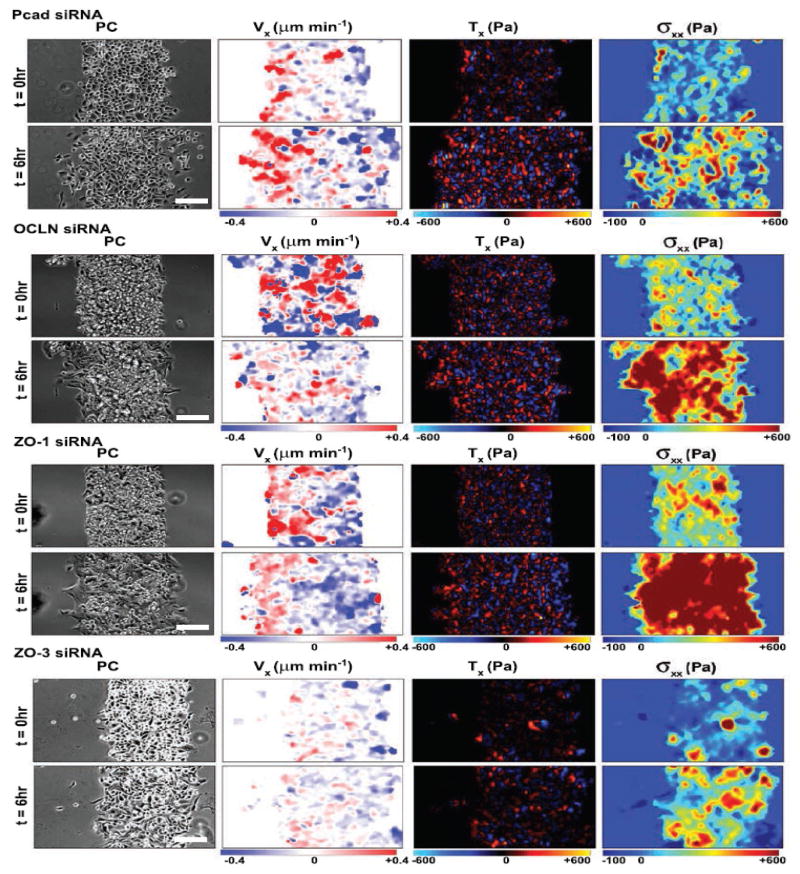
The success of tying plithotaxis to a specific mechanotransduction pathway raises hope that similar meetings at the mesoscale can be found. In the epithelial cell sheet, for example, adhesion molecules associated with tight junctions, adherens junctions, desmosomes, and gap junctions have a role in the development of monolayer stresses that keep the layer continuous, intact and advancing to fill a wound or grow a tissue. Identification of distinct roles of specific molecules is complicated experimentally because knocking down one adhesion molecular could, in principle, cause overexpression of another by compensatory mechanisms. Taking that issue into account, Bazellieres et al.33 identified distinct mechanical phenotypes that could be tied to groups of adhesion molecules, but in ways that proved to be most unexpected. For example, despite distinct loci in adherens junctions versus tight junctions, knocking down of P-cadherin versus occludin results in quite similar phenotypes, both being characterized by augmented migration speed together with reduced intracellular stress (top panels Figure 1). And despite similar loci –both within tight junctions, knocking down of ZO-1 versus ZO-3 results in highly dissimilar phenotypes; knocking down of ZO-3 reduces intracellular stress as might have been anticipated, whereas knocking down of ZO-1 causes just the opposite, increasing intracellular stress even as it increases migration speed (bottom panels, Figure 1). The knocking down of ZO-1 produces a distinct mechanical phenotype compared to all other adhesome proteins. This raises the natural question, could ZO-1 be directly tied to a mechanotransduction pathway in much the same way merlin is tied to plithotaxis?
Figure 1.
Bazellieres et al. 33 measured the effect of knocking down different proteins on monolayer mechanics. Cells were confined to a known geometry as then released at t=0 hr to migrate into free space as the investigators monitored monolayer spread area in phase contrast (PC), average velocity along the spreading direction (Vx), the traction stress exerted by each cell upon its substrate (Vx), and intercellular stress exerted by each cell upon is neighbors (σxx). Knocking down of P-cadherin (Pcad) and occludin (OCLN) results in a mechanical phenotype with faster migration velocity and somewhat weaker monolayer stresses compared to control cells. Knocking down ZO-1 results in a phenotype with faster migration and higher monolayer stress, whereas knocking down ZO-3 results in a phenotype with slower migration and weaker monolayer stress compared to control cells. Reprinted by permission from Macmillan Publishers Ltd: Nature Cell Biology33, copyright 2015.
Bazellieres et al.33 were also able to highlight the unanticipated roles of some specific molecules, P-cadherin and E-cadherin in particular, in the development of monolayer stresses across these different phenotypes. Surprisingly, P-cadherin but not E-cadherin is linked to the magnitude of intercellular tensile stress, whereas E-cadherin is linked to the temporal build-up of intercellular stress. Although these roles extend across mechanical phenotypes, if E-cadherin is removed then P-cadherin assumes the role of E-cadherin in mechanotransduction.
Cell sorting and differential adhesion
Another meeting at the mesoscale involves organogenesis, cell sorting and the differential adhesion hypothesis (DAH)34,35. If distinct cell types are mixed in vitro, they segregate reproducibly into different homogeneous domains with one cell type becoming engulfed by the other. While chemical cues are clearly involved, this demixing of cells has striking similarities to the phase separation of immiscible Newtonian fluids34,35. Indeed, the rate of demixing and the topology of the inner versus outer domains can be predicted by measuring a tissue surface tension. For adherent, contractile cells, this tissue surface tension is set by a competition between cell-cell adhesion and cortical contractile force36,37. Interestingly, in embryonic cells where cell sorting is thought to play a key role in organogenesis, the tissue surface tension correlates with total amount of cadherins and provides a direct link between the molecular origin and the physics observed. Recently, however, this tidy picture has been upset, and found to embody much richer physics than previously thought. Pawlizak et al.20 reexamined the DAH in certain cancer cell lines across the epithelial-to-mesenchymal transition (EMT). During EMT, the amount of cadherins expressed at the cell-cell junction declines and the ratio of different types of cadherins changes, suggesting the tissue surface tension should also change. While Pawlizak et al.20 found tissue surface tension changes after EMT, they observed that cell-cell adhesive stresses fail to correlate with the amount of cadherins. Surprisingly, they found that after EMT neither cell-cell adhesive stresses nor cadherin amount predicts cell sorting. Moreover, some cell types no longer behave as Newtonian fluids at all, thus violating the fundamental premise upon which the DAH rests. To understand cell sorting in these cases, Pawlizak et al. 20 implicated an altogether different physical principle, namely, cell jamming.
Cell jamming and the case of the bronchial epithelium
Like coffee beans in a chute, collective cellular systems can sometimes flow but can sometimes jam; the macroscopic properties of the system arise from changes of groups of particles or cells at the mesoscale. Whether living or inert, when the collective is jammed it develops shape stability and elastic stresses much as does a solid. But when the collective is unjammed its shape stability is lost, its elastic stresses vanish, and it can flow like a fluid. Transitions of a collective system between jammed and unjammed states remain poorly understood, even in inert granular systems. Investigation of cell jamming in certain diseases, including cancer19,20 and asthma17, are just now beginning appearing in the literature.
In asthma, the injury-repair response of the airway epithelium is known to be aberrant and impaired, and has been implicated in early stages of disease pathogenesis. Underlying mechanisms remain unknown, however. Recent findings by Park et al.17 suggest that cell jamming may play a central role. When airway epithelial cells from healthy human donors are cultured in air-liquid interface, they are initially unjammed, but as they mature and differentiate over 14 days they eventually jam. This transition to the jammed state can be disturbed, however, by external stimuli or disease conditions. For example, compressive stresses that mimic the effects of bronchoconstriction, a process that typifies an asthmatic exacerbation, provoke the transition of the mature epithelial layer from the jammed state back to the unjammed state. Furthermore, when cells are cultured from asthmatic donors, the transition to jamming is significantly delayed, and this delay potentially reflects dysmaturity of the asthmatic cell layer and thus helps to explain why the airway epithelium in the asthmatic has an impaired injury-repair process and is susceptible to injury.
One might logically expect that cell jamming is promoted by increased cell-cell adhesion but, paradoxically, just the opposite trend prevails. Cells from asthmatic donors exert greater intercellular stresses across their junction with neighboring cells, a finding that suggests that asthmatic cells exhibit greater cell-cell adhesive stresses. This paradox can be explained by the vertex model of cell jamming36,38, which considers energy barriers that impede mutual cellular rearrangements. The theory holds that these mechanical energy barriers are attributable in part to the cell-cell junction and can be expressed in terms of a net line tension. This net line tension, in turn, has two competing components: a contractile energy that is always positive and adhesive energy that is always negative (Figure 2). When adhesive energy is smaller than contractile energy, the net line tension is positive, the energy barriers to mutual cellular rearrangements are appreciable, and as a result the system jams. But when adhesive energy exceeds contractile energy, then the net line tension becomes negative, the energy barriers to mutual cellular rearrangements vanish, and as a result the system unjams and can flow. Curiously, this transition is tied uniquely to a critical parameter describing cell shape. In experiments performed independently of this theoretical prediction, this and other counterintuitive predictions of the theory of cell jamming have been confirmed17. Taken together, these findings highlight the need to account for the mesoscopic physics at play in collective cellular systems and the challenge of assigning disease to specific molecular actors. Jamming in the bronchial epithelium is controlled by the competition between adhesion and contractility, and would thus be influenced by molecules as wide ranging as E-cadherin, myosin motors, or actin cross-linkers. Because these molecules likely act in concert to precipitate disease, tying cell jamming to the role of specific molecules is likely to prove challenging.
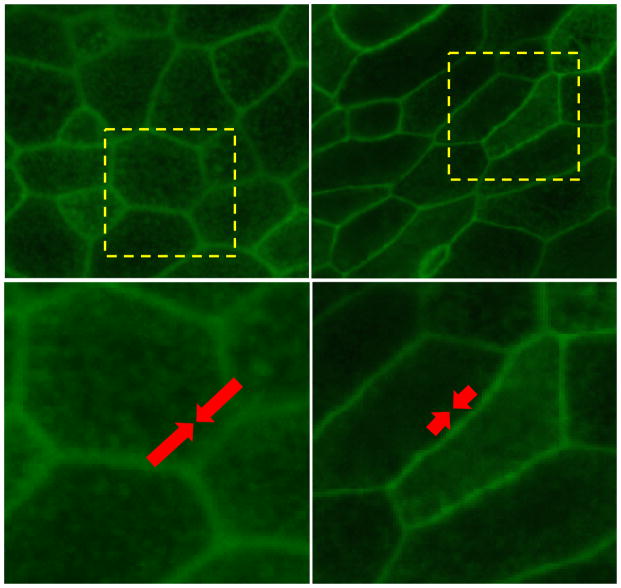
Figure 2.
Paradoxically, as cell-cell adhesive stresses increase (white arrows), the cellular collective unjams and undergoes a transition from a solid-like to a fluid-like state. This paradox explained by the vertex model and the theory of cell jamming 17,36,38,39. In this theory, mechanical energy barriers are attributable in part to the cell-cell junction and can be expressed in terms of a net line tension acting at the cell-cell junction. This line tension is composed of two components; one is positive and the other is negative. The positive component (indicated by red arrows) captures the energy associated with actin-myosin contractility and acting to minimize the area of cell-cell contact. The negative component (indicated by white arrows) captures the energy associated with cell-cell adhesion and acting to maximize the area of cell-cell contact. These two components compete. When line tension is positive, contractile line tension dominates, energy barriers to cellular rearrangements are finite, and the cellular collective becomes solid-like and jammed (A, C). When line tension is negative, however, cell-cell adhesion dominates, energy barriers to cellular rearrangements are vanished, and the cellular collective becomes fluid-like and unjammed (B, D). Well-differentiated primary human bronchial epithelial cells are jammed (A, C), but compressive stress induces the transition toward to the jammed state (B, C). Cells were stained with phalloidin conjugated with Alexa 488. The dotted areas in A and B are magnified in C and D.
Conclusions
The surprising findings highlighted here, when taken together, now establish that the phenotypes of collective cellular migration, as well as the underlying physics, are far richer than previously anticipated. In that connection, we call attention to the classical paper by the Nobel laureate Kenneth G. Wilson14, in which he emphasizes that events distinguished by great disparities in scale often have little direct influence upon one another, and therefore each scale can be treated independently of the others. He uses the example of an ocean wave, which can be well described as a disturbance in a continuous liquid without regard to molecular structure of water. But in certain classes of collective systems, he points out, events at many scales of length conspire to make contributions of equal importance, and even interact directly and strongly across those scales. Just as the inert collective systems that were his focus are typified by spontaneous fluctuations and critical transitions between phases of matter, so too we now understand that cellular collectives display many of these same features17,36,38–40. These features innately span many scales of length, and will likely come increasingly into play in the understanding development, wound healing, cardiovascular disease, asthma, and cancer.
Acknowledgments
The authors thank to Dr. Jennifer Mitchel for kindly providing the F-actin stained images of HBE cells used in Figure 2. This work was supported by the National Cancer Institute (U01CA202123) and the National Heart, Lung and Blood Institute (R01HL107561, P01HL120839), the American Heart Association (13SDG14320004), and the Francis Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bausch AR, Kroy K. A bottom-up approach to cell mechanics. Nature Physics. 2006 [Google Scholar]
- 2.Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Bio. 2014;15:802–12. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature. 2011;478:260–U154. doi: 10.1038/nature10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prager-Khoutorsky M, Lichtenstein A, Krishnan R, et al. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nature Cell Biololog. 2011;13:1457–65. doi: 10.1038/ncb2370. [DOI] [PubMed] [Google Scholar]
- 6.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 7.Thompson DAW. On Growth and Form. 1917 [Google Scholar]
- 8.Wolff J. The Law of Bone Remodeling. Berlin Heidelberg New York: Springer; 1986; 1896. [Google Scholar]
- 9.Murray C. The physiological principle of minimum work. I. The vascular system and the cost of blood volume. Proceedings of the National Academy of Sciences USA. 1926;12:207–14. doi: 10.1073/pnas.12.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon T. Size and shape in biology. Science. 1973;179:1201–4. doi: 10.1126/science.179.4079.1201. [DOI] [PubMed] [Google Scholar]
- 11.McMahon T, Bonner J. On Size and Life. Scientific American Library; 1983. [Google Scholar]
- 12.McMahon TA. Muscles, reflexes, and locomotion. Princeton, NJ: Princeton University Press; 1984. [Google Scholar]
- 13.Cook JR, Carta L, Benard L, et al. Abnormal muscle mechanosignaling triggers cardiomyopathy in mice with Marfan syndrome. J Clin Invest. 2014;124:1329–39. doi: 10.1172/JCI71059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson K. Problems in physics with many scales of length. Scientific American. 1979;241:158–79. [Google Scholar]
- 15.Tambe D, Fredberg J. And I hope you like jamming too. New Journal of Physics. 2015;17:091001. doi: 10.1088/1367-2630/17/9/091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JA, Fredberg JJ, Drazen JM. Putting the Squeeze on Airway Epithelia. Physiology (Bethesda) 2015;30:293–303. doi: 10.1152/physiol.00004.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JA, Kim JH, Bi D, et al. Unjamming and cell shape in the asthmatic airway epithelium. Nat Mater. 2015;14:1040–8. doi: 10.1038/nmat4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadati M, Nourhani A, Fredberg JJ, Taheri Qazvini N. Glass-like dynamics in the cell and in cellular collectives. Wiley interdisciplinary reviews Systems biology and medicine. 2014;6:137–49. doi: 10.1002/wsbm.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haeger A, Krause M, Wolf K, Friedl P. Cell jamming: collective invasion of mesenchymal tumor cells imposed by tissue confinement. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbagen.2014.03.020. [DOI] [PubMed]
- 20.Pawlizak S, Fritsch AW, Grosser S, et al. Testing the differential adhesion hypothesis across the epithelial-mesenchymal transition. New Journal of Physics. 2015 In press. [Google Scholar]
- 21.Holmes S. The Behavior of Epidermis of Amphibians when Cultivated Outside the Body. Journal of Experimental Zoology. 1914;17:281–95. [Google Scholar]
- 22.Trepat X, Wasserman M, Angelini T, et al. Physical forces during collective cell migration. Nature Physics. 2009;5:426–30. [Google Scholar]
- 23.Saez A, Anon E, Ghibaudo M, et al. Traction forces exerted by epithelial cell sheets. J Phys Condens Matter. 2010;22:194119. doi: 10.1088/0953-8984/22/19/194119. [DOI] [PubMed] [Google Scholar]
- 24.Tambe DT, Croutelle U, Trepat X, et al. Monolayer stress microscopy: limitations, artifacts, and accuracy of recovered intercellular stresses. PloS one. 2013;8:e55172. doi: 10.1371/journal.pone.0055172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tambe DT, Hardin CC, Angelini TE, et al. Collective cell guidance by cooperative intercellular forces. Nature materials. 2011;10:469–75. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23:1024–30. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angelini TE, Hannezo E, Trepat X, Marquez M, Fredberg JJ, Weitz DA. Glass-like dynamics of collective cell migration. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4714–9. doi: 10.1073/pnas.1010059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trepat X, Fredberg JJ. Plithotaxis and emergent dynamics in collective cellular migration. Trends in cell biology. 2011;21:638–46. doi: 10.1016/j.tcb.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrahan JP. Dynamic heterogeneity comes to life. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4701–2. [Google Scholar]
- 30.Kim JH, Serra-Picamal X, Tambe DT, et al. Propulsion and navigation within the advancing monolayer sheet. Nat Mater. 2013;12:856–63. doi: 10.1038/nmat3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dufresne ER, Schwartz MA. Cell migration: Towards the void. Nature Materials. 2013;12:783–4. doi: 10.1038/nmat3743. [DOI] [PubMed] [Google Scholar]
- 32.Das T, Safferling K, Rausch S, Grabe N, Boehm H, Spatz JP. A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat Cell Biol. 2015;17:276–87. doi: 10.1038/ncb3115. [DOI] [PubMed] [Google Scholar]
- 33.Bazellieres E, Conte V, Elosegui-Artola A, et al. Control of cell-cell forces and collective cell dynamics by the intercellular adhesome. Nat Cell Biol. 2015;17:409–20. doi: 10.1038/ncb3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255–63. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Amack JD, Manning ML. Knowing the Boundaries: Extending the Differential Adhesion Hypothesis in Embryonic Cell Sorting. Science. 2012;338:212–5. doi: 10.1126/science.1223953. [DOI] [PubMed] [Google Scholar]
- 36.Farhadifar R, Roper JC, Aigouy B, Eaton S, Julicher F. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr Biol. 2007;17:2095–104. doi: 10.1016/j.cub.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 37.Brodland GW. The Differential Interfacial Tension Hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J Biomech Eng. 2002;124:188–97. doi: 10.1115/1.1449491. [DOI] [PubMed] [Google Scholar]
- 38.Bi D, Lopez JH, Schwarz JM, Manning ML. A density-independent rigidity transition in biological tissues. Nat Phys. 2015 advance online publication. [Google Scholar]
- 39.Glazier JA, Graner F. Simulation of the differential adhesion driven rearrangement of biological cells. Physical Review E. 1993;47:2128–54. doi: 10.1103/physreve.47.2128. [DOI] [PubMed] [Google Scholar]
- 40.Vicsek T, Zafeirisa A. Collective motion. Physics Reports. 2012;517:71–140. [Google Scholar]