Abstract
Objectives
There is a strong association between supportive ties and health. However most research has focused on the health benefits that come from the support one receives while largely ignoring the support giver and how giving may contribute to good health. Moreover, few studies have examined the neural mechanisms associated with support giving or how giving support compares to receiving support.
Method
The current study assessed the relationships: 1) between self-reported receiving and giving social support and vulnerability for negative psychological outcomes and 2) between receiving and giving social support and neural activity to socially rewarding and stressful tasks. Thirty-six participants (M age=22.36, SD=3.78, 44% female) completed three tasks in the fMRI scanner: (1) a stress task (mental arithmetic under evaluative threat), (2) an affiliative task (viewing images of close others), and (3) a prosocial task.
Results
Both self-reported receiving and giving social support were associated with reduced vulnerability for negative psychological outcomes. However, across the three neuroimaging tasks, giving, but not receiving support was related to reduced stress-related activity (dorsal anterior cingulate cortex, (r=−.27), left (r=−.28) and right anterior insula (r=−.33), and left (r=−.32) and right amygdala (r=−.32) to a stress task, greater reward-related activity (left (r=.42) and right ventral striatum (VS; r=.41) to an affiliative task, and greater caregiving-related activity (left VS (r=.31), right VS (r=.31), and septal area (r=.39) to a prosocial task.
Conclusion
These results contribute to an emerging literature suggesting that support giving is an overlooked contributor to how social support can benefit health.
Keywords: social support, helping, caregiving, providing social support, social relationships and health
Social relationships are critical to health and well-being (1–3). Those without close ties fare poorly across a range of important outcomes (1,4,5) including mental and physical health (2–6), whereas those with more social ties show enhanced mental and physical health. To date, the research literature has focused on how “social support”—the perception or experience that one is loved and cared for by others, esteemed and valued, and part of a social network of mutual assistance and obligations (7)—relates to positive health outcomes. Since social support, by definition, focuses more on the consequences of receiving support, studies linking social support and health have largely focused on the support recipient while ignoring the individual giving support and care. Therefore the following study aimed to examine the associations between both receiving and giving support with: 1) vulnerability to negative psychological outcomes and 2) neural activity to stressful and socially rewarding tasks.
Since the 1970’s, studies have shown associations between supportive ties and reduced morbidity and mortality (8). Theories on social support suggest that these health benefits may come from the support we receive from others, which then helps to reduce stress responding (9–11). For example, undergoing stressful tasks with a friend present (vs. alone) leads to reduced cardiovascular responses to the stressors (12, see 13 for a review). In addition, self-reports of social support have been associated with reduced distress-related neural activity (in the dorsal anterior cingulate cortex (dACC)) to an experience of social rejection (14,15) and receiving support during a painful experience is associated with reduced pain-related neural activity (16,17). Though the benefits of receiving support are well established, receiving support can sometimes be ineffective, or in worst cases, backfire (e.g. 18,19). Therefore, it is possible that the support that one receives may not explain the relationship between social support and health in its entirety.
There has been increasing interest in the support giver, and the health benefits that may be accrued by giving to others. Thus, support giving, much like support receiving, is associated with a number of beneficial mental and physical health outcomes (20–24). Specifically, giving to others is associated with lower mortality rates over a 5-year period (20), fewer sick days (24), and reduced cardiovascular activity over a 24-hour period (22). The mechanisms leading to support giving’s beneficial effects may have evolved out of a caregiving system that helped nurture and support infants and others in need (25–30). In particular, giving to others may rely on distinct neural regions that serve to reinforce the caregiving behavior and are also involved in the regulation of stress responses (31,32).
In fact, it has been shown that the neural regions involved in maternal caregiving behavior in animals and the provision of social support in humans are also known for processing basic rewards; these include the ventral striatum (VS) and septal area (SA; 31,32). Moreover, these caregiving regions have inhibitory connections with regions known to be involved in threat and stress responding such as the amygdala (33,34). This activity in caregiving regions may dampen stress responding in order to facilitate adaptive care during times of stress, which over time may have health benefits. For example, giving support to a romantic partner in need (vs. not giving support) activated both the VS and SA (32). Furthermore, one of these regions, the SA, was negatively correlated with amygdala activity when participants gave support to their partners, suggesting that caregiving-related circuitry may help dampen stress responding. Indeed, in a separate study, giving support to a friend (vs. a control condition) led to reduced sympathetic-related responding to a laboratory-based stress task (21). Together, these findings suggest that giving to others may be stress-reducing on its own and therefore may be an overlooked contributor to the mental and physical health benefits of social support.
Still, little work has examined both receiving and giving support in terms of how they relate to stress-related neural activity. Furthermore, no studies have assessed social support and neural activity to positive, stress-free experiences. Hence, the current study examined the associations between both receiving support and giving support with neural activity in response to stressful and socially rewarding tasks. Specifically, we examined how both individual differences in receiving and giving support related to neural activity in response to a stress task, an affiliative task (in which participants viewed images of their close others), and a prosocial task.
There were two main goals. First, we examined whether self-reported support receiving related to negative psychological outcomes and neural activity to the three tasks. The current literature on receiving social support and health-related outcomes suggest that receiving social support is sometimes helpful, particularly at reducing stress, and sometimes not. Therefore, receiving support was hypothesized to be associated with less negative psychological outcomes, however, given the mixed findings in the literature, no directional hypotheses were made for the neural outcomes. Second, we examined the associations between self-reported support giving and negative psychological outcomes and neural activity to the three tasks. Based on emerging findings that giving to others might be good for health and relationships, support giving was hypothesized to be associated with less negative psychological outcomes, decreased stress-related activity to the stressful task, increased reward-related activity to viewing close others, and increased caregiving-related activity to the prosocial task.
Method
Participants
Forty-seven individuals participated as part of a larger study on the neural mechanisms associated with social support. The sample was then constrained to those who completed all 3 neuroimaging tasks. A single outlier who was consistently greater than 3 SD’s away from the mean (both below and above) across multiple tasks was removed leaving a final sample of 36 participants (Table 1). The self-reported ethnic composition included 8.3% Black/African American, 22.2% Latino/Chicano, 33.3% White, 30.6% Asian, and 5.6% Other. Procedures were run between June and October of 2014 in accordance with Institutional Review Board guidelines and all participants provided informed consent.
Table 1.
Participant characteristics. (n=36)
| Variable | Value |
|---|---|
|
| |
| Age (years) | 22.36 (3.78) |
| Sex | 16 females/20 males |
| Self-reported receiving support | 23.74 (3.12) |
| Self-reported giving support | 19.19 (3.46) |
Self-report Measures
Support Receiving and Giving
Support receiving and giving was assessed with the 21-item 2-way social support scale (35). Questions are rated on a 0-not at all to 5-always scale with higher numbers indicating a greater amount of support received and given. Sample items to assess receiving support include: “when I am feeling down there is someone I can lean on,” and “I have someone to help me if I am physically unwell.” Giving support is evaluated by items such as: “I look for ways to cheer people up when they are feeling down,” and “I have helped someone with their responsibilities when they were unable to fulfill them.” Participants reported receiving more support than they gave (t(35)=8.26, p<.001). Reports of receiving support were also moderately associated with reports of support giving (r=.501, p=.002) suggesting that both forms of social support are related, but separate constructs.
Vulnerability Index
Since receiving social support has historically been associated with less stress, we examined the associations between self-reported receiving and giving social support and a broad array of negative psychological outcomes. A “vulnerability index” was created that included depression (Beck Depression Inventory,36), sensitivity to social rejection (Mehrabian Rejection Sensitivity,37), perceptions of stress (Perceived Stress Scale,38), and feelings of loneliness (UCLA Loneliness Scale,39). The scales were standardized to the same scale by converting the raw scores to z-scores. Scores were averaged to create a single value per participant (α=.82).
Neuroimaging Measures
The association between questionnaire measures of receiving and giving social support and neural activity was assessed across 3 separate tasks
Stress Task
Stress-related neural activity was manipulated with a modified version of the Montreal Imaging Stress Task (MIST;40) where participants calculated math problems of varying difficulty under the guise that the experimenters were comparing their performance to other students at UCLA. As a control condition, participants completed “practice” blocks where they answered easy math problems (e.g. 1+2+4=) silently in their heads. Participants pushed a button once they came up with an answer. No answer choices or responses were displayed. During the “test” blocks, the blocks designed to elicit stress, participants mentally calculated more difficult math problems (e.g. 14 × 3/6 =) under time pressure and then selected an answer from among a set of possible answers. The subsequent screen provided the participant with feedback about whether or not they answered correctly and their performance relative to the “typical UCLA student.” Over time, the feedback indicated that participants’ performance (based on how quickly and accurately they responded relative to the average student) became increasingly worse as the typical student’s performance grew better, thus amplifying the social evaluative nature and uncontrollability of the situation.
Participants completed a total of 8 56-second blocks separated by 5-seconds of fixation crosshair: 4 practice blocks and 4 test blocks. Within each block, 8 math problems were presented for 5-seconds each. For the practice blocks, math problems were followed by 2-seconds of rest, but for the test blocks, math problems were followed by 2-seconds of feedback (1-second indicating whether the participant correctly answered and 1-second indicating how they performed relative to others). After each block participants rated how stressful they found the previous block (“how stressful was that”), rated from 1-not at all stressed to 4-extremely stressed. As expected, participants found the test blocks more stressful (M=2.90, SD=.73) than the practice blocks (M=1.98, SD=.72, t(35)=8.74, p<.001) indicating that the test blocks were indeed stressful. Stress-related neural activity was evaluated by comparing the test blocks to the practice blocks.
Affiliative Task
To examine associations between social support and reward-related activity in the ventral striatum to viewing images of close others, participants completed a modified affiliative task previously shown to activate reward-related neural circuitry (41–43). Prior to their experimental session, participants emailed 2 digital photographs of 2 different close others and ratings to two items using a 1–10 scale: “how close are you to this person,” anchored by “not at all” and “extremely” and “to what extent is this person a source of social support to you” anchored by “not a source of support” and “tremendous source of support.” Participants were indeed able to identify people with whom they had close, supportive relationships (M closeness=9.21, SD=1.31; M supportiveness=9.03, SD=1.26). One outlier, who rated one of their close others a 4 on closeness and supportiveness, was removed from any analyses evaluating the affiliative task. Therefore, the affiliative task is based on a sample of 35 participants.
Images of close others were reformatted into standard space and presented along with gender, race, and age-matched strangers in a block design. For each block, 2 images of a single person (either a close other or a stranger) were presented for a total of 16 seconds (8 seconds for each of the 2 images). In between blocks of images, participants completed easy mental arithmetic silently in their heads (e.g. count back by 3’s from 639) in 12-second blocks. This was included as a neutral baseline condition intended to prevent participants from continuing to think about their close others in between trials. In total, 16 blocks were presented, 8 mental arithmetic, 4 close other, and 4 stranger. Neural activity to the affiliative task was evaluated by comparing blocks of close others to blocks of strangers.
Prosocial Task
In addition to exploring associations between social support and neural activity to a stressful and affiliative task, we wanted to understand the associations between social support and neural responses to acting prosocially. Thus, participants completed a modified version of a donation task (44,45). In this task, participants had the chance to earn raffle tickets for themselves and for “someone you know that could use some money right now.” Before the task began, participants were asked to name the person they would be playing for and to provide the email and phone number of this person so that the experimenters could later contact them directly if they won. After the study was over, the tickets were entered into a drawing (including subjects as well as those they were playing for), in which 2 people won $300 each. The more tickets a participant won, the greater chance that they or the person they were playing for could win the $300.
During the task, participants saw 4 different trial types. On each trial, participants viewed a proposed distribution of raffle tickets split across themselves and the other person they chose to play for (3.5 seconds followed by a jittered fixation period, lasting 3 seconds on average (range=2.28–6.07)), and participants could then choose whether to accept or reject the proposed split. During the key prosocial trials—costly giving trials, participants chose whether to accept offers where they could give raffle tickets to the other person at a cost of tickets for themselves (e.g. YOU −10, OTHER +50). During the self-reward trials, participants could win raffle tickets for themselves without any cost to the other person (e.g. YOU +40, OTHER +0). On control trials, participants saw trials in which no tickets could be won or lost (YOU +0, OTHER +0). Costly reward (e.g. YOU +30, OTHER −10) trials were also included in the task to keep participants engaged and interested in the task, but were not analyzed.
The prosocial task included 90 trials (40 costly giving—to account for the fact that some trials would not be accepted, 20 pure reward, 20 control, 10 costly reward). The range of tickets that participants could win or lose varied between −30 tickets and +70 tickets. The running total of tickets was not shown. During data analysis, trials were binned based on the acceptance of an offer. Thus, if a participant accepted a costly giving trial, that trial was modeled as a prosocial giving trial, otherwise it was modeled separately and not examined (based on prior procedures; 44,45). To assess caregiving-related neural activity in response to acting prosocially, costly giving trials were compared to both control and self-reward trials.
fMRI Data Acquisition
Participants were scanned at UCLA’s Staglin IMHRO Center for Cognitive Neuroscience on a Siemens 3 Telsa “Tim Trio” MRI scanner. Two anatomical scans, a high-resolution T1-weighted echo-planar imaging volume (spin-echo, TR=5000ms; TE=33ms; matrix size 128×128; 36 axial slices; FOV =20cm; 3mm thick, skip 1mm) and T2-weighted matched-bandwidth (slice thickness=3mm, gap=1mm, 36 slices, TR=5000ms, TE=34ms, flip angle=90°, matrix=128×128, FOV=20 cm) were collected. Functional scans for each of the three tasks (stress task: 9 minutes, 31 seconds; affiliative task: 4 minutes, 14 seconds; prosocial task: 10 minutes, 12 seconds) were then acquired (echo planar T2* weighted gradient-echo, TR=2000ms, TE=25ms, flip angle=90°, matrix size 64×64, 36 axial slices, FOV=20 cm; 3-mm thick, skip 1mm). The affiliative and prosocial tasks were counterbalanced across participants, but the stress task was always presented after these other two tasks to ensure that the stressfulness of completing the stress task did not alter the potentially pleasant experience of completing the other two tasks.
Statistical Analyses
Self-report correlations
Self-report measures of receiving and giving social support were correlated with the vulnerability index, the measure of current negative psychological outcomes, using SPSS version 20.0.
fMRI Data
Data was preprocessed using the DARTEL procedure in SPM8 (Wellcome Department of Imaging Neuroscience, London). Images were realigned, then normalized to the T2-weighted matched bandwidth and warped into Montreal Neurologic Institute (MNI) space, and finally smoothed with an 8mm Gaussian kernel, full width at half maximum. The general linear model was used to estimate first-level effects to each condition of interest compared to their respective controls. Group level analyses were then computed using the first-level contrast images for each participant.
Region-of-Interest (ROI) Analyses
Given that the main goals of the current study were to examine the association between self-reported social support and stress, reward, and caregiving-related neural circuitry to three separate tasks, analyses were constrained to a-priori hypothesized regions-of-interest (ROI, see Fig. 1). In addition, two regions that were unrelated to the hypotheses tested in the current study were created in order to test the specificity of the associations.
Figure 1.
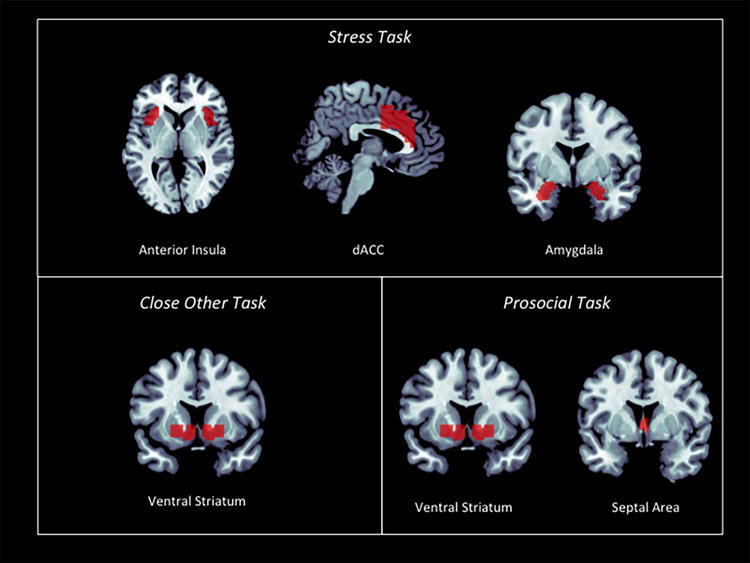
Regions of Interest (ROI) for each of the three tasks. Analyses were targeted to reward-related activity in the ventral striatum (VS) for the close other task, stress-related activity in the anterior insula (AI), dorsal anterior cingulate cortex (dACC), and amygdala for the stress task, and caregiving-related activity in the VS and septal area (SA) for the prosocial task.
For the stress task, we examined regions known to signal threat and stress including the dorsal anterior cingulate cortex (dACC), left and right anterior insula (AI), and left and right amygdala. Stress-related ROIs were structurally defined using the Automated Anatomical Labeling (AAL) Atlas (46). To further refine the dACC ROI, we constrained the region at 32<y<0 on the basis of summary data on cingulate activations to physical pain (47) and for the AI ROI, we divided the insula at y=8 the approximate boundary between the dysgranular and granular sectors.
For the affiliative task, left and right ventral striatum (VS) ROI’s were structurally defined by combining the caudate and putamen from the AAL Atlas and then constraining the regions at −24<x<24, 4<y<18, −12<z<0. Based on previous work on the neural correlates of support giving (32), the anatomical ROIs of the VS and an additional ROI of the septal area (SA) were used to explore activity in maternal caregiving regions to the prosocial task. The SA ROI was previously defined for a study examining prosocial emotions (48) according to microscopic sections between y=0 and y=14 that show the location of the septal nuclei through the anterior commissure and anterior to the optic chiasma. Though the septal nuclei are too small to be imaged with the 3T fMRI, the SA ROI used in the current study encompasses the larger surrounding area. The SA has also been imaged and reported on previously (49–51) although it is still a relatively small region and thus results should be interpreted with caution.
Finally, “control” regions were created based on their irrelevance to the current tasks in order to establish whether the associations between social support and neural activity were specific to the hypothesized regions. The supplementary motor area (SMA) was chosen as a control region for the affiliative task because this task did not involve any motor actions (i.e. there were no button responses required) and the olfactory cortex, including the olfactory tubercle and Broca’s olfactory cortex located under the corpus callosum, was chosen as a control region for the stress and prosocial tasks because there is no hypothesized function of this region in stress or prosocial processes. Both regions were defined using the AAL atlas using the Wake Forest University Pickatlas (52).
All ROI analyses were run in Marsbar (http://marbar.sourceforge.net) and thresholded at p<.05.
Results
Correlation between receiving/giving social support and the vulnerability index
In line with the current literature linking social support with well-being, both self-reported receiving support (r=−.60, p<.001) and self-reported giving support (r=−.64, p<.001) were negatively correlated with the vulnerability index (Fig. 2). That is, both receiving and giving more support were related to lower reported negative psychological outcomes.
Figure 2.
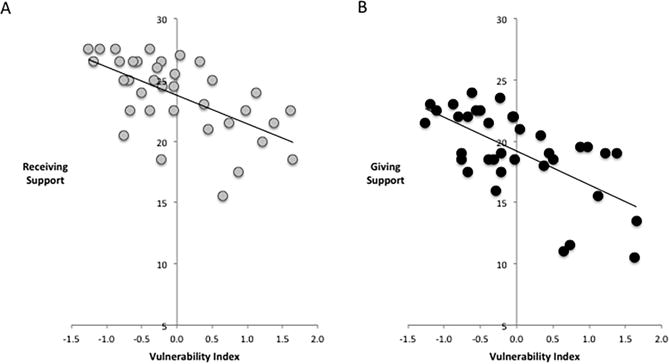
Associations between self-reported social support and the vulnerability index. Self-reported negative psychological outcomes were negatively correlated with receiving support (A) and giving support (B) such that less vulnerability was associated with greater support receiving and giving.
Neural Results
Stress Task
Main effect of task
For the stressful math task, we looked for activity in stress and threat-related regions (dACC, AI, amygdala) when participants completed test blocks compared to when participants completed practice blocks. Validating the stressfulness of the task, dACC (M=.21, SD=.61) and left (M=.14, SD=.51) and right AI (M=.08, SD=.55) activity was greater during the test blocks compared to the practice blocks (M dACC=.06, SD=.48, t(35)=2.07, p=.023; M L AI=.03, SD=.42, t(35)=1.63, p=.056; M R AI=−.07, SD=.43, t(35)=2.29, p=.014). Amygdala activity, however, did not differ between test (L amyg M=−.25, SD=.59; R amyg M=−.30, SD=.63) and practice blocks (L amyg M=−.30, SD=.63, t(35)=.74, p=.23; R amyg M=−.32, SD=.48, t(35)=.16, p=.44).
Correlations between receiving/giving support and neural activity
We then examined whether questionnaire measures of social support were related to neural activity to the stress task. Although receiving support was not associated with dACC (r=.07), AI (left r=.08, right r=.03) or amygdala activity (left r=−.04, right r=−.02), support giving was negatively correlated with dACC (r=−.27, p=.054), left (r=−.28, p=.048) and right AI (r=−.33, p=.024), and left (r=−.32, p=.028) and right amygdala (r=−.32, p=.028 activity to test (vs. practice) blocks (Fig. 3). The correlations between neural activity and self-reported support giving were significantly different from the correlations with self-reported support receiving across all of these regions (dACC Z=2.01, p=.022; left AI Z=2.13, p=.016; right AI Z=2.02, p=.014; left amygdala Z=1.71, p=.043; right amygdala Z=1.81, p=.035). Thus, those who reported the most support giving also displayed the least amount of threat-related activity to the socially evaluative stressor, which is consistent with hypotheses that support giving might be stress-reducing.
Figure 3.
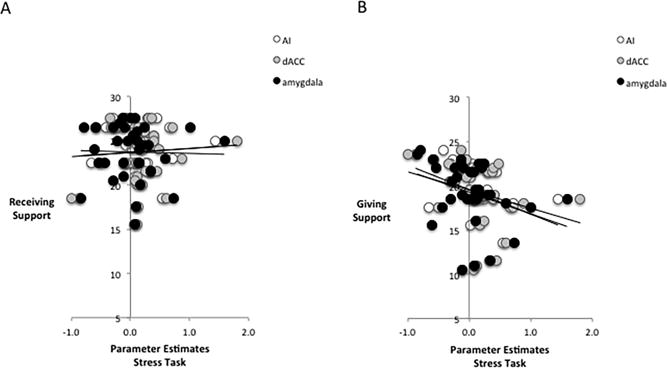
Associations between stress and threat-related activity and self-reported social support. (A) Parameter estimates from the anterior insula (AI), dorsal anterior cingulate cortex (dACC), and amygdala while participants completed stressful blocks of mental arithmetic (vs. practice blocks) were not associated with how much support they reported receiving. (B) Stress-related activity was, however, negatively correlated with support giving such that less AI, dACC, and amygdala to stressful blocks (vs. practice blocks) was associated with greater support giving.
Supporting the specificity of this effect, there were no associations between olfactory cortex activity (the chosen control region) and either self-reported receiving support (r=.164, p=.170) or self-reported support giving (r=.007, p=.491) in response to the stress task (test vs. practice blocks).
Affiliative Task
Main effect of task
Ventral striatum activity was assessed as participants viewed images of close, supportive others and strangers. Consistent with the importance of reward-related processing to close social relationships (42), viewing images of close others (vs. strangers) led to increased activity in the left (M close other=.04, SD=.22, M stranger=−.03, SD=.20, t(34)=1.88, p=.034) and right VS (M close other=−.03, SD=.20, M stranger=−.10, SD=.21, t(34)=1.71, p=.049).
Correlations between receiving/giving support and neural activity
Correlational analyses revealed that while support receiving was not associated with VS activity (r left=.03, r right=−.02), support giving was associated with greater left (r=.42, p=.006) and right VS (r=.41, p=.008) to viewing images of close others (vs. strangers). Moreover, these associations were significantly different from those between receiving support and VS activity (left Z=2.34, p=.009; right Z=2.58, p=.005; Fig. 4).
Figure 4.
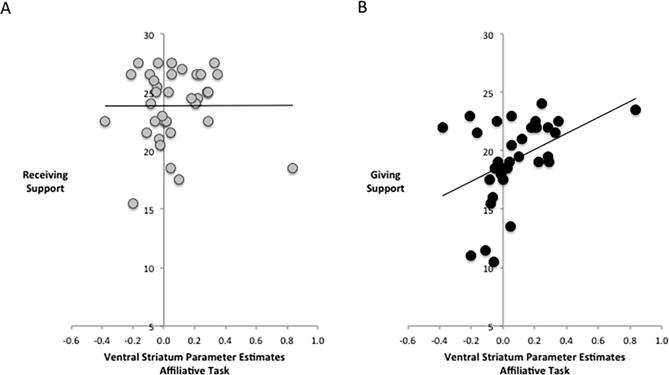
Associations between ventral striatum (VS) activity to the affiliative task and social support. (A) VS to viewing images of close others (vs. strangers) was not associated with receiving support. (B) However, parameter estimates from the VS ROI to viewing images of close others (vs. strangers) was associated with more support giving.
To examine the specificity of these effects, we examined the correlation between activity in the control region (SMA) and questionnaire measures of social support. As expected, there were no associations between SMA activity and either self-reported receiving support (r=−.024, p=.446) or self-reported support giving (r=.191, p=.136) in response to the affiliative task.
Prosocial Task
Main effect of task
To ensure that the prosocial task activated the hypothesized caregiving regions, we evaluated differences in the left and right VS and SA activity to costly giving and reward trials (vs. control) separately. Consistent with prior studies (44,45), VS activity was greater during the costly giving trials, when participants chose to give raffle tickets to someone else (M left VS=.26, SD=.50; M right VS=.42, SD=.62), compared to the control condition (M left VS=.04, SD=.20, t(35)=2.795, p=.004; M right VS=.07, SD=.24, t(35)=3.937, p<.001). Moreover, in response to self-reward (vs. control) trials, there was greater activity in the right VS (M=.31, SD=.75, t(35)=2.193, p=.018), but not the left VS (M=.16, SD=.72, t(35)=1.15, p=.13). SA activity was not significantly different between costly giving trials (M=−.18, SD=1.55) and control trials (M=−.18, SD=.55, t(35)=−.01, p=.50) or between self-reward trials (M=−.50, SD=1.78) compared to control trials (t(35)= −1.34, p=.094). There were no differences in VS and SA to costly giving vs. self-reward (ps>.14), indicating that both giving and receiving lottery tickets elicited reward-related activity.
Correlations between receiving/giving support and neural activity
Correlational analyses showed no association between self-reported receiving support and either left (r=.11, p=.26) or right VS activity (r=.09, p=.31) in response to costly giving (vs. self-reward) trials. The association between receiving support and SA activity during costly giving (vs. self-reward) was marginal (r=24, p=.084). However, there was a significant association between support giving and left VS (r=.31, p=.033), right VS (r=.31, p=.034), and SA (r=.39, p=.095) activity (Fig. 5). That is, those who reported giving the most support to others demonstrated the greatest VS and SA activity to costly giving trials relative to self-reward trials. Thus, the more participants reported giving support to others, the more caregiving-related neural activity they showed to trials in which they chose to give to another person in need, but no such association was found between how much support people received and neural activity to the prosocial task. The correlations between self-reported support giving and the VS were marginally different from the correlation between self-reported receiving support and the VS (left: Z=−1.32, p=.094; right Z=−1.19, p=.12), however, the correlation between self-reported support giving and SA activity was not significantly different from the correlation between self-reported receiving support and SA activity (p=.17).
Figure 5.
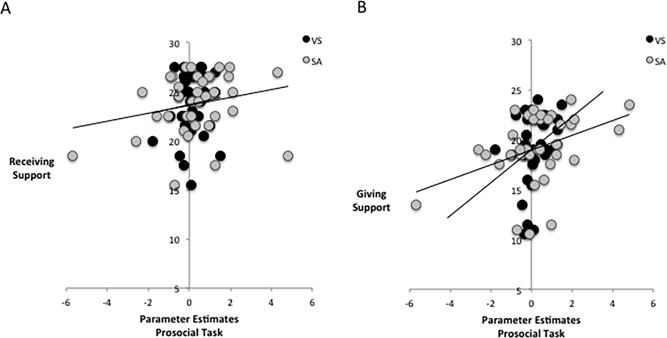
Correlations between parameter estimates from caregiving-related neural regions during the prosocial task and social support. (A) Whereas activity in the ventral striatum (VS) and septal area (SA) when participants acted prosocially (vs. selfishly) was not significantly associated with receiving support, (B) greater VS and SA activity during prosocial (vs. selfish) decisions was associated with more support giving.
Finally, we examined the correlation between activity in the control region (olfactory cortex) and questionnaire measures of social support. There were no associations between olfactory cortex activity and either the questionnaire measure of receiving support (r=.004, p=.491) or the questionnaire measure of support giving (r=.021, p=.452).
Discussion
To date, our understanding of the association between social ties and health has been incomplete insofar as there is a fair amount known about the benefits of receiving support, but less is known about the benefits that may come from giving to others. The current study examined the associations between questionnaire measures of receiving and giving support to others, self-reported negative psychological outcomes, and neural activity to stressful and socially rewarding tasks. Although both receiving and giving social support were related to lower reported negative psychological outcomes, at the level of the brain, only support giving was associated with beneficial outcomes. Specifically, support giving was associated with lower threat-related activity to a stress task, greater activity in the ventral striatum to viewing images of close others (vs. strangers), and greater caregiving-related neural activity to acting prosocially (vs. selfishly). These results add to the accumulating literature suggesting that giving to others might be beneficial for health and well-being (30–24,32,44,53).
The current findings suggest that giving to others may act through multiple routes: 1) via reduced stress-related mechanisms, 2) via increased reward-related activity in response to viewing close others, and 3) via increased caregiving-related activity in response to acting prosocially. The findings from each of the three tasks are discussed below.
Although physical health outcomes were not measured in this study, the current results shed light on possible neural pathways by which giving to others may be associated with better health outcomes (20,22), namely by reducing activity in stress and threat-related regions during stressful experiences. Indeed, stressor-evoked dACC, AI, and amygdala activity, as studied here, have all been associated with increased autonomic (SNS) responding (see 31 for review).
Why might giving support, but not receiving support, be associated with reduced stress-related neural activity? Giving support might bypass some of the conditions that result in the detrimental effects associated with receiving support. For instance, receiving support sometimes backfires because it mismatches one’s personal preferences for support (54), leaves one feeling indebted (18), and signals that something distressing or negative has happened (55). Giving support, on the other hand, allows an individual to control when and how support is given, can lead to a greater sense of autonomy and self-efficacy (56), is associated with increased feelings of social connection (32), and increased happiness (57). Thus, to the extent that giving to others simultaneously bypasses the problems that receiving support sometimes elicits and increases one’s psychological resources, giving, relative to receiving support, may result in more effective stress reduction. The current results are correlational, however, and so experimental work that directly compares both receiving and giving support on physical health-related outcomes is needed to understand when and how each kind of support is uniquely beneficial or whether those who give support are simply less prone to stress-related responding to socially evaluative stressors, as examined in the current study.
The hypothesis that support giving might be stress reducing on its own appears to contradict the large and well-known literature on the negative health effects of chronic caregiving (e.g. 58,59). It is possible that when the caregiving needs exceed the demands of psychological, material, or social resources available to an individual, giving support may no longer be health-protective. However, studies linking chronic caregiving to poor health outcomes have thus far failed to control for the emotional distress of witnessing the deterioration of a loved one. Therefore it remains unclear whether the support giving component of chronic caregiving is the “active ingredient” in the association between caregiving and bad health or if there are other factors at play. Some studies suggest that caregiving can be associated with beneficial, rather than detrimental, health outcomes (60,61), pointing to the possibility that the caregiving itself may not be the cause of the negative health effects. Thus, much like receiving support, there are a number of factors that contribute to whether and when giving to others is or is not beneficial for health.
In addition to the relationship between support giving and reduced stress-related neural activity, support giving was also associated with increased VS activity to viewing images of close others. Those who reported giving more support to others also displayed greater reward-related activity to images of their own loved ones. There was no such association between VS activity and reports of receiving support. The ventral striatum is particularly sensitive to social (vs. nonsocial) rewards (62), and also to the degree of closeness between two individuals (63). For instance, sharing monetary rewards with a friend activates the VS more than sharing rewards with a stranger. Furthermore, those reporting higher closeness ratings with the friend display greater VS to the shared wins with the friend (vs. a stranger). Similarly, the number of years married is associated with increased VS to images of romantic partners (vs. close friends; 41). Based on these findings it is possible that VS activity to “social rewards” might also signal the degree of closeness and connection with others. Thus the positive correlation between support giving and VS to images of close others may suggest that there is something unique about giving support, but not necessarily receiving support, that binds people closer together (26).
The prosocial task assessed caregiving-related neural activity as participants had the opportunity to give to another individual. Self-reported support giving, but not support receiving, was positively correlated with VS and SA activity in response to acting prosocially (vs. selfishly). These findings suggest that those who tend to give more support might also be more rewarded by acting prosocially toward a known other, which may encourage more subsequent support giving behavior. Although the current task assessed prosocial behavior toward a single target, caregiving-related activity while acting prosocially toward others more broadly may predict better health outcomes on its own. Consistent with this possibility, one recent study found that ventral striatum activity to giving, but not to receiving monetary rewards for oneself, was associated with reduced depression levels a year later (45). Future work directly linking caregiving-circuitry during prosocial behavior and health outcomes outside of the scanner will clarify the role of the VS and SA in any health benefits that may come from giving to others.
An important future direction for understanding how support giving relates to beneficial outcomes is to isolate the unique contribution of support giving itself from other individual difference factors, such as extraversion or general positive affect. Previous work exploring the health benefits of support giving has shown that the associations between support giving and health remain after controlling for subjective well-being, baseline health factors, and extraversion (20,23) suggesting that there is something uniquely beneficial about giving to others. However, controlling for these additional personality factors will help clarify the unique role that support giving plays in health.
In sum, the results of the current study suggest that examining the psychological and physical health benefits of giving support to others deserves greater empirical attention and that gaining a full understanding of how and why social ties are so important to well-being requires the consideration of both the support that is received and given.
Acknowledgments
This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors also acknowledge the Staglin IMHRO Center for Cognitive Neuroscience.
Glossary
- VS
ventral striatum
- SA
septal area
- dACC
dorsal anterior cingulate cortex
- AI
anterior insula
- ROI
region-of-interest
Footnotes
Conflicts of Interest and Source of Funding: None declared
References
- 1.Bowlby J A secure base: Parent-child attachment and healthy human development. New York, NY: Basic Books; 1988. [Google Scholar]
- 2.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 3.Holt-Lundstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunnar MR, Bruce J, Grotevant HD. International adoption of institutionally reared children: Research and policy. Dev Psychopathol. 2000;12:677–693. doi: 10.1017/s0954579400004077. [DOI] [PubMed] [Google Scholar]
- 5.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosom Med. 2011;73:724–30. doi: 10.1097/PSY.0b013e318235be76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wills TA. Social support and interpersonal relationships. In: Clark MS, editor. Prosocial Behavior. Newbury Park: Sage; 1991. pp. 265–289. [Google Scholar]
- 8.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of alameda county residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 9.Cassel JC. The contribution of the social environment to host resistance. Am J Epidemiol. 1976;104:107–123. doi: 10.1093/oxfordjournals.aje.a112281. [DOI] [PubMed] [Google Scholar]
- 10.Cobb S. Social support as a moderator of life stress. Psychosomatic Med. 1976;38:300–314. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–57. [PubMed] [Google Scholar]
- 12.Kamarck TW, Manuck SB, Jennings JR. Social support reduces cardiovascular reactivity to psychological challenge: a laboratory model. Psychosom Med. 1990;52:42–58. doi: 10.1097/00006842-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Thorsteinsson EB, James JE. A meta-analysis of the effects of experimental manipulations of social support during laboratory stress. Psychol & Health. 1999;14:869–886. [Google Scholar]
- 14.Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1602. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masten CL, Telzer EH, Fuligni A, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Soc Cogn Affect Neurosci. 2012;7:106–114. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberger NI, Master SL, Inagaki TI, Taylor SE, Shirinyan D, Lieberman MD, Naliboff B. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proc Nat Acad Sci USA. 2011;108:11721–11726. doi: 10.1073/pnas.1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: Involvement of neural reward systems. PLoS ONE. 2010;5:e133309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gleason MEJ, Iida M, Bolger N, Shrout PE. Daily supportive equity in close relationships. Pers Soc Psychol Bull. 2003;29:1036–1045. doi: 10.1177/0146167203253473. [DOI] [PubMed] [Google Scholar]
- 19.Bolger N, Amarel D. Effects of social support visibility on adjustment to stress: experimental evidence. J Pers Soc Psychol. 2007;92:458–475. doi: 10.1037/0022-3514.92.3.458. [DOI] [PubMed] [Google Scholar]
- 20.Brown SL, Nesse RM, Vinokur AD, Smith DM. Providing social support may be more beneficial than receiving it: Results from a prospective study of mortality. Psychol Sci. 2003;14:320–327. doi: 10.1111/1467-9280.14461. [DOI] [PubMed] [Google Scholar]
- 21.Inagaki TK, Eisenberger NI. Giving support to others reduces sympathetic nervous system-related responses to stress. Psychophysiology in press. [DOI] [PubMed] [Google Scholar]
- 22.Piferi RL, Lawler KA. Social support and ambulatory blood pressure: an examination of both receiving and giving. Int J Psychophysiol. 2006;62:328–336. doi: 10.1016/j.ijpsycho.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Poulin MJ, Brown SL, Dillard AJ, Smith DM. Giving to others and the association between stress and mortality. Am J Public Health. 2013;103:1649–55. doi: 10.2105/AJPH.2012.300876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaananen A, Buunk BP, Kivimaki M, Pentti J, Vahtera J. When it is better to give than to receive: long-term health effects of perceived reciprocity in support exchange. J Pers Soc Psychol. 2005;89:176–193. doi: 10.1037/0022-3514.89.2.176. [DOI] [PubMed] [Google Scholar]
- 25.Brown SL, Brown RM. Connecting prosocial behavior to improved physical health: Contributions from the neurobiology of parenting. Neurosci Biobehav Rev. 2015;55:1–17. doi: 10.1016/j.neubiorev.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Brown SL, Brown RM. Selective investment theory: recasting the functional significance of close relationships. Psychol Inq. 2006;17:1–29. [Google Scholar]
- 27.Brown S, Brown M, Preston S. The human caregiving system. In: Brown SL, Brown M, Penner LA, editors. Moving Beyond Self Interest: Perspectives from Evolutinoary Biology, Neuroscience, and the Social Sciences. Oxford University Press; 2011. pp. 76–88. [Google Scholar]
- 28.Preston S. The Origins of Altruism in Offspring Care. Psychol Bull. 2013;139:1305–41. doi: 10.1037/a0031755. [DOI] [PubMed] [Google Scholar]
- 29.Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 30.Feeney BC, Collins NL. Predictors of caregiving in adult intimate relationships: an attachment theoretical perspective. J Pers Soc Psychol. 2001;80:972–994. [PubMed] [Google Scholar]
- 31.Eisenberger NI, Cole SW. Social neuroscience and health: neuropsychological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- 32.Inagaki TK, Eisenberger NI. Neural correlates of giving support to a loved one. Psychosom Med. 2011;74:3–7. doi: 10.1097/PSY.0b013e3182359335. [DOI] [PubMed] [Google Scholar]
- 33.Adolphs R, Tranel D, Damasio A. Fear and the human amygdala. J Neurosci. 1995;15:5879–5892. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 35.Shakespeare-Finch J, Obst PL. The development of the 2-way social support scale: a measure of giving and receiving emotional and instrumental support. J Pers Assess. 2011;93:483–490. doi: 10.1080/00223891.2011.594124. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 37.Mehrabian A. Questionnaire measures of affiliative tendency and sensitivity to rejection. Psychol Rep. 1976;38:199–209. [Google Scholar]
- 38.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 39.Russell DW. UCLA loneliness scale (version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- 40.Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The montreal imaging stress task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci. 2005;30:319–25. [PMC free article] [PubMed] [Google Scholar]
- 41.Acevedo BP, Aron A, Fisher HE, Brown LL. Neural correlates of long-term intense romantic love. Soc Cogn Affect Neurosci. 2012;7:145–159. doi: 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aron A, Fisher H, Mashek D, Strong G, Li H, Brown L. Reward, motivation and emotion systems associated with early-stage intense romantic love. J Neurophysiology. 2005;93:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- 43.Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I, Breen EC, Eisenberger NI. The role of the ventral striatum in sickness-induced approach toward support figures. Brain Behav Immun. 2015;44:247–252. doi: 10.1016/j.bbi.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci U S A. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Telzer EH, Fuligni AJ, Lieberman MD, Gálvan A. Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proc Natl Acad Sci U S A. 2014;111:6600–5. doi: 10.1073/pnas.1323014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 47.Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–44. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED, Grafman J. The neural basis of human social values: evidence from functional MRI. Cereb Cortex. 2009;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho SS, Konrath S, Brown S, Swain JE. Empathy and stress related neural responses in maternal decision making. Front Neurosci. 2014;8:152. doi: 10.3389/fnins.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krueger F, McCabe K, Moll J, Kriegeskorte N, Zahn R, Strenziok M, Heinecke A, Grafman J. Neural correlates of trust. Proc Natl Acad Sci USA. 2007;104:20084–20089. doi: 10.1073/pnas.0710103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morelli SA, Rameson LT, Lieberman MD. The neural components of empathy: predicting daily prosocial behavior. Soc Cogn Affect Neuroci. 2014;9:39–47. doi: 10.1093/scan/nss088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 53.Dunn EW, Aknin LB, Norton MI. Spending money on others promotes happiness. Science. 2008;319:1687–1688. doi: 10.1126/science.1150952. [DOI] [PubMed] [Google Scholar]
- 54.Cutrona CE. Stress and social support: In search of optimal matching. J Soc Clin Psychol. 1990;9:3–14. [Google Scholar]
- 55.Seidman G, Shrout PE, Bolger N. Why is enacted social support associated with increased distress? Using simulation to test two possible sources of spuriousness. Pers Soc Psychol Bull. 2006;32:52–65. doi: 10.1177/0146167205279582. [DOI] [PubMed] [Google Scholar]
- 56.Gruenewald TL, Liao DH, Seeman TE. Contributing to others, contributing to oneself: perceptions of generativity and health later in life. J Gerontol B Psychol Sci Soc Sci. 2012;67:660–5. doi: 10.1093/geronb/gbs034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- 58.Schulz R, Beach SR. Caregiving as a risk factor for mortality: The caregiver health effects study. J Am Med Assoc. 1999;22:15–19. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 59.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 60.Brown SL, Smith DM, Schulz R, Kabeto MU, Ubel PA, Poulin M, Yi J, Kim C, Langa KM. Caregiving behavior is associated with decreased mortality risk. Psychol Sci. 2009;20:488–494. doi: 10.1111/j.1467-9280.2009.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amirkhanyan AA, Wolf DA. Caregiver stress and noncaregiver stress: Exploring the pathways of psychiatric morbidity. Gerontologist. 2003;48:817–827. doi: 10.1093/geront/43.6.817. [DOI] [PubMed] [Google Scholar]
- 62.Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–94. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 63.Fareri DS, Niznikiewicz MA, Lee VK, Delgado MR. Social network modulation of reward-related signals. J Neurosci. 2012;32:9045–9052. doi: 10.1523/JNEUROSCI.0610-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


