Abstract
Obesity is an increasingly urgent global problem, yet, little is known about its causes and less is known how obesity can be effectively treated. We showed previously that the aryl hydrocarbon receptor (AHR) plays a role in the regulation of body mass in mice fed Western diet. The AHR is a ligand-activated nuclear receptor that regulates genes involved in a number of biological pathways, including xenobiotic metabolism and T cell polarization. This study was an investigation into whether inhibition of the AHR prevents Western diet-based obesity. Male C57Bl/6J mice were fed control and Western diets with and without the AHR antagonist α-naphthoflavone or CH-223191, and a mouse hepatocyte cell line was used to delineate relevant cellular pathways. Studies are presented showing that the AHR antagonists α-naphthoflavone and CH-223191 significantly reduce obesity and adiposity and ameliorates liver steatosis in male C57Bl/6J mice fed a Western diet. Mice deficient in the tryptophan metabolizing enzyme indoleamine 2,3-dioxygenase 1 (IDO1) were also resistant to obesity. Using an AHR-directed, luciferase-expressing mouse hepatocyte cell line, we show that the transforming growth factor β1 (TGFβ1) signaling pathway via PI3K and NF-κB and the toll-like receptor 2/4 (TLR2/4) signaling pathway stimulated by oxidized low-density lipoproteins via NF-κB, each induce luciferase expression; however, TLR2/4 signaling was significantly reduced by inhibition of IDO1. At physiological levels, kynurenine but not kynurenic acid (both tryptophan metabolites and known AHR agonists) activated AHR-directed luciferase expression. We propose a hepatocyte-based model, in which kynurenine production is increased by enhanced IDO1 activity stimulated by TGFβ1 and TLR2/4 signaling, via PI3K and NF-κB, to perpetuate a cycle of AHR activation to cause obesity; and inhibition of the AHR, in turn, blocks the cycle's output to prevent obesity. The AHR, with its broad ligand binding specificity, is a promising candidate for a potentially simple therapeutic approach for the prevention and treatment of obesity and associated complications.
Keywords: Aryl Hydrocarbon Receptor, Obesity, Liver steatosis, α-Naphthoflavone and CH-223191, TLR2/TGFβ/PI3K/NF-κB /IDO1/AHR axis
Graphical abstract
1. Introduction
One of the accepted causes for the worldwide rise in obesity and associated problems is the increased global consumption of the high-calorie, high-fat, high-carbohydrate, high-salt, low-fiber Western diet. In 2011–2012, nearly 35% of adults aged were obese (Ogden et al., 2014). Obesity is a contributor to inflammation (De Nardo and Latz, 2011), diabetes and metabolic syndrome (Wang et al., 2011b), cardiovascular disease (Poirier et al., 2006), and cancer (van den Brandt et al., 2000). It has been estimated that 25–70% of obesity is gene based (Maffeis, 2000), and twins studies suggest that 25–40% of individual differences in obesity are genetic (Stunkard et al., 1986). A few genes have been identified that influence obesity, such as the Leptin (Ob) (Zhang et al., 1994) and Adiponectin (Yamauchi et al., 2001) genes; however, it is clear that many genes are involved in the complex interactions that have given rise to the global obesity explosion (Woods et al., 1998). Furthermore, non-genetic factors are a major contributor to obesity in that a heightened exposure to environmental toxicants or obesogens (Grun and Blumberg, 2006) is strongly associated with the sharp increase in obesity and associated diseases (Baillie-Hamilton, 2002), in which the aryl hydrocarbon receptor (AHR) may be playing a large role (Wang et al., 2011a; Kerley-Hamilton et al., 2012; Xu et al., 2015).
The AHR is a ligand/toxicant-activated nuclear receptor that regulates hundreds of genes (Kerley-Hamilton et al., 2012) and many cellular pathways, and mice with the Ahr gene deleted suffer several developmental and metabolic anomalies (Fernandez-Salguero et al., 1995; Lahvis et al., 2000; Quintana et al., 2008). Upon agonist binding, the AHR translocates to the nucleus where it complexes with the AHR nuclear translocator (Hoffman et al., 1991). The AHR is best known for the induction by environmental toxicants of genes in the cytochrome P450 Cyp1 family and several Phase II detoxification genes (Nebert et al., 1993; Hankinson, 1995). Like all cellular signaling pathways, the AHR signaling pathway is known to interact with many other signaling pathways (Puga et al., 2005), including TGFβ (Gaido et al., 1992). In summation, the AHR plays vital roles in vascular patterning, organ modeling, extracellular matrix deposition, cell proliferation, apoptosis, and the cardiac system.
In addition to exogenous ligands, such as dioxin and benzo[a]pyrene, several tryptophan (Trp) catabolites have been identified as endogenous AHR ligands (Zelante et al., 2014). One such Trp catabolite is L-kynurenine (Kyn), an AHR agonist that activates AHR-directed, naive T cell polarization to the anti-inflammatory Treg phenotype (Veldhoen et al., 2009; Mezrich et al., 2010; Nguyen et al., 2010; Nguyen et al., 2013). Trp is an essential amino acid of which 95% is metabolized in a tissue-specific manner by the rate-limiting enzymes tryptophan 2,3-dioxygenase 2 (TDO2) and indoleamine 2,3-dioxygenase (IDO1 and IDO2) (Mangge et al., 2013).
Low-density lipoproteins (LDLs) have also been identified as an endogenous activator of AHR signaling (McMillan and Bradfield, 2007), although not as an AHR-binding ligand. LDLs are one of several types of lipoprotein particles in forms by which diet-derived fats are transported in the blood. An LDL particle is composed of an apolipoprotein B protein, 50–60 ancillary proteins, and ~5,000 fat molecules that includes variable amounts of cholesterol, phospholipids, and triglycerides (Dashty et al., 2014). LDL particles are oxidized (ox-LDL) over time by free radicals, transition metals, and enzymatic reactions (Yoshida and Kisugi, 2010), in which ox-LDL can serve as a ligand to stimulate TLR2 and TLR4 signaling (Chavez-Sanchez et al., 2014). Toll-like receptors (TLRs) are a class of pattern recognition receptors that respond broadly to molecular structures such as bacterial cell walls, and are an integral part of the innate immune response (O'Neill et al., 2013). TLR2 is known to play a role in obesity because the lack of functional TLR2 signaling prevented obesity and adiposity in C57Bl/6J (B6) mice on fatty diets (Himes and Smith, 2010).
We had shown previously that the AHR is involved in the regulation of body mass gain in mice, in which B6 mice were significantly more obese when fed Western diet than were B6.D2 mice, a congenic mouse strain that encodes an AHR with a lower ligand-binding affinity (Kerley-Hamilton et al., 2012). We extended those studies here and show that AHR antagonism prevents obesity and ameliorates liver steatosis, and we propose a model linking Western diet to AHR activation via signaling pathways stimulated by diet-derived compounds. The results indicate new roles for the AHR in obesity, and our demonstration that obesity may be dependent on AHR signaling, which can be inhibited by an array of compounds, could lead to simple therapeutic and preventative approaches in the treatment of obesity.
2. Materials and methods
2.1 Materials
The control, low-fat diet (catalog #D12450B) contained 20% kcal protein, 70% kcal carbohydrates (35% kcal from sucrose), 10% kcal fat (4.5% kcal from lard). The Western diet (catalog #D12071702) contained 20% kcal protein, 35% kcal carbohydrates (17.5% kcal from sucrose), 45% kcal fat (40% kcal from lard), and 2% cholesterol). Both diets were purchased from Research Diets, Inc. (New Brunswick, NJ). The diets contained no detectable phytoestrogens or xenobiotics. The α-naphthoflavone was purchased from Sigma-Aldrich (St. Louis, MO) and the CH-223191 from R&D Systems, Inc. (Minneapolis, MN). The TLR2/4 inhibitor oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (ox-PAPC) was purchased from InVivoGen (San Diego, CA), PI3K inhibitor LY294002 from Cell Signaling Technology (Danvers, MA), NF-κB inhibitor BAY11-7085 from Sigma-Aldrich, and IDO1 inhibitor Norharmane from Santa Cruz Biotechnology (Dallas, TX).
2.2 Mice
An overview of the rationale and experimental design for the mouse and in vitro studies is depicted in Fig. S1. Only male mice were used in the studies reported here. Male mouse strains B6 (C57Bl/6J, stock# 000664), B6.AhrKO (B6;129-Ahrtm1Bra/J, stock# 002727), and B6.Ido1−/− (B6.129-Ido1tm1Alm/J, stock# 005867) were purchased from The Jackson Laboratory (Bar Harbor, ME). The mice were reared in 12-hr light/dark cycles, and to minimize exposure to exogenous toxicants, the cage was bedded with chemical-free shredded paper (Pure-o-cell, The Andersons Lab Bedding, Maumee, OH). Food consumption was determined during the 5-wk and 26-wk diet regimens with four mice selected from different cages for each experimental group at wk 3 or 15, respectively (Fig. S2). The mice were housed individually in cages containing a tightly-accessible, chow-holding device, in which the chow was replaced and weighed each day. Food consumption for mice in the 5-wk diet regimen was measured for 7 days, and for mice in the 26-wk diet regimen food consumption was measured for 10–14 days. The calculated amount of AHR antagonist added to the diet that was to be consumed by the mice was based on the assumption that adult mice on average consume ~4.5 gm of chow/day (http://www.researchdiets.com/) and that the average body mass for males is 30 gm. All animals were treated humanely following the regulations and specifications of the Dartmouth IACUC.
A power analysis was carried out to determine the number of mice per experimental group required to reach an alpha probability of 0.01. Based on the previous results showing the differential weight gains of B6 vs. B6.D2 mice, we could accurately estimate the mean mass and standard deviation. We set the power probability at 0.8 to arrive at a minimum 14 mice per experimental group. We used 14–21 mice per experimental group. If obviously sick or dead, the mouse was eliminated from the study (~4–5 from a total of ~256 mice). Pups from a given litter of a given Ahr genotype and gender were placed in different experimental groups of the appropriate genotype and sex. The study was not blinded. The variance appeared similar among the groups that were statistically compared.
2.3 Cell culture and luciferase assays
H1L7.5c3 mouse hepatocytes, which have a stably transfected luciferase reporter gene regulated by a promoter with multiple AHR response elements (courtesy of Dr. Michael Denison, University of California, Davis, CA) (He et al., 2011) were cultured in alpha Minimum Essential Medium (Corning, Manassas, VA), supplemented with 10% FBS (Hyclone Laboratory, Logan, UT), 2mM L-glutamine, 0.2% penicillin/streptomycin, and 2.2g/L sodium bicarbonate (Sigma Aldrich, St. Louis, MO). The cells were maintained at 37°C and 5% CO2. H1L7.5c3 cells were seeded in white-walled, white-bottomed 96-well plates (Corning, Manassas, VA) at 4000 cells/well and incubated for 24hr in culture medium. After the 24-hr incubation, the medium was removed, and the cells were washed once with Dulbecco’s Phosphate Buffered Saline (Corning, Manassas, VA). Cells were treated for an additional 24hr with the indicated reagents, all of which were dissolved in DMSO unless otherwise noted, and in which the DMSO did not exceed 1% concentration in the culture medium.
Luciferase assays were carried out using the H1L7.5c3 cells (He et al., 2011). At the indicated times and concentrations of exposures, cells were removed from incubation and allowed to equilibrate to room temperature for 15min. After equilibration, the medium was removed and the cells were washed twice with at room temperature with DPBS. The cells were lysed with 20µl/well 1× Passive Lysis Buffer (Promega, Madison, WI) and shaken for 20min at room temperature. Luciferase activity was recorded using an LMax Luminometer Microplate Reader (Molecular Devices, Sunnyvale, CA) programmed to inject 50µl Luciferase Assay Reagent (Promega, Madison, WI) per well with a 10sec integration of emitted luminescence.
2.4 Magnetic resonance imaging
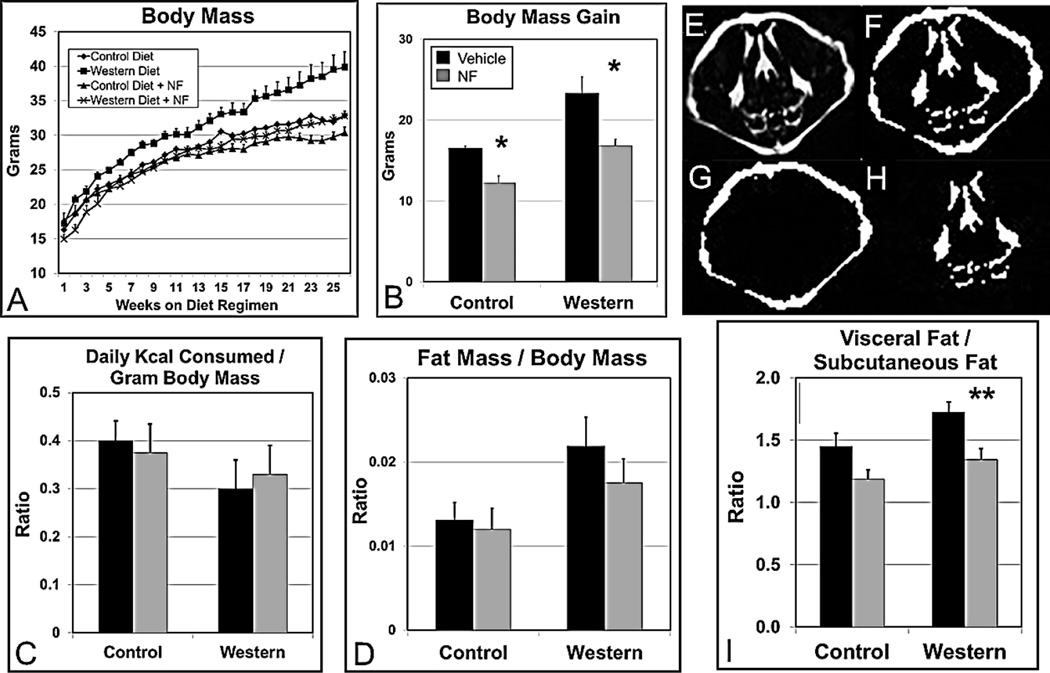
Magnetic resonance imaging (MRI) was carried out in the Dartmouth Irradiation, Preclinical Imaging & Microscopy Shared Resource. At wks 8, 14, and 24 following weaning, mice (n=4–6 per experimental group) were anaesthetized with 2% isoflourane via nosecone prior to imaging. MR images were acquired on an Agilent 9.4T scanner equipped with triple axes gradient coils and VnmrJ software (Agilent Technologies, Santa Clara, CA, www.agilent.com). Temperature and respiration rates were monitored using the small animal physiological monitoring system (Small Animal Instruments Inc., Stony Brook, NY, www.i4sa.com,). Images were acquired using a spin echo sequence, with an additional water suppression pulse for fat imaging. The acquisition parameters were: TR=700 ms, TE=13 ms, field of view=4 cm×4 cm, acquisition matrix=126 pixels×126 pixels, slice thickness=2 mm and 2 signal averages. Only sections including the peritoneal cavity were included in the analysis. In each image, visible fat was scored as being either visceral or subcutaneous based on its location (Fig. 2E–H). The ratios of visceral fat to subcutaneous fat were calculated per section, and the total mean ratio for the entire mouse was calculated.
Fig. 2.
The AHR antagonist NF (~3mg/kg/day) prevents Western diet-based obesity over an extended time period and reduces and redistributes body fat. (A) Male B6 mice (n=15–22 mice per experimental group) were fed control, Western, control+NF, or Western+NF diet ad libitum for 26 weeks beginning at weaning. (B) Total body mass gain was determined at the end of the 26-wk diet regimen. (C) Food consumption for each experimental group (n=4) was determined over a 10–14-day period at week 15 during the 26-wk diet regimen. (D) Gonadal fat mass/total body mass ratios were determined by weighing at the end of the 26-wk diet regimen. (E) Magnetic resonance imaging (MRI) images were acquired by quantifying pixel density of (F) total fat, (G) subcutaneous fat, and (H) visceral fat. (I) A plot of the pixel quantifications of approximately 25 cross-section MRI images of the thoracic and abdominal cavities per mouse (n=4/experimental group). p-value of the NF-treated group to the corresponding vehicle-treated control group: *, ≤0.03; **, ≤0.01. Error bars represent SEM.
2.5 Histology
The histology procedures were carried out by the Pathology Shared Resource at Dartmouth Hitchcock Medical Center. Liver samples taken at sacrifice were fixed in 10% neutral-buffered formalin. Tissue was subsequently processed, paraffin-embedded, and sectioned at a thickness of 5µM onto glass slides. Slides were stained using Masson’s Trichrome stain to determine relative differences in liver fibrotic content and fat vacuole volume (Takahashi and Fukusato, 2014). Each liver had 4 sections stained and examined with each examined section taken at least 30µM apart and representative sections were chosen. The stained slides were examined at 100× magnification using a Olympus BX51 microscope (Waltham, MA). Images were generated using identical settings with a QImaging Micro Publisher 5.0 RTV camera (Surrey, British Columbia, Canada). Pathological assessment was done with the assistance of Drs. James Gorham, M.D., Ph.D. and Arief Suriawata, M.D. of the Dartmouth Hitchcock Pathology department.
2.6 Serum protein and lipid measurements
Serum was obtained from collected blood samples via retro-orbital bleeding by centrifugation and stored at −80°C from mice fasted for 5 hr and from 5-hr fasted mice at sacrifice. The serum lipid and protein concentration determinations were carried out by the Serology/Clinical Pathology Division of Charles River Laboratory (Wilmington, MA).
2.7 Data analysis
Differences were considered statistically significant with a p-value ≤0.05. A 2-way ANOVA was performed on the body mass data for mice on the 5-wk (p-value <0.002) and 26-wk (p-value <0.0001) diet regimens to determine whether there were significant differences among the groups regarding diet and AHR antagonist (Table S1). The analysis was performed using the statistical software JMP version 10.0.1 with a full factorial design to examine main effects and interactions among the two independent variables. The ANOVA showed that there was statistical significance among the mouse groups for both diet regimens. An Effects test was carried out on the results from the 26-wk diet regimen (Table S2), which showed there was no statistically significant interaction between diet and AHR antagonist, i.e., the effect of diet and AHR antagonist on body mass was independent. For direct comparisons, statistical significance was calculated using two-sided paired Student’s t-test, and dependent upon the data at hand, equal or unequal variance analysis was applied. Error bars represent standard error of the mean (SEM).
3. Results
3.1 The AHR is required for Western diet-based weight gain
In previous work we demonstrated links among the AHR, Western diet, obesity, and non-alcoholic fatty liver disease (NAFLD) suggesting that the AHR may play a role in regulating metabolism, adiposity, and obesity (Kerley-Hamilton et al., 2012). To further explore the role of AHR signaling underlying obesity, we carried out a series of experiments to test the hypothesis that if AHR activation is required for Western diet-based obesity, then the lack of a functional AHR or the inhibition of AHR activity should prevent diet-based weight gain. We found that B6.Ahr−/− mice on Western diet did not become obese and were not significantly different in size than B6 mice on control diet (Fig. S3A), in agreement with published work on B6.Ahr−/− mice (Xu et al., 2015).
We next examined AHR inhibition. The AHR is a promiscuous nuclear receptor that can bind hundreds of known chemicals with ligand-specific effects (Soshilov and Denison, 2014), including many antagonists (Guyot et al., 2013), the AHR antagonist α-naphthoflavone (NF) was selected as an AHR antagonist for initial testing in preventing obesity. NF (i) is non-toxic at effective doses (Nebert and Jensen, 1979; Nazarenko et al., 2001); (ii) has been tested in vivo in mice and has sufficient bioactivity and bioavailability (Patel et al., 2009); and (iii) is relatively affordable. A dose-response experiment was carried out to determine whether NF could act as an AHR-based inhibitor of obesity and to determine an effective dosage. Weaned male B6 mice were fed for 5 wks Western diet containing by mass 0.1%, 0.2%, and 2% NF. NF at increased amounts in Western diet caused a corresponding decrease in body mass gain (Fig. S3B) and adiposity (Fig. S3C). The highest NF dose also caused a substantial and significant drop in adiposity (Fig. S3C). No overt toxicity was apparent in the mice from any of the NF treatments, but it was observed that the liver mass to body mass ratio was dependent on the magnitude of the NF dose (Fig. S3D).
3.2 Obesity is prevented by AHR inhibition
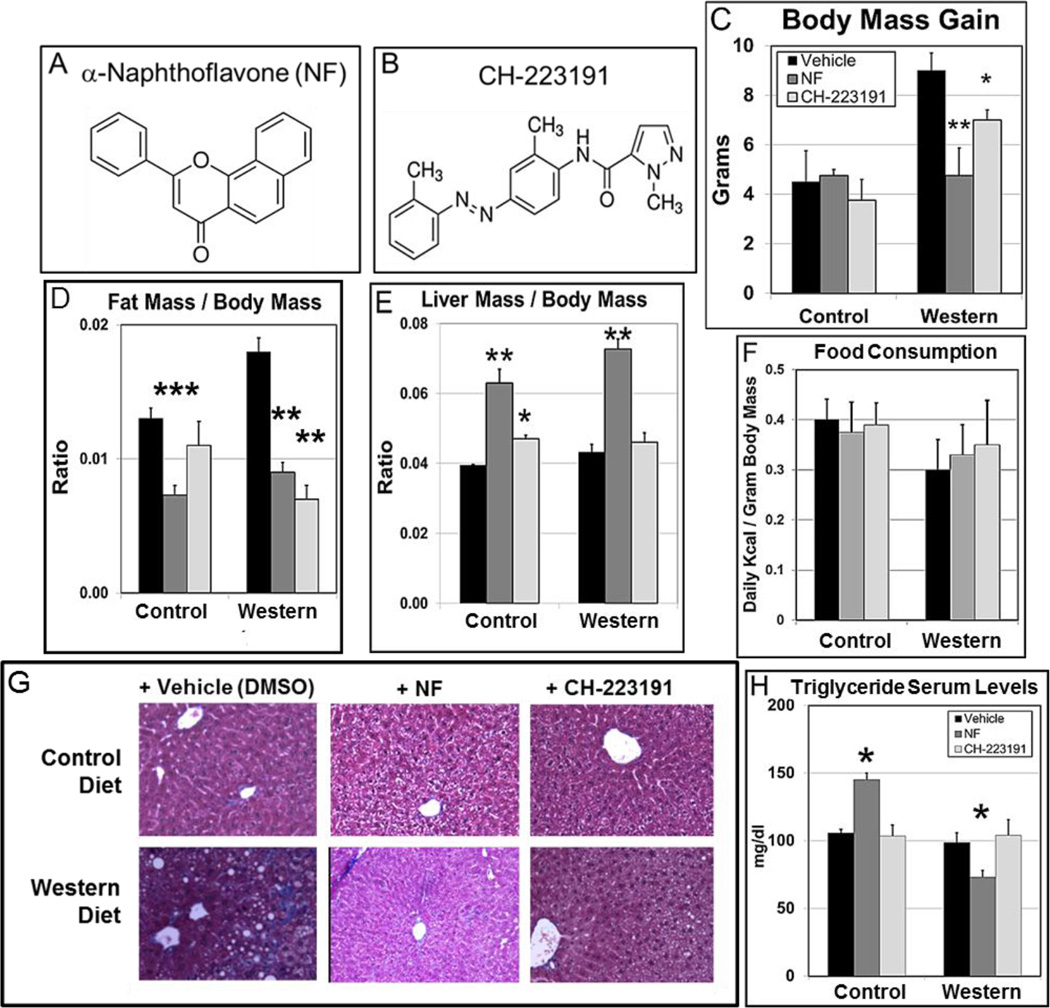
The dose-response results with NF demonstrated that AHR antagonism may prevent obesity at an achievable dose. Furthermore, the demonstration that a second and more specific AHR antagonist, such as CH-223191 (Kim et al., 2006; Zhao et al., 2010; Smith et al., 2011) could effectively reduce obesity and its complications would strengthen the hypothesis that AHR signaling plays a central role in diet-based obesity. Whereas NF (Fig. 1A) acts as a weak agonist to compete out more potent AHR ligands (Santostefano et al., 1993), CH-223191 (Fig. 1B) acts as a bonefied AHR antagonist (Zhao et al., 2010). NF (~3mg/day/kg) and ~10mg/kg/day CH-223191, an amount shown to be effective in vivo as an AHR inhibitor (Kim et al., 2006), were added to the control and Western diets.
Fig. 1.
AHR antagonists prevent diet-based obesity, adiposity, and hepatic steatosis. Chemical structure of (A) α-naphthoflavone (NF) and (B) CH-223191. A comparison of the effects of NF and CH-223191 on B6 male mice (n=4/experimental group) fed ad libitum at weaning control and Western diets ± NF (~3mg/kg/day) or ± CH-223191 (~10mg/kg/day) on (C) total body mass gain and (D) gonadal fat mass to total body mass ratio. (E) Food consumption for each experimental group was determined over a 5–7-day period at week 3 during the 5-wk diet regimen. (F) Total liver mass to total body mass ratio at the conclusion of the 5-wk diet regimen. (G) Representative liver sections stained with Masson’s trichrome and (H) plot of triglyceride serum levels of the same experimental groups. p-values to the corresponding vehicle-treated control group: *, ≤0.05; **, ≤0.02; ***, ≤0.002. Error bars represent standard error of the mean (SEM).
The diets were administered ad libitum to male B6 mice over a span of 5 wks starting at weaning. Both NF and CH-223191 significantly reduced body mass for mice on Western diet (Fig. 1C and Table S3). We then asked whether the increased body mass in the B6 mice on Western diet to those fed Western diet+AHR antagonist was due to an increase in the relative accumulation of body fat rather than an overall proportional increase in body size. It is known that the ratio of gonadal fat pad mass to total body mass correlates highly to the ratio of total body fat mass to total body mass (Rogers and Webb, 1980). Using this metric, we found that the fat mass to body mass ratio was reduced significantly in mice fed Western diet for both antagonists (Fig. 1D). These results support the notion that inhibition of AHR signaling not only prevents obesity but may also promote leanness. There were no significant differences in the amount of food consumed for a given diet±AHR antagonist (Fig. S2A) nor in the daily consumed Kcal per gram body mass (Fig. 1E).
Hepatomegaly independent of diet was observed with treatment by NF; but in contrast, CH-223191 did not affect liver size (Fig. 1F), suggesting somewhat different modes of action by the two antagonists. Noting the contrasting effects of NF and CH-223191 on liver size, we asked whether the two antagonists had differential effects on liver steatosis. Masson’s trichrome-stained liver sections from mice on the 5-wk diet regimen fed control diet, control diet+NF, and control diet+CH (CH-223191) showed little evidence of fat vesicles, while the Western diet-fed mice showed incipient liver steatosis. (Fig. 1G). Both experimental groups of mice fed Western diet+NF and Western diet+CH showed few if any fat vesicles, further supporting the notion that AHR inhibition ameliorates liver steatosis. NF and CH-223191 had minimal effects on the serum levels of total cholesterol, low- and high-density lipoprotein (LDL and HDL), alkaline phosphatase (ALK), and total proteins (TP) for all the experimental groups (Fig. S4, Table S4). There was increased variability in the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) among the groups fed Western diet with or without an AHR antagonist but not to statistically significant levels (Fig. S4, Table S4). In contrast, serum triglyceride levels were paradoxically increased significantly by NF in mice fed control diet and decreased significantly in mice fed Western diet (Fig. 1H), although the triglyceride serum concentrations, like all the measured serum markers, were within or near normal ranges (http://phenome.jax.org/) (Table S4). Thus, although there were some significant AHR antagonist-dependent changes and some experimental groups that displayed a high degree of variability, the serum marker values did not appear to reside in a range that was pathological for any of the experimental groups.
3.3 α-Naphthoflavone prevents obesity over a longer time span
The efficacy of NF as an AHR-based obesity preventative was determined by testing on an expanded time scale. Note that CH-223191 was not tested further due to prohibitive cost. At weaning, B6 male mice were fed control diet or Western diet with and without NF (Fig. 2A). The total body mass gain of mice fed Western diet was consistently significantly greater by week 18 than that of mice fed control diet, and at the end of the 26-wk regimen had gained 40% more body mass (Table S5). Also at week 18, body mass gain was significantly less in mice fed Western diet+NF to mice fed Western diet alone and were only 5% larger than mice on control diet at the end of the study (Table S5). Although no ill effects were observed, mice fed control diet+NF were significantly smaller (18%) than mice on control diet (Fig. 2B, Table S5).
In order to determine whether the significant differences in body mass observed among the experimental groups were due to the amount of food consumed, food intake for four mice from each experimental group was measured. There were no significant differences in the amount of food consumed for a given diet±NF (Fig. S2B), and there were no significant differences for a given diet±NF in daily Kcal consumed per gram body mass (Fig. 2C). Thus, the difference in body mass between the mice on Western diet vs. Western diet+NF was not due to differences in the amount of food consumed and indicates an AHR-dependent metabolic basis for Western diet-induced obesity.
3.4 AHR inhibition reduces visceral fat
We again determined whether the increased body mass in the B6 mice on Western diet to those fed Western diet+NF was due to an increase in the relative accumulation of body fat or due to an overall proportional increase in body size using the ratio of gonadal fat pad mass to total body mass (Rogers and Webb, 1980). We found that the inferred total fat mass to body mass ratio was reduced by NF treatment (Fig. 2D), but unlike the mice on the 5-wk diet regimen (Fig. 1D), was not significantly different for mice on Western diet to those on Western diet+NF. We next asked whether the reduction in body mass by NF treatment was associated with a change in the distribution of visceral and subcutaneous fat depots. Increased abdominal or visceral fat is associated with a higher risk of metabolic syndrome, heart disease, diabetes, and many other diseases (Cameron et al., 2009). Although the total fat mass to body mass ratio was not significantly different (Fig. 2D), magnetic resonance imaging analysis (Fig. 2E–H) revealed that NF caused a significant shift in fat from the visceral compartment to the less unhealthy subcutaneous compartment (Fig. 2I).
3.5 AHR antagonism improves liver steatosis
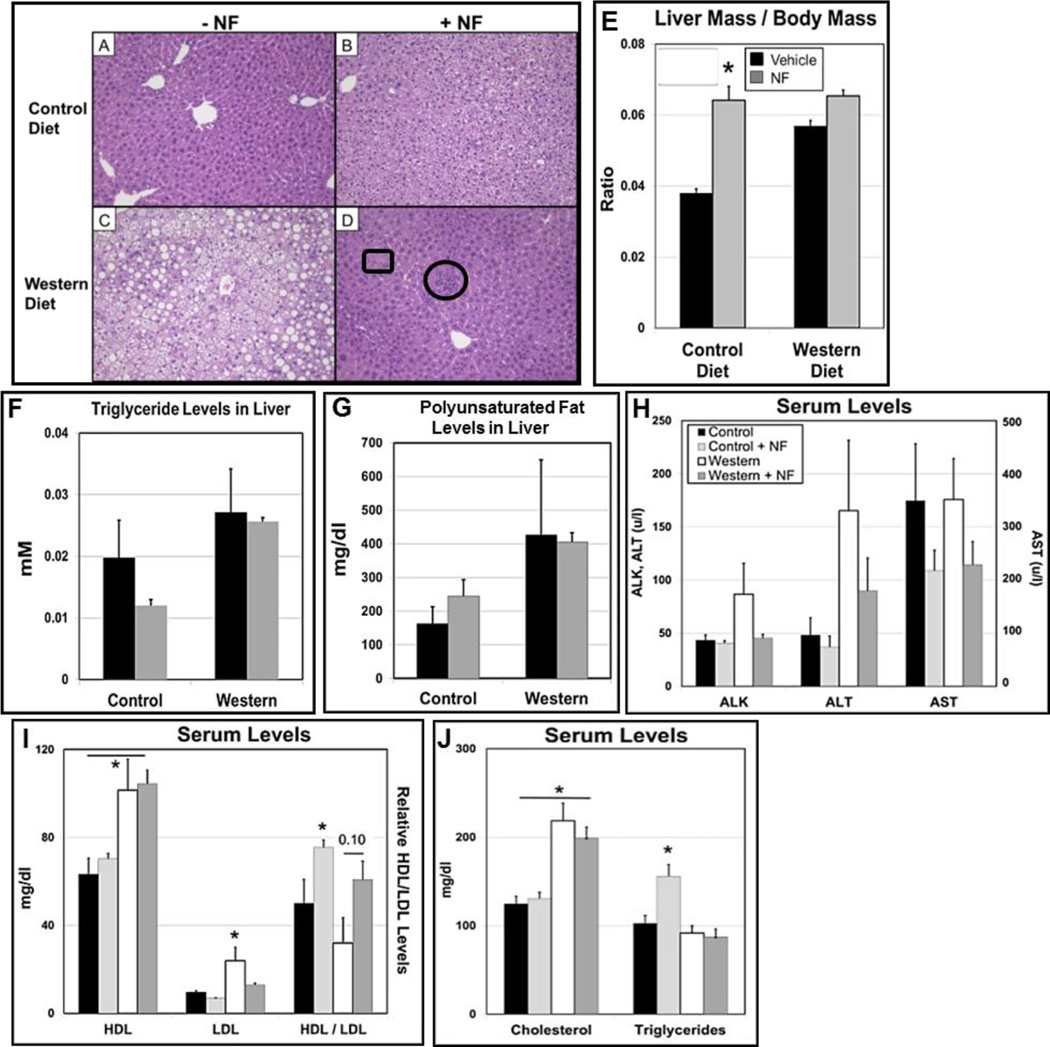
Liver steatosis and the disruption of liver function are strongly associated with obesity (Chien et al., 2006). Liver sections from B6 male mice were stained with Masson’s trichrome. Mice fed control diet and control diet+NF, as expected, showed no fat vesicles (Fig. 3A,B), while mice fed Western diet showed obvious fat accumulation (Fig. 3C). The mice fed Western diet+NF showed little if any fat vesicles (Fig. 3D), suggesting that AHR inhibition by NF prevented longer-term, diet-induced liver steatosis.
Fig. 3.
Longer-term exposure to the AHR antagonist α-naphthoflavone (NF) (~3mg/kg/day) ameliorates Western diet-based liver steatosis and increases liver size independently of diet in B6 male mice. Representative liver sections stained with Masson’s trichrome from mice fed (A) control, (B) control+NF, (C) Western, or (D) Western+NF diet ad libitum for 26 weeks beginning at weaning. The circle in (D) includes a collection of eosinophils and the rectangle an example of a Mallory-Denk body. (E) Liver mass to body mass ratio (n=15–22 mice per experimental group). Plots of (F) triglyceride and (G) polyunsaturated fat levels from the liver (n=4/experimental group); plots of serum levels of (H) alkaline phosphatase (ALK), alanine aminotransferase (ALT), and aspartate aminotransferase (AST); (I) high-density lipoproteins (HDL), low-density lipoproteins (LDL), and HDL/LDL ratios, and (J) cholesterol and triglycerides levels at the conclusion of the 26-wk diet regimen. p-value of the NF-treated group to the corresponding vehicle-treated control group: *, ≤0.05. Error bars represent SEM.
Although NF appeared to greatly ameliorate diet-based hepatic steatosis, some ill effects were noted. In contrast to the effects of NF on liver size in the shorter-term studies (Fig. 1E), a similar outcome was apparent only in the mice fed control diet+NF (Fig. 3E), in which there was an NF-dependent increase in the liver mass to body mass ratio. The NF also caused ballooning degeneration in the control+NF group (Fig. 3B), and a prevalence of low-grade inflammation was seen in the Western+NF group based on the presence of Mallory-Denk bodies and collections of eosinophils (Fig. 3D). Triglyceride (Fig. 3F) and polyunsaturated fat levels (Fig. 3G) were not reduced in the mice fed Western-diet+NF suggesting that the accumulated fat in the liver vesicles of mice fed Western diet contain primarily saturated fats.
The hepatomegaly and higher fat levels in the livers of mice treated with NF relative to that of control mice on the 26-wk diet regimen was not associated with typical liver damage markers, as ALK, ALT, and AST serum levels were either near control levels or reduced, although not significantly (Fig. 3H). LDL and LDL/HDL ratio serum levels were significantly decreased by NF (Fig. 3I). Serum cholesterol and triglyceride levels were unaffected for mice on Western diet+NF to those on Western diet alone, but similar to the results of the shorter-term 5-wk study (Fig. 1H), NF caused a significant increase in triglyceride accumulation in the liver of mice on control diet (Fig. 3J).
3.6 Kynurenine is an AHR ligand in hepatocytes
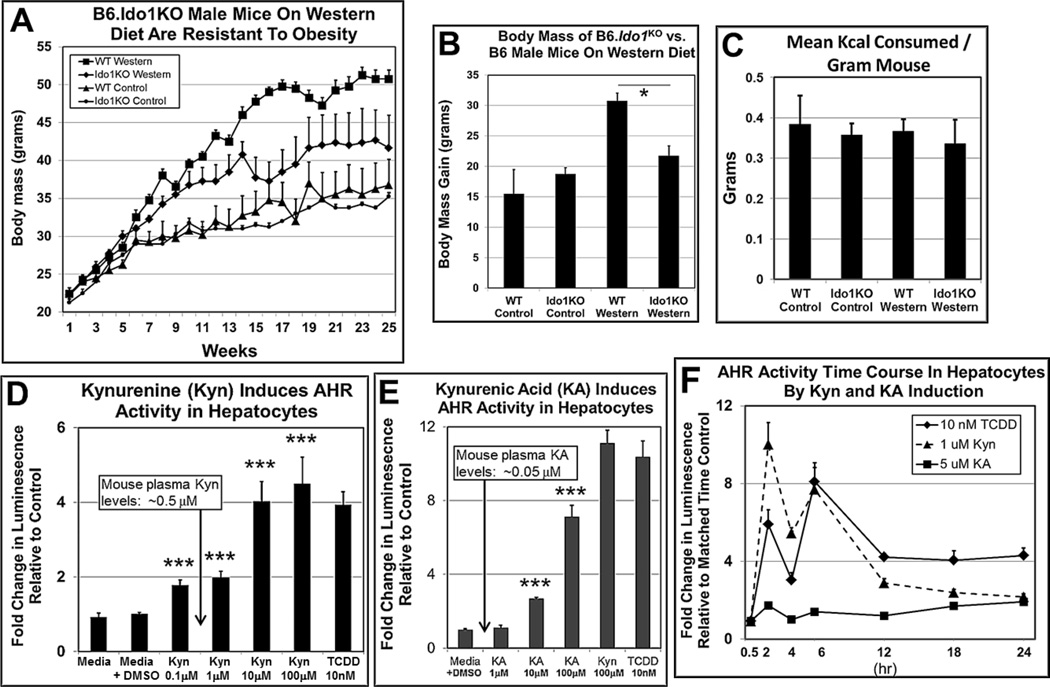
IDO1, IDO2, and TDO2 catabolize Trp to Kyn and play roles in obesity. B6 mice on a low-fat diet and treated with the IDO1 and IDO2 inhibitor 1-methyltryptophan gained significantly less body mass than placebo-treated control mice (Corona et al., 2013). Stronger evidence that Kyn may be essential for maximal body weight gain was obtained using B6.Ido1−/− null mice (Nagano et al., 2013), in which a Trp metabolite was shown to be required for weight gain in mice on a high-fat diet. We repeated those studies with B6.Ido1−/− mice using a comparable Western diet, but in our study, included experimental groups on control diets (Fig. 4A). We obtained similar results, in which the body mass of male B6.Ido1−/− vs. wild-type B6 mice on Western diet for 26 wks was significantly less (Fig. 4B) without any significant differences in the amount of food consumed (Fig. S2C) nor in consumed daily Kcal per gram body mass (Fig. 4C). These results confirmed a key role for IDO1 in obesity.
Fig. 4.
IDO1 may play a key role in AHR-based obesity. Body mass of B6.Ido−/− male mice vs. B6 male mice on Western diet is significantly less. (A) Growth curve over 26 wks (n=4 per experimental group), (B) body mass gain at the end of the 26-wk diet regimen, and (C) Kcal food/gram mouse intake among the experimental groups. (D) Kynurenine (Kyn) activates AHR signaling at physiological concentrations in H1L7.5c3 cells and (E) Kynurenic acid (KA) acts as an AHR agonist after a 24-hr exposure by inducing the AHR-regulated luciferase gene in H1L7.5c3 mouse hepatocyte cells (n=5 biological replicates). Data are plotted as fold change in luminescence relative to the control (media+vehicle (DMSO)). (F) Time-course plot of Kyn-, KA-, and TCDD-induced, AHR-regulated luciferase activity in H1L7.5c3 cells (n=5 biological replicates). Data points for each time point were plotted as fold change in luminescence relative to matched cells exposed to vehicle alone. *, p-value ≤0.02; ***, ≤0.001. Error bars represent SEM.
Kyn is a known AHR agonist that activates the AHR-directed polarization of naive T cells to the anti-inflammatory Treg phenotype (Veldhoen et al., 2009; Mezrich et al., 2010; Nguyen et al., 2010; Nguyen et al., 2013). We asked whether Kyn at physiological levels may also be a candidate agonist capable of activating AHR-directed transcription using the mouse hepatocyte cell line H1L7.5c3, which expresses an AHR transcriptionally-dependent, luciferase reporter gene system in Hepa-1c1c7 cells (He et al., 2011). We found that increasing amounts of Kyn induced increasing levels of luciferase activity indicating that Kyn is a proficient AHR agonist in hepatocytes as reported (Mezrich et al., 2010) (Fig. 4D). We carried out similar studies using kynurenic acid (KA), a downstream Trp metabolite that is also a known AHR agonist (DiNatale et al., 2010), and which also appeared to be an effective AHR inducer (Fig. 4E). However, Kyn was an effective AHR agonist at the physiological level in plasma of ~0.5µM, while an elicited AHR response by KA was not observed at the lowest tested concentration of 1µM, which is ~20 fold greater than physiological plasma levels (Giorgini et al., 2013). AHR-based luciferase activity was induced by 2hr at the physiologically relevant dose of 1µM, with peak induction at 6hr (Fig. 4F). The time-course profile of Kyn was similar to that of 2,3,7,8 Tetrachlorodibenzo-p-dioxin (TCDD), while KA elicited considerably reduced AHR induced luminescence over the same time period. These results are consistent with the contention that Kyn but not KA is the Trp metabolite that may accumulate to cellular levels to cause an elevated rate of AHR activation.
3.7 TLR2/4 and TGFβ1 activate the AHR
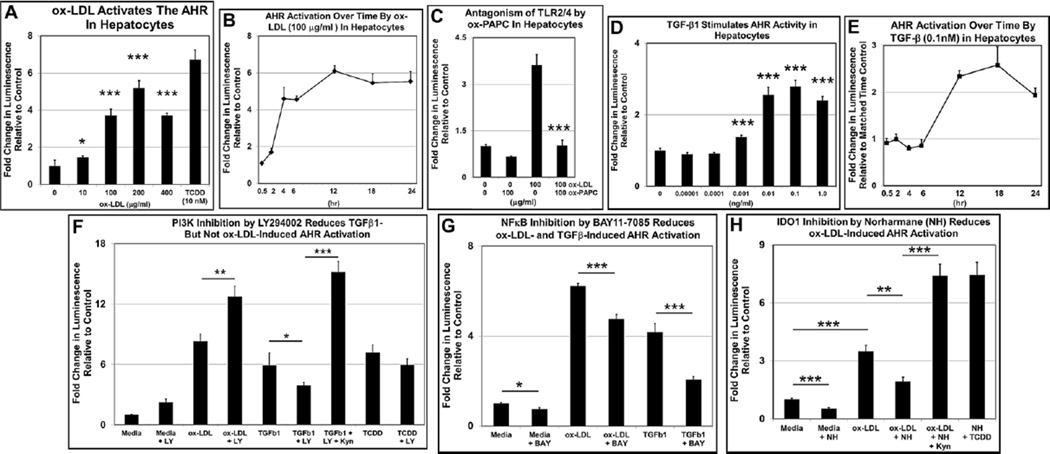
The Western diet-derived byproduct ox-LDL stimulates AHR transcriptional activity in Hepa-1c1c7 cells (McMillan and Bradfield, 2007) and lipopolysaccharides (LPS) activate the AHR in mouse liver (Wu et al., 2011). Both ox-LDL and LPS are ligands for TLR2 and TLR4 in immune cells (Erridge et al., 2008; Li et al., 2010; Chavez-Sanchez et al., 2014). The demonstration that TLR2 deficiency in B6 mice on Western diet prevented obesity (Himes and Smith, 2010) made ox-LDL, via TLR2/4 binding, a reasonable candidate for initiating the signaling cascade that ultimately activates the AHR. To test this hypothesis, we first carried out a dose-response experiment, in which higher concentrations of ox-LDL added to H1L7.5c3 cells caused correspondingly increased levels of AHR-directed luciferase activity (Fig. 5A). The effective ox-LDL concentrations were at levels falling in the normal range of ox-LDL plasma concentrations in mice of 3–300µg/ml (Kato et al., 2009). A time-course experiment revealed that the ox-LDL induced AHR transcriptional activity by 4hr and peaked at 12hr (Fig. 5B). Inclusion of the TLR2/4 antagonist ox-PAPC (Erridge et al., 2008) significantly reduced AHR transcriptional activity to near control levels (Fig. 5C) supporting a role for TLR2/4 signaling in AHR activation.
Fig. 5.
ox-LDL via NF-κB and TGFβ1 via PI3K and NF-κB stimulate AHR transcriptional activity in H1L7.5c3 cells. H1L7.5c3 cells were seeded, cultured for 24hr, exposed to ox-LDL and TGFβ1 at the indicated amounts and durations, lysed, and assayed for luciferase activity (n=5–10 biological replicates). (A) A dose-response plot and (B) time-course plot of H1L7.5c3 cells exposed to ox-LDL. (C)The TLR2/4 inhibitor ox-PAPC was added at the indicated concentrations 1hr prior to a 24-hr incubation with ox-LDL. (D) A dose-response plot and (E) time-course plot of H1L7.5c3 cells exposed to TGFβ1. For the inhibitor studies, H1L7.5c3 cells were seeded, cultured for 24hr, and incubated for 24hr with 100µg/ml ox-LDL or 0.1ng/ml TGFβ1 with the co-administration of (F) 10µg/ml LY294002 (LY), (G) 10µg/ml BAY11-7085 (BAY), or (H) 10µM Norharmane (NH). The cells were lysed and assayed for luciferase activity. Data are plotted as fold change in luminescence relative to the media control cells (media + vehicle (DMSO)). For the time-course studies, data points for each time point were plotted as fold change in luminescence relative to matched cells exposed to vehicle alone. p-values: *, ≤0.05, **, ≤0.01; ***, ≤0.001. Error bars represent SEM.
TGFβ1 levels are elevated in the adipose tissue of obese subjects (Samad et al., 1997), the serum of diabetic patients (Olivieri et al., 2010), and in obese mice (Tan et al., 2012). More to the point, TGFβ1 signaling stimulates Ido1 transcription, which eventually leads to the enzymatic production of Kyn from Trp (Chen, 2011). Thus, we asked whether TGFβ1 may also be an endogenous agent that induces AHR-directed luciferase activity in H1L7.5c3 cells. We found that TGFβ1 activated AHR transcriptional activity in a dose-responsive manner (Fig. 5D) at concentrations less than that measured in human plasma (2–12ng/ml) (Wakefield et al., 1995) but at a relatively slower time-course to that of Kyn (Fig. 5E). Thus, as expected, the direct-binding AHR agonists Kyn and TCDD (positive control) stimulated AHR transcriptional activity relatively quickly, while ox-LDL and TGFβ1, which act on the AHR via signal transduction, produced more delayed responses.
3.8 The AHR signaling axes include PI3K and NF-κB
The PI3K and NF-κB complexes are part of critical signal transduction pathways for multiple essential cellular functions, including the TGFβ1 to IDO1 (Chen, 2011; Pallotta et al., 2011) and TLR2/4 (Kawai and Akira, 2007) signaling pathways. The inhibition in mice of PI3K was shown to reduce adiposity (Ortega-Molina et al., 2015), and the inhibition of NF-κB by amlexanox prevented obesity (Reilly et al., 2013). We asked whether PI3K and NF-κB may be part of the TLR2/4 and TGFβ1 signal transduction pathways by co-incubating the respective PI3K and NF-κB inhibitors LY294002 (LY) and BAY11-7085 (BAY) with ox-LDL and TGFβ1 for 24hr in H1L7.5c3 cells. The PI3K inhibitor LY in the presence of TGFβ1 caused a significant reduction in AHR transcriptional activity (Fig. 5F), suggesting that signal transduction initiated by TGFβ1 for AHR activation is via PI3K. In contrast, in the presence of ox-LDL, LY did not inhibit AHR activity and may in fact act as a weak AHR agonist (see media+LY). However, the NF-κB inhibitor BAY caused a significant reduction in the presence of ox-LDL and TGFβ1 (Fig. 5G), suggesting that signal transduction for AHR activation by both ox-LDL and TGFβ1 is through NF-κB.
3.9 AHR transcriptional activation by TLR2/4 signaling is linked to IDO1
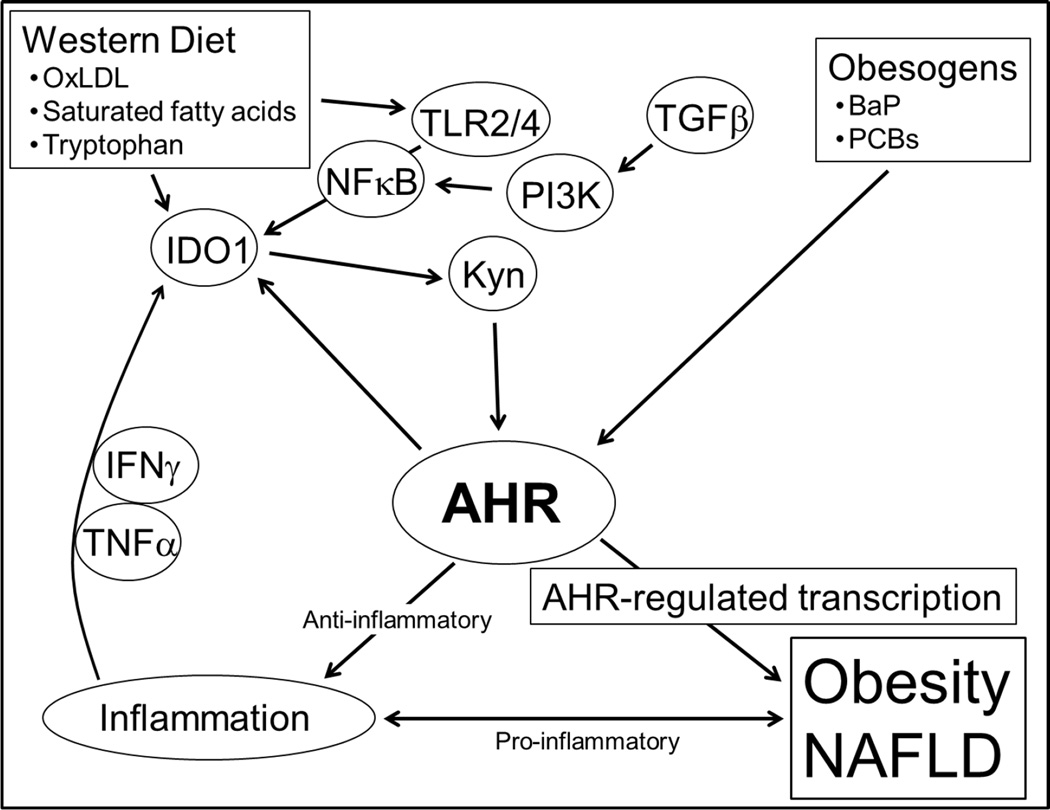
In our model (Fig. 6), an excess production of Kyn via increased cellular IDO1 levels and activity leads to amplified AHR signaling ultimately giving rise to obesity. There is a known association between TLR2/4 signaling and IDO1 gene expression in human immune and mesenchyme cells (Koga et al., 2006; Opitz et al., 2009), and we asked whether the induction of AHR transcriptional activity in hepatocytes by ox-LDL was, at least in part, also dependent on IDO1. The IDO1 inhibitor Norharmane (NH) (Sono and Cady, 1989; Chiarugi et al., 2000) and ox-LDL were added to H1L7.5c3 hepatocytes and incubated for 24hr (Fig. 5H). NH in the presence of ox-LDL caused a significant reduction in AHR transcriptional activity. To demonstrate that the treatment with NH did not interfere with AHR signaling and instead blocked upstream signaling components, the addition of Kyn or TCDD to the cells co-treated with NH re-induced AHR transcriptional activity to levels exceeding that of cells incubated with ox-LDL alone These results indicate that the ox-LDL-stimulated TLR2/4 signaling pathway activates AHR transcriptional activity by increasing the production of IDO1-generated Kyn.
Fig. 6.
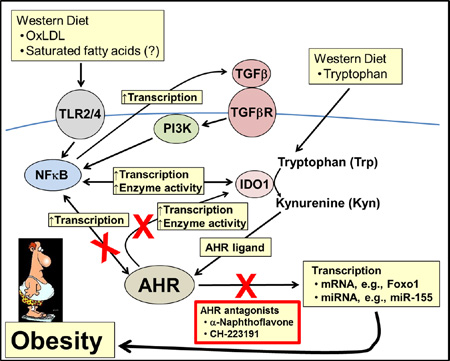
Model depicting AHR-dependent, diet-Induced signaling pathways in liver. AHR-based obesity requires the stimulation of signaling pathways by Western diet-derived ox-LDL and saturated fatty acids that induce Ido1 transcription and activate IDO1-mediated kynurenine production; allowing the activated AHR to serve as a hub connecting environmental obesogen exposures, Western diet, obesity, and chronic inflammation. BaP, benzo[a]pyrene; PCBs, polychlorinated biphenyls.
4. Discussion
4.1 Kynurenine and IDO1 in obesity
We had several questions regarding the role of the AHR in obesity, the first of which was the identity of a diet-derived agonist for AHR-based obesity, and Kyn was an obvious candidate. Kyn levels and the ratio of Kyn to Trp, which reflects IDO1, IDO2, and/or TDO2 activities, are elevated in the plasma and adipose tissue of obese women to that in lean women (Wolowczuk et al., 2012; Favennec et al., 2015). In contrast, Kyn and Kyn/Trp plasma levels are depressed in mice on high-fat diet (Poulain-Godefroy et al., 2013); in which both species are perhaps revealing the dysregulation of TDO and IDO enzymatic activities caused by an obese state. Ido1 and Ido2 gene expression is regulated in an AHR-dependent manner (Mezrich et al., 2010) and pro-inflammatory cytokines, especially INFγ (Brandacher et al., 2007). The elevated Kyn levels in human obesity are presumably due in part to constitutive IDO transcription induced by chronic inflammation (Mangge et al., 2013). Because most nutrient and fat metabolism occurs in the liver (Chien et al., 2006), and the liver is the site of most AHR activity (Tuteja et al., 1985), we hypothesized that the bulk of AHR-based obesity is due to the activity in that organ.
We repeated, in part, studies with B6.Ido1−/− mice (Nagano et al., 2013), but in addition, included mice on low-fat control diet to establish basal body weight levels. Like Nagano et al., we showed that the body mass of B6.Ido1−/− vs. wild-type B6 male mice on Western diet was significantly less (Fig. 4), but we also showed that the B6.Ido1−/− mice on Western diet was nonetheless significantly greater than the mice on control diet suggesting that other Trp-catabolizing activities are perhaps in play regarding obesity, i.e., IDO2 and/or TDO2. We suggest that the amount of Trp in the Western diet for the mice studies described here provides the presumed increased levels of IDO1 (via cytokine and AHR induction) with more than enough substrate to generate greater amounts of Kyn, that in turn, escalates the level of AHR signaling leading to obesity. By inhibiting AHR signaling with antagonists, we propose that the Trp-IDO1-Kyn-AHR-obesity cycle is blocked.
The question arises as to how IDO1 deficiency has such a profound effect on body fat and body mass when presumably the primary modulator of systemic Trp levels is via hepatic TDO2 activity (Kanai et al., 2009). Plasma levels of Kyn and Trp increased approximately 2.5- and 9-fold, respectively, in B6.Tdo2−/− mice relative to wild type mice (Kyn/Trp ratio of ~0.3) suggesting that systemic Kyn levels are more than sustained by the activity of the IDO enzymes in B6.Tdo2−/− mice. However, B6.Ido1−/− mice showed an even greater drop in the plasma Kyn/Trp ratio as well as a resistance to obesity (Nagano et al., 2013). Thus, IDO1, like its many roles in immune cell regulation, may be the key regulator of systemic Kyn levels in the context of obesity and obesity-associated inflammation (Watcharanurak et al., 2014), while the role of TDO2 in obesity and its regulation by glucocorticoids remain obscure (Poulain-Godefroy et al., 2013) and need to be explored further.
The increased levels of Trp that usually accompany a Western diet did not in itself seem sufficient to induce and maintain an AHR dysregulated/obese state. Thus, we asked what other possible Western diet components may contribute to increased AHR activation and how could they be mechanistically linked. A model is proposed for AHR-directed obesity based on TLR2/4-initiated signaling and TGFβ perpetuated positive feedback loops (Fig. 6), which in turn, promote IDO1 expression and activity. We contend that Western diet, in addition to supplying sufficient Trp substrate amounts, provides an overabundance of ox-LDL and saturated fatty acids (SFA) as agonists for sustained TLR2/4 signaling (Lee et al., 2004; Chavez-Sanchez et al., 2014).
4.2 Feedback loops link Western diet to IDO1 and AHR
Recent studies in dendritic cells have shown that IDO1 has signaling as well as enzymatic functions. The signaling role is carried out by the IDO1-SHP (PTPN6 and PTPN11) complex, which activates the non-canonical NF-κB transcriptional pathway (Pallotta et al., 2014), which in turn, induces transcription of the Tgfb1 (Pallotta et al., 2011) and Ido1 (Puccetti and Grohmann, 2007) genes, setting up positive feedback loops (Volpi et al., 2014). In agreement with other studies, our results demonstrate the importance of NF-κB in TLR2/4-induced AHR signaling (Wu et al., 2011; Vogel et al., 2014) and of TGFβ1 for AHR regulation (Wolff et al., 2001). Further support that these components contribute to obesity is that inhibition of NF-κB by amlexanox (Reilly et al., 2013) or the loss of Ido1 (Nagano et al., 2013) (Fig. 4) in mice prevented obesity. Other contributors to IDO1 activity is the chronic inflammation associated with obesity, in which IFNγ (Chen, 2011) and TNFα (Robinson et al., 2005) are major positive regulators of Ido1 gene expression..
Functional associations of the AHR with SRC and IDO1 in immune cells are recent findings. Activation of the AHR by a ligand (e.g., possibly Kyn) stimulates the AHR-associated SRC kinase to auto-phosphorylate and subsequently phosphorylate IDO1 to convert IDO1 to an active form capable of metabolizing Trp to Kyn (Bessede et al., 2014). Because Kyn is an AHR agonist, and the AHR is a positive transcription factor for the Ido1 gene, another positive feedback loop in the IDO1-AHR axis may play a role in the obese state.
4.3 Conclusions
We propose a crucial role for the AHR in obesity by providing a hub that links obesity, inflammation, obesogens, and Western diet (Fig. 6). Stimulation by components in Western diet, e.g., ox-LDL, activates TLR2/4 signaling to initiate a process that ultimately provides levels of activated IDO1 that produce excess Kyn from the available Trp. In our model, the increased Kyn levels upregulate AHR signaling, which leads to obesity and associated diseases by a yet to be determined mechanism, that likely involves increased transcription of AHR-regulated genes, e.g., possibly Foxo1 and/or miR-132/212 (Kerley-Hamilton et al., 2012). Lastly and perhaps most importantly, the demonstration that obesity may be dependent on AHR signaling, which can be inhibited by numerous AHR antagonists, may pave the way for treatment with known dietary compounds, such as the natural compounds curcumin (Ciolino et al., 1998) and resveratrol (Casper et al., 1999) as well as synthetic drugs (Smith et al., 2011) leading to simple therapeutic and preventative approaches.
Supplementary Material
Highlights.
The AHR acts as a hub in Western diet-based obesity.
Inhibition of AHR signaling by antagonists prevents obesity and liver steatosis.
ox-LDL stimulates AHR activity via a TLR2/4, NF-kB, IDO1, kynurenine axis.
TGFβ stimulates AHR activity in Hepa-1c1c7 cells via PI3K and NF-kB.
The AHR offers a simple and promising approach for treating obesity.
Acknowledgments
We thank the Bioinformatics, Clinical Pharmacology, Genomics & Molecular Biology, Immune Monitoring & Flow Cytometry, Irradiation & Pre-clinical Imaging & Microscopy, and Pathology Shared Resources at Dartmouth for their advice and services; and the reviewers and editor for their thoughtful comments. We thank Dr. Michael Denison at UC Davis for the H1L7.5c3 cells. We also thank Carrie Freitag and Drs. Chris Amos, Darcy Bates, Rick Enelow, Jim Gorham, Paul Guyre, Mark Israel, Lionel Lewis, Jason Moore, Richard Rothstein, Arief Suriawinata, and Yoli Sanchez for their support and criticisms. This work was supported by funding from NIH/NCRR award 5P20RR024475-02 and NIH/NIGMS award 8P20GM103534-02, Norris Cotton Cancer Center Prouty award P30CA023108, and Department of Medicine Research Award.
Abbreviations
- AHR
Aryl hydrocarbon receptor
- ALK
Alkaline phosphatase
- ALT
Alanine aminotransferase
- ASP
Aspartate aminotransferase
- B6
C57BL/6 mouse strain harboring the gene encoding the higher-affinity AHR
- B6.D2
C57BL/6 mouse strain harboring the gene encoding the lower-affinity AHR
- BaP
Benzo[a]pyrene
- CH
CH-223191
- CHOL
Cholesterol
- HDL
High-density lipoprotein
- IDO
Indoleamine 2,3-dioxygenase
- Kcal
Kilocalories
- Kyn
Kynurenine
- LDL
Low-density lipoprotein
- MRI
Magnetic resonance imaging
- NF
α-Naphthoflavone
- PCBs
Polychlorinated biphenyls
- SEM
Standard error of the mean
- SFA
Saturated fatty acids
- TCDD
2,3,7,8 Tetrachlorodibenzo-p-dioxin
- TDO
Tryptophan 2,3-dioxygenase
- TRIG
Triglycerides
- Trp
Tryptophan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have declared no conflict of interest.
Transparency document
The Transparency document associated with this article can be found in the online version.
Author contributions
BJM, IYR, JSK-H and CRT conceived and designed the study. BJM, IYR, HFH, HWT, KVN, BG, RJW, LL, and AJC performed the experiments and acquired the data. BJM, IYR, JSKH, CSR, KVN, BG, and CRT analyzed the data. BJM, IYR, JSK-H, WBK, RJW, and CRT wrote, edited, and approved the manuscript.
The authors approved the manuscript and declare they have no actual or potential competing financial interests.
References
- Baillie-Hamilton P. Chemical Toxins: A Hypothesis to Explain the Global Obesity Epidemic. The Journal of Alternative and Complementary Medicine. 2002;8:185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, Iannitti R, Tissi L, Volpi C, Belladonna ML, Orabona C, Bianchi R, Lanz TV, Platten M, Della Fazia MA, Piobbico D, Zelante T, Funakoshi H, Nakamura T, Gilot D, Denison MS, Guillemin GJ, DuHadaway JB, Prendergast GC, Metz R, Geffard M, Boon L, Pirro M, Iorio A, Veyret B, Romani L, Grohmann U, Fallarino F, Puccetti P. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandacher G, Hoeller E, Fuchs D, Weiss HG. Chronic immune activation underlies morbid obesity: is IDO a key player? Curr Drug Metab. 2007;8:289–295. doi: 10.2174/138920007780362590. [DOI] [PubMed] [Google Scholar]
- Cameron AJ, Dunstan DW, Owen N, Zimmet PZ, Barr EL, Tonkin AM, Magliano DJ, Murray SG, Welborn TA, Shaw JE. Health and mortality consequences of abdominal obesity: evidence from the AusDiab study. Med J Aust. 2009;191:202–208. doi: 10.5694/j.1326-5377.2009.tb02753.x. [DOI] [PubMed] [Google Scholar]
- Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56:784–790. [PubMed] [Google Scholar]
- Chavez-Sanchez L, Garza-Reyes MG, Espinosa-Luna JE, Chavez-Rueda K, Legorreta-Haquet MV, Blanco-Favela F. The role of TLR2, TLR4 and CD36 in macrophage activation and foam cell formation in response to oxLDL in humans. Hum Immunol. 2014;75:322–329. doi: 10.1016/j.humimm.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Chen W. IDO: more than an enzyme. Nat Immunol. 2011;12:809–811. doi: 10.1038/ni.2088. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Sbarba PD, Paccagnini A, Donnini S, Filippi S, Moroni F. Combined inhibition of indoleamine 2,3-dioxygenase and nitric oxide synthase modulates neurotoxin release by interferon-γ-activated macrophages. Journal of Leukocyte Biology. 2000;68:260–266. [PubMed] [Google Scholar]
- Chien KL, Hsu HC, Chao CL, Lee BC, Chen MF, Lee YT. Interaction of obesity, metabolic syndrome and Framingham risk on steatohepatitis among healthy Taiwanese: population-based nested case-control study. Cardiovasc Diabetol. 2006;5:12. doi: 10.1186/1475-2840-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolino HP, Daschner PJ, Wang TT, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochemical pharmacology. 1998;56:197–206. doi: 10.1016/s0006-2952(98)00143-9. [DOI] [PubMed] [Google Scholar]
- Corona AW, Norden DM, Skendelas JP, Huang Y, O'Connor JC, Lawson M, Dantzer R, Kelley KW, Godbout JP. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX(3)CR1)-deficient mice. Brain Behav Immun. 2013;31:134–142. doi: 10.1016/j.bbi.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashty M, Motazacker MM, Levels J, de Vries M, Mahmoudi M, Peppelenbosch MP, Rezaee F. Proteome of human plasma very low-density lipoprotein and low-density lipoprotein exhibits a link with coagulation and lipid metabolism. Thrombosis and Haemostasis. 2014;111:518–530. doi: 10.1160/TH13-02-0178. [DOI] [PubMed] [Google Scholar]
- De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends in immunology. 2011;32:373–379. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erridge C, Kennedy S, Spickett CM, Webb DJ. Oxidized Phospholipid Inhibition of Toll-like Receptor (TLR) Signaling Is Restricted to TLR2 and TLR4: Roles for CD14, LPS-Binding Protein, and MD2 as Targets for Specficity of Inhibition. Journal of Biological Chemistry. 2008;283:24748–24759. doi: 10.1074/jbc.M800352200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favennec M, Hennart B, Caiazzo R, Leloire A, Yengo L, Verbanck M, Arredouani A, Marre M, Pigeyre M, Bessede A, Guillemin GJ, Chinetti G, Staels B, Pattou F, Balkau B, Allorge D, Froguel P, Poulain-Godefroy O. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity. 2015;23:2066–2074. doi: 10.1002/oby.21199. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Maness SC, Leonard LS, Greenlee WF. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-dependent regulation of transforming growth factors-alpha and -beta 2 expression in a human keratinocyte cell line involves both transcriptional and post-transcriptional control. The Journal of biological chemistry. 1992;267:24591–24595. [PubMed] [Google Scholar]
- Giorgini F, Huang S-Y, Sathyasaikumar KV, Notarangelo FM, Thomas MAR, Tararina M, Wu H-Q, Schwarcz R, Muchowski PJ. Targeted Deletion of Kynurenine 3-Monooxygenase in Mice: A NEW TOOL FOR STUDYING KYNURENINE PATHWAY METABOLISM IN PERIPHERY AND BRAIN. Journal of Biological Chemistry. 2013;288:36554–36566. doi: 10.1074/jbc.M113.503813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- Guyot E, Chevallier A, Barouki R, Coumoul X. The AhR twist: ligand-dependent AhR signaling and pharmaco-toxicological implications. Drug Discovery Today. 2013;18:479–486. doi: 10.1016/j.drudis.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- He G, Tsutsumi T, Zhao B, Baston DS, Zhao J, Heath-Pagliuso S, Denison MS. Third-Generation Ah Receptor–Responsive Luciferase Reporter Plasmids: Amplification of Dioxin-Responsive Elements Dramatically Increases CALUX Bioassay Sensitivity and Responsiveness. Toxicological Sciences. 2011;123:511–522. doi: 10.1093/toxsci/kfr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes RW, Smith CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. The FASEB Journal. 2010;24:731–739. doi: 10.1096/fj.09-141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EC, Reyes H, Chu FF, Sander F, Conley LH, Brooks BA, Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Kanai M, Funakoshi H, Takahashi H, Hayakawa T, Mizuno S, Matsumoto K, Nakamura T. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Molecular brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato R, Mori C, Kitazato K, Arata S, Obama T, Mori M, Takahashi K, Aiuchi T, Takano T, Itabe H. Transient Increase in Plasma Oxidized LDL During the Progression of Atherosclerosis in Apolipoprotein E Knockout Mice. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:33–39. doi: 10.1161/ATVBAHA.108.164723. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kerley-Hamilton JS, Trask HW, Ridley CJ, Dufour E, Ringelberg CS, Nurinova N, Wong D, Moodie KL, Shipman SL, Moore JH, Korc M, Shworak NW, Tomlinson CR. Obesity is mediated by differential aryl hydrocarbon receptor signaling in mice fed a Western diet. Environmental health perspectives. 2012;120:1252–1259. doi: 10.1289/ehp.1205003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH, Suh PG. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69:1871–1878. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- Koga R, Hamano S, Kuwata H, Atarashi K, Ogawa M, Hisaeda H, Yamamoto M, Akira S, Himeno K, Matsumoto M, Takeda K. TLR-Dependent Induction of IFN-β Mediates Host Defense against Trypanosoma cruzi. The Journal of Immunology. 2006;177:7059–7066. doi: 10.4049/jimmunol.177.10.7059. [DOI] [PubMed] [Google Scholar]
- Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, Bentz M, Southard J, Bradfield CA. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci U S A. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- Li M, Lin J, Wang Z, He S, Ma X, Li D. Oxidized Low-density Lipoprotein-induced Proinflammatory Cytokine Response in Macrophages are Suppressed by CD4+CD25+Foxp3+ Regulatory T Cells Through Downregulating Toll Like Receptor 2-mediated Activation of NF-κB. Cellular Physiology and Biochemistry. 2010;25:649–656. doi: 10.1159/000315084. [DOI] [PubMed] [Google Scholar]
- Maffeis C. Aetiology of overweight and obesity in children and adolescents. European Journal of Pediatrics. 2000;159:S35–S44. doi: 10.1007/pl00014361. [DOI] [PubMed] [Google Scholar]
- Mangge H, Summers KL, Meinitzer A, Zelzer S, Almer G, Prassl R, Schnedl WJ, Reininghaus E, Paulmichl K, Weghuber D, Fuchs D. Obesity-related dysregulation of the Tryptophan-Kynurenine metabolism: Role of age and parameters of the metabolic syndrome. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20491. [DOI] [PubMed] [Google Scholar]
- McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor is activated by modified low-density lipoprotein. Proc Natl Acad Sci U S A. 2007;104:1412–1417. doi: 10.1073/pnas.0607296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano J, Shimizu M, Hara T, Shirakami Y, Kochi T, Nakamura N, Ohtaki H, Ito H, Tanaka T, Tsurumi H, Saito K, Seishima M, Moriwaki H. Effects of indoleamine 2,3-dioxygenase deficiency on high-fat diet-induced hepatic inflammation. PloS one. 2013;8:e73404. doi: 10.1371/journal.pone.0073404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarenko DA, Dertinger SD, Gasiewicz TA. In vivo antagonism of AhR-mediated gene induction by 3'-methoxy-4'-nitroflavone in TCDD-responsive lacZ mice. Toxicol Sci. 2001;61:256–264. doi: 10.1093/toxsci/61.2.256. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Jensen NM. Benzo[a]pyrene-initiated leukemia in mice. Association with allelic differences at the Ah locus. Biochemical pharmacology. 1979;28:149–151. doi: 10.1016/0006-2952(79)90284-3. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Puga A, Vasiliou V. Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Ann N Y Acad Sci. 1993;685:624–640. doi: 10.1111/j.1749-6632.1993.tb35928.x. [DOI] [PubMed] [Google Scholar]
- Nguyen NT, Hanieh H, Nakahama T, Kishimoto T. The roles of aryl hydrocarbon receptor in immune responses. Int Immunol. 2013;25:335–343. doi: 10.1093/intimm/dxt011. [DOI] [PubMed] [Google Scholar]
- Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LAJ, Golenbock D, Bowie AG. The history of Toll-like receptors [mdash] redefining innate immunity. Nat Rev Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. PRevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri A, De Angelis S, Dionisi S, D'Annunzio G, Locatelli M, Marinaro M, Bonato V, Amendola A, Songini M, Velluzzi F, Schirru C, Cotichini R, Stazi MA, Dotta F, Lorini R, Bottazzo GF, Boirivant M. Serum transforming growth factor β1 during diabetes development in non-obese diabetic mice and humans. Clinical & Experimental Immunology. 2010;162:407–414. doi: 10.1111/j.1365-2249.2010.04253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Köppel A, Tolosa E, Hoberg M, Anderl J, Aicher WK, Weller M, Wick W, Platten M. Toll-Like Receptor Engagement Enhances the Immunosuppressive Properties of Human Bone Marrow-Derived Mesenchymal Stem Cells by Inducing Indoleamine-2,3-dioxygenase-1 via Interferon-β and Protein Kinase R. Stem Cells. 2009;27:909–919. doi: 10.1002/stem.7. [DOI] [PubMed] [Google Scholar]
- Ortega-Molina A, Lopez-Guadamillas E, Mattison JA, Mitchell SJ, Munoz-Martin M, Iglesias G, Gutierrez VM, Vaughan KL, Szarowicz MD, Gonzalez-Garcia I, Lopez M, Cebrian D, Martinez S, Pastor J, de Cabo R, Serrano M. Pharmacological Inhibition of PI3K Reduces Adiposity and Metabolic Syndrome in Obese Mice and Rhesus Monkeys. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta MT, Orabona C, Bianchi R, Vacca C, Fallarino F, Belladonna ML, Volpi C, Mondanelli G, Gargaro M, Allegrucci M, Talesa VN, Puccetti P, Grohmann U. Forced IDO1 expression in dendritic cells restores immunoregulatory signalling in autoimmune diabetes. J Cell Mol Med. 2014;18:2082–2091. doi: 10.1111/jcmm.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, Mazza EMC, Boon L, Grassi F, Fioretti MC, Fallarino F, Puccetti P, Grohmann U. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- Patel RD, Murray IA, Flaveny CA, Kusnadi A, Perdew GH. Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Laboratory investigation; a journal of technical methods and pathology. 2009;89:695–707. doi: 10.1038/labinvest.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss: An Update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease From the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- Poulain-Godefroy O, Eury E, Leloire A, Hennart B, Guillemin GJ, Allorge D, Froguel P. Induction of TDO2 and IDO2 in Liver by High-Fat Feeding in Mice: Discrepancies with Human Obesity. International Journal of Tryptophan Research. 2013;6:29–37. doi: 10.4137/IJTR.S11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- Puga A, Tomlinson CR, Xia Y. Ah receptor signals cross-talk with multiple developmental pathways. Biochemical pharmacology. 2005;69:199–207. doi: 10.1016/j.bcp.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Reilly SM, Chiang S-H, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, Downes M, Yu RT, Liddle C, Evans RM, Oh D, Li P, Olefsky JM, Saltiel AR. An inhibitor of the protein kinases TBK1 and IKK-[epsiv] improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19:313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Hale PT, Carlin JM. The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J Interferon Cytokine Res. 2005;25:20–30. doi: 10.1089/jir.2005.25.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers P, Webb GP. Estimation of body fat in normal and obese mice. Br J Nutr. 1980;43:83–86. doi: 10.1079/bjn19800066. [DOI] [PubMed] [Google Scholar]
- Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Molecular Medicine. 1997;3:37–48. [PMC free article] [PubMed] [Google Scholar]
- Santostefano M, Merchant M, Arellano L, Morrison V, Denison MS, Safe S. alpha-Naphthoflavone-induced CYP1A1 gene expression and cytosolic aryl hydrocarbon receptor transformation. Molecular Pharmacology. 1993;43:200–206. [PubMed] [Google Scholar]
- Smith KJ, Murray IA, Tanos R, Tellew J, Boitano AE, Bisson WH, Kolluri SK, Cooke MP, Perdew GH. Identification of a high-affinity ligand that exhibits complete aryl hydrocarbon receptor antagonism. The Journal of pharmacology and experimental therapeutics. 2011;338:318–327. doi: 10.1124/jpet.110.178392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sono M, Cady SG. Enzyme kinetic and spectroscopic studies of inhibitor and effector interactions with indoleamine 2,3-dioxygenase. 1. Norharman and 4-phenylimidazole binding to the enzyme as inhibitors and heme ligands. Biochemistry. 1989;28:5392–5399. doi: 10.1021/bi00439a012. [DOI] [PubMed] [Google Scholar]
- Soshilov AA, Denison MS. Ligand Promiscuity of Aryl Hydrocarbon Receptor Agonists and Antagonists Revealed by Site-Directed Mutagenesis. Molecular and Cellular Biology. 2014;34:1707–1719. doi: 10.1128/MCB.01183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard A, Foch T, Hrubec Z. A twin study of human obesity. JAMA. 1986;256:51–54. [PubMed] [Google Scholar]
- Takahashi Y, Fukusato T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World Journal of Gastroenterology : WJG. 2014;20:15539–15548. doi: 10.3748/wjg.v20.i42.15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CK, Chong HC, Tan EHP, Tan NS. Getting /`Smad/' about obesity and diabetes. Nutrition and Diabetes. 2012;2:e29. doi: 10.1038/nutd.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N, Gonzalez FJ, Nebert DW. Developmental and tissue-specific differential regulation of the mouse dioxin-inducible P1-450 and P3-450 genes. Dev Biol. 1985;112:177–184. doi: 10.1016/0012-1606(85)90131-9. [DOI] [PubMed] [Google Scholar]
- van den Brandt PA, Spiegelman D, Yaun S-S, Adami H-O, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Pooled Analysis of Prospective Cohort Studies on Height, Weight, and Breast Cancer Risk. American Journal of Epidemiology. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CFA, Khan EM, Leung PSC, Gershwin ME, Chang WLW, Wu D, Haarmann-Stemmann T, Hoffmann A, Denison MS. Cross-talk between Aryl Hydrocarbon Receptor and the Inflammatory Response: A ROLE FOR NUCLEAR FACTOR-κB. Journal of Biological Chemistry. 2014;289:1866–1875. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi C, Mondanelli G, Puccetti P, Grohmann U. TLRs and tryptophan metabolism at the crossroad of immunoregulatory pathways. Immunometabolism. 2014;1:28–50. [Google Scholar]
- Wakefield LM, Letterio JJ, Chen T, Danielpour D, Allison RS, Pai LH, Denicoff AM, Noone MH, Cowan KH, O'Shaughnessy JA. Transforming growth factor-beta1 circulates in normal human plasma and is unchanged in advanced metastatic breast cancer. Clinical Cancer Research. 1995;1:129–136. [PubMed] [Google Scholar]
- Wang C, Xu CX, Krager SL, Bottum KM, Liao DF, Tischkau SA. Aryl Hydrocarbon Receptor Deficiency Enhances Insulin Sensitivity and Reduces PPAR-alpha Pathway Activity in Mice. Environmental health perspectives. 2011a;119:1739–1744. doi: 10.1289/ehp.1103593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011b;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- Watcharanurak K, Zang L, Nishikawa M, Yoshinaga K, Yamamoto Y, Takahashi Y, Ando M, Saito K, Watanabe Y, Takakura Y. Effects of upregulated indoleamine 2, 3-dioxygenase 1 by interferon [gamma] gene transfer on interferon [gamma]-mediated antitumor activity. Gene Ther. 2014;21:794–801. doi: 10.1038/gt.2014.54. [DOI] [PubMed] [Google Scholar]
- Wolff S, Harper PA, Wong JM, Mostert V, Wang Y, Abel J. Cell-specific regulation of human aryl hydrocarbon receptor expression by transforming growth factor-beta(1) Mol Pharmacol. 2001;59:716–724. doi: 10.1124/mol.59.4.716. [DOI] [PubMed] [Google Scholar]
- Wolowczuk I, Hennart B, Leloire A, Bessede A, Soichot M, Taront S, Caiazzo R, Raverdy V, Pigeyre M, Consortium A, Guillemin GJ, Allorge D, Pattou F, Froguel P, Poulain-Godefroy O. Tryptophan metabolism activation by indoleamine 2,3-dioxygenase in adipose tissue of obese women: an attempt to maintain immune homeostasis and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2012;303:R135–R143. doi: 10.1152/ajpregu.00373.2011. [DOI] [PubMed] [Google Scholar]
- Woods S, Seeley R, Porte D, Schwartz M. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- Wu D, Li W, Lok P, Matsumura F, Vogel CF. AhR deficiency impairs expression of LPS-induced inflammatory genes in mice. Biochem Biophys Res Commun. 2011;410:358–363. doi: 10.1016/j.bbrc.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CX, Wang C, Zhang ZM, Jaeger CD, Krager SL, Bottum KM, Liu J, Liao DF, Tischkau SA. Aryl hydrocarbon receptor deficiency protects mice from diet-induced adiposity and metabolic disorders through increased energy expenditure. Int J Obes. 2015 doi: 10.1038/ijo.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kisugi R. Mechanisms of LDL oxidation. Clinica Chimica Acta. 2010;411:1875–1882. doi: 10.1016/j.cca.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, Fallarino F, De Luca A, Moretti S, Gargaro M, Bartoli A, Romani L. Tryptophan feeding of the IDO1-AhR axis in host-microbial symbioses. Frontiers in Immunology. 2014;5 doi: 10.3389/fimmu.2014.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–441. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhao B, DeGroot DE, Hayashi A, He G, Denison MS. CH223191 Is a Ligand-Selective Antagonist of the Ah (Dioxin) Receptor. Toxicological Sciences. 2010;117:393–403. doi: 10.1093/toxsci/kfq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.