Abstract
Objectives
This study aimed to evaluate the cost-effectiveness of the CardioMems device in patients with chronic heart failure.
Background
The CardioMems device, an implantable pulmonary artery pressure monitor, was shown to reduce heart failure hospitalizations and improve quality of life in the CHAMPION trial.
Methods
We developed a Markov model to determine the hospitalization, survival, quality of life, cost, and incremental cost-effectiveness ratio of CardioMems implantation compared with usual care among a CHAMPION trial cohort of heart failure patients. We obtained event rates and utilities from published trial data; we used costs from literature estimates and Medicare reimbursement. We performed subgroup analyses of preserved and reduced ejection fraction and an exploratory analysis in a lower-risk cohort based on the CHARM trials.
Results
CardioMems reduced lifetime hospitalizations (2.18 versus 3.12), increased QALYs (2.74 versus 2.46) and increased costs ($176,648 versus $156,569), yielding a cost of $71,462 per QALY gained and $48,054 per life-year gained. The cost per QALY gained was $82,301 in patients with reduced ejection fraction and $47,768 in those with preserved ejection fraction. In the lower-risk CHARM cohort, the device would need to reduce heart failure hospitalizations by 41% in order to cost less than $100,000 per QALY gained. The cost-effectiveness was most sensitive to the device’s durability.
Conclusion
In populations similar to the CHAMPION trial, the CardioMems device is cost-effective if the trial effectiveness is sustained over long periods. Post-marketing surveillance data on durability will further clarify its value.
Keywords: Heart failure, monitoring, cost-effectiveness, CardioMems, technology
Heart failure treatment costs over $20.9 billion in total healthcare expenditures.(1) Most of these costs are incurred from treating clinical decompensations of patients with heart failure that result in over 1 million hospital admissions annually.(1,2) The CHAMPION trial, a randomized, single-blinded, multicenter trial, investigated the use of an implantable, wireless pulmonary artery pressure monitoring system to decrease heart-failure-related hospitalizations.(3) In this study, 550 patients with NYHA Class III heart failure and a heart failure hospitalization within the previous year underwent pulmonary artery sensor implantation. Patients were randomized to a treatment group in which providers were given access to the pressure readings or a control group in which the provider could not access the pressure readings. The treatment group was found to have fewer heart failure hospitalizations and improved quality of life.
New management strategies such as CardioMems that reduce costly heart failure hospitalizations may decrease the substantial clinical and economic burden of heart failure. However, the high device cost (listed as $17,750 with Medicare) raises questions regarding its value.(4) We performed an independent analysis of the cost-effectiveness of this device in a cohort based on the trial, as well as in subgroups defined by ejection fraction. Additionally, we performed an exploratory analysis of the device in an alternative, larger trial-based cohort of heart failure patients using the CHARM trials.(5)
METHODS
Decision model
We developed a Markov model to determine the cost-effectiveness of the CardioMems device compared with usual care from a societal perspective in a CHAMPION trial cohort over a lifetime horizon. This included adults (average age of 62) with NYHA Class III heart failure, hospitalized within 1 year with preserved (21.7%) or reduced ejection fraction (78.3%). We used hospitalization and mortality rates from the CHAMPION trial.(3) We performed subgroup analyses of reduced ejection fraction (average age of 60) and preserved ejection fraction (average age of 66) cohorts from the CHAMPION trial utilizing overall trial event rates and subgroup-specific rate ratios for each event from trials with larger sample sizes than CHAMPION.(6–9) Subgroup-specific device efficacy was extracted from the CHAMPION trial.(3)
In the model, individuals had CardioMems device placement at the outset, which could involve a procedural complication or device deployment failure. In subsequent monthly intervals, individuals could experience heart failure hospitalizations, non-heart failure hospitalizations, device complications, and all-cause mortality (Supplement Figure S1). Individuals had an increased mortality risk during the heart failure hospitalization and for two months post-hospitalization. The model followed all patients over their lifetimes. We matched the mortality rates over the mean duration of the trial for the control arm of the CHAMPION trial (17 months). After the trial period of 17 months, all event rates are extrapolated. We extrapolated an age-based increase in overall mortality from a previous retrospective analysis.(10)
Heart failure hospitalization rate and efficacy of the CardioMems device
We matched the trial rates of hospitalizations secondary to heart failure for each cohort. We modeled a declining rate of hospitalization over the CHAMPION trial duration. We modeled the CardioMems reduction in the rate of heart failure hospitalizations on the reduction over the entire trial (hazard ratio 0.63). We assumed that preventing a hospitalization prevented an inpatient and a two-month post-hospitalization increase in mortality.(11–13) We did not model any additional CardioMems-associated mortality reduction in the base case. For our base case, we assumed the benefit of the CardioMems device would continue lifelong, and examined shorter durations in sensitivity analyses.
CardioMems device events
We modeled periprocedural complications as a composite of the procedure-related serious adverse events and major bleeding during the thirty-day post-procedure anticoagulation period.(3) We additionally modeled procedural placement failure and CardioMems-related serious adverse events that occurred after the initial month.
Quality of life and costs
We included quality of life estimates for the patient’s baseline health, the use of the CardioMems device, hospitalizations, and complications using utilities. We calculated utility values by converting the 6-month Minnesota Living with Heart Failure (MLWHF) questionnaire score for the control arm in the CHAMPION trial into EQ-5D scores.(14) The difference-in-difference in EQ-5D score between groups from baseline to 6 months was applied as the quality of life benefit for the CardioMems device for the first year. The difference-in-difference between groups from baseline to 12 months was applied thereafter. The six-month differences were utilized for the entire first year because 226 of 550 patient scores were missing at 12 months. Disutilities were applied for the initial procedure, hospitalizations, and complications. Comparisons of patient utility during and after a heart failure hospitalization showed an 11% lower utility during hospitalization, for a decrement of approximately 3 days.(15) These assumptions were tested in sensitivity analyses, including an analysis in which we alternatively assumed long-term utility change secondary to heart failure hospitalizations.(16)
We included all healthcare-related costs. Hospitalization costs were taken from the AHRQ National Inpatient Sample(17) with physician costs calculated using the 2014 Medicare Professional Fees (CPT Codes 99212, 992213, 99222, 99223, 99254, and 99255).(18) Age-adjusted outpatient medical costs for patients with heart failure were taken from the literature.(2) The cost of CardioMems implantation, in addition to the device cost, is the cost for the right heart catheterization and angiography ($1,129) (from base Medicare 2014 Professional Fees, CPT Codes 93451 and 93568) along with the fee for device placement, currently an unlisted fee, which we assumed would approximate that of inserting a temporary single chamber cardiac electrode ($185, Medicare CPT 33210).(18,19) This assumption was based on selecting reimbursement for a simple intracardiac procedure given this is additional to catheterization and angiography reimbursement. We based the monthly cost of CardioMems management on the estimated time required to monitor the device by physicians and nurses and average provider wage.(20,21,Klein L, personal communication, November 2015) All costs were updated to 2014 USD using the medical component of the consumer price index.(22)
Sensitivity analyses
We evaluated the effect of uncertainty in all model inputs (Table 1). We focused on device-specific uncertainties and characteristics of the patient population. Given the single clinical trial currently available, we varied estimates of the efficacy of the device extensively by adjusting the reduction in hospitalizations, the effect of the device on mortality, the effect on quality of life, and the duration of the device’s effectiveness. With its recent market introduction, we also varied the costs of the device, implantation, and monitoring substantially. Given the large heterogeneous heart failure population, we also conducted analyses on a range of hospitalization costs, baseline utility values, and baseline hospitalization and mortality rates. This included an exploratory analysis of the intervention in a cohort based on the CHARM trials, which was a lower-risk cohort with greater quality-of-life and lower event rates. This cohort included adults (average age of 62) with NYHA Class II (45%), Class III (52%) and Class IV (3%) heart failure, as opposed to patients in CHAMPION who exclusively had NYHA Class III disease. We used published CHARM trial data to estimate hospitalization and mortality rates along with utility scores.(5,6,23) We adjusted the rates to match the ejection fraction subgroup composition of the CHAMPION cohort and to include only patients with a previous heart failure hospitalization. We estimated the device effectiveness needed to meet important cost-effectiveness thresholds in this cohort.
Table 1.
Selected Input Parameters*
| Base Case | Range | Source | |
|---|---|---|---|
| Event Probabilities (Monthly)† | |||
| CHAMPION cohort | |||
| Baseline All-Cause Mortality (%)‡ | 0.99 | 0.66–1.31 | (3) |
| Baseline Heart Failure Hospitalization (HFH)(%)§ | 8.76 | 4.38–13.15 | (3) |
| Inpatient HFH Mortality (%) | 3.90 | 3.60–4.20 | (11,12) |
| Relative Risk of Death after HFH** | 3.32 | 1.00–4.98 | (13) |
| Non-heart Failure Hospitalization (%) | 8.30 | 6.99–9.60 | (3) |
| Relative Risk (RR) of Preserved Ejection Fraction (pEF) Subgroup, compared to Reduced Ejection Fraction (rEF) Subgroup | |||
| RR of All-Cause Mortality, pEF vs. rEF | 0.52 | 0.43–1.00 | (6–9) |
| RR of HFH, pEF vs. rEF | 0.64 | 0.54–1.00 | (6–9) |
| RR of HFH Inpatient Mortality, pEF vs. rEF | 0.74 | 0.67–1.00 | (6–9) |
| CHARM Cohort†† | |||
| Heart Failure Mortality (%)‡‡,‡ | 0.66 | 0.43–0.89 | (5,6) |
| Baseline HFH (%)‡‡,§ | 3.11 | 2.32–3.89 | (5,6) |
| CardioMems Arm Specific Parameters | |||
| RR of HFH, compared to usual care | 0.63§§ | 0.52–0.77 | (3) |
| Placement Failure (%) | 4.35 | 2.68–6.01 | (3) |
| Costs ($) | |||
| Cost of Heart Failure Hospitalization | 12,832 | 8,341–16,750 | (17) |
| Cost of CardioMems Device | 17,750 | 8,875–35,500 | (4) |
| Cost of CardioMems Placement*** | 1,129 | 564–2,258 | (4,19) |
| Monthly Cost of CardioMems Device Management | 68 | 34–136 | ††† |
| Utilities | |||
| Baseline Utility, CHAMPION Cohort‡‡‡ | 0.55 | 0.51–0.75 | (3,14) |
| Baseline Utility, CHARM Cohort‡‡‡ | 0.66 | 0.64–0.68 | (14,23) |
| Disutility of Heart Failure Hospitalization | 0.059 | 0–0.11 | (15) |
| Utility of CardioMems Device for first 12 months‡‡‡ | 0.010 | 0–0.019 | (3,14,15) |
| Utility of CardioMems Device after first 12 months‡‡‡ | 0.004 | 0–0.019 | (3,14,15) |
Abbreviations: HFH: heart failure hospitalizations; RR: relative risk; pEF: preserved ejection fraction; rEF: reduced ejection fraction; MLWHF: Minnesota Living with Heart Failure.
Listed probabilities refer to the probabilities for patients with rEF. Probabilities of pEF calculated via the RR between pEF and rEF groups.
Adjusted for age with an exponential model (Supplement for further details).
Heart failure hospitalization probability adjusted by a monthly decreasing exponential model based on model stage to adjust for decreasing hospitalization rate with increasing time from initial hospitalization. This was set as constant after 17 months (Supplement for further details).
Increased risk for two months prior to returning to baseline.
Only differed from CHAMPION cohort with regards to hospitalization probability, mortality probability, and baseline quality of life. Used the same exponential models as CHAMPION cohort to adjust hospitalization and mortality probabilities.
Estimated from patients from all three CHARM trials and adjusted for those with a previous HFH and ejection fraction composition.
rEF subgroup RR of 0.67; pEF subgroup RR of 0.48.
Consists of Medicare professional reimbursement for right-heart catheterization, angiography, and CardioMems placement. CardioMems placement reimbursement not defined; estimated to be equal to a temporary transvenous cardiac electrode placement.
Estimated secondary to time associated with monitoring program (Dr. Liviu Klein, personal communication, November, 2015) and provider wages (20,21) (see Supplement for details)
MLWHF scores converted into EQ-5D scores.
We performed a probabilistic sensitivity analysis by performing 10,000 simulations in which we simultaneously sampled from the distributions of each input parameter with each simulation (Supplement for details).
We discounted future costs and benefits at 3% annually, and adhered to best practices.(24,25) The main outcome measure was cost per QALY gained. Cost-effectiveness thresholds followed ACC/AHA guidelines, with a threshold of less than $50,000 indicating highly cost-effective and greater than $150,000 not cost-effective.(26)
RESULTS
Comparison to the CHAMPION trial results
In the first six months, modeled rates of hospitalizations per patient-six months for the usual care and the intervention groups of 0.44 and 0.29 matched the CHAMPION trial’s 0.44 (CI 0.36–0.53) and 0.32 (0.26–0.40). The modeled annual rates over the mean trial period for the two groups were 0.68 and 0.45, matching trial results of 0.69 (0.61–0.78) and 0.46 (0.40–0.53). The modeled annual mortality probabilities over the mean trial period for the usual care and intervention groups were 14.8% and 13.2% matching the trial’s 14.7% (11.7–17.9) and 11.9% (9.5–16.3). Further details are included in the Supplement.
Base Case
In the CHAMPION trial cohort, the modeled CardioMems arm has a total of 2.18 hospitalizations per patient compared to 3.12 in the usual care arm, an absolute reduction of 0.94 hospitalizations over a patient’s lifetime (Table 2). The CardioMems arm increased life expectancy by 0.42 years and quality-adjusted life expectancy by 0.28 QALYs. The CardioMems arm achieved its health benefits at an increased cost of $20,079. Taken together, the CardioMems intervention costs $71,462 per QALY gained or $48,054 per life-year gained.
Table 2.
Base Case Results
| Strategy | HFH (# per patient) |
Survival (Years) |
Cost (2014 USD) |
QALY | $/Life Year | $/QALY (ICER) |
|
|---|---|---|---|---|---|---|---|
| CHAMPION Cohort | Usual Care | 3.12 | 5.31 | 156,569 | 2.46 | --- | --- |
| CardioMems | 2.18 | 5.72 | 176,648 | 2.74 | 48,054 | 71,462 | |
| Reduced EF Subgroup | Usual Care | 3.10 | 4.74 | 148,724 | 2.18 | --- | --- |
| CardioMems | 2.31 | 5.10 | 168,987 | 2.43 | 55,547 | 82,301 | |
| Preserved EF Subgroup | Usual Care | 3.17 | 7.35 | 184,877 | 3.48 | --- | --- |
| CardioMems | 1.74 | 7.96 | 204,289 | 3.88 | 31,865 | 47,768 |
Ejection fraction subgroups
Patients receiving usual care in the preserved ejection fraction subgroup had a longer average survival than the reduced ejection fraction subgroup (Table 2). The reduction in hospitalizations with CardioMems was greater for patients with preserved ejection fraction compared with those with reduced ejection fraction, which also resulted in lower incremental costs. With more QALYs gained and a smaller difference in costs, CardioMems cost $47,768 per QALY gained in the preserved ejection fraction cohort compared to $82,301 in the reduced ejection fraction cohort.
Costs
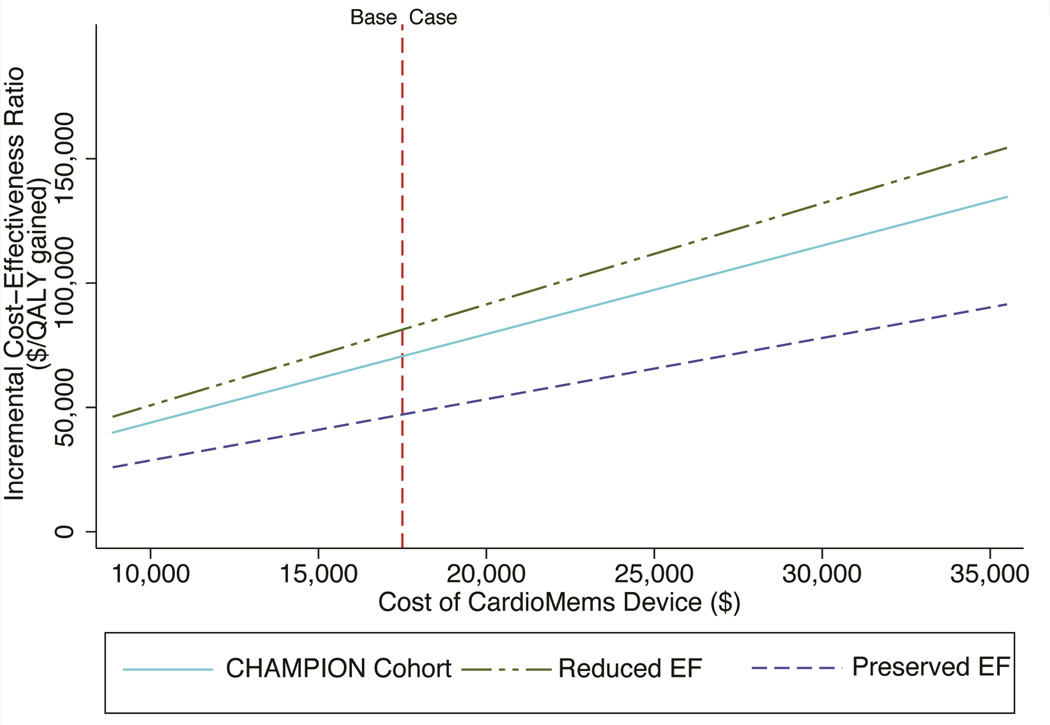
The base case used a device cost of $17,750. The device cost less than $50,000 per QALY gained if the cost is less than $9,798 in those with reduced ejection fraction and less than $18,657 in those with preserved ejection fraction (Figure 1). Use of the device would cost more than $150,000 per QALY gained if it cost more than $34,418 in the reduced ejection fraction subgroup or $59,296 in the preserved ejection fraction subgroup. The cost of a heart failure hospitalization ($12,832) was based on the national average; however, there is significant hospital variation. In a large, urban, public, teaching hospital with a higher predicted cost of hospitalization ($16,750), the CardioMems device costs $62,121 per QALY gained. In a small, rural, private, non-teaching hospital with lower predicted costs of hospitalization ($8,341) the device becomes less favorable, costing $82,169 per QALY gained (Figure 2).
Figure 1. Cost-effectiveness of CardioMems as a function of device cost.
This one-way sensitivity analysis demonstrates the effect of the price of the CardioMems device on its cost-effectiveness in both the CHAMPION cohort and the preserved EF and reduced EF subgroups.
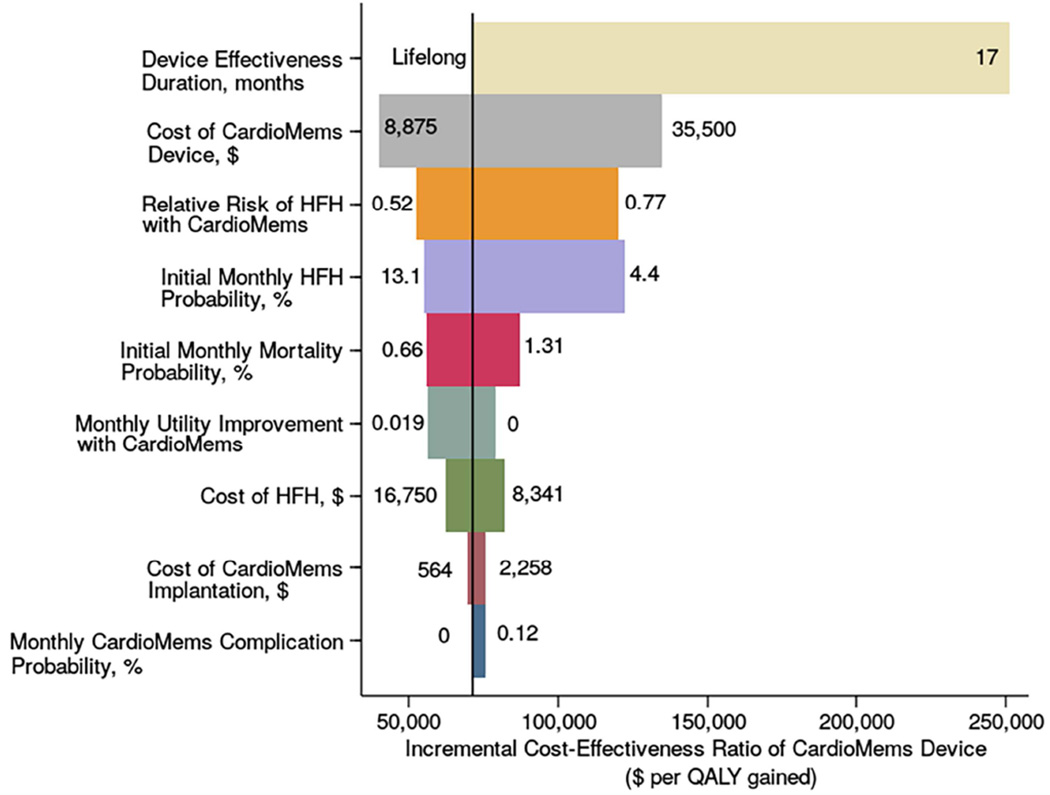
Figure 2. Tornado Diagram: series of one-way sensitivity analyses.
The bars indicate the range of cost per QALY gained with the CardioMems device compared with usual care in 1-way sensitivity analyses of the input parameters across the range of values, listed to the left and right of the bars. The above variables were selected because they had the largest effect on the cost-effectiveness ratio of the device. The solid line demonstrates the cost-effectiveness in the CHAMPION cohort of $71,462 per QALY gained. The dotted line represents the cost-effectiveness threshold of $150,000 per QALY gained.
In the base case, we assumed a monthly cost of $68 to monitor the CardioMems device. This cost would need to be under $190 monthly for the device to cost less than $100,000 per QALY and below $403 monthly for a cost less than $150,000 per QALY.
Device efficacy
The efficacy of the CardioMems device is modeled via reduction in hospitalizations, the risk of mortality associated with hospitalizations, and the effect on baseline quality of life. While CardioMems had a 0.63 hazard ratio for hospitalizations over the entire randomized period of the CHAMPIONS trial, the confidence interval was 0.52–0.77. Over this range, CardioMems costs between $52,556 and $120,143 per QALY gained. The effect of the CardioMems device on mortality is uncertain. In the base case, we assumed that prevented hospitalizations had a similar inpatient mortality risk to all heart failure hospitalizations and that preventing these hospitalizations would also avert the associated increase in post-hospitalization mortality, which led to an 11% relative reduction in mortality in the CardioMems arm compared with usual care over the trial period. If we had instead assumed a 20% relative reduction, the value of the CardioMems device would improve to $55,378 per QALY gained. However, if preventing hospitalizations did not reduce mortality, the cost would be $159,984 per QALY gained. For the device to cost less than $100,000 per QALY gained, the relative reduction in mortality must be at least 4%.
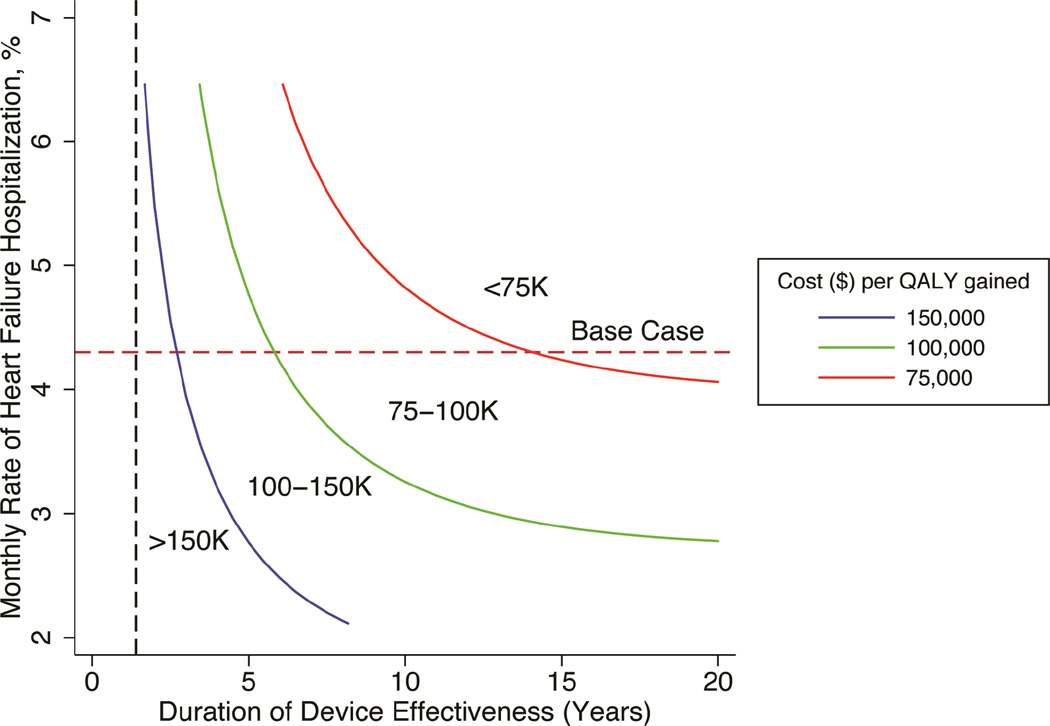
Though the CHAMPION trial followed patients for 17 months on average, we assumed a lifelong duration. If CardioMems’ effectiveness ceased after 17 months, its cost per QALY gained was $214,879. Cost per QALY gained declined as the duration of effectiveness increased, dropping below $150,000 at 34 months and $100,000 at 72 months. In two-way sensitivity analyses, we found that the device is much less cost-effective if its duration of effect is shorter in populations with lower monthly hospitalization rates (Figure 3).
Figure 3. Cost-effectiveness of CardioMems device as a function of hospitalization risk and duration of device effect.
This graph is a two-way sensitivity analysis of the monthly rate of heart failure hospitalizations and the duration of clinical effect. The probability of heart failure hospitalizations has been varied and the average monthly rate of hospitalizations (discounted) of the usual care arm has been displayed on the y-axis. The duration that the CardioMems device is assumed to function (years) is on the x-axis. The colored lines represent the cost per QALY gained at that point. The cost per QALY gained decreased with increases in the hospitalization rates and the duration of effectiveness. The red dotted horizontal line displays the base case hospitalization rate (4.3%). The black vertical dotted line is the average patient follow-up in the CHAMPION study (17 months).
Adjusting the peri-procedural complication rate, chronic complication rate, or placement failure rate did not substantially alter our findings (Supplement).
Utilities
The impact of the CardioMems device on quality of life did not substantially change our main findings (Supplement). Neither alternative assumptions of baseline utility nor duration of disutility from hospitalization substantially altered our results (Supplement). Adjusting long-term baseline utility based on the number of heart failure hospitalizations also did not substantially affect our findings (Supplement).
Severity of illness
Baseline rates of hospitalization and mortality matched trial rates. However, individual healthcare systems will have heterogeneous patient groups with different rates of readmission and mortality. We found decreases to the hospitalization rate and increases to the mortality rate both increased the cost per QALY of CardioMems, but the cost did not exceed $150,000 per QALY gained (Supplement Figure S2).
In the CHARM cohort, the usual care arm had greater survival (7.9 years), more QALYs (4.67), and fewer heart failure hospitalizations (1.71) compared with the CHAMPION cohort. If the device had a similar effect on quality of life in the CHARM cohort as in the CHAMPION trial, the device would need to prevent 26.5% of failure hospitalization to cost less than $150,000 per QALY gained and 41.1% to cost less than $100,000 per QALY gained (Supplement Figure S3).
Time horizon
In the base case, we used a lifetime horizon. Over a five-year horizon, which was used in the trial cost-effectiveness, we found a $15,029 difference in costs and a 0.11 difference in QALYs, yielding a cost of $138,466 per QALY gained (Supplement Figure S4).
Probabilistic sensitivity analysis
In the probabilistic sensitivity analysis, we found 17.3% of simulations showed CardioMems was the preferred intervention at a willingness-to-pay threshold of $50,000, 76.9% at a threshold of $100,000 and 95.1% at a threshold of $150,000 (Supplement Figure S5). Additional sensitivity analyses are available in the Supplement.
DISCUSSION
Our analysis demonstrates that the use of the CardioMems device is cost-effective in patients with NYHA Class III heart failure and a history of heart failure hospitalization in the preceding year. Our base case analysis finds a cost of $71,462 per QALY gained in a CHAMPION trial population. The device value is most sensitive to its durability and the association between the reduction in hospitalizations and survival. The device must provide benefits over at least 34 months to cost less than $150,000 per QALY. With randomized data of 17 months and open-access data for an additional 13 months, the device’s effectiveness over a longer period is unknown. Although heart failure hospitalizations are associated with an increased mortality risk, it is unclear how preventing hospitalizations with CardioMems affects survival. The CHAMPION trial was underpowered to detect a mortality difference; a survival analysis submitted to the FDA demonstrated substantial uncertainty (HR 0.8, CI: 0.55–1.15). We demonstrate that the device would need to reduce mortality by 4% to cost less than $100,000 per QALY. While this seems likely, it is possible that prevented hospitalizations may be lower risk and mortality may be relatively unaffected. Future studies that follow hospitalization trends and refine estimates of the effect of the device on mortality can reduce the uncertainty regarding its clinical and economic value.
Our analysis indicates that the CardioMems device provides better value in patients with preserved ejection fraction. There are few evidence-based treatments available for patients with preserved ejection fraction; thus, the CardioMems device represents a rare evidence-supported intervention for this important group. Our model predicted greater value in this group due to the longer survival and increased device effectiveness in this group; however, the estimate of effectiveness in this group is based on only 119 trial patients and should also be refined with future research.
The incremental cost-effectiveness ratio found in the CHAMPION trial was more favorable than our estimate.(3) Their study, performed over a five-year time horizon, found a larger difference in QALYs and a much smaller cost difference between the two arms ($4,282) to lead to an incremental cost-effectiveness of $13,379 per QALY. We do not have access to their assumptions to analyze these differences. The lifetime horizon used in our model captures longer-term benefits and costs and the impact of device durability.
The importance of the CardioMems device is tied to the scope of the problem. Healthcare expenditures secondary to heart failure are expected to rise from $20.9 billion in 2012 to $53.1 billion in 2030, with 80% of these costs attributed to heart failure hospitalizations.(27) These hospitalizations are not only costly but also markers of a worsening clinical prognosis, being associated with high rates of rehospitalization and mortality. Strategies to reduce this clinical and economic burden are needed. We demonstrate that the CardioMems device may be a cost-effective intervention for outpatient heart failure management. However, our analysis also shows that the savings from reduction in hospitalization costs are exceeded by the intervention costs, which could thereby still have a large budgetary impact. We also illustrate the value decreases in lower risk patients; with the substantial heterogeneity in morbidity of heart failure patients, ensuring patients fit the trial criteria will be important. Although it is a costly intervention that should be reserved for appropriately selected patients and still requires further evaluation, the value of this device compares favorably to other technologies used in similar patient groups, such as left-ventricular assist devices.(28)
Study limitations
There are a number of limitations to our analysis. First, a single trial has evaluated the intervention; the effectiveness seen in this trial should be confirmed in post-surveillance evaluation. Second, there may be treatment benefits that are not captured in our model, such as identifying patients who need to initiate advanced therapy. Third, although there were no serious device-related complications outside of the procedural period, long-term safety data is not currently available. Finally, we attempted to capture additional costs of using the device using the time required at a heart failure center, but the average national cost of the monitoring program is currently unclear.
Conclusions
This analysis shows that the use of the CardioMems device is a cost-effective means of improving heart failure quality of life and reducing rehospitalizations. It is a better value in patients with preserved ejection fraction, a group with few effective therapies. The cost-effectiveness of CardioMems is most sensitive to the duration of effectiveness; therefore, further research on the continued hospitalization trends of patients with the device will be important for future evaluations.
Supplementary Material
Clinical Perspectives
In patients with NYHA Class III heart failure and a heart failure hospitalization in the preceding year, the CardioMems device reduced heart failure readmissions and improved quality of life at a cost below commonly accepted U.S. willingness-to-pay thresholds. The device was cost-effective for both patients with reduced ejection fraction and preserved ejection fraction.
Translational Outlook
The clinical effectiveness of the device has only been demonstrated in a single randomized clinical trial with a mean follow-up of 17 months. Evaluation of the long-term heart failure hospitalization rate and the relationship between averted hospitalizations and changes in mortality in individuals after CardioMems implantation will refine estimates of the value of the device. Additional data regarding the cost of maintaining and monitoring the device in the community will also inform future economic evaluations.
Acknowledgments
The authors thank Iris H. Ma, MD for her thoughtful comments on the manuscript draft.
Funding Sources
Dr. Sandhu, Dr. Owens, Dr. Turakhia, Dr. Kaiser, and Dr. Heidenreich were supported by the Department of Veteran Affairs. Dr. Goldhaber-Fiebert is supported was supported by an NIH NIA Career Development Award (K01 AG037593-01A1: PI: Goldhaber-Fiebert). This study was also supported in part by the Department of Veterans Affair's Quality Enhancement and Research Initiative 04-326.
Abbreviations
- ACC/AHA
American College of Cardiology/American Heart Association
- AHRQ
Agency for Health Research and Quality
- CHAMPION trial
CardioMems Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients trial
- CHARM trials
Candesartan in Heart failure: Reduction in Mortality and morbidity trials
- CPT
Current Professional Technologies
- MLWHF
Minnesota Living with Heart Failure
- NYHA
New York Heart Association
- QALYs
Quality-adjusted life years
- USD
United States Dollars
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Dr. Turakhia has consulted for St. Jude Medical.
Conflicts of Interest: None
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4:68–75. doi: 10.1161/CIRCOUTCOMES.110.957225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services. Federal Register: August 22, 2014: 42 CFR Parts 405, 412, 413, et al. [Accessed January 14, 2015]; Available at: http://www.facs.org/~/media/files/advocacy/regulatory/fy 2015 ipps final rule_fr.ashx. [Google Scholar]
- 5.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 6.Bello NA, Claggett B, Desai AS, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail. 2014;7:590–595. doi: 10.1161/CIRCHEARTFAILURE.113.001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson F, Torp-Pedersen C, Seibaek M, Burchardt H, Kober L group Ds. Effect of age on short and long-term mortality in patients admitted to hospital with congestive heart failure. Eur Heart J. 2004;25:1711–1717. doi: 10.1016/j.ehj.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) J Am Coll Cardiol. 2008;52:347–356. doi: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor CM, Abraham WT, Albert NM, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2008;156:662–673. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on healthrelated quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–251. doi: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Jaagosild P, Dawson NV, Thomas C, et al. Outcomes of acute exacerbation of severe congestive heart failure: quality of life, resource use, survival. SUPPORT Investigators. The Study to Understand Prognosis and Preferences for Outcomes and Risks of Treatments. Arch Intern Med. 1998;158:1081–1089. doi: 10.1001/archinte.158.10.1081. [DOI] [PubMed] [Google Scholar]
- 16.Gohler A, Geisler BP, Manne JM, et al. Utility estimates for decision-analytic modeling in chronic heart failure--health states based on New York Heart Association classes and number of rehospitalizations. Value Health. 2009;12:185–187. doi: 10.1111/j.1524-4733.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 17.Ziaeian B, Sharma PP, Yu TC, Johnson KW, Fonarow GC. Factors associated with variations in hospital expenditures for acute heart failure in the United States. Am Heart J. 2015;169:282–289. e15. doi: 10.1016/j.ahj.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Center for Medicare and Medicaid. Physician Fee Schedule. 2015 Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html?redirect=/PhysicianFeeSched/. Accessed.
- 19.St. Jude Medical. St. Jude Medical Reimbursement Guide. [Accessed March 1, 2015];2015 Available at: http://professional.sjm.com/resources/reimbursement/crm-ep/cardiomems. [Google Scholar]
- 20.Medical Group Management Association. Physician Compensation and Production Survey: 2014 Report Based on 2013 Data. 2014. Englewood, CO: Medical Group Management Association; 2014. [Google Scholar]
- 21.Occupational Employment Statistics. Occupational Employment and Wages, May 2014, 29-1141 Registered Nurses. [Accessed November 20, 2015]; Available at: http://www.bls.gov/oes/current/oes291141.htm.
- 22.United States Department of Labor, Bureau of Labor Statistics. Consumer Price Index. [Accessed March 1, 2015]; Available at: http://www.bls.gov/bls/inflation.htm.
- 23.Lewis EF, Lamas GA, O'Meara E, et al. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail. 2007;9:83–91. doi: 10.1016/j.ejheart.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 25.Caro JJ, Briggs AH, Siebert U, Kuntz KM Force I-SMGRPT. Modeling good research practices--overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med Decis Making. 2012;32:667–677. doi: 10.1177/0272989X12454577. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–2322. doi: 10.1016/j.jacc.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller LW, Guglin M, Rogers J. Cost of ventricular assist devices: can we afford the progress? Circulation. 2013;127:743–748. doi: 10.1161/CIRCULATIONAHA.112.139824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.