Abstract
Drug addiction is a syndrome of dysregulated motivation, evidenced by intense drug craving and compulsive drug-seeking behavior. In the search for common neurobiological substrates of addiction to different classes of drugs, behavioral neuroscientists have attempted to determine the neural basis for a number of motivational concepts and describe how they are changed by repeated drug use. Here, we describe these concepts and summarize previous work describing three major neural systems that play distinct roles in different conceptual aspects of motivation: (1) a nigrostriatal system that is involved in two forms of instrumental learning, (2) a ventral striatal system that is involved in Pavlovian incentive motivation and negative reinforcement, and (3) frontal cortical areas that regulate decision making and motivational processes. Within striatal systems, drug addiction can involve a transition from goal-oriented, incentive processes to automatic, habit-based responding. In the cortex, weak inhibitory control is a predisposing factor to, as well as a consequence of, repeated drug intake. However, these transitions are not absolute, and addiction can occur without a transition to habit-based responding, occurring as a result of the overvaluation of drug outcomes and hypersensitivity to incentive properties of drug-associated cues. Finally, we point out that addiction is not monolithic and can depend not only on individual differences between addicts, but also on the neurochemical action of specific drug classes.
Keywords: Drug Addiction, Motivation, Neural Systems, Incentive Sensitization, Allostatsis, Habit, Drug Learning
1 Introduction
Drug addiction is characterized by intense drug craving and compulsive drug use, with an excessive amount of time and effort spent procuring drug1 (Jaffe 1975; Koob and Le Moal 2008; Tiffany 1999; Skinner and Aubin 2010; Gass et al. 2014; American Psychiatric Association 2013). In many cases, the drug has no direct benefit to the subject’s survival, and drug-taking occurs despite adverse consequences to one’s health, livelihood, and family. Smoking cigarettes is a classic example of this, and becoming addicted to prescription opioids highlights the complex balance between the benefits and harms of many drugs. So what drives drug-taking in the first place, and what shifts motivational systems into “overdrive,” resulting in addiction? The obvious starting point is that the individual must first try the drug. This initiation depends on a number of factors, including individual personality traits, cultural norms, social context, and interactions with comorbid mental illness (Bucholz 1999; Compton et al. 2007; Homberg et al. 2014). Following this initiation, a drug is taken repeatedly to obtain some perceived positive effect, be it physiological or psychological (e.g., euphoria or social acceptance), or to relieve a perceived negative state (e.g., to reduce anxiety). The experienced outcome of drug use reinforces the drug-taking behavior; that is, the experience of a positive outcome or the removal of a negative outcome increases the likelihood that the drug will be used again. These forms of positive and negative reinforcement, respectively (Khantzian 1997; Wise and Bozarth 1987), help maintain drug-taking behaviors, particularly in early, often experimental stages.
The transition from initial controlled drug use to uncontrolled use and addiction is not well understood, in part because this transition is gradual, and is not defined by a precise behavioral or biological tipping point (Ahmed and Koob 1998). However, it is clear that most drug users begin by experimenting with a variety of drugs, that multiple drug experiences are needed, and that not all drug-taking experiences will result in addiction (even if use is prolonged; think of four years on an American college campus). Below, we describe several key psychological processes that underlie initial drug-taking, how they are altered in the addicted state, and the neurobiological substrates thought to underlie these processes. Because these ideas have been presented in many reviews (Robinson et al. 2014; Belin et al. 2009; Wise and Koob 2014; Hogarth et al. 2013; Wiers et al. 2007; Kalivas and Volkow 2005; Redish et al. 2008), we will focus on core motivational processes involved in addiction and their key neurobiological substrates. In addition, we highlight how different motivational processes may be involved in different aspects of addiction depending on the individual, the drug class, and the duration of drug-taking behavior.
1.1 Rodent Models of Addiction
Much of what is known about the psychological and neurobiological substrates of addictive behavior has been learned using animal models (Lesscher and Vanderschuren 2012; Deroche-Gamonet et al. 2004). Here, we review a few key approaches to orient the reader to studies referred to in subsequent sections. Early studies used repeated administration of potentially addictive drugs to an animal (usually rats or mice) and measured the consequences on behavior and brain function. This approach was, and still is, very useful for understanding how drug exposure changes the brain and influences behavior, but lacks an aspect of voluntary drug-taking (Wolf and Ferrario 2010; Vezina and Leyton 2009; Schmidt and Pierce 2010). Ultimately, self-administration approaches became the gold standard not only for studying drug addiction, but also for assessing how likely it is that a drug will be abused (Schuster and Thompson 1969; Weeks 1962). More recently, self-administration models that attempt to differentiate “casual” drug-taking from “addiction-like” overconsumption have been developed. These models typically use extended access procedures in which drug is freely available over prolonged periods of time. These procedures induce escalated drug self-administration and incorporate the idea of continued pursuit of the drug despite adverse consequences (e.g., crossing an electrified grid to obtain the drug). Indeed, a number of studies have found that extended access self-administration procedures produce “addiction-like” behaviors and neuroadaptations that are distinct from those associated with limited access “drug-taking” behaviors (Loweth et al. 2014; Wolf and Ferrario 2010; Deroche-Gamonet et al. 2004; Lesscher and Vanderschuren 2012; Ahmed 2010). In addition to modeling different aspects of addiction, self-administration models have been informed by concepts from learning theory and psychology in order to ask questions not only about drug-taking and drug-seeking behaviors, but also to determine how these behaviors can be influenced and modified by non-drug experiences (e.g., stressors) and stimuli in the environment that are associated with drug (i.e., Pavlovian-conditioned cues; referred to as drug cues throughout). For example, neuroadaptations induced by drug self-administration have long-term behavioral consequences that can be measured using rodent models of relapse or “reinstatement.” These relapse tests involve “extinguishing” drug self-administration by omitting drug delivery for several sessions and then presenting the rat with stressors, drug cues, or the drug itself. The degree to which these stimuli “reinstate” drug-seeking behavior is a model of relapse severity. Not surprisingly, there are different neurobiological systems underlying these different forms of reinstatement, which are themselves distinct from those involved in drug-taking (Torregrossa and Kalivas 2008; Shalev et al. 2002).
2 Overview of Motivational Processes and Their Neurobiological Substrates
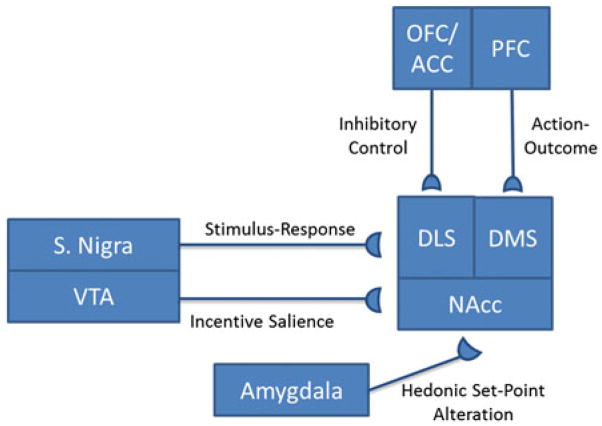
Shifts in behavioral and psychological responses to drugs and drug cues underlie a transition from controlled or causal drug use to addiction. Experimental evidence from human and animal studies shows that drugs produce alterations in mesocorti-colimbic circuits that underlie drug-seeking and drug-taking behaviors, particularly within basal ganglia circuits (Fig. 1; Nestler 2005; Koob and Nestler 1997; Belin et al. 2009; Fields et al. 2007; Sesack and Grace 2010; Vanderschuren and Kalivas 2000; Pierce and Kalivas 1997; Wolf 1998; Steketee 2003). The basal ganglia can be divided into a ventral subsystem in which the nucleus accumbens (NAcc) receives dopaminergic input from the ventral tegmental area (VTA) and glutamatergic input from the prefrontal cortex and amygdala, and a dorsal subsystem (comprised of dorsolateral and dorsomedial subregions) which receives input from the substantia nigra. The NAcc and dorsal striatum project to the ventral pallidum and globus pallidus, respectively, and both have reciprocal connections with frontal cortical areas, including the prefrontal, anterior cingulate, and orbitofrontal cortices. These reciprocal connections form parallel cortical–striatal–pallidal–cortical loops (Humphries and Prescott 2010; Alexander et al. 1990; Haber 2003) that are intimately involved in the initiation and maintenance of drug-taking and drug-seeking behaviors (as well as other motivated behaviors). This system, particularly the ventral subsystem, projects to and receives input from limbic areas including the amygdala and hippocampus (Schmidt et al. 2005; Berendse et al. 1992; Brog et al. 1993; Klitenick et al. 1992). Because of its connectivity with these emotion and learning centers, as well as the hypothalamus, the ventral subsystem of the basal ganglia is often referred to as a “functional interface” between motivation and action (Mogenson et al. 1980; Kelley et al. 2005). In comparison, the dorsal subsystem has been more tightly linked to the processing of goal-directed behavior and habit, which is discussed below (Belin et al. 2009; Yin et al. 2004; Faure et al. 2005). For further discussions of the role of these circuits in motivation, see chapters by Learning and Motivational Processes Contributing to Pavlovian–Instrumental Transfer and Their Neural Bases: Dopamine and Beyond, Multiple Systems for the Motivational Control of Behavior and Associated Neural Substrates in Humans, The Computational Complexity of Valuation and Motivational Forces in Decision-Making Processes, and Neurophysiology of Reward-Guided Behavior: Correlates Related to Predictions, Value, Motivation, Errors, Attention, and Action, in this volume.
Fig. 1.
An oversimplified diagram of key neural projections underlying motivational aspects of addiction. Note that this diagram does not reflect that these projections participate in multiple processes. For example, the amygdala is also involved in the hedonic response to drug rewards and in incentive–salience attribution to reward cues. Also, reciprocal connections between the cortex and midbrain areas are involved in all the concepts listed in the figure
Addiction is mediated by several different motivational processes that can be dissociated experimentally and neurobiologically. For example, the maintenance of drug-taking by negative reinforcement processes results from the removal of a negative drug withdrawal state by the drug (Koob and Le Moal 2001). This hedonic allostasis model also emphasizes that drugs produce a reward deficit in which previously pleasurable events now fail to produce the same hedonic effect. Thus, addiction is maintained by a need both to increase the amount of drug used in order to reach the new hedonic threshold and to overcome anhedonia (i.e., a generalized inability to feel pleasure) induced by repeated drug exposure. From a biological standpoint, this new set point is mediated by reduced activity within dopamine, opioid, and GABA neurotransmitter systems, while anhedonia is mediated by alterations in the function of the amygdala and corticotropin-releasing hormone (CRH) systems (Koob and Le Moal 2008; George et al. 2012) (Fig. 1). In comparison, the incentive–sensitization model of addiction (Robinson and Berridge 1993) proposes that in the addicted state, drugs and drug cues have a heightened ability to direct and influence behavior, ultimately focusing behavior toward drug-seeking and drug-taking. This concept also stresses that in the addicted state, drugs and drug cues are better able to direct behavior, not because they are pleasurable or “liked,” but because they are highly noticeable, attractive, and “wanted” (for more detailed information on the distinct roles of wanting and liking in motivating behavior, please see Robinson et al., in this volume). Neurobiologically, the enhanced influence of drug cues over behavior (i.e., incentive sensitization) is linked to increased responsivity of mesolimbic dopamine systems, including inputs into this system from cortical areas (Fig. 1). Thus, alterations in dopamine- and glutamate-mediated transmission, particularly in the NAcc, drive incentive sensitization that underlies cue-triggered drug-seeking behaviors.
These first two models of addiction propose bivalent changes in brain reward systems to increase motivation: one in which the function of mesolimbic systems and hedonic responses are reduced and drug is needed to return to a “normal” state and one in which heightened responsivity of mesocorticolimbic systems to drug cues drives drug-seeking and drug-taking. These ideas describe different aspects of motivation that may be involved in different phases of addiction.2 For example, hedonic allostasis may maintain drug-taking behaviors during early withdrawal or attempts to reduce drug use, whereas incentive sensitization may promote relapse even after physiological and emotional withdrawal symptoms have subsided.
Additional concepts of addiction focus on the relationship between behavioral actions (e.g., lighting a cigarette) and an outcome (e.g., smoking a cigarette). Within this framework, initial drug-taking involves learning that an action (drug-seeking) results in a given outcome (euphoria, anxiety reduction, etc.), but the nature of the relationship between the action and outcome changes as a consequence of drug use to produce addiction. Goal-directed (i.e., action–outcome) models posit that the expected drug outcome is improperly overvalued such that behaviors directed toward obtaining drug override all others. Similarly, drug-induced anhedonia may also contribute to this improper valuation through the underestimating of the value of alternative goals. On the other hand, habit-based models of addiction (i.e., stimulus–response) posit that after initial learning and repeated drug exposure, the outcome no longer drives drug-seeking behavior, but instead, drug-seeking is an automated response triggered by drug cues, emotional states, stress, etc. Like hedonic allostasis and incentive sensitization, goal-directed and habit models of addiction also propose that changes in mesolimbic function underlie these behaviors. However, habit-based ideas emphasize a transition from behaviors mediated by the ventral subsystem of the basal ganglia to behaviors mediated by the dorsal subsystem (Fig. 1). In addition, goal-directed, habit-based, and incentive sensitization all incorporate the idea that the relationship between drug-seeking behavior and consumption of the drug itself changes as addiction develops, though they differ in the nature of the relationship that is thought to change. Below, we provide more specific details of the processes involved in each of these models described above as well as evidence in support of and contrary to them.
3 Hedonic Allostasis
The hedonic allostasis model of addiction (Koob and Le Moal 2001) is based on Solomon’s opponent process theory, but has been modified to account for the chronic, pathological nature of drug-taking and relapse (Solomon and Corbit 1974). In Solomon’s original work, he proposed that drug-taking consists of two dissociable processes: a short-lived a-process, which might include elevated heart rate, a state of well-being or euphoria (i.e., a hedonic affective state), or motoric effects (e.g., punding or chewing that are common with psychostimulants). At the same time, the drug also produces a b-process that has two key features: (1) It is in opposition to the a-process (e.g., reduced heart rate) and (2) it has a slower onset and offset relative to the a-process. The b-process can thus counteract aspects of the a-process. Solomon argued that the b-process, because it is longer lasting than the a-process, could explain the phenomenon of drug withdrawal. Further, he suggested that the b-process becomes larger after repeated drug administration. This change in the b-process has two effects: first, to further counteract the a-process (resulting in drug tolerance and a need to increase drug intake to compensate) and second, to worsen the withdrawal syndrome.
The hedonic allostasis model adds to this idea by positing that a portion of the b-process involves a reduction in the hedonic affective state (to counter euphoria induced by drug) that is maintained after drug use is discontinued. This maintained anhedonic b-process changes the “set point” for activation of the a-process such that the person remains in a relatively anhedonic state in the absence of drug. Koob and colleagues call this “allostatic state … a new equilibrium, a state of chronic deviation [from the] normal (homeostatic) operating level to a pathological (allo-static) operating level” (George et al. 2012).3 This anhedonic deficit creates a motivational drive to take drugs in order to reverse anhedonia (through negative reinforcement). However, because the set point for the desired a-process has changed, people must now take larger doses of the drug to activate the a-process once more and overcome the enhanced b-process. In this manner, a shift from positive to negative reinforcement underlies drug addiction. In addition, the imbalance between a- and b-processes produces an unending “downward anhe-donic spiral” where the b-process (anhedonia) continually grows stronger due to drug use and thus does not produce the desired a-process effect (euphoria to remove anhedonia). The motivational drive to relieve this hedonic deficit becomes ever stronger and leads to more drug-taking (Ahmed and Koob 1998; Koob and Le Moal 2001). Thus, drug withdrawal induces both physiological withdrawal (e.g., shakes in alcoholism or temperature dysregulation in the case of opioids) and “hedonic” withdrawal (i.e., anhedonia and malaise).
Drug-induced alterations in a- and b-processes are mediated by within-systems adaptations, in which changes occur in systems responsible for initial drug effects, and between-systems adaptations, which involve the recruitment of additional systems not initially engaged by the drug (Koob and Le Moal 2008). Neurobiologically, within-systems changes mediating a reduction in the set point a-process are due to drug-induced reductions in mesolimbic dopamine. For example, acute administration of alcohol stimulates VTA dopamine input into the NAcc (Brodie et al. 1999; Meyer et al. 2009; Nimitvilai et al. 2012; Gonzales et al. 2004). However, repeated alcohol exposure reduces the activity of VTA neurons (Diana et al. 1993; Shen 2003; Shen et al. 2007), including during withdrawal from chronic alcohol drinking (Bailey et al. 1998). This is consistent with the idea that increased alcohol intake is needed to restore reduced function of the mesolimbic system to prealcohol exposure levels. In another study, Barak et al. (2014) used a chronic intermittent alcohol procedure to induce escalated intake; this escalation was associated with a decrease in the firing rate of VTA dopamine neurons. When administered glial-derived neurotrophic factor (GDNF), the escalation of alcohol intake and the decrease in neural firing rates were blocked. These data suggest that normalizing an alcohol-induced deficit in VTA neuron firing (and by extension NAcc dopamine levels) resulted in a reversal of escalated alcohol intake. Additional evidence for a-process set point alterations comes from animal studies of intracranial self-stimulation (ICSS), in which lever pressing is reinforced by electrical stimulation of the medial forebrain bundle (Edmonds and Gallistel 1974; Miliaressis et al. 1986). ICSS reward thresholds are defined as the intensity of stimulation needed to support self-stimulation; increases in ICSS thresholds are interpreted as a need for stronger stimulation to achieve the same level or reinforcement (Diana 2011). Indeed, chronic exposure to many drugs increases ICSS thresholds (Kenny et al. 2006; Epping-Jordan et al. 1998; Kenny et al. 2003; but see Kenny and Markou 2006). Importantly, increased ICSS thresholds are long-lasting and persist even during protracted abstinence (Ahmed et al. 2002; Kenny et al. 2003). ICSS activates dopamine neurons (Yeomans 1989), and maintenance of ICSS requires activation of the hypothalamus (Kempadoo et al. 2013; Olds 1962). Thus, these experiments match electrophysiological data from VTA neurons supporting the hypothesis that a reduction in the activity of dopamine neurons drives the escalation of drug intake, but also engage additional hypothalamic circuits and transmitter systems.
Between-systems b-process adaptations that lead to enhanced anhedonia after repeated drug use may involve CRH within the extended amygdala as well as the dynorphin–κ opioid system (George et al. 2012). CRH receptor antagonists block withdrawal-induced anxiety (Basso et al. 1999; Logrip et al. 2011), withdrawal-induced increases in nicotine and alcohol self-administration (George et al. 2007; Finn et al. 2007) and escalated cocaine and heroin self-administration during extended access tests (Specio et al. 2008; Greenwell et al. 2009). Further, blockade of κ receptors reduces negative hedonic aspects of drug withdrawal and decreases drug-seeking (Koob 2013; Koob et al. 2014; Chartoff et al. 2012). After extended drug intake, input from CRH and dynorphin systems into the striatum is strengthened (e.g., see Turchan et al. 1997), leading to a further increase in the anhedonic b-process. Ultimately, these changes in b-processes are mediated by between-systems adaptations in CRH that lead to a further masking of the a-processes mediated by within-systems adaptations in mesolimbic dopamine systems and the persistence of drug-taking behavior (Aston-Jones et al. 1999; Nestler 2001; Koob 2003; George et al. 2012).
The negative reinforcement process of the hedonic allostasis model can account for drug-taking during early and late phases of addiction (especially in the case of alcoholics who can experience shakes and life-threatening seizures if alcohol use is stopped abruptly) and addresses why drug users may continually increase the amount of drug used. While the physiological, flu-like symptoms of withdrawal for most commonly abused drugs (shakes, nausea, malaise) subside within two to three weeks after drug use is discontinued, the hedonic effects, including mood alterations that manifest as depression and anxiety, may last longer (Soto et al. 1985; Ashton 1991; Rothwell et al. 2012; Hughes 2007; Satel et al. 1993). This may explain why many recovering addicts relapse weeks to months after drug use has stopped and when physical withdrawal symptoms are absent. However, it is important to note that drug-taking behavior can occur even in the absence of subjective, hedonic effects (Fischman and Foltin 1992; Fischman 1989; Lamb et al. 1991; Robinson and Berridge 2001), suggesting that the reinforcing properties of drugs are not mediated by their hedonic impact alone (Robinson and Berridge 2001). In addition, drug-seeking can often be context specific (e.g., only occurring in particular places) and triggered by drug cues (like hearing the click of a lighter when trying to quit smoking). An increase in hedonic set point is intrinsic and thus should not be modified by drug cues and drug contexts (Crombag et al. 2000, 2001; Badiani 2013). Thus, the hedonic allostasis model does not explain cue-induced reinstatement, context-dependent self-administration behaviors, or craving reported by recovering addicts when faced with a drug cue (Fox et al. 2005; Witteman et al. 2015; Celentano et al. 2009; Bossert et al. 2013). Further, a decrease in the a-process would be expected to generalize to other types of rewards, because there is a partial overlap in the neural circuits underlying the responses to “natural” rewards, such as food and sex, and drugs. However, it is not clear whether addicts are impaired in their ability to experience pleasure from non-drug sources (DiLeone et al. 2012; Hone-Blanchet and Fecteau 2014; Ziauddeen and Fletcher 2013; Volkow et al. 2012). Thus, the idea that drug history produces a generalized, long-lasting anhedonia cannot explain all instances of addiction, and is unlikely to drive relapse after long-term abstinence.
4 Incentive Sensitization
The incentive–sensitization concept of addiction proposes that addiction is due to adaptations in brain regions within the mesocorticolimbic systems that mediate a specific aspect of “incentive motivation”: the process of “incentive–salience attribution” (Robinson and Berridge 1993, 2001; also see Robinson et al., this volume). Drug-induced neuroadaptations result in the sensitization of incentive–motivational processes such that drugs and drug-related stimuli acquire enhanced (sensitized) salience. Ultimately, it is sensitization of the incentive–motivational properties of drugs and drug-related stimuli that govern compulsive pursuit of drug.
Incentive motivation refers to a multi-component processes by which behavior is directed toward “incentives” such as food, a potential mate, drugs, and stimuli associated with these incentives through Pavlovian learning. During early drug use, the activation of neural systems that mediate pleasure triggers incentive–motivational processes that become progressively stronger across repeated drug use. An association between the act, event, or cues in the environment in which the pleasure occurs is then formed through Pavlovian learning processes. These Pavlovian cues can themselves gain motivational properties through the separate process of incentive–salience attribution. In this process, after the initial association has been formed, salience is then attributed to the mental representation of the reward/drug-associated cue. Incentive sensitization posits that the process of incentive–salience attribution occurs in a neural system that is separable from the system responsible for the pleasurable effects of the initial incentive (liking) and separable from the neural systems responsible for the initial Pavlovian associative learning processes. Thus, cues that have incentive salience are able to direct behavior, not because they are pleasurable or “liked,” but because they are highly noticeable, attractive, and “wanted.” Within this conceptual framework, addiction develops and persists due to drug-induced neuroadaptations in the brain systems that mediate incentive salience (wanting), making these systems hypersensitive (sensitized) to drugs and drug cues. Further, sensitization of brain circuits that mediate incentive salience, a component of incentive motivation, results in drug-seeking behavior. The sensitization within these neural circuits persists even after long periods without drug use, making addicts susceptible to relapse long after drug use has ceased. Furthermore, progressive alterations in incentive salience through associative learning mechanisms engaged by repeated drug-taking experiences enhance the overall incentive–motivational properties of drug cues leading to increasingly compulsive patterns of drug-seeking and drug-taking. Rodent studies have shown that “liking” and “wanting” processes can be dissociated neurobiologically (Berridge and Kringelbach 2015). This is discussed further by Robinson et al., in this volume.
Neurobiologically, incentive sensitization has traditionally been attributed to progressive enhancement (i.e., sensitization) of mesolimbic responses to drugs and drug cues (Berridge and Robinson 2003; Di Chiara et al. 1999; Wise 1987, 1988). Many studies have focused on the role of dopamine projections from the VTA to the NAcc in this process (Vanderschuren and Kalivas 2000; Flagel et al. 2008; Vezina 2004; Vezina and Leyton 2009), as well as the contribution of enhanced glutamatergic transmission particularly within the NAcc (Wolf and Ferrario 2010; Loweth et al. 2014; Kalivas and Nakamura 1999; Vanderschuren and Kalivas 2000; Cardinal et al. 2002). The focus on dopamine systems arose because many commonly abused drugs share the ability to enhance dopaminergic neurotransmission within the mesocorticolimbic system (Vanderschuren and Kalivas 2000; Vezina 2004; Wolf 2002) and to produce psychomotor sensitization (Babbini and Davis 1972; Joyce and Iversen 1979; Kita et al. 1992; Benwell and Balfour 1992; Littleton 1998; Nestby et al. 1997), an enduring hypersensitivity to the psy-chomotor activating effects of drugs that includes locomotion and stereotyped sniffing and rearing behaviors (Robinson and Becker 1986; Segal 1975).
The development of psychomotor sensitization is a progressive, incremental process, much like the development of addiction itself. Furthermore, psychomotor sensitization can persist for months to years after drug use has stopped (Paulson et al. 1991; Robinson and Becker 1986). Thus, incentive sensitization underlying addiction is thought to be mediated by sensitization-related adaptations in brain systems that mediate the incentive–salience and incentive–motivational properties of drug and drug cues.4 Consistent with this, drug pretreatments that produce psychomotor sensitization facilitate the acquisition of drug self-administration in the monkey, rat, and mouse (Woolverton et al. 1984; Horger et al. 1990; Lessov et al. 2001), increase motivation for drug assessed by breakpoint on a progressive ratio schedule (Mendrek et al. 1998; Lorrain et al. 2000; Deroche et al. 1999), and enhance the escalation of drug intake in extended access models (Ferrario and Robinson 2007). Thus, psychomotor sensitization is linked to enhanced motivation for drugs and with addiction-like behaviors (Ferrario et al. 2005; Morgan and Roberts 2004 though see also Ahmed and Koob 1998). The incentive–sensitization model additionally proposes that drug-induced increases in incentive salience contribute to aberrant motivation underlying addiction. In support of this, drug cues themselves can support conditioned approach behavior (Meyer et al. 2012b; Uslaner et al. 2006; Yager and Robinson 2013), and pretreatment with a sensitizing regimen of amphetamine enhances the subsequent acquisition of Pavlovian-conditioned approach behavior (Harmer and Phillips 1998).
Although VTA-NAcc circuitry is heavily involved in psychomotor and incentive sensitization, alterations in glutamatergic transmission within the NAcc mediated by both cortical and amygdalar inputs play important roles (Ma et al. 2014; MacAskill et al. 2014; Everitt and Wolf 2002). For example, cocaine self-administration increases glutamatergic transmission in the NAcc, drug exposure increases the number of excitatory inputs from the basolateral amygdala to the NAcc shell, and remodeling of glutamatergic synapses from the PFC to NAcc contributes to cue-triggered drug-seeking behavior (MacAskill et al. 2014; Wolf 2003; Cornish et al. 1999; Wolf and Ferrario 2010; Robinson and Kolb 2004; Ma et al. 2014). Furthermore, the central nucleus of the amygdala (CeN) is necessary for Pavlovian-conditioned approach to reward predictive incentive stimuli (Everitt et al. 2003), and activation of dopaminergic input into the CeN is sufficient to elicit approach to drug cues (Cardinal et al. 2002).
The induction and expression of psychomotor sensitization is modulated by learning and the circumstances surrounding drug administration (Robinson et al. 1998). This concept is particularly important to the incentive–sensitization model of addiction because it posits that associative learning processes focus “wanting” onto drug cues enabling the expression of sensitization-related neuroadaptations to be influenced by factors that contribute to associative learning (Robinson and Berridge 1993). For example, even when neural sensitization is induced by repeated drug exposure, the expression of sensitization can be context specific. That is, if the drug is given in an environment that is different from where initial drug treatments were received, the sensitized response is not evoked (Post et al. 1981; Anagnostaras and Robinson 1996; Terelli and Terry 1999). However, these same animals will express a sensitized response when given drug in the initial treatment environment (Anagnostaras and Robinson 1996; Post et al. 1981). Thus, although neural sensitization occurred, the behavioral expression of sensitization is context dependent, i.e., it is influenced by conditioned stimuli paired with drug administration (Anagnostaras and Robinson 1996). Conversely, animals placed in a drug-paired environment will show conditioned locomotor hyperactivity in the absence of any drug (see Stewart 1992 for review). Enhanced incentive salience of drug cues and increased incentive–motivational power of these cues in rodent models are consistent with behavioral studies in human drug addicts, as well as self-reports of enhanced cue-triggered craving during abstinence (Childress et al. 1993; Gawin and Kleber 1986; for review, see Veilleux and Skinner 2015). In addition, incentive sensitization accounts for the context dependence of drug craving. More recent studies of incentive sensitization have focused on individual differences in the attribution of incentive salience in order to address why some individuals are able to maintain controlled drug use, while others develop addiction (Robinson et al. 2014; Meyer et al. 2012a).
In summary, at the heart of incentive sensitization is the concept that drugs produce neuroadaptations in systems that mediate the attribution of incentive salience to drug cues. Through these sensitization-related processes, Pavlovian cues associated with drug-taking become increasingly able to direct and focus behaviors, inducing cue-triggered motivational states that drive pathological drug “wanting” and addiction. Several other concepts of addiction presented below incorporate the idea that drug cues influence drug-seeking behaviors. However, they differ from incentive sensitization in that not all concepts include the induction of a motivational state by Pavlovian cues.
5 Addiction as a Disorder of Learning and Decision Making
In the above sections, we discussed ideas about addiction that focused on hedonic responses to drug- and emotion-driven states, versus conditioned incentive–motivational states elicited by drug cues. Addiction can also be described in the context of aberrant decision-making processes in which the drug becomes irresistible and leads to persistent drug-taking even in the face of negative consequences. Decision-making conceptualizations of motivated behavior are based on the idea that there is some value, either real or expected, to the outcome of a particular action. These actions have potential costs and rewards, and individuals vary in their ability to estimate these costs and rewards, just as they differ in how much they value these costs and rewards in the first place. Thus, making a decision to engage in some action depends on the individual’s ability to weigh out the costs, benefits, and consequences of their actions. There are at least two strategies in forming this appreciation. In the forward-search strategy, the outcome of each possible action is independently considered every time the person makes a decision. In caching, the person learns essentially through trial and error that a particular action in a given situation is likely to produce a certain outcome; then, the appropriate action is chosen when that particular situation is encountered. Thus, the forward-search strategy involves planning, is flexible, and is sensitive to changes in the action–outcome relationship, while the cache strategy is inflexible, is fast, and leads to habitual responding (Redish et al. 2008; also see Redish et al., in this volume). In many instances, these strategies occur sequentially; the initial phase of motivated decision making involves forward-search planning and evolves later into a caching strategy. These forward-search and caching strategies are analogous to reflective (goal-directed) control and reflexive (habitual) control of decision-making processes (Balleine and Dickinson 1998; Dolan and Dayan 2013; Doll et al. 2012).
Many studies of drug addiction consider the transition from forward-search goal-directed behavior to caching or habitual behaviors a hallmark of addiction (Corbit et al. 2012; Zapata et al. 2010; Everitt and Robbins 2015; Redish et al. 2008). Below, we describe general processes involved in goal-directed and habit-based responding, and how alterations in these processes may contribute to addiction. The main distinction between these two ideas is that for goal-directed behavior, the outcome (i.e., the subjective or physiological drug experience) maintains addiction, whereas for habit-based responding, the outcome itself is no longer relevant; drug-taking behavior is instead maintained by automated processes elicited by drug cues, stress, etc.
5.1 Goal- Versus Habit-Based Responding
During initial drug-taking experiences, an individual learns that an action (drug-seeking) produces some outcome (drug experience). This action–outcome relationship, or A-O relationship, can be dependent on the presence of environmental stimuli (or, as the notation goes, S:A-O). In other words, the individual learns that drug-seeking actions produce drug-taking outcomes only in the presence of certain stimuli such as drug cues. As addiction develops, the drug-taking outcome, either actual or expected, becomes overvalued. This overvaluation can be a result of drug withdrawal (Hutcheson et al. 2001), or an inflated expectation of the euphoric effects of the drug, even when the euphoric event is actually decreased in magnitude (Kennett et al. 2013). Overvaluation of the drug-taking outcome (O) can lead to pathological drug-seeking (A), especially when triggered by drug cues (S). In this manner, goal-directed responding can lead to addiction when the expected drug outcome is improperly overvalued such that behaviors directed toward obtaining drug override all others.
In contrast, habit-based ideas of addiction suggest that a drug-seeking action is an automated response (R) elicited by a drug cue (S). This stimulus–response, or S-R, learning process leads to habitual drug-seeking and drug-taking, despite changes in the outcome of the drug-seeking action. Further, habitual, S-R drug-taking behaviors are automatically elicited in the absence of craving or urges, and occur independently of antecedent motivational states or the actual outcome of drug use itself (Hogarth et al. 2013; Belin et al. 2013; Ostlund and Balleine 2008; Everitt et al. 2001; Tiffany 1990). As frequency of use increases, more automated and routine actions are prompted that can further perpetuate drug-taking. These automatic drug-taking behaviors are initiated by drug cues and have been conceptualized as “incentive habits,” or habit processes prompted by incentive stimuli (Pavlovian cues) that have undergone incentive sensitization (Belin et al. 2013). Importantly, incentive habits differ from incentive motivation because cue-driven S-R responses occur in the absence of an explicit motivational state, whereas Pavlovian stimuli with incentive motivational properties induce motivational states that produce drug-taking.
S-R habits are further characterized by the blunting of executive function such that conscious decision making (i.e., ability to weigh costs, benefits, and potential consequences) becomes increasingly compromised. A decline in function of executive control processes further enhances habit-based behaviors and reduces outcome-based decision-making processes (Jentsch and Taylor 1999). These features of habitual use develop over prolonged drug use, further drive drug-taking behavior, and likely occur in parallel with the other features of addiction. Therefore, through learning routine drug-taking patterns, automatic drug-use behaviors crystallize, becoming highly efficient and repetitive behaviors that are resilient to intervention.
In humans, many aspects of drug-taking behavior can be categorized as habitual, such as the tapping and packing of a pack of cigarettes, or the preparation of a needle, which can occur in an automated fashion analogous to driving the same route to work each day. The automation of complex behaviors can be adaptive, but these processes can also be usurped by drugs. Thus, habitual behaviors in the addicted state are aberrant not because these behaviors have become automated, but because these behaviors are fixed in a habitual state and do not move back into a goal-directed system (Everitt and Robbins 2005; Dickinson et al. 2002; Miles et al. 2003; Robbins and Everitt 1999). However, some aspects of human addiction do not fit as well with habit-based ideas of drug-taking. For example, addicts often engage in novel and even creative behaviors in order to maintain their drug use (e.g., a prescription opioid pill abuser that switches from oral administration to intravenous street drug use). This transition requires an entirely different set of actions that were never previously used to obtain drug and thus do not fit well with an automated, habitual behavioral response.
Another layer can be added to these concepts of goal-directed and habitual behaviors; that is the ability of Pavlovian-conditioned motivational states5 (as described above in the Incentive Sensitization section) to invoke the retrieval of specific outcomes, such as drug-specific effects. Thus, while addiction can transition from A-O to S-R, addiction may also be marked by a transition from the ability of Pavlovian stimuli to elicit the representations of specific drug outcomes to elicit general motivational states (Hogarth 2012; Hogarth et al. 2013). As discussed next, these instrumental and Pavlovian processes are subserved by different neurobio-logical systems and thus may interact to produce craving and drug addiction.
5.2 Neural Circuits Governing Goal- and Habit-Based Decision Making
Studies of food reinforcement show that prefrontal cortical inputs to the dorso-medial section of the striatum are critical for the formation of associations between actions and outcomes (Shiflett and Balleine 2011; Balleine 2005). The neuro-chemical basis for goal-directed responding may be related to dopamine signals, where drug-induced dopamine release maladaptively increases the value of the outcome (i.e., drug intake; Redish 2004; Naude et al. 2014; Belin et al. 2009). Because the likelihood of engaging in a drug-seeking action is a function of the perceived value of the outcome (O), this “hijacking” of the dopamine system would increase drug-seeking progressively. A change in the valuation of an outcome as a result of drug exposure involves alterations in the amygdala and hippocampus (Wassum et al. 2011; Mahler and Berridge 2012), and the retrieval of this now altered outcome likely involves a circuit connecting areas of the prefrontal cortex (including the orbitofrontal cortex) and the NAcc (Shiflett and Balleine 2010; Jentsch and Taylor 1999; Puumala and Sirvio 1998; Simon et al. 2013; Winstanley et al. 2004; Johnson et al. 2007; Schoenbaum et al. 2006; Kalivas and Volkow 2005).
As described above, initial drug-seeking responses are mediated by dopamine transmission in the ventral striatum. However, as goal-directed drug-taking transitions to habitual drug-taking, prolonged activation of dopamine neurotransmission triggers a transition to behavior mediated by the dorsolateral striatum (Balleine et al. 2014; Yin et al. 2006). This transition from ventral to dorsal striatal activation reduces goal-directed behaviors and supports habit learning such that behavior is driven by drug cues, but no longer requires a representation of or motivational state driven by the outcome (Corbit et al. 2012; Vanderschuren et al. 2005; Willuhn et al. 2012). Dorsal and ventral regions of the striatum are connected through a complex “spiraling” or “looping” of reciprocal connections to the VTA and substantia nigra that allow one striatal subregion to regulate dopaminergic tone in adjacent regions. For example, inputs from the NAcc shell to the VTA can inhibit dopamine release within the NAcc core (Belin and Everitt 2008). Similarly, the NAcc core regulates dopaminergic tone within the dorsal striatum through the substantia nigra. The dorsal striatum is divided into multiple domains as well, and dopaminergic input is regulated through reciprocal nigrostriatal projections that are organized anatomically from ventral to dorsal. Each domain regulates the dopaminergic tone of the adjacent domain and, through their spatial location, produces a “spiral” of reciprocal connections that can regulate dopaminergic tone throughout the striatum. This anatomical arrangement allows for the transition from goal-directed behaviors mediated by the NAcc to habit-based behaviors mediated by the dorsal striatum. Specifically, initial exposure to a drug would induce dopaminergic responses and neuroadaptations in the ventral striatum, but because of the spiraling reciprocal connections, these responses and neuroadaptations would engage and alter dorsal striatal projections during extended drug-taking (e.g., Porrino et al. 2004; Letchworth et al. 2001; Ito et al. 2000, 2002; Belin and Everitt 2008). Consistent with the idea of a transition between goal-directed and habit-based processes, an intact connection between NAcc core and the dorsal striatum is required to elicit habit behaviors (Belin and Everitt 2008). The involvement of the dorsal striatum in habit learning is also dynamic throughout prolonged drug use, with activity shifting from the dorsal–medial striatum (DMS) to the dorsal–lateral striatum (DLS) in driving habit-based, stimulus–response behaviors (Murray et al. 2012). Consistent with this, inactivation of the DLS after prolonged cocaine exposure renders rats sensitive to changes in goal value and hence restores goal-directed behavior while attenuating automated stimulus–response processes (Zapata et al. 2010). Thus, reciprocal connections between these circuits allow for transitions between habitual and goal-directed behaviors as needed, whereas drug-induced alterations within these circuits perturb this flexibility. This is further compounded by drug-induced cortical dysregulation that compromises executive function and planning.
5.3 Pavlovian Cues: Reward Prediction, Incentive Salience, and Transfer to Instrumental Responding
Pavlovian cues play an important role in these processes by triggering sequences of actions that can be goal-directed and habitual, or by inducing a conditioned motivational state (incentive motivation). The ability of cues to guide goal-directed behavior likely involves the dopamine system as well, in which changes in dopa-mine neuron activity signal prediction error—outcomes that have a perceived value that was either greater or less than expected based on the presence of reward-predicting cues (Schultz et al. 1997). Therefore, increases in dopamine neuron activity, and the resultant increases in dopamine (e.g., Hart et al. 2014), would lead to more rapid associations between drugs and their cues. However, dopamine is also involved in the attribution of incentive salience to drug cues (Flagel et al. 2011; Saddoris et al. 2015). In other words, drug-associated cues acquire incentive salience as a consequence of drug-induced increases in dopamine. The incentive motivational state induced by these cues can then transfer to instrumental drug-taking, thereby increasing the drug-seeking and drug-taking behavior. These Pavlovian transfer effects during food self-administration are dependent on the orbitofrontal cortex (Ostlund and Balleine 2007a, b), while the devaluation of an instrumental outcome during A-O learning is dependent on the prelimbic cortex (Corbit and Balleine 2003). However, whether Pavlovian transfer effects during drug-taking also involve these brain areas is not known.
6 Impulsive Action and Impaired Executive Function
Layered on top of the concept of enhanced goal-directed behavior described above is the contribution of impulsivity and impulsive action. One consequence of the overvaluation of a drug is impulsive responding in which individuals (1) value immediate drug delivery over more adaptive, but long-term, outcomes and/or (2) value less probable drug availability over more certain, alternative outcomes. These patterns of responding could be due to the overvaluation of the drug itself and/or to altered discounting of the temporal delay between the action and receipt of the outcome, the probability of drug availability, or the negative consequences of drug-taking. This aberrant outcome valuation, in the form of impulsivity, may explain the correlation between impulsive behavior and drug-taking (Moeller and Dougherty 2002; de Wit and Richards 2004; Perry and Carroll 2008). However, impulsivity may also reflect a loss of inhibitory control over drug urges, including those elicited by drug cues (Jentsch and Taylor 1999; Belin et al. 2013). In this case, in contrast to overvaluing drug outcomes, individuals are simply unable to make choices based on long-term outcomes (Bechara 2001, 2005).
The crystallization of habit appears to be further exacerbated by cortical dys-regulation, particularly in the prefrontal areas involved in planning (Volkow et al. 2003). Under normal circumstances, habitual behaviors can be inhibited by cortical systems. However, repeated drug use also produces neural alterations in prefrontal cortical systems that exacerbate habitual behaviors. These adaptations in human addicts occur within the orbitofrontal cortex and anterior cingulate cortex (Lubman et al. 2004; van Huijstee and Mansvelder 2014). In addition, dysregulations in these regions are implicated in other behavioral control disorders involving impulsivity, evaluation and planning, and inhibitory control (Verdejo-Garcia et al. 2006, 2007; Sakagami et al. 2006). Dampened cortical activation and reduced executive function after repeated drug exposure may explain the addict’s failure to control habitual behaviors as well as the inflexibility in response to changing outcomes (Jentsch and Taylor 1999). Together, the loss of prefrontal inhibitory control and consolidation of dopaminergic signaling in the dorsal striatum are thought to perpetuate responses to drug cues by bypassing goal-directed behaviors entirely, and prompting drug use in a stimulus-dependent manner. These changes may also explain the ineffectiveness of cognitive control approaches to reducing drug-taking behaviors.
7 Models that Integrate Motivational Concepts
7.1 Dual-Process Models
Dual-process models of drug addiction describe drug-taking behavior as the output of two competing forces: an automatic, impulse-generating system that is approach-oriented and generates drug-taking behavior, and a controlled, reflective system that uses cognitive control to regulate the impulse-generating system. For example, in the case of alcoholism, Wiers et al. (2007) argues that alcohol-use disorders occur from an imbalance between automatic and controlled processes. In adolescence, where much of the evidence for this model is derived, this controlled process is weak—as any parent can attest. Upon repeated alcohol exposure, the appetitive/approach system is sensitized, and recreational users are attracted to and influenced by drug-associated cues (Duka and Townshend 2004; Field et al. 2005). In contrast, the controlled process degrades as a result of chronic alcohol use (White et al. 2000). Thus, adolescents with already weak control systems are particularly prone to the further sensitization and weakening of approach and control systems, respectively.
While these ideas may seem analogous to the habit-based and goal-directed response systems described above, they should not be conflated. Instead, incentive motivation, action–outcome systems, and habit-based systems together produce drug-seeking behaviors. However, because each process can be localized to some extent to different neural circuits, they can be independently modified by drug exposure. Specifically, cue-triggered behaviors rely heavily on the ventral and dorsal striatum, as well as prelimbic and orbitofrontal cortices (Daw et al. 2011; de Wit et al. 2009, 2012). These systems are in turn under negative control of a regulatory gating system mediated by cortical regions. This cortical regulatory system can then influence whether forward-search or cached response patterns (discussed earlier in this section) are employed. While such an arbiter of response strategy selection has not been identified, it may involve the infralimbic and dor-solateral components of the prefrontal cortex, as manipulations of these regions can induce switches from one strategy to the other (McClure and Bickel 2014; Jentsch and Taylor 1999; Coutureau and Killcross 2003; Killcross and Coutureau 2003; Daw et al. 2005).
7.2 Transitions from Drug-Taking to Addiction
Given the neural overlap and interactions between the different motivational concepts described above, it seems likely that these concepts are explaining different aspects or stages of addiction (Piazza and Deroche-Gamonet 2013). For example, several have suggested that transition into addiction is characterized by a slow yet categorical change in the motivational processes underlying drug-taking. Koob and colleagues have argued that addiction transitions from positive reinforcement, to incentive sensitization, to allostasis (Koob et al. 2014). Goldstein and Volkow (2002) propose a model, based on neuroimaging data, where the intoxicating effects of drugs initially involve the frontal cortex, particularly the anterior cingulate and prefrontal cortices. As addiction develops, the degree of incentive motivation attributed to drugs (as measured by drug craving) is paralleled by activity in the orbitofrontal and anterior cingulate cortices.
7.3 Drug Addiction: A Compendium of Vulnerabilities
Redish (2004) and Redish et al. (2008) have utilized the goal-directed and habit distinction to describe how “vulnerabilities” in these decision-making processes can lead to addiction (Redish et al. 2008). Redish describes 10 such vulnerabilities or “failure points” in which the decision-making process can become maladaptive. These failure points can be grouped into general categories, including negative reinforcement, positive reinforcement, learning processes (including reward prediction and action–outcome learning), response inhibition and discounting, and habit. However, it should be mentioned here that even though we have grouped these vulnerabilities together for discussion purposes, they are to an extent neurobiologically dissociable and thus represent separate psychological processes.
Each of these failure points can lead to addiction. For example, a person may form incorrect action–outcome relationships, or improperly categorize situations in which action–outcome relationships occur. They would thus develop an “illusion of control” in which they view the success in achieving a positive outcome as result of their own actions, or because of changing situations, even if this is not the case. In this case, they would be less likely to select alternatives to drug-taking. For habit-based system vulnerabilities, behavior becomes inflexible, and insensitive to changes in the consequences of behavioral actions. While a non-addict may be able to switch from habitual to goal-directed responding without difficulty, an addict that suffers from improper response inhibition vulnerability will have difficulty doing so.
These vulnerabilities can be applied to positive and negative reinforcement as well. For negative reinforcement, the increase in “need” that happens as a result of withdrawal increases the probability that a person will take drugs to satisfy the need, and the allostatic load that accumulates (strengthened opponent process) is a result of the neuroadaptive response to repeated drug exposure. For positive reinforcement, the euphoric effect of drugs creates an intense reward signal that is stored in memory and becomes a goal to be sought after. Stimuli that induce recall of this euphoric event induce craving. Action–outcome vulnerabilities are also related to the reward signal. For example, incentive sensitization leads to a sought-after event, but the expected reward is much greater than the actual reward, and thus the distinction between “wanting” and “liking” arises, as discussed earlier. Impulsivity, then, is a similar improper valuation of future events in favor of choices that result in immediate outcomes. In this sense, Redish’s conceptualization is unique in that there is no single “culprit,” such as the habit system, that is responsible for the transition into addiction (Ahmed 2008; see chapters by Redish et al., and Cornwell et al., in this volume for further discussions of multiple vulnerabilities in motivational systems). Each of the predominant models of addiction explains one or more, but not all, of vulnerabilities to addiction. The advantage of describing multiple vulnerabilities is that it acknowledges that individuals likely become addicts for different reasons and that the biological underpinnings of drug-taking may depend on which vulnerability is being measured. Further, different drugs may be influenced by these vulnerabilities to different degrees. For example, opioid drugs may exploit the opponent process and positive reinforcement (reward-mimicking) vulnerabilities more than psychostimulant drugs, which may act more on the incentive salience and habit vulnerabilities. Nicotine, which facilitates learning by enhancing the reinforcing properties of cues (Caggiula et al. 2009), may drive drug-taking and relapse by enhancing initial learning about these cues.
8 Conclusion
There has been a great deal of focus on how personality traits or other pre-existing factors can influence drug-taking behavior, including sensation-seeking, impulsivity, and incentive–salience attribution (Flagel et al. 2009; Bickel et al. 2012; Bardo et al. 1996; Everitt 2014). These studies demonstrate that the predisposition to take drugs is not due to a single factor. Similarly, in this chapter, we have summarized some basic motivational concepts underlying drug-taking and how these processes are dysregulated as addiction develops. Behavioral neuroscience views largely ignore, or even avoid, the heterogeneity of motivational processes in addiction and are limited by studies in which only a single concept is tested. This point is illustrated in part by Fig. 2, in which we graphed the collaboration network of the references cited in this chapter. Each researcher cited is a node, and two researchers are connected if they have coauthored a paper together. A few prominent groups emerge that correspond to the main concepts discussed in the chapter. What is apparent is that there are very few connections between these groups and only a few researchers bridge these concepts. Of course, this is not a complete representation of the entire addiction field. However, the absence of cross-concept collaborations and integration is a major impediment to progress. Human studies strongly support the idea that different addicts take different drugs for different reasons and that these reasons may change throughout the course of the addict’s lifetime. Thus, ideas including hedonic allostasis, incentive sensitization, decision making and learning, impulsivity, and dual-process theory are applicable in some but not all situations. For example, much of what is known about negative reinforcement and allostasis comes from studies on opiates and alcohol, while incentive sensitization stems primarily from studies of psychostimulants (though see Robinson and Berridge 2000). Therefore, it is perhaps not surprising that none of these theories explain all aspects of addiction. Furthermore, it is likely that the transition into addiction is not driven by a single process, and people may transition to compulsive drug-taking for different reasons, both behavioral and neural. We believe that a unified framework of addiction is not a red herring. Instead, a framework that is flexible and adaptable to different drug classes, personality types, and environmental situations will be enormously useful in the development of specialized treatment for the wide variety of addicted individuals in the population.
Fig. 2.
Collaboration network of the references cited in this chapter. Each researcher cited is a node, and two researchers are connected if they have coauthored a paper together
Acknowledgments
The authors thank MEJ Newman for making the collaboration network depicted in Fig. 2.
Footnotes
Note that “substance use disorder” in humans is diagnosed on a continuum from mild to severe based on 11 behavioral criteria laid out in the DSM V, which does not mention “addiction.” This choice is in part intended to help identify and treat overuse of drugs (e.g., alcohol and nicotine which can cause or worsen a number of other conditions) and also to avoid social stigmas associated with the term “addiction,” which may result from the lack of clear biological markers that identify the addicted state. We avoid this symptomatological approach to describing addiction in favor of focusing on the core motivational processes involved in drug-taking, in a manner analogous to the National Institute of Mental Health’s Research Domain Criteria (RDoC) initiative (Cuthbert 2014; Litten et al. 2015; NIMH 2015).
These models also disagree regarding the relationship between dopamine and hedonia, whereas an altered hedonic set point is proposed to be mediated in part by changes in dopamine; the “liking” reaction to rewards is dopamine independent (Berridge and Robinson 1998; Koob and Le Moal 2008). This discrepancy may lie in the definition of hedonia, which may be referred to generally as affect or pleasure, or operationalized into a specific subjective experience or behavioral measure.
Note that this is a deviation from McEwen’s definition of allostasis, which states that allostasis consists of the adaptive physiological changes that are evoked in order to return a system to homeostasis. Thus, this Koob’s allostatic state is better referred to as allostatic load, which McEwen defines as the long-term cost of allostasis that accumulates over time (McEwen 1998).
Note that neuroadaptations accompanying psychomotor sensitization are thought to overlap with the neuroadaptations underlying the incentive sensitization that drives drug-seeking and drug-taking behavior. However, psychomotor sensitization and incentive sensitization are neuro-biologically dissociable processes that mediate different aspects of behavior. Thus, the presence of psychomotor sensitization is indicative of changes underlying incentive sensitization, but they are not one and the same.
The notation for action–outcome (A-O) and stimulus–response (S-R) responding can be confusing because the drug-seeking action is denoted as either A or R in each of these conceptualizations. Indeed, notations such as (action–outcome), (action–outcome in the presence of environmental stimuli), and (stimulus–response) are sometimes used to avoid this confusion (Redish et al. 2008). The notation for the activation of a conditioned motivational state by Pavlovian cues is , where O is the conditioned motivational or affective state elicited by drug stimuli S and a is the behavioral response elicited by this state. However, we avoid this notation because O has quite different meanings depending on whether it is the outcome of an action (as in A-O) or the motivational state elicited by a Pavlovian stimulus (as in is ).
Contributor Information
Paul J. Meyer, Email: pmeyer@buffalo.edu, Behavioral Neuroscience Program, Department of Psychology, University at Buffalo, Park Hall B72, Buffalo, NY 14260, USA
Christopher P. King, Behavioral Neuroscience Program, Department of Psychology, University at Buffalo, Park Hall B72, Buffalo, NY 14260, USA
Carrie R. Ferrario, Email: ferrario@umich.edu, Department of Pharmacology, University of Michigan Medical School, A220B MSRB III, 1150 West Medical Center Drive, Ann Arbor, MI 48109-5632, USA
References
- Ahmed SH. The origin of addictions by means of unnatural decision. Behav Brain Sci. 2008;31(4):437–438. [Google Scholar]
- Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35(2):172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5(7):625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- American Psychiatric Association, editor. Diagnostic and statistical manual of mental disorders: DSM-5. 2013. [Google Scholar]
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor activating stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110(6):1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Ashton H. Protracted withdrawal syndromes from benzodiazepines. J Subst Abuse Treat. 1991;8(1–2):19–28. doi: 10.1016/0740-5472(91)90023-4. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Babbini M, Davis WM. Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. Br J Pharmacol. 1972;46(2):213–224. doi: 10.1111/j.1476-5381.1972.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A. Substance-specific environmental influences on drug use and drug preference in animals and humans. Curr Opin Neurobiol. 2013;23(4):588–596. doi: 10.1016/j.conb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Manley SJ, Watson WP, Wonnacott S, Molleman A, Little HJ. Chronic ethanol administration alters activity in ventral tegmental area neurons after cessation of withdrawal hyperexcitability. Brain Res. 1998;803(1–2):144–152. doi: 10.1016/s0006-8993(98)00654-4. [DOI] [PubMed] [Google Scholar]
- Balleine BW. Neural bases of food-seeking: affect, arousal and reward in corticostriatolim-bic circuits. Physiol Behav. 2005;86(5):717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37(4–5):407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Morris RW, Leung BK. Thalamocortical integration of instrumental learning and performance and their disintegration in addiction. Brain Res. 2014 doi: 10.1016/j.brainres.2014.12.023. http://dx.doi.org/10.1016/j.brainres.2014.12.023. [DOI] [PubMed]
- Barak S, Wang J, Ahmadiantehrani S, Ben Hamida S, Kells AP, Forsayeth J, Bankiewicz KS, Ron D. Glial cell line-derived neurotrophic factor (GDNF) is an endogenous protector in the mesolimbic system against excessive alcohol consumption and relapse. Addict Biol. 2014;20:629. doi: 10.1111/adb.12152. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77(1–2):23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Koob GF, Rivier J, Vale W. Corticotropin-releasing factor antagonist attenuates the ‘anxiogenic-like’ effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology. 1999;145(1):21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Bechara A. Neurobiology of decision-making: risk and reward. Semin Clin Neuropsychiatry. 2001;6(3):205–216. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, Everitt BJ. Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol. 2013;23(4):564–572. doi: 10.1016/j.conb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199(1):89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105(4):849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Graaf YG-D, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316(3):314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Pleasure systems in the Brain. Neuron. 2015;86(3):646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134(3):287–297. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229(3):453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23(11):1848–1852. [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338(2):255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Bucholz KK. Nosology and epidemiology of addictive disorders and their comorbidity. Psychiatr Clin N Am. 1999;22(2):221–240. doi: 10.1016/s0193-953x(05)70073-3. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Celentano M, Caprioli D, Dipasquale P, Cardillo V, Nencini P, Gaetani S, Badiani A. Drug context differently regulates cocaine versus heroin self-administration and cocaine-versus heroin-induced Fos mRNA expression in the rat. Psychopharmacology. 2009;204(2):349–360. doi: 10.1007/s00213-009-1467-x. [DOI] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62(1):167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64(5):566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Instrumental and Pavlovian incentive processes have dissociable effects on components of a heterogeneous instrumental chain. J Exp Psychol Anim Behav Process. 2003;29(2):99–106. doi: 10.1037/0097-7403.29.2.99. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry. 2012;72(5):389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93(4):1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Coutureau E, Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav Brain Res. 2003;146(1–2):167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Chan J, Dell’Orco J, Dineen SP, Robinson TE. The ability of environmental context to facilitate psychomotor sensitization to amphetamine can be dissociated from its effect on acute drug responsiveness and on conditioned responding. Neuropsychopharmacology. 2001;24(6):680–690. doi: 10.1016/S0893-133X(00)00238-4. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116(1):1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69(6):1204–1215. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8(12):1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Sym Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- de Wit S, Corlett PR, Aitken MR, Dickinson A, Fletcher PC. Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. J Neurosci. 2009;29(36):11330–11338. doi: 10.1523/JNEUROSCI.1639-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit S, Watson P, Harsay HA, Cohen MX, van de Vijver I, Ridderinkhof KR. Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci. 2012;32(35):12066–12075. doi: 10.1523/JNEUROSCI.1088-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305(5686):1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11(8):2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999;46(12):1624–1633. doi: 10.1016/s0006-3223(99)00236-x. [DOI] [PubMed] [Google Scholar]
- Diana M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry. 2011;2:64. doi: 10.3389/fpsyt.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysio-logical and biochemical evidence. Proc Natl Acad Sci. 1993;90(17):7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Wood N, Smith JW. Alcohol seeking by rats: action or habit? Q J Exp Psychol Sect B. 2002;55(4):331. doi: 10.1080/0272499024400016. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci. 2012;15(10):1330–1335. doi: 10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Dayan P. Goals and habits in the brain. Neuron. 2013;80(2):312–325. doi: 10.1016/j.neuron.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Simon DA, Daw ND. The ubiquity of model-based reinforcement learning. Curr Opin Neurobiol. 2012;22(6):1075–1081. doi: 10.1016/j.conb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology. 2004;176(3–4):353–361. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- Edmonds DE, Gallistel CR. Parametric analysis of brain stimulation reward in the rat: III. Effect of performance variables on the reward summation function. J Comp Physiol Psychol. 1974;87(5):876–883. doi: 10.1037/h0037217. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories—indications for novel treatments of addiction. Eur J Neurosci. 2014;40:2163. doi: 10.1111/ejn.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36(2–3):129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2015;67:8.1–8.28. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22(9):3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25(11):2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58(9):751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Robinson TE. Amphetamine pretreatment accelerates the subsequent escalation of cocaine self-administration behavior. Eur Neuropsychopharmacol. 2007;17(5):352–357. doi: 10.1016/j.euroneuro.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Craving and cognitive biases for alcohol cues in social drinkers. Alcohol Alcohol. 2005;40(6):504–510. doi: 10.1093/alcalc/agh213. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31(6):939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Fischman MW. Relationship between self-reported drug effects and their reinforcing effects: studies with stimulant drugs. NIDA Res Monogr. 1989;92:211–230. [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Self-administration of cocaine by humans: a laboratory perspective. In: Bock GR, Whelan J, editors. Cocaine: scientific and social dimensions. Vol. 166. CIBA Foundation Symposium; 1992. pp. 165–180. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186(1):48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30(9):880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Gass JC, Motschman CA, Tiffany ST. The relationship between craving and tobacco use behavior in laboratory studies: a meta-analysis. Psychol Addict Behav. 2014;28(4):1162–1176. doi: 10.1037/a0036879. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43(2):107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF. CRF–CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci. 2007;104(43):17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiol Behav. 2012;106(1):58–64. doi: 10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103(2):121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long-but not short-access rats. Addict Biol. 2009;14(2):130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behav Pharmacol. 1998;9(4):299–308. [PubMed] [Google Scholar]
- Hart AS, Rutledge RB, Glimcher PW, Phillips PE. Phasic dopamine release in the rat nucleus accumbens symmetrically encodes a reward prediction error term. J Neurosci. 2014;34(3):698–704. doi: 10.1523/JNEUROSCI.2489-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L. Goal-directed and transfer-cue-elicited drug-seeking are dissociated by pharmacotherapy: Evidence for independent additive controllers. J Exp Psychol Anim Behav Process. 2012;38(3):266–278. doi: 10.1037/a0028914. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Balleine BW, Corbit LH, Killcross S. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann N Y Acad Sci. 2013;1282:12–24. doi: 10.1111/j.1749-6632.2012.06768.x. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Karel P, Verheij MM. Individual differences in cocaine addiction: maladaptive behavioural traits. Addict Biol. 2014;19(4):517–528. doi: 10.1111/adb.12036. [DOI] [PubMed] [Google Scholar]
- Hone-Blanchet A, Fecteau S. Overlap of food addiction and substance use disorders definitions: analysis of animal and human studies. Neuropharmacology. 2014;85:81–90. doi: 10.1016/j.neuropharm.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Horger BA, Shelton K, Schenk S. Preexposure sensitizes rats to the rewarding effects of cocaine. Pharmacol Biochem Behav. 1990;37(4):707–711. doi: 10.1016/0091-3057(90)90552-s. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9(3):315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90(4):385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 2001;4(9):943–947. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20(19):7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22(14):6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JH. Drug addiction and drug abuse. In: Goodman LS, Gilman A, editors. The pharmacological basis of therapeutics. MacMillan; New York: 1975. pp. 284–324. [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146(4):373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johnson A, van der Meer MA, Redish AD. Integrating hippocampus and striatum in decision-making. Curr Opin Neurobiol. 2007;17(6):692–697. doi: 10.1016/j.conb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce EM, Iversen SD. The effect of morphine applied locally to mesencephalic dopamine cell bodies on spontaneous motor activity in the rat. Neurosci Lett. 1979;14(2–3):207–212. doi: 10.1016/0304-3940(79)96149-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9(2):223–227. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86(5):773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci. 2013;33(18):7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett J, Matthews S, Snoek A. Pleasure and addiction. Front Syst Neurosci. 2013;4:117. doi: 10.3389/fpsyt.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26(22):5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31(6):1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Polis I, Koob GF, Markou A. Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur J Neurosci. 2003;17(1):191–195. doi: 10.1046/j.1460-9568.2003.02443.x. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry. 1997;4(5):231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13(4):400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Kita T, Okamoto M, Nakashima T. Nicotine-induced sensitization to ambulatory stimulant effect produced by daily administration into the ventral tegmental area and the nucleus accumbens in rats. Life Sci. 1992;50(8):583–590. doi: 10.1016/0024-3205(92)90370-5. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, DeWitte P, Kalivas PW. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J Neurosci. 1992;12(7):2623–2632. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27(2):232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, Jr, George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9(3):482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, Henningfield JE, Goldberg SR. The reinforcing and subjective effects of morphine in post-addicts: a dose-response study. J Pharmacol Exp Ther. 1991;259(3):1165–1173. [PubMed] [Google Scholar]
- Lesscher HM, Vanderschuren LJ. Compulsive drug use and its neural substrates. Rev Neurosci. 2012;23(5–6):731–745. doi: 10.1515/revneuro-2012-0066. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Palmer AA, Quick EA, Phillips TJ. Voluntary ethanol drinking in C57BL/6 J and DBA/2 J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacology. 2001;155(1):91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21(8):2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 2015;39(4):579–584. doi: 10.1111/acer.12669. [DOI] [PubMed] [Google Scholar]
- Littleton J. Neurochemical mechanisms underlying alcohol withdrawal. Alcohol Health Res World. 1998;22(1):13–24. [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Koob GF, Zorrilla EP. Role of corticotropin-releasing factor in drug addiction: potential for pharmacological intervention. CNS Drugs. 2011;25(4):271–287. doi: 10.2165/11587790-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Arnold GM, Vezina P. Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res. 2000;107(1–2):9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 2014;76(Pt B):287–300. doi: 10.1016/j.neuropharm.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Pantelis C. Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction (Abingdon, England) 2004;99(12):1491–1502. doi: 10.1111/j.1360-0443.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schluter OM, Huang YH, Dong Y. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83(6):1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type-and input-specific connectivity in the nucleus accumbens. Nat Neurosci. 2014;17(9):1198–1207. doi: 10.1038/nn.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology. 2012;221(3):407–426. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Bickel WK. A dual-systems perspective on addiction: contributions from neuroimaging and cognitive training. Ann N Y Acad Sci. 2014;1327:62–78. doi: 10.1111/nyas.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann NY Acad Sci. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology. 1998;135(4):416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS ONE. 2012a;7(6):e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2012b;219(4):999–1009. doi: 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Meshul CK, Phillips TJ. Ethanol- and cocaine-induced locomotion are genetically related to increases in accumbal dopamine. Genes Brain Behav. 2009;8(3):346–355. doi: 10.1111/j.1601-183X.2009.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FJ, Everitt BJ, Dickinson A. Oral cocaine seeking by rats: action or habit? Behav Neurosci. 2003;117(5):927–938. doi: 10.1037/0735-7044.117.5.927. [DOI] [PubMed] [Google Scholar]
- Miliaressis E, Rompre PP, Laviolette P, Philippe L, Coulombe D. The curve-shift paradigm in self-stimulation. Physiol Behav. 1986;37(1):85–91. doi: 10.1016/0031-9384(86)90388-4. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM. Impulsivity and substance abuse: what is the connection? Addict Disord Treat. 2002;1(1):3–10. [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2–3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Morgan D, Roberts DC. Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev. 2004;27(8):803–812. doi: 10.1016/j.neubiorev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 2012;37(11):2456–2466. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naude J, Dongelmans M, Faure P. Nicotinic alteration of decision-making. Neuropharmacology. 2014;96:244. doi: 10.1016/j.neuropharm.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Nestby P, Vanderschuren LJ, De Vries TJ, Hogenboom F, Wardeh G, Mulder AH, Schoffelmeer AN. Ethanol, like psychostimulants and morphine, causes long-lasting hyperreactivity of dopamine and acetylcholine neurons of rat nucleus accumbens: possible role in behavioural sensitization. Psychopharmacology. 1997;133(1):69–76. doi: 10.1007/s002130050373. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2(2):119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- NIMH. Research domain criteria (RDoC) 2015 http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml.
- Nimitvilai S, Arora DS, McElvain MA, Brodie MS. Ethanol blocks the reversal of prolonged dopamine inhibition of dopaminergic neurons of the ventral tegmental area. Alcohol Clin Exp Res. 2012;36(11):1913–1921. doi: 10.1111/j.1530-0277.2012.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J. Hypothalamic substrates of reward. Physiol Rev. 1962;42:554–604. doi: 10.1152/physrev.1962.42.4.554. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. The contribution of orbitofrontal cortex to action selection. Ann N Y Acad Sci. 2007a;1121:174–192. doi: 10.1196/annals.1401.033. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007b;27(18):4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. On habits and addiction: an associative analysis of compulsive drug seeking. Drug Discov Today Dis Models. 2008;5(4):235–245. doi: 10.1016/j.ddmod.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. The time coures of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200(1):1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology. 2013;229(3):387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci. 1997;17(9):3254–3261. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24(14):3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Lockfeld A, Squillace KM, Contel NR. Drug-environment interaction: context dependency of cocaine-induced behavioral sensitization. Life Sci. 1981;28(7):755–760. doi: 10.1016/0024-3205(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Puumala T, Sirvio J. Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience. 1998;83(2):489–499. doi: 10.1016/s0306-4522(97)00392-8. [DOI] [PubMed] [Google Scholar]
- Redish AD. Addiction as a computational process gone awry. Science. 2004;306(5703):1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci. 2008;31(4):415–437. doi: 10.1017/S0140525X0800472X. discussion 437–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398(6728):567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Part B 0):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Thomas MJ, Gewirtz JC. Protracted manifestations of acute dependence after a single morphine exposure. Psychopharmacology. 2012;219(4):991–998. doi: 10.1007/s00213-011-2425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Cacciapaglia F, Wightman RM, Carelli RM. Differential dopamine release dynamics in the nucleus accumbens core and shell reveal complementary signals for error prediction and incentive motivation. J Neurosci. 2015;35(33):11572–11582. doi: 10.1523/JNEUROSCI.2344-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Pan X, Uttl B. Behavioral inhibition and prefrontal cortex in decision-making. Neural Networks Official J Int Neural Network Soc. 2006;19(8):1255–1265. doi: 10.1016/j.neunet.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Satel SL, Kosten TR, Schuckit MA, Fischman MW. Should protracted withdrawal from drugs be included in DSM-IV? Am J Psychiatry. 1993;150(5):695–704. doi: 10.1176/ajp.150.5.695. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526(1–3):65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29(2):116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- Segal DS. Behavioral and neurochemical correlates of repeated d-amphetamine administration. Adv Biochem Psychopharmacol. 1975;13:247–262. [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54(1):1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shen RY. Ethanol withdrawal reduces the number of spontaneously active ventral tegmental area dopamine neurons in conscious animals. J Pharmacol Exp Ther. 2003;307(2):566–572. doi: 10.1124/jpet.103.053371. [DOI] [PubMed] [Google Scholar]
- Shen RY, Choong KC, Thompson AC. Long-term reduction in ventral tegmental area dopamine neuron population activity following repeated stimulant or ethanol treatment. Biol Psychiatry. 2007;61(1):93–100. doi: 10.1016/j.biopsych.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Balleine BW. At the limbic-motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation. Eur J Neurosci. 2010;32(10):1735–1743. doi: 10.1111/j.1460-9568.2010.07439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett MW, Balleine BW. Molecular substrates of action control in cortico-striatal circuits. Prog Neurobiol. 2011;95(1):1–13. doi: 10.1016/j.pneurobio.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Beas BS, Montgomery KS, Haberman RP, Bizon JL, Setlow B. Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. Eur J Neurosci. 2013;37:1779. doi: 10.1111/ejn.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MD, Aubin HJ. Craving’s place in addiction theory: contributions of the major models. Neurosci Biobehav Rev. 2010;34(4):606–623. doi: 10.1016/j.neubiorev.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81(2):119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Soto CBD, O’Donnell WE, Allred LJ, Lopes CE. Symptomatology in alcoholics at various stages of abstinence. Alcohol Clin Exp Res. 1985;9(6):505–512. doi: 10.1111/j.1530-0277.1985.tb05592.x. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology. 2008;196(3):473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res Brain Res Rev. 2003;41(2–3):203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Stewart J. Conditioned stimulus control of the expression of sensitization of the behavioral activating effects of opiate and stimulant drugs. In: Gormezano I, Wasserman EA, editors. Learning and memory: the behavioral and biological substrates. Erlbaum; Hillsdale, NJ: 1992. pp. 917–923. [Google Scholar]
- Terelli E, Terry P. Amphetamine induced conditioned activity and sensitization: the role of habituation to the test context and the involvement of Pavlovian processes. Behav Pharmacol. 1999;9:409–419. doi: 10.1097/00008877-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychol Rev. 1990;97(2):147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. Cognitive concepts of craving. Alcohol Res Health. 1999;23(3):215–224. [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Kalivas PW. Neurotensin in the ventral pallidum increases extracellular gamma-aminobutyric acid and differentially affects cue- and cocaine-primed reinstatement. J Pharmacol Exp Ther. 2008;325(2):556–566. doi: 10.1124/jpet.107.130310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan J, Lason W, Budziszewska B, Przewlocka B. Effects of single and repeated morphine administration on the prodynorphin, proenkephalin and dopamine D2 receptor gene expression in the mouse brain. Neuropeptides. 1997;31(1):24–28. doi: 10.1016/s0143-4179(97)90015-9. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res. 2006;169(2):320–324. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- van Huijstee AN, Mansvelder HD. Glutamatergic synaptic plasticity in the mesocorticol-imbic system in addiction. Front Cell Neurosci. 2014;8:466. doi: 10.3389/fncel.2014.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25(38):8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151(2–3):99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Veilleux JC, Skinner KD. Smoking, food, and alcohol cues on subsequent behavior: a qualitative systematic review. Clin Psychol Rev. 2015;36:13–27. doi: 10.1016/j.cpr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Negative emotion-driven impulsivity predicts substance dependence problems. Drug Alcohol Depend. 2007;91(2–3):213–219. doi: 10.1016/j.drugalcdep.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Rivas-Perez C, Lopez-Torrecillas F, Perez-Garcia M. Differential impact of severity of drug use on frontal behavioral symptoms. Addict Behav. 2006;31(8):1373–1382. doi: 10.1016/j.addbeh.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27(8):827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56(Suppl 1):160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111(10):1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Cely IC, Balleine BW, Maidment NT. Micro-opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. J Neurosci. 2011;31(5):1591–1599. doi: 10.1523/JNEUROSCI.3102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JR. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;138(3537):143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24(8):1251–1256. [PubMed] [Google Scholar]
- Wiers RW, Bartholow BD, van den Wildenberg E, Thush C, Engels RC, Sher KJ, Grenard J, Ames SL, Stacy AW. Automatic and controlled processes and the development of addictive behaviors in adolescents: a review and a model. Pharmacol Biochem Behav. 2007;86(2):263–283. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, Phillips PE. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci USA. 2012;109(50):20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24(20):4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35(1–2):227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- Wise RA. The neurobiology of craving: Implications for the understanding and treatment of addiction. J Abnorm Psychol. 1988;97(2):118–132. doi: 10.1037//0021-843x.97.2.118. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39(2):254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteman J, Post H, Tarvainen M, de Bruijn A, Perna ES, Ramaekers JG, Wiers RW. Cue reactivity and its relation to craving and relapse in alcohol dependence: a combined laboratory and field study. Psychopharmacology (Berl) 2015;232(20):3685–3696. doi: 10.1007/s00213-015-4027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54(6):679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interventions. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Wolf ME. LTP may trigger addiction. Mol Interventions. 2003;3(5):248–252. doi: 10.1124/mi.3.5.248. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35(2):185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Cervo L, Johanson CE. Effects of repeated methamphetamine administration on methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav. 1984;21:737–741. doi: 10.1016/s0091-3057(84)80012-x. [DOI] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2013;226(2):217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS. Two substrates for medial forebrain bundle self-stimulation: myelinated axons and dopamine axons. Neurosci Biobehav Rev. 1989;13(2–3):91–98. doi: 10.1016/s0149-7634(89)80016-8. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19(1):181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166(2):189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci Official J Soc Neurosci. 2010;30(46):15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Fletcher PC. Is food addiction a valid and useful concept? Obes Rev. 2013;14(1):19–28. doi: 10.1111/j.1467-789X.2012.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]