Abstract
Despite substantial progress in implementing human immunodeficiency virus testing, challenges remain in achieving widespread uptake particularly in rural resource-limited settings. We sought to understand motivations for human immunodeficiency virus testing in a community-based human immunodeficiency virus testing programme in rural South Africa. We conducted a questionnaire survey in participants undergoing voluntary human immunodeficiency virus testing within an ongoing community-based integrated human immunodeficiency virus/TB intensive case finding programme at congregate rural settings. Participants responded to a six-item non-mutually exclusive motivations survey which included the topics of feeling ill, recent HV exposure, risky lifestyle, illness in a family member, and pregnancy. Among 2068 respondents completing the survey, 1393 (67.4%) were women, median age was 40 years (IQR 19–56), and 1235 (59.7%) were first time testers. Among all testers, 142 (6.9%) were human immunodeficiency virus-positive with median CD4 count 346 (IQR 218–542). Community-based testing for human immunodeficiency virus is acceptable and meets the needs of community members in rural South Africa. Motivations for human immunodeficiency virus testing at the community level are complex and differ according to gender, age, site of community testing, and human immunodeficiency virus status. These differences can be utilised to improve the focus and yield of community-based human immunodeficiency virus screening.
Keywords: Africa, diagnosis, human immunodeficiency virus, screening, epidemiology
Introduction
Human immunodeficiency virus (HIV) testing is the first step in the cascade of services leading to successful HIV treatment and prevention.1 Despite great strides in improving access to HIV testing, highly affected countries in Africa still face challenges in achieving high uptake of voluntary counselling and testing for HIV (VCT). Testing for HIV is lowest in rural areas; a 2012 South African study found that only 32% of rural adults had been tested for HIV.2 Intensive case finding (ICF) employing community-based VCT has been proposed as a strategy to expand acceptability of and access to VCT services in rural settings.3,4 If successful, the strategy could increase the number of individuals diagnosed with HIV. This would be an important first step to achieving earlier initiation of antiretroviral therapy, reducing morbidity and mortality, and potentially reducing HIV incidence among rural populations in sub-Saharan Africa.5–8
Research on barriers to and motivations for VCT in Africa has been predominantly limited to urban areas and in health care facility-based testing sites. Studies of urban facility-based VCT describe recent knowledge about HIV as the most common motivation for testing; other motivations include feeling ill, self-perceived risk, perceived infidelity, and confirmatory testing.9,10 In these settings, logistical9 and social10,11 barriers can interfere with uptake of HIV testing. Existing rural studies of testing motivations have focused on health facility-based testing, and very little information has been published regarding reasons for HIV testing at community settings.10,11 Impoverished and difficult to access rural populations, identified through community-based VCT, may differ significantly from those in urban areas and/or those self-selected by their use of health facility-based testing.11 Finally, although information from existing studies is of value, none correlate motivations with the subsequent HIV test result.
To maximise the implementation of community-based VCT, it is important to assess motivations for HIV testing as well as to understand motivations that correlate with HIV seropositivity. These may allow programme directors and health planners to further focus testing strategies, particularly in difficult to reach rural communities. To improve the characterisation of populations using rural community-based VCT and maximise acceptability and yield, we evaluated motivations for HIV testing and explored their associations with demographic characteristics and HIV seropositivity in a community-based integrated HIV/TB screening programme in rural South Africa.
Methods
Setting
This study was performed in the rural Msinga subdistrict of KwaZulu-Natal, home to 180,000 Zulu people, living in widely dispersed family compounds within a 2000 km2 mountainous area. The district has an estimated HIV prevalence of 24.6%.12 The area also suffers from high rates of HIV-associated drug susceptible and drug-resistant tuberculosis.13 The population is under-served and with difficult access to existing community health care facilities. The community is served by a 350-bed government district hospital, 16 primary care clinics, and three mobile clinics.
This study collected information regarding motivations for HIV testing nested within an ongoing community-based integrated HIV/TB intensive case finding (ICF) programme at congregate rural settings beginning in March 2010.14 A team of nurses, VCT counsellors, and educators travelled to community sites throughout the subdistrict including social grant disbursement points, municipal events/health fairs, taxi/bus ranks, schools, and events organised by home-based care personnel. The team provided health education before inviting community members to be voluntarily tested for HIV and screened for TB. The population participating in the screening services represents a convenience sample of this rural population who attended these community congregate setting events. The TB DOTS programme and the HIV/AIDS/STD programme also provide services intermittently in the community. However, these community services remain separate and do not routinely integrate their TB and HIV services in the same manner as our programme. The Department of Health supported the planning and development of our community-based services to increase reach into the community for HIV and TB screening but did not contribute materially to its implementation.
This report will focus on the HIV screening component of the programme. Community members who chose to participate in the HIV screening were asked to provide verbal consent by a trained Zulu speaking community health worker. After verbal consent was obtained, a standard questionnaire in Zulu to all screening participants collecting information on demographics, prior HIV testing, and current TB symptoms. Trained lay VCT counsellors conducted HIV counselling in isiZulu, the local language of the area, in a private, confidential location. After obtaining written consent, the counsellors performed HIV testing by rapid fingerstick; if positive, this was followed by a rapid confirmatory test. HIV-positive community members were offered phlebotomy for CD4 count by a nurse; blood specimens were brought to the hospital lab and processed. When the staff obtained results, community members were notified by phone or in person. Community members were counselled and referred for clinical care according to national guidelines. Community members who underwent voluntary HIV testing in this community-based service were requested to respond to a series of questions regarding their motivation for testing. Six non-exclusive potential motivations were each individually assessed in a yes–no format: (1) using the community-based service to know HIV status, (2) feeling ill, (3) having known/suspected recent HIV exposure, (4) leading a risky lifestyle, (5) knowing family member who was sick/dead, and (6) being pregnant. These six items were initially developed as indicators for monitoring and evaluation reporting to a funding agency of the community-based screening programme. During creation of the funder’s report, the findings were identified as noteworthy, leading to the current paper. Although the monitoring and evaluation indicators were not initially based on a validated behavioural model, the questionnaire itself was patterned on a well established and validated information, motivation, and behavioural model.15
Ethical approval was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee and Yale University School of Medicine.
Analysis
Responses from individuals 12 years and older undergoing HIV testing in the parent community-based screening programme who answered all items were included in the analysis (Figure 1). Individuals were classified as HIV-negative and HIV-positive after testing. Among those testing HIV-positive, individuals were further classified as known HIV-positive, defined as those individuals who reported a previous positive HIV test, or new HIV-positive, defined as those individuals who denied a previous positive HIV test, either because they were first-time testers or they had previously tested negative.
Figure 1.
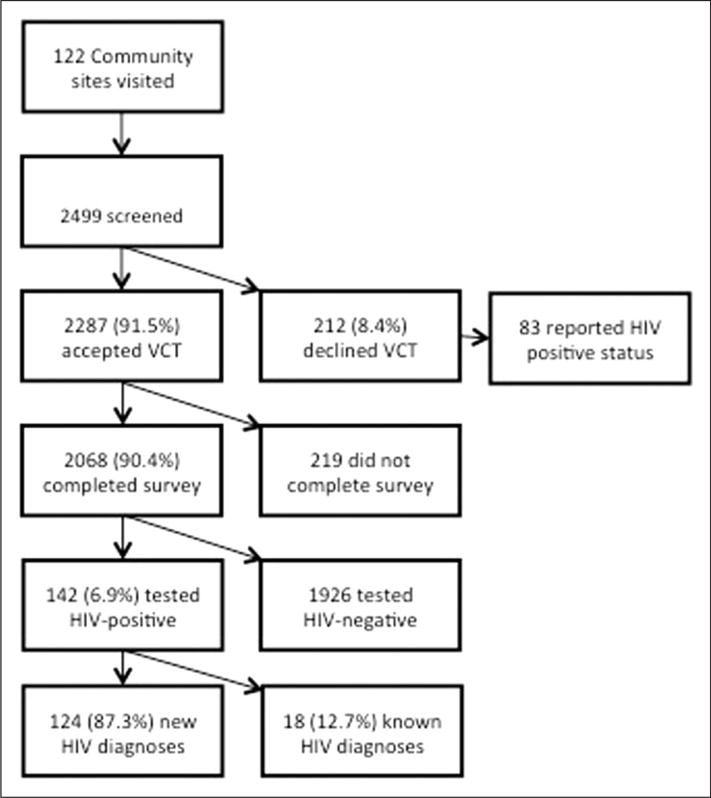
Flow diagram showing HIV testing, motivation survey respondents, and HIV testing results.
All data analysis was conducted using SAS version 9.2 software (SAS Institute, Cary, NC). Descriptive analyses were conducted to determine the frequencies of each motivation overall and according to age, gender, community site, and HIV status. Univariate testing using t-tests and Chi-square tests was conducted to compare motivations by gender and age; Cochran–Mantel–Haenszel statistic was used to compare motivation by site of testing. A p-value <0.05 was considered significant.
Results
Respondent characteristics and HIV status
During the study period, 122 community sites were visited including 44 (36.1%) municipal events, 34 (27.9%) social grant disbursement points, 16 (13.1%) home-based care events, 7 (5.7%) taxi ranks, 10 (8.2%) school events, and 11 (9.0%) other events. Among 2499 community members who were seen for integrated HIV and TB screening, 2287 (91.5%) accepted VCT while 212 (8.5%) declined VCT (Figure 1); there were no significant demographic differences between these groups.
Of the 2287 HIV testers, 2068 (90.4%) responded to the motivations survey (Figure 1). Of these, 1393 (67.4%) were women. Compared to the 219 individuals who tested for HIV but did not complete the motivations survey, survey respondents (n = 2068) were younger (median age 40 versus 54, p < 0.01), less likely to be female (67% versus 79%, p = 0.03), and less likely to be HIV-positive (6.9% versus 11.9%; p < 0.01). Overall, 142 individuals (6.9%) were found to be HIV-positive with median CD4 count 346 cells/mm3 (IQR 218–542). Comparing the 142 HIV-positive testers who took the survey with the 26 HIV-positive testers who did not, there were no significant demographic or CD4 cell count differences.
Among the survey respondents (n = 2068), the median age was 40 (IQR 19–56), 67.4% were women, and 40.3% had tested for HIV previously. There was no significant difference between the 142 HIV-positive and 1926 HIV-negative respondents for median age, proportion of women, or history of HIV testing (Table 1). Women were more likely than men to have tested previously (43.5% versus 33.6%; p < 0.01).
Table 1.
Characteristics of respondents.
| All respondents (n = 2068) | HIV-negative n = 1926 (93.1%) | HIV-positive n = 142 (6.9%) | p-value | |
|---|---|---|---|---|
| Age, median (IQR) | 40 (19–56) | 40 (18–56) | 39.5 (25–49) | 0.99 |
| Women (%) | 1393 (67.4) | 1295 (67.2) | 98 (69.0) | 0.63 |
| First-time testers (%) | 1235 (59.7) | 1151 (59.8) | 84 (59.2) | 0.89 |
| TB symptoms (%) | 799 (38.6) | 732 (38.0) | 67 (47.2) | 0.03 |
Of 2068 screeners who completed the motivations survey, 97.4% reported wanting to use this community-based service to know their HIV status (Table 2). Additional reasons for testing included feeling ill (14.6%), leading a risky lifestyle (12.5%) and known/suspected recent HIV exposure (9.4%), illness or death in a family member (5.3%), and pregnancy (0.5%). More than a quarter (26.3%) of respondents reported more than one motivation for HIV testing.
Table 2.
Survey respondents’ motivations for HIV testing (n = 2068).
| Motivation | All respondents n = 2068 (%) | HIV-negative n = 1926 (%) | HIV-positive n = 142 (%) | p-value |
|---|---|---|---|---|
| > 1 motivation affirmed | 505 (24.4) | 437 (22.7) | 68 (47.9) | <0.01 |
| Using the ICF service to know HIV status | 2015 (97.4) | 1877 (97.5) | 138 (97.2) | 0.84 |
| Feeling ill | 302 (14.6) | 267 (13.9) | 35 (24.6) | <0.01 |
| Known/suspected recent HIV exposure | 195 (9.4) | 162 (8.4) | 33 (23.2) | <0.01 |
| Risky lifestyle | 258 (12.5) | 214 (11.1) | 44 (31.0) | <0.01 |
| Family member sick or died | 110 (5.3) | 94 (4.9) | 16 (11.3) | <0.01 |
| Pregnant | 11 (0.5) | 10 (0.5) | 1 (0.7) | 0.77 |
Women were more likely to be motivated to test because of feeling ill (16.0% versus 11.7% men, p < 0.01) and because a family member was sick or had died (6.2% versus 3.6% men, p = 0.01) (Figure 2). Men (15.1%) were more likely to be motivated by their risky lifestyle compared to women (11.2%, p = 0.01). However, there was no difference between men and women’s interest in being tested for HIV due to a known or suspected recent HIV exposure.
Figure 2.
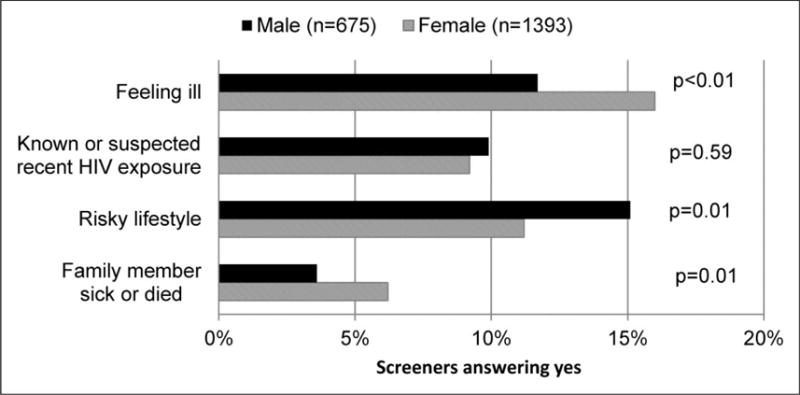
Survey respondents’ motivations for HIV testing, by gender (n = 2068).
Predominant motivations also varied significantly by age (Figure 3). Among those 50 years and older, the most common motivation for HIV testing was feeling ill (26.6%). Respondents aged 35–49 ascribed their decision to test to feeling ill, risky lifestyle, and known/suspected recent HIV exposure in approximately equal proportions. For younger respondents age 25–34 and 12–24 years, risky lifestyle was the predominant motivator (21.9% and 11.0%, respectively).
Figure 3.
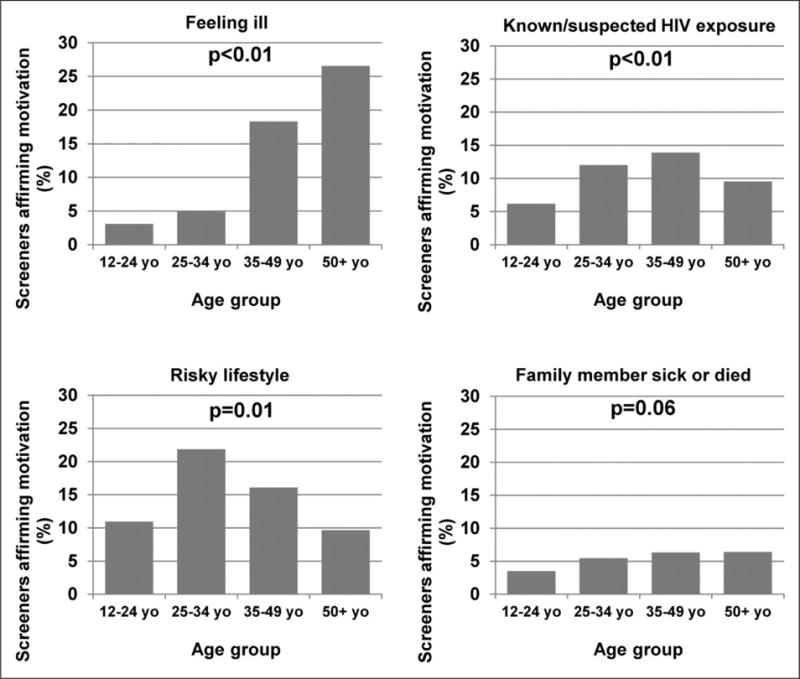
Survey respondents’ motivations for HIV testing, by age group (n = 2068). (a) Feeling ill, (b) known/suspected HIV exposure, (c) risky lifestyle and (d) family member sick or died.
Motivations for HIV testing also varied by community site (Figure 4). Compared to all other sites, community members screened at taxi/bus ranks were more likely to report recent HIV exposure (27.8% versus 8.3%; p < 0.01) and risky lifestyle (36.5% versus 11.1%; p < 0.01). After adjustment for age group, gender, and HIV-positivity, the increased frequencies of these motivations among taxi/bus rank screeners remained significant.
Figure 4.
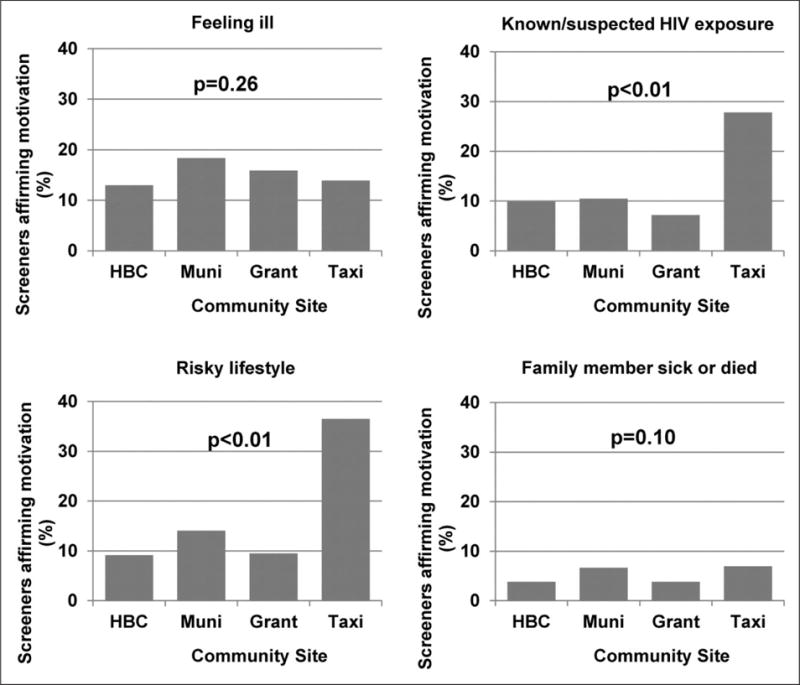
Survey respondents’ motivations for HIV testing, by community site (n = 2068). (a) Feeling ill, (b) known/suspected HIV exposure, (c) risky lifestyle and (d) family member sick or died.
Among the 142 (6.9%) individuals who tested HIV-positive, 124 (87.3%) were newly diagnosed and 18 (12.7%) were known to be HIV-positive (Figure 1). There were no significant differences in median age (37 [IQR 24–49] versus 42 [IQR 38–46], p = 0.2), proportion of women (70.2% versus 61.1%, p = 0.44), presence of TB symptoms (45.2% versus 61.1%, p = 0.21), or median CD4 cell count (342 [IQR 221–525] versus 440 [IQR 169–563], p = 0.68) between these groups. Among the 124 newly diagnosed HIV respondents, 84 (67.7%) were undergoing HIV testing for the first time. The repeat testers were more likely to be HIV-positive (6.8% versus 4.9%, p = 0.08). Motivations for HIV testing did not differ by history of previous HIV testing.
Importantly, motivations varied by HIV status. Compared to HIV-negative testers (Figure 5), HIV-positive testers were significantly more likely to be motivated by feeling ill, having known/suspected recent HIV exposure, having a risky lifestyle, and having a family member who was sick or dead. HIV-positive testers were also significantly more likely to report greater than one motivation for testing (47.9% versus 22.7%; p < 0.001). HIV testers who already knew they were HIV-positive more frequently reported feeling ill compared to those newly diagnosed with HIV but this difference did not reach statistical significance (38.9 versus 22.6%; p = 0.13).
Figure 5.
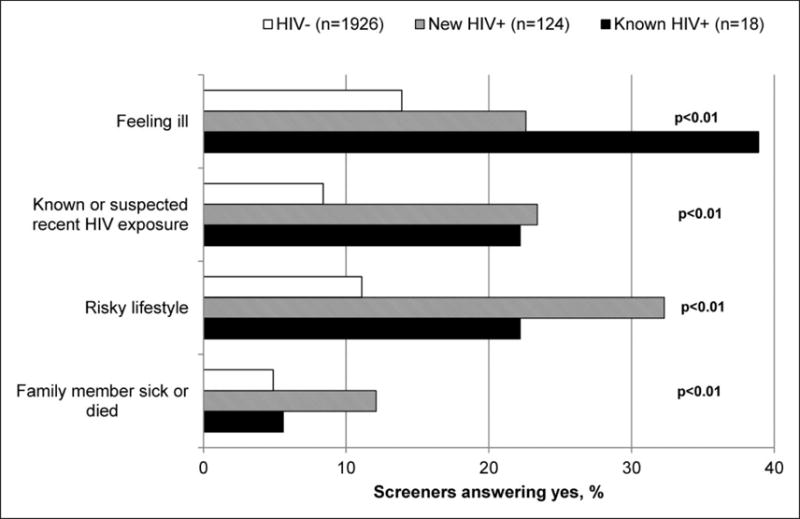
Survey respondents’ motivations for HIV testing, by HIV status.
Of note, a substantial proportion of both HIV-positive and HIV-negative screeners endorsed a positive symptom screen for tuberculosis. Presence of TB symptoms was significantly higher among those who, when screened, were HIV-positive (47.2% versus 38.0%; p < 0.03) (Table 1).
Discussion
This study assessed motivations for HIV testing in a community-based integrated HIV and TB screening programme in rural South Africa. The findings demonstrated that providing community-based testing for HIV meets the needs of community members and that HIV testing is acceptable in this environment, confirming the work of van Rooyen and others.16,17 However, this study also importantly demonstrated that motivations for testing are complex and differ according to gender, age, site of community testing, and HIV status. Further, the findings identified a small proportion of individuals who are aware of their HIV-positive status but repeatedly sought testing services. This information may have value in targeting an array of venues for reaching different populations with HIV services and improving HIV screening yield.
This survey of motivations highlighted differences by gender, age group, and community site. With regards to gender, women were largely motivated by symptoms in themselves or in family members, compared to men who were frequently motivated because of risk behaviours, consistent with gender-specific motivations found in other studies.18,19 This is also consistent with local cultural patterns where individuals often have multiple concurrent sexual partners; men tend to have higher rates of such partnerships than women.20,21 Gender-specific health education and designing gender-specific risk reduction interventions may be helpful. VCT services should adapt their approach in different demographic groups to target higher risk populations. Motivation data also varied considerably by community site, which may yield screeners with different demographic characteristics. For example, VCT services at taxi ranks tended to attract younger people who were motivated by perceptions of risky behaviour, while grant disbursement sites were characterised by older individuals who primarily reported feeling ill. This highlights the potential value of exploring a broad array of venues for community-based VCT in order to reach a maximal group of interested screeners. The results highlight the fact that despite the presence of the traditional facility-based government health care system, community members are missed for HIV screening through mostly health facility-based HIV testing strategies; local residents in this rural setting sought and used services from our community-based team. Future research should explore the different demographic groups at each of these sites and the impact on yield of HIV-positive individuals in order to design the most effective community-based VCT initiatives.
Another important contribution of this study is a quantification of motivations for screening. The presence of a large number of screeners in our study with multiple screening motivations indicates that the reasons for testing among community members in rural South Africa are complex. Although literature exists about the yield of community-based HIV testing in sub-Saharan Africa, reports of motivations for HIV testing other than desiring to know serostatus are limited.2 Mulogo et al. in rural Uganda found a <2% prevalence of any motivation other than desire to know serostatus among facility-based testers and a <4% prevalence of any motivation other than desire to know serostatus or being asked to participate in the study among home-based testers.11 The other choices available for testing motivation were similar in content to those in our study. In our study, however, over 25% reported other motivations for testing; among those found to be HIV-positive, close to 50% affirmed other motivations. These findings may reflect differences in survey methodology and execution in our community-based setting or a greater appreciation in our community of complexity of motivations and self-recognition of reasons to test.
Of note, the oldest age group ascribed interest in testing to feeling ill, while those who were 35–49 years of age attributed interest in testing in similar proportions to feeling ill, risky lifestyle, and recent HIV exposure. This may reflect the differences in understanding of HIV transmission among different age groups or may reflect increases in risk behaviour in younger age groups.
The greater frequency of risk-related motivations among those screeners found to be HIV-positive indicates that rural community members recognise risk factors for HIV transmission and perceive this risk in their own behaviour. Previous data from the same study site indicate that both users and non-users of the ICF service have high levels of knowledge of HIV cause (>80% affirming that it is a virus) and transmission (>70% affirming that it can be spread by sex, blood, childbirth, and breastfeeding).22 Similarly, other studies have demonstrated the connection between acceptance of testing and both self-perception of risk and knowledge of HIV.23–25 Pregnancy was a very rare motivation for testing, which may indicate that antenatal clinic services have achieved widespread HIV testing in this high-risk population.
Another important finding of this study is that two HIV-positive populations were motivated to use the community-based HIV testing service: those not previously known to be HIV-positive and a second poorly appreciated population of community members who were attracted to the service although already aware of their HIV-positive status. This is one of few studies to describe a substantial sub-population of previously known HIV-positive individuals accessing a community-based HIV testing service. In our study, nearly 13% of individuals testing HIV-positive already knew their status but still requested a repeat HIV test. Anecdotally, community members reported that they wished to confirm their diagnosis after previous testing or after attempting “cure” with traditional medicine, but a more systematic evaluation of this group’s testing motivations is needed. A substantial number (n = 83) of users of this community-based integrated TB and HIV screening service reported positive HIV status and declined an HIV test (Figure 1); since they did not test for HIV, their motivations for using the community-based service were not assessed. However, these patients requested services including blood draw for CD4 cell count, tuberculosis screening, and other general health consultations/referrals (including STI symptoms and TB symptoms) despite having access to these services through the government health care system.
The information obtained in this study contributes to the limited literature on community-based VCT in rural and resource-limited settings in sub-Saharan Africa and has implications for programme service design, implementation, and cost-effectiveness estimates. The fact that a substantial number of HIV-positive community members sought out these services from a community-based programme indicates that there may be value to bundling a broader array of primary care services with community-based HIV testing. In particular, the patients identified with HIV had a high frequency of having a positive TB symptom screen, supporting programmatic integration of HIV and TB screening services, and emphasising the need for widespread TB symptom screening in high prevalence areas. Community members may value additional primary care services including those for noncommunicable diseases, but this requires further study.
This study is unique in that we are able to correlate motivations for community-based HIV testing with the subsequent HIV test result. The HIV testing motivations reported and their correlation with HIV-positive status offer important insights into both the community member’s self-perception of HIV risk and the value of community-based case finding for HIV. The reasons why community members in resource-limited settings decide to pursue HIV testing are critical to understand in order to more widely implement the community-based HIV testing strategy and improve overall HIV awareness, uptake of HIV testing, identification, and linkage to care. In addition to the exploration of demography and motivation of screeners, the strengths of this study include the focus on neglected and difficult to reach rural populations, the large number of respondents, survey completion in the vast majority of HIV testers (90.4%), the variety of community sites visited, and the consistent documentation of patients’ previous HIV status.
There are limitations to this study. Socioeconomic variables that may have affected testing behaviour were not assessed at the time of HIV testing. Although highly informative, the motivation questions were developed from indicators required by a funder which were not grounded in a behavioural model. In addition, the use of the screening service by known HIV-positive community members was not anticipated, and, as a consequence, the survey was not designed to explore this sub-population’s motivations for using our service. Nevertheless, it is important that this group has been identified and that the need for more rigorous future study of their HIV testing motivations be appreciated. This programme was not designed to sample a representative proportion of the population but represents a convenience sample; therefore the results may not be generalisable. In addition, since we have not collected information from non-participants, we cannot comment on acceptability of the services beyond the large number of local residents who chose to participate.
Conclusions
The expansion of community-based VCT services in high HIV burden countries should take into account the motivations and needs of community members in order to most effectively serve hard to reach populations such as those in rural areas. By better defining and exploring the reasons why community members were interested in and used our community-based screening strategy, we have provided new information to improve the focus and yield of community-based screening in this rural area and beyond. In this study, we determined that a diverse population with a variety of motivations for screening is attracted to and accepting of community-based VCT services. In addition, motivations for testing differ by demographic characteristics, which may inform design and more efficient targeting of community-based testing services. Further, the study identified two HIV-positive populations, newly and previously diagnosed HIV-positive community members; distinctions between them and the needs of each require further study. Lastly, the results suggest the need to broaden and expand community-based services beyond HIV testing alone to a more comprehensive continuum of HIV and non-HIV primary care services, with strengthened linkage to existing primary care and HIV services.
Acknowledgments
This article was presented in part at South African AIDS Conference, Durban, South Africa, June 2013.
Funding
This research was supported by NIAID (K23AI089260), Doris Duke Clinical Research Foundation (2010073), Gilead Foundation (157201), USAID/URC (674-C-00-09-00121-00), PEPFAR (U62 CCU 223540), and Fogarty International Center R24 (TWOO7988-02S1).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bassett IV, Regan S, Chetty S, et al. Who starts antiretroviral therapy in Durban, South Africa? … not everyone who should. AIDS. 2010;24:S37–44. doi: 10.1097/01.aids.0000366081.91192.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabana H, Doherty T, Swanevelder S, et al. Knowledge of HIV status prior to a community HIV counseling and testing intervention in a rural district of south Africa: results of a community based survey. BMC Infect Dis. 2012;12:73. doi: 10.1186/1471-2334-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawichai S, Celentano D, Srithanaviboonchai K, et al. NIMH Project Accept (HPTN 043) HIV/AIDS community mobilization (CM) to promote mobile HIV voluntary counseling and testing (MVCT) in rural communities in Northern Thailand: modifications by experience. AIDS Behav. 2012;16:1227–1237. doi: 10.1007/s10461-011-0099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweat M, Morin S, Celentano D, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis. 2011;11:525–532. doi: 10.1016/S1473-3099(11)70060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matovu JK, Makumbi FE. Expanding access to voluntary HIV counselling and testing in sub-Saharan Africa: alternative approaches for improving uptake, 2001–2007. Trop Med Int Health. 2007;12:1315–1322. doi: 10.1111/j.1365-3156.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 6.Palella FJ, Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003;138:620–626. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Tanser F, Barnighausen T, Grapsa E, et al. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bor J, Herbst AJ, Newell ML, et al. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339:961–965. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jereni BH, Muula AS. Availability of supplies and motivations for accessing voluntary HIV counseling and testing services in Blantyre, Malawi. BMC Health Serv Res. 2008;8:17. doi: 10.1186/1472-6963-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou L, Guo J, Fan L, et al. Survey of motivation for use of voluntary counseling and testing services for HIV in a high risk area of Shenyang, China. BMC Health Serv Res. 2009;9:23. doi: 10.1186/1472-6963-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulogo EM, Abdulaziz AS, Guerra R, et al. Facility and home based HIV counseling and testing: a comparative analysis of uptake of services by rural communities in southwestern Uganda. BMC Health Serv Res. 2011;11:54. doi: 10.1186/1472-6963-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.South African National Department of Health. The 2011 National Antenatal Sentinel HIV & Syphilis Prevalence Survey in South Africa. 2011 [Google Scholar]
- 13.Moodley P, Shah NS, Tayob N, et al. Spread of extensively drug-resistant tuberculosis in KwaZulu-Natal province, South Africa. PLoS One. 2011;6:e17513. doi: 10.1371/journal.pone.0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shenoi SV, Moll A, Mntambo AQ, et al. High yield from community-based intensive case finding (CBICF) for TB-HIV in rural South Africa. Kuala Lumpur: International Union Against TB & Lung Disease; 2012. [Google Scholar]
- 15.Fisher JD, Fisher WA. The information motivation-behavioral skills model of HIV preventive behavior. In: Diclemente RJ, Crosby RA, Kegler MC, editors. Emerging theories in health promotion practice and research. 2nd. San Francisco: Jossey Bass; 2009. pp. 21–64. [Google Scholar]
- 16.van Rooyen H, McGrath N, Chirowodza A, et al. Mobile VCT: reaching men and young people in urban and rural South African pilot studies (NIMH Project Accept, HPTN 043) AIDS Behav. 2013;17:2946–2953. doi: 10.1007/s10461-012-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tumwebaze H, Tumwesigye E, Baeten JM, et al. Household-based HIV counseling and testing as a platform for referral to HIV care and medical male circumcision in Uganda: a pilot evaluation. PLoS One. 2012;7:e51620. doi: 10.1371/journal.pone.0051620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chirawu P, Langhaug L, Mavhu W, et al. Acceptability and challenges of implementing voluntary counselling and testing (VCT) in rural Zimbabwe: evidence from the Regai Dzive Shiri Project. AIDS Care. 2010;22:81–88. doi: 10.1080/09540120903012577. [DOI] [PubMed] [Google Scholar]
- 19.Zachariah R, Spielmann MP, Harries AD, et al. Motives, sexual behaviour, and risk factors associated with HIV in individuals seeking voluntary counselling and testing in a rural district of Malawi. Trop Doct. 2003;33:88–91. doi: 10.1177/004947550303300211. [DOI] [PubMed] [Google Scholar]
- 20.Harrison A, O’Sullivan LF. In the absence of marriage: long-term concurrent partnerships, pregnancy, and HIV risk dynamics among South African young adults. AIDS Behav. 2010;14:991–1000. doi: 10.1007/s10461-010-9687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mah TL, Halperin DT. Concurrent sexual partnerships and the HIV epidemics in Africa: evidence to move forward. AIDS Behav. 2010;14:11–16. doi: 10.1007/s10461-008-9433-x. (discussion 34–17) [DOI] [PubMed] [Google Scholar]
- 22.Sani S, Shenoi S, Myende H, et al. TB knowledge is inferior to HIV knowledge in rural South Africa. Third South African TB conference; Durban, South Africa. 2012. [Google Scholar]
- 23.Morin SF, Khumalo-Sakutukwa G, Charlebois ED, et al. Removing barriers to knowing HIV status: same-day mobile HIV testing in Zimbabwe. J Acquir Immune Defic Syndr. 2006;41:218–224. doi: 10.1097/01.qai.0000179455.01068.ab. [DOI] [PubMed] [Google Scholar]
- 24.deGraft-Johnson J, Paz-Soldan V, Kasote A, et al. HIV voluntary counseling and testing service preferences in a rural Malawi population. AIDS Behav. 2005;9:475–484. doi: 10.1007/s10461-005-9018-x. [DOI] [PubMed] [Google Scholar]
- 25.Sherr L, Lopman B, Kakowa M, et al. Voluntary counselling and testing: uptake, impact on sexual behaviour, and HIV incidence in a rural Zimbabwean cohort. AIDS. 2007;21:851–860. doi: 10.1097/QAD.0b013e32805e8711. [DOI] [PubMed] [Google Scholar]


