Abstract
Background
Though enzyme-replacement therapy (ERT) with alglucosidase alfa has significantly improved the prospects for patients with classic infantile Pompe disease, some 50 % of treated infants do not survive ventilator-free beyond the age of 3 years. We investigated whether higher and more frequent dosing of alglucosidase alfa improves outcome.
Methods
Eight cross-reactive immunological material (CRIM) positive patients were included in the study. All had fully deleterious mutations in both GAA alleles. Four received a dose of 20 mg/kg every other week (eow) and four received 40 mg/kg/week. Survival, ventilator-free survival, left-ventricular mass index (LVMI), motor outcome, infusion-associated reactions (IARs), and antibody formation were evaluated.
Results
All eight patients were alive at study end, seven of them remained ventilator-free. The patient who became ventilator dependent was treated with 20 mg/kg eow. Three of the four patients receiving 20 mg/kg eow learned to walk; two of them maintained this ability at study end. All four patients receiving 40 mg/kg/week acquired and maintained the ability to walk at study end (ages of 3.3–5.6 years), even though their baseline motor functioning was poorer. There were no apparent differences between the two dose groups with respect to the effect of ERT on LVMI, the number of IARs and antibody formation.
Conclusions
Our data may suggest that a dose of 40 mg/kg/week improves outcome of CRIM positive patients over that brought by the currently recommended dose of 20 mg/kg eow. Larger studies are needed to draw definite conclusions.
Background
Pompe disease (glycogen storage disease type II, OMIM #232300) is a rare, autosomal recessive lysosomal storage disorder caused by deficiency of acid α-glucosidase and characterized by lysosomal glycogen storage, mainly in muscle tissue (Hirschhorn and Reuser 2001). Depending largely on how much enzyme activity is preserved, it can present at different ages, from soon after birth to late adulthood. Patients with the classic infantile form present in the first months of life with generalized muscle weakness, hypertrophic cardiomyopathy, respiratory problems, and feeding difficulties (van den Hout et al 2003). If untreated, they usually die before one year of age due to cardio-respiratory insufficiency.
Patients’ prospects were significantly improved in 2006, when enzyme-replacement therapy (ERT) with recombinant human acid α-glucosidase (Myozyme®, alglucosidase alfa) was approved. ERT prolongs lifespan, improves cardiac hypertrophy, and enables patients to reach previously unmet motor milestones (Van den Hout et al 2000, 2004; Kishnani et al 2007, 2009; Chakrapani et al 2010; Hahn et al 2015). However, response to treatment varies between patients. When treated with either 20 or 40 mg/kg every other week (eow), approximately half of patients with classic infantile Pompe disease do not survive ventilator-free beyond the age of 3 years (Kishnani et al 2009). Similarly, a substantial proportion of patients do not learn to walk, and nearly all retain residual muscle weakness (Muller et al 2009; Case et al 2012; van Gelder et al 2012). Effective clearance of glycogen from skeletal muscle is reported in only a small number of patients (Winkel et al 2003; Van den Hout et al 2004; Thurberg et al 2006; Kishnani et al 2007, 2009). Preclinical studies in mice (Bijvoet et al 1999; Raben et al 2003) and clinical studies in infantile patients (Van den Hout et al 2000, 2004; Kishnani et al 2007, 2009; McVie-Wylie et al 2008) have shown that the reduction in glycogen levels in skeletal muscle is dose-dependent. On the basis of these findings and of the published intracellular half-life of alpha-glucosidase (Van der Ploeg et al 1988, 1991; Kamphoven 2004; Maga et al 2013), we estimated that patients might benefit from a higher and more frequent dose. We therefore treated affected infants with a dose of 40 mg/kg/week, i.e., the dose previously administered to four infants treated with recombinant human acid α-glucosidase from rabbit milk (Van den Hout et al 2000, 2004). The safety and efficacy of this higher and more frequent dosing regimen was compared with that of the recommended dose of 20 mg/kg eow.
Methods
Patients
Classic infantile Pompe disease was defined as symptoms of muscle weakness within six months of birth, hypertrophic cardiomyopathy, and confirmation of total deficiency of acid α-glucosidase (GAA) activity combined with the finding of pathogenic mutations in both GAA alleles. From 2009 on we treated new patients with 40 mg/kg/week. In the current study we compared patients who started treatment with the recommended dose of 20 mg/kg eow (start before 2009) to patients who started with a dose of 40 mg/kg/week (start after 2009) and who had received the treatment for at least 3 years. Data of this ongoing investigator driven study were included until April 1 2014; or until a dose change. The study was performed independent from industry. The Medical Ethical Committee at Erasmus MC University Medical Center approved the protocols and all parents gave written informed consent.
None of the patients received immunomodulation. Only CRIM positive classic infantile patients were included, which means that the combined set of very severe mutations led to the production of at least some in-active alpha-glucosidase protein. Due to the small number of patients no comparative statistics were applied.
Clinical efficacy
Clinical efficacy was measured by assessing survival, ventilator-free survival, number of hospitalizations for respiratory infections, cardiac dimensions, and motor function. Cardiac dimensions were measured by 2D-guided M-mode echocardiographic tracings (using a Philips iE33 xMAtrix Echocardiography System, Philips Medical Systems, Andover, MA, USA), at baseline and at regular intervals thereafter. Left-ventricular mass index (LVMI) was calculated as a measure for hypertrophic cardiomyopathy (LVMI > +2z-scores (Poutanen and Jokinen 2007)) and left ventricular internal dimension (LVID) as a measure for ventricular dilatation. Motor function was examined using the Alberta Infant Motor Scale (AIMS) (Piper and Darrah 1994) and the achievement of motor milestones was examined at regular clinical assessments.
Safety
Safety assessments included the monitoring of infusion-associated reactions (IARs). Adverse events that were judged to be possibly, probably or definitely related to ERT were considered to be IARs. The severity of each IAR was indexed by clinical judgment as mild, moderate or severe (Van den Hout et al 2004).
Before enzyme infusions, blood samples were drawn at regular intervals to measure antibodies to ERT with an enzyme-linked immunosorbent assay (ELISA) (van Gelder et al 2014).
Pharmacokinetic analysis
To determine the activity of acid α-glucosidase in the blood circulation and the rate of alglucosidase alfa clearance in relation to dosing, we measured the activity in plasma during enzyme infusions with 20 mg/kg and 40 mg/kg. Blood samples were drawn before the start of the infusion, at 2 and 3 h after start, at 15 min before the end, at the end of infusion, and then 15, 30, 60, and 120 min thereafter.
To determine the percentage of the enzyme in the blood that was antibody-bound, patients’ plasma samples were incubated in the presence of Protein-A Sepharose beads to bind antibody-bound alglucosidase alfa, and in parallel in the presence of Sepharose beads only (control). After removal of the beads by centrifugation, acid α-glucosidase activity was measured in the supernatant (de Vries et al 2010). Pre-infusion serum samples were collected to determine the corresponding patients’ antibody titers by ELISA (van Gelder et al 2014).
Results
Patients
We included eight patients with classic infantile Pompe disease, four of whom were treated with alglucosidase alfa in a dose of 20 mg/kg eow and four with 40 mg/kg/week. The patients’ characteristics are summarized in Table 1. Patients in the 20 mg/kg eow dose group started ERT at a median age of 0.9 months (range 0.1–2.2 months) vs. a median age of 3.1 months (range 0.3–4.6 months) in the 40 mg/kg/week group. The median age at study end was 4.1 years (range 1.7–9.4 years) in the 20 mg/kg eow dose group and 3.5 years (range 3.3–5.6 years) in the 40 mg/kg/week dose group. All patients had very severe mutations in the GAA gene (Table 1, www.pompecenter.nl).
Table 1.
Patient characteristics related to infusion-associated reactions (IARs)
| Patient | Gender | Age at start of ERT in months | Age at study end in months (years) | Mutation I | Mutation II | Total no. of IARs possibly related to ERT (no. severe) | ERT duration at first IAR in months (in years) | ERT duration at last IAR in months (in years) |
|---|---|---|---|---|---|---|---|---|
| 20 mg/kg eow | ||||||||
| 1 | M | 0.1 | 33 (2.7)# | c.1460 T > C | c.1460 T > C | 18 (1) | 2.9 (0.2) | 29.5 (2.5) |
| 2 | F | 0.5 | 113 (9.4) | c.2481 + 102_2646 + 31del | c.2481 + 102_2646 + 31del | None | NA | NA |
| 3 | M | 1.2 | 66 (5.5) | c.1933G > T | c.525delT | 27 (0) | 3.2 (0.3) | 19.9 (1.7) |
| 4 | M | 2.2 | 20 (1.7) | c.2481 + 102_2646 + 31del | c.525delT | 3 (1) | 8.1 (0.7) | 17.3 (1.4) |
| Total | 48 (2) | |||||||
| 40 mg/kg/week | ||||||||
| 5 | F | 0.3 | 42 (3.5) | c.525delT | c.1933G > A | 2 (0) | 1.4 (0.1) | 9.4 (0.8) |
| 6 | F | 2.4 | 67 (5.6) | c.2481 + 102_2646 + 31del | c.2481 + 102_2646 + 31del | 70 (6) | 0.7 (0.1) | 37.6 (3.1) |
| 7 | M | 3.8 | 39 (3.3) | c.2481 + 102_2646 + 31del | c.525delT | 10 (0) | 0.9 (0.1) | 10.3 (0.9) |
| 8 | F | 4.6 | 41 (3.4) | c.378_379del | c.2104C > T | 5 (0) | 9.7 (0.8) | 12.2 (1.0) |
| Total | 87 (6) | |||||||
M male; F female; eow every other week; IAR infusion-associated reaction; ERT enzyme-replacement therapy, NA not applicable
aPatient developed respiratory insufficiency
Clinical efficacy
Survival and ventilator-free survival
At baseline, four of the eight patients required supplemental oxygen; 50 % in both dose groups. Oxygen supply was discontinued in all patients within months after start of treatment.
At study end, one of the four patients in the 20 mg/kg eow dose group had developed respiratory insufficiency and became ventilator dependent at the age of 2.7 years during a pneumonia incident (Table 1). In the 40 mg/kg/week group none had developed respiratory insufficiency. All patients are alive.
Hospital admissions for respiratory infections
After the start of ERT, three of the four patients treated with 20 mg/kg eow were repeatedly hospitalized for respiratory infections or aspiration pneumonias, the number of admissions ranged from 3 to 5. In the 40 mg/kg/week group none of the patients were admitted for respiratory infections or aspiration pneumonias since the start of ERT and all were discharged from hospital within 3 weeks after the start of ERT.
Cardiac outcome
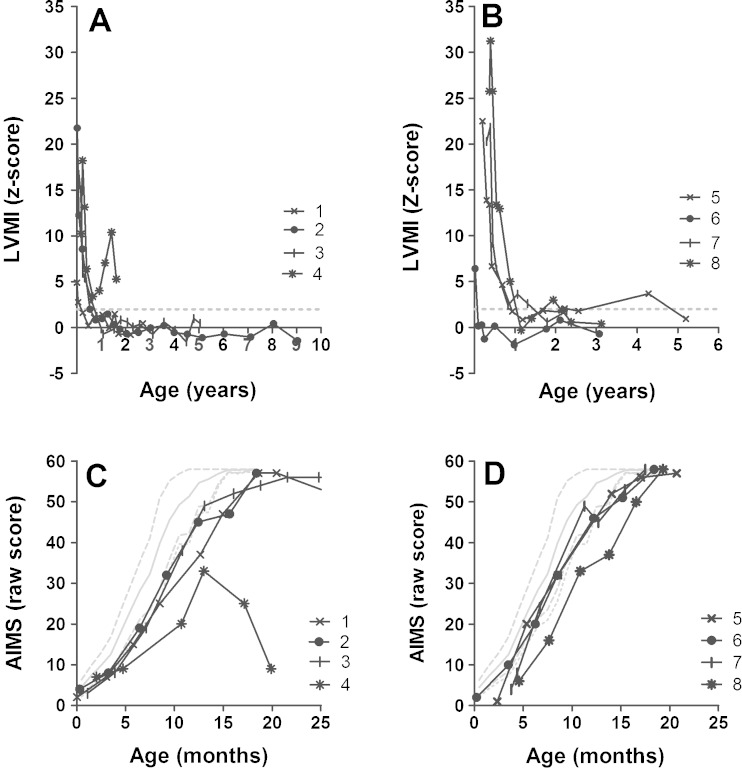
Median baseline LVMI was similar in both groups; in the 20 mg/kg eow group (median z-score +13.5, range z-score 4.9–21.8) and in the 40 mg/kg/week dose group (median z-score +21.4, range z-score 6.4–25.8). LVMI steadily decreased in both dose groups (Fig. 1a, b). At study end, LVMI was within normal limits in three of four patients in the 20 mg/kg eow dose group and in all patients in the 40 mg/kg/week dose group.
Fig. 1.
Left-ventricular mass index (LVMI) z-scores and Alberta infant motor scale scores over time. The different symbols represent different patients. LVMI 20 mg/kg eow (a) and 40 mg/kg/week group (b); The dashed gray line represents the upper limit of normal (+2 z-scores). AIMS 20 mg/kg eow (c) and 40 mg/kg/week group (d). The different symbols represent different patients. Gray solid line: p50; dashed gray line: p90 and p10; dotted gray line: p5
Important to note is that one of the patients, treated with 40 mg/kg/week, had severe left-ventricular dilatation and severe mitral valve regurgitation at baseline, which was considered to be life threatening by the treating cardiologist. After 1.6 years of treatment this patient’s LVMI had normalized and mitral regurgitation had become moderate. End-diastolic left ventricular internal dimension (LVIDd) and shortening fraction had also become normal (Kampmann et al 2000; Park 2008).
Motor function
At baseline, all eight patients showed symptoms of muscle weakness, including head lag and axial hypotonia; six had AIMS scores below the 5th percentile (2/4 in the 20 mg/kg eow and 4/4 in the 40 mg/kg/week dose groups (Fig. 1c, d)). During treatment, seven of the eight patients ultimately approached the maximal AIMS score and learned to walk: 3/4 in the 20 mg/kg eow dose group (median age at walking 16 months, range 15–17 months), and 4/4 in the 40 mg/kg/week dose group (median age at walking 15 months, range 14–17 months). Over time, some patients lost motor milestones (Fig. 1c). One patient who had initially learned to walk lost this skill after becoming ventilator-dependent at the age of 2.7 years. The only patient who did not learn to walk temporarily lost the ability to attain a sitting position after a respiratory syncytial virus infection at the age of 1.3 years. The loss of motor milestones was observed only in the 20 mg/kg eow group and not in the 40 mg/kg/week dose group. At study end, two of the four patients in the 20 mg/kg eow group were able to walk compared to all four patients in the 40 mg/kg/week group. Yet, muscular problems such as facial-muscle weakness, weakness of the neck flexors, and ankle dorsiflexors weakness were observed in patients treated with 40 mg/kg/week.
Safety
Infusion-associated reactions
IARs were experienced by 3/4 patients treated with 20 mg/kg eow and by 4/4 patients treated with 40 mg/kg/week (Table 1). The number of IARs per patient varied substantially. One patient in the 40 mg/kg/week dose group had 70 IARs (over 50 % of all IARs), six of them were severe. Remarkably, the IARs started within minutes of the start of the infusion, when the infusion rate was still slow. Total IgE, serum tryptase, and complement levels were within the normal range. Two patients treated with 20 mg/kg eow had one severe IAR each. The most common IARs were exanthema, fever, and decreased oxygen saturation. All IARs could be controlled by slowing the infusion rates and prolonging the duration of the infusion, with or without the administration of antihistamines and/or steroids. No patients discontinued treatment because of IARs, all recovered without sequelae, and premedication could be stopped. At the end of the study, 4/4 patients treated with 40 mg/kg/week had been IAR-free for at least 1.5 years and all received infusions at home.
Antibody formation
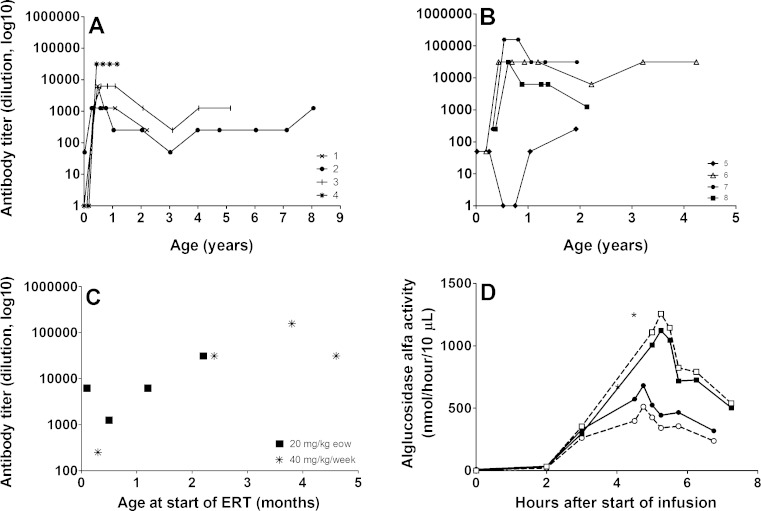
Figure 2a, b shows the antibody titers to alglucosidase alfa of the two groups over the entire study period. In the 20 mg/kg eow dose group the median peak antibody titer was 1:6250 (range 1:1250–1:31,250); in the 40 mg/kg/week dose group the median peak was 1:31,250 (range 1:250–1:156,250).
Fig. 2.
Antibody titers to alglucosidase alfa and enzyme activity in plasma using doses of either 20 mg/kg or 40 mg/kg. Antibody titers to alglucosidase alfa over time in 20 mg/kg eow (a) and 40 mg/kg/week group (b). Peak antibody titers in relation to age at start of ERT. Patients received either 20 mg/kg eow (squares) or 40 mg/kg/week (asterisks) (c). Enzyme activity (d): blood samples were collected just before the start of infusion (0 h) and at regular time intervals thereafter. A dose of 20 mg/kg (circles) and 40 mg/kg (squares) were given to the same patient (patient 3) at 1 week interval after 5.5 years of therapy (titer 1:6250). Closed symbols represent total acid α-glucosidase activity in the plasma; open symbols represent the amount of activity that was not antibody-bound. The activity in the supernatant was measured with MUGlc and is expressed in nmol 4 MU liberated per 10 μl supernatant per hour. NB: Even though the enzyme-activity assay is a standardized and validated assay there is always a slight variation in the figures obtained. All samples were analyzed as part of one experiment. The total set of analyses were performed three times with comparable results
Peak antibody titers of patients who started ERT before the age of 2 months ranged from 1:50–1:6250, those of patients who started ERT later ranged from 1:31,250–1:156,250 (Fig. 2c).
Pharmacokinetic profile
We studied differences in the pharmacokinetics of alglucosidase alfa administrations of 20 mg/kg and 40 mg/kg by giving both doses to the same patient at an interval of 1 week. A 40 mg/kg infusion led to approximately twice the enzyme activity in plasma as compared to a 20 mg/kg infusion (Fig. 2d). The plasma half-life seemed independent of the dose.
Around the time that these experiments were performed, this patient’s antibody titer was 1:6250. Using a Protein-A Sepharose based precipitation method, we could not detect substantial amounts of antibody-bound alglucosidase alfa during enzyme infusion (Fig. 2d). Neither could we detect antibody-bound alglucosidase alfa in the plasma of three other patients who received 40 mg/kg/week and had antibody titers ranging from 1:1250 to 1:31,250 (patients 6, 7, and 8) at the time of investigation.
Dose increase at time of clinical deterioration
In three of the four patients in the 20 mg/kg eow dose group the dose was increased to 40 mg/kg/week (ages 1.7, 2.7 and 5.5 years). This decision was made because the patients experienced life threatening respiratory infections leading to respiratory insufficiency in one of them.
After dose increase, respiratory infections disappeared in two patients. In the third patient, who had become ventilator dependent, ventilation remained required in supine position and during respiratory infections during the day. The two patients who were not able to walk did not regain walking ability. Patients are all alive 5 years after dose increase.
Discussion
It is unquestionable that the introduction of enzyme replacement therapy has significantly improved the life expectancy of patients with classic infantile Pompe disease (Van den Hout et al 2000, 2004; Kishnani et al 2007, 2009; Chakrapani et al 2010). Nevertheless, nearly 50 % of the infants treated do not survive ventilator-free (Kishnani et al 2007, 2009). In this study we evaluated the efficacy and safety of a higher and more frequent dosing regimen, which we hoped would improve the patients’ clinical outcome.
Preclinical studies in mice have shown a dose dependent uptake of alglucosidase alfa in the range from 10 to 100 mg/kg (Raben et al 2003; Kamphoven 2004; McVie-Wylie et al 2008; Khanna et al 2012). In the very first clinical study in which we treated classic infantile patients with recombinant human alpha-glucosidase from rabbit milk, we observed a similar dose dependent effect in that the alpha-glucosidase activity in the skeletal muscle only normalized when the dose was increased from 15 to 20 mg/kg/week to 40 mg/kg/week (Van den Hout et al 2000, 2004, Winkel et al 2003). It is known that muscle cells are hard to treat since only a small fraction of infused enzyme actually reaches the muscle cells. Further it is by now generally accepted that treatment needs to be started before irreversible muscle damage has occurred. This combined experience was reason for us to treat patients with a dose of 40 mg/kg/week from start and not to wait until patients deteriorated. Earlier no difference in clinical response was found between infantile patients treated with either 20 mg/kg/eow and 40 mg/kg/eow (Kishnani et al 2009). This might be attributed to the lower dose and larger dose interval. Another factor that may have played a role is that there were more CRIM negative patients in the higher dose group (Kishnani et al 2009; Banugaria et al 2011, van Gelder et al 2014). CRIM negative patients tend to perform poorer. We therefore excluded CRIM negative patients from the current study.
The most notable contrast we observed between the two dose groups was the difference in overall clinical condition, which was reflected in the difference in hospital admissions for the two groups: while none of the patients treated with 40 mg/kg/week had ever had respiratory infections requiring hospitalization, 3/4 patients treated with 20 mg/kg eow required frequent readmissions. Consequently, one of these patients developed respiratory insufficiency at the age of 2.7 years. Our study results suggest that the 40 mg/kg/week dosing regimen helps to stabilize or improve the respiratory condition of affected infants better. Similarly, motor function appeared to be better in the 40 mg/kg/week dose group, all of whom learned to walk and maintained the ability to do so. Unlike 3/4 patients treated with 20 mg/kg eow learned to walk and only 2/4 could still walk at the end of the study. The loss of motor milestones in the 20 mg/kg eow dose group was preceded by infections requiring hospital admissions. Importantly walking was not regained in our patients when the dose was increased after deterioration. Recently two studies also reported minor effects of dose increase when patients perform poorly (Case et al 2015; Hahn et al 2015). It should also be noted that response to ERT varies between patients treated with the same dose. This is illustrated by 1/4 patients treated with the lower dose of 20 mg/kg eow, who performed well until the end of the follow-up at the age of 9 years.
With regard to cardiac hypertrophy, both dosing regimens worked equally well, which is explained by the fact that a lower dose is required to correct or prevent the cardiac hypertrophy compared to the skeletal muscle weakness (Bijvoet et al 1999; Van den Hout et al 2000; Raben et al 2003). For the same reason, adults with Pompe disease with residual α-glucosidase activities of up to 25 % do not generally develop hypertrophic cardiomyopathy, while they do have skeletal muscle weakness (Hirschhorn and Reuser 2001).
Although we observed no clear differences in safety parameters, the small numbers do not allow us to draw firm conclusions. While nearly all patients in each dose group experienced IARs the overall number of IARs was higher in the 40 mg/kg/week dose group. This was largely due to a single patient that had had over 50 % of the total number of IARs. A similar pattern was observed in the pivotal trials (Kishnani et al 2007, 2009). The patient with most IARs in our study had recurrent episodes of exanthema, coughing and vomiting, occasionally accompanied by saturation drops. Remarkably, the IARs started within minutes of the start of the infusion, when the infusion rate was still slow. Total IgE, serum tryptase and complement levels were within the normal range. While this patient had a relatively high sustained antibody titer, the titer was similar to that of other patients who did not develop as many IARs. At the time of writing, the patient was receiving home-based enzyme therapy without problems, and time since last IAR was over 2 years.
It is well recognized that therapeutic proteins can induce an immunological response that neutralizes the effect of ERT. Three of the four patients treated with 40 mg/kg/week and two of the four treated with 20 mg/kg eow developed a peak antibody titer of 31,250 which was estimated to be the highest titer without significant consequences for ERT at a dose of 40 mg/kg (van Gelder et al 2014). Using pharmacokinetic studies in the present study, we could not detect substantial amounts of antibody-bound alglucosidase alfa during enzyme infusion in patients whose antibody titers ranged from 1:6250 to 1:31,250. One patient receiving 40 mg/kg/week had a peak antibody titer of 1:156,250, which later declined to 1:31,250. According to earlier estimates, as much as 54 % of the administered enzyme (about 10 mg/kg) is antibody-bound at a dose of 20 mg/kg and a titer of 1:156,250 (van Gelder et al 2014). If a similar amount (10 mg/kg) were bound upon administration of 40 mg/kg, about 30 mg/kg would theoretically still be available for uptake in the target tissues.
Overall, we found no apparent correlation between the level of antibodies and the dose of ERT, although patients treated with 40 mg/kg/week tended to develop higher antibody titers than those receiving 20 mg/kg eow. This is consistent with a previous study that compared the level of antibody titers between patients treated with 20 or 40 mg/kg eow (Banugaria et al 2011). In line with previous observations (Khallaf et al 2013; van Gelder et al 2014) the patient's peak antibody titer seemed to be related to the age at start of therapy.
A further point that requires attention is that muscular problems were still observed in patients treated with 40 mg/kg/week. This may be due to insufficient glycogen clearance. As glycogen also accumulates in neural tissues, including motor neurons of the spinal cord and peripheral nerves (Gambetti et al 1971), we cannot exclude the possibility that neurological damage plays a role as well.
Our study describes a limited number of CRIM positive patients. We have chosen to report on children who received at least 3 years ERT in a dose of 40 mg/kg/week. Inclusion of more patients and longer follow-up will be needed to get the full picture. So far our group of CRIM positive children starting on 40 mg/kg/week seems to have a better outcome than those who started on 20 mg/kg eow. Our data suggest that the 40 mg/kg/week has the best effect when applied from the start.
Abbreviations
Eow, every other week; LVMI, left-ventricular mass index; IAR, infusion-associated reaction; ERT, enzyme-replacement therapy; GAA, acid α-glucosidase; AIMS, Alberta Infant Motor Scale; ELISA, enzyme-linked immunosorbent assay; MUGlc, 4-methylumbelliferyl-α-D-glucopyranoside; MU, 4-methylumbelliferon; CRIM, cross-reactive immunological material.
Acknowledgments
The authors would like to thank all patients and their parents for participating in this study. We are grateful to R. Nelisse, and J. Koemans-Schouten for material and data collection, to L. Özkan for technical assistance, and to D. Alexander for his critical reading of the manuscript. Financial support was obtained from ZonMw—Dutch Organization for Healthcare Research and Innovation of Care (Grant 152001005), ‘Prinses Beatrix Fonds (project number OP07-08)’, and the 7th Frame Program ‘EUCLYD—a European Consortium for Lysosomal Storage Diseases’ of the European Union (health F2/2008 grant agreement 201678).
Compliance with ethical guidelines
Competing interests
AT van der Ploeg, AJJ Reuser and NAME van der Beek have provided consulting services for various industries in the field of Pompe’s disease under agreements with Erasmus MC.
Conflict of interest
None.
Study in human subjects
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all parents of patients for being included in the study.
References
- Banugaria SG, Prater SN, Ng YK, et al. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: Lessons learned from infantile Pompe disease. Genet Med. 2011;13:729–736. doi: 10.1097/GIM.0b013e3182174703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijvoet AG, Van Hirtum H, Kroos MA, et al. Human acid alpha-glucosidase from rabbit milk has therapeutic effect in mice with glycogen storage disease type II. Hum Mol Genet. 1999;8:2145–2153. doi: 10.1093/hmg/8.12.2145. [DOI] [PubMed] [Google Scholar]
- Case LE, Beckemeyer AA, Kishnani PS. Infantile Pompe disease on ERT: update on clinical presentation, musculoskeletal management, and exercise considerations. Am J Med Genet C: Semin Med Genet. 2012;160:69–79. doi: 10.1002/ajmg.c.31321. [DOI] [PubMed] [Google Scholar]
- Case LE, Bjartmar C, Morgan C, et al. Safety and efficacy of alternative alglucosidase alfa regimens in Pompe disease. Neuromuscul Disord. 2015;25:321–332. doi: 10.1016/j.nmd.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Chakrapani A, Vellodi A, Robinson P, Jones S, Wraith JE. Treatment of infantile Pompe disease with alglucosidase alpha: the UK experience. J Inherit Metab Dis. 2010;33:747–750. doi: 10.1007/s10545-010-9206-3. [DOI] [PubMed] [Google Scholar]
- de Vries JM, van der Beek NA, Kroos MA, et al. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol Genet Metab. 2010;101:338–345. doi: 10.1016/j.ymgme.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Gambetti P, DiMauro S, Baker L. Nervous system in Pompe's disease. Ultrastructure and biochemistry. J Neuropathol Exp Neurol. 1971;30:412–430. doi: 10.1097/00005072-197107000-00008. [DOI] [PubMed] [Google Scholar]
- Hahn A, Praetorius S, Karabul N, et al. Outcome of patients with classical infantile pompe disease receiving enzyme replacement therapy in Germany. JIMD Rep. 2015;20:65–75. doi: 10.1007/8904_2014_392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R, Reuser AJJ (2001) Glycogen storage disease type II: acid alpha-glucosidase (acid maltase) deficiency. In: Scriver C, Beaudet A, Valle D, Sly W (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New-York, pp 3389–3420
- Kamphoven J (2004) Pompe's disease; the mouse model as model in the development of enzyme therapy. PhD Thesis. Erasmus MC University Medical Center, Department of Clinical Genetics
- Kampmann C, Wiethoff CM, Wenzel A, et al. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart. 2000;83:667–672. doi: 10.1136/heart.83.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khallaf HH, Propst J, Geffrard S, Botha E, Pervaiz MA. CRIM-negative pompe disease patients with satisfactory clinical outcomes on enzyme replacement therapy. JIMD Rep. 2013;9:133–137. doi: 10.1007/8904_2012_192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Flanagan JJ, Feng J, et al. The pharmacological chaperone AT2220 increases recombinant human acid alpha-glucosidase uptake and glycogen reduction in a mouse model of Pompe disease. PLoS One. 2012;7:e40776. doi: 10.1371/journal.pone.0040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani PS, Corzo D, Nicolino M et al (2007) Recombinant human acid alpha-glucosidase — major clinical benefits in infantile-onset Pompe disease. Neurology 68:99–109 [DOI] [PubMed]
- Kishnani PS, Corzo D, Leslie ND, et al. Early treatment with alglucosidase alpha prolongs long-term survival of infants with Pompe disease. Pediatr Res. 2009;66:329–335. doi: 10.1203/PDR.0b013e3181b24e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga JA, Zhou JH, Kambampati R, et al. Glycosylation-independent lysosomal targeting of acid alpha-glucosidase enhances muscle glycogen clearance in pompe mice. J Biol Chem. 2013;288:1428–1438. doi: 10.1074/jbc.M112.438663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVie-Wylie AJ, Lee KL, Qiu H, et al. Biochemical and pharmacological characterization of different recombinant acid alpha-glucosidase preparations evaluated for the treatment of Pompe disease. Mol Genet Metab. 2008;94:448–455. doi: 10.1016/j.ymgme.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CSJH, O’Grady G, Suarez AH, Heller JH, Kishnani PS. Language and speech function in children with infantile Pompe disease. J Pediatr Neurol. 2009;7:147–156. [Google Scholar]
- Park MK (2008) Pediatric cardiology, 5th edn. Elsevier, Amsterdam
- Piper MC, Darrah J (1994) Motor assessment of the developing infant. Saunders, Philadelphia
- Poutanen T, Jokinen E. Left ventricular mass in 169 healthy children and young adults assessed by three-dimensional echocardiography. Pediatr Cardiol. 2007;28:201–207. doi: 10.1007/s00246-006-0101-5. [DOI] [PubMed] [Google Scholar]
- Raben N, Danon M, Gilbert AL, et al. Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab. 2003;80:159–169. doi: 10.1016/j.ymgme.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Thurberg BL, Maloney CL, Vaccaro C, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for pompe disease. Lab Investig. 2006;86:1208–1220. doi: 10.1038/labinvest.3700484. [DOI] [PubMed] [Google Scholar]
- Van den Hout H, Reuser AJ, Vulto AG, Loonen MC, Cromme-Dijkhuis A, Van der Ploeg AT. Recombinant human alpha-glucosidase from rabbit milk in Pompe patients. Lancet. 2000;356:397–398. doi: 10.1016/S0140-6736(00)02533-2. [DOI] [PubMed] [Google Scholar]
- van den Hout HM, Hop W, van Diggelen OP, et al. The natural course of infantile Pompe's disease: 20 original cases compared with 133 cases from the literature. Pediatrics. 2003;112:332–340. doi: 10.1542/peds.112.2.332. [DOI] [PubMed] [Google Scholar]
- Van den Hout JM, Kamphoven JH, Winkel LP, et al. Long-term intravenous treatment of Pompe disease with recombinant human alpha-glucosidase from milk. Pediatrics. 2004;113:e448–e457. doi: 10.1542/peds.113.5.e448. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg AT, Loonen MC, Bolhuis PA, Busch HM, Reuser AJ, Galjaard H. Receptor-mediated uptake of acid alpha-glucosidase corrects lysosomal glycogen storage in cultured skeletal muscle. Pediatr Res. 1988;24:90–94. doi: 10.1203/00006450-198807000-00021. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg AT, Kroos MA, Willemsen R, Brons NH, Reuser AJ. Intravenous administration of phosphorylated acid alpha-glucosidase leads to uptake of enzyme in heart and skeletal muscle of mice. J Clin Invest. 1991;87:513–518. doi: 10.1172/JCI115025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder CM, van Capelle CI, Ebbink BJ, et al. Facial-muscle weakness, speech disorders and dysphagia are common in patients with classic infantile Pompe disease treated with enzyme therapy. J Inherit Metab Dis. 2012;35:505–511. doi: 10.1007/s10545-011-9404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder CM, Hoogeveen-Westerveld M, Kroos MA, Plug I, van der Ploeg AT, Reuser AJ. Enzyme therapy and immune response in relation to CRIM status: the Dutch experience in classic infantile Pompe disease. J Inherit Metab Dis. 2014;38(2):305–314. doi: 10.1007/s10545-014-9707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel LP, Kamphoven JH, van den Hout HJ, et al. Morphological changes in muscle tissue of patients with infantile Pompe's disease receiving enzyme replacement therapy. Muscle Nerve. 2003;27:743–751. doi: 10.1002/mus.10381. [DOI] [PubMed] [Google Scholar]