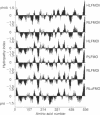
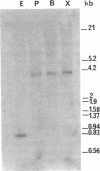
Abstract
Complementary DNA (cDNA) clones encoding the adult human liver flavin-containing monooxygenase (FMO; dimethylaniline N-oxidase, EC 1.14.13.8) were isolated from lambda gt10 and lambda gt11 libraries. The cDNA libraries were screened with three synthetic 36-mer oligonucleotide probes derived from the nucleic acid sequence of the pig liver FMO cDNA. The deduced amino acid sequence for the adult human liver FMO was quite distinct from the pig liver FMO, and adult human liver FMO was designated form II (HLFMO II). The full-length cDNA sequence of HLFMO II [2119 base pairs (bp)] had an open reading frame of 1599 nucleotides, which encoded a 533-amino acid protein of Mr 59,179, a 5'-noncoding region of 136 nucleotides and a 3'-noncoding region of 369 nucleotides excluding the poly(A) tail. The deduced amino acid sequence of HLFMO II had 80% similarity with the rabbit liver FMO II but only a 52%, 55%, and 53% amino acid similarity with the rabbit liver (form I), the pig liver (form I), and fetal human liver (form I) FMOs, respectively. RNA analysis of adult human liver RNA showed that there was one HLFMO II mRNA species. Analysis of genomic DNA indicated that HLFMO II was the product of a single gene. These results indicated that the deduced amino acid sequence for HLFMO II contained highly conserved residues and suggested that FMO enzymes were closely related and, undoubtedly, derived from the same ancestral gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Peoples O. P., Walsh C. T. Acinetobacter cyclohexanone monooxygenase: gene cloning and sequence determination. J Bacteriol. 1988 Feb;170(2):781–789. doi: 10.1128/jb.170.2.781-789.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dannan G. A., Guengerich F. P. Immunochemical comparison and quantitation of microsomal flavin-containing monooxygenases in various hog, mouse, rat, rabbit, dog, and human tissues. Mol Pharmacol. 1982 Nov;22(3):787–794. [PubMed] [Google Scholar]
- Dolphin C., Shephard E. A., Povey S., Palmer C. N., Ziegler D. M., Ayesh R., Smith R. L., Phillips I. R. Cloning, primary sequence, and chromosomal mapping of a human flavin-containing monooxygenase (FMO1). J Biol Chem. 1991 Jul 5;266(19):12379–12385. [PubMed] [Google Scholar]
- Gasser R., Tynes R. E., Lawton M. P., Korsmeyer K. K., Ziegler D. M., Philpot R. M. The flavin-containing monooxygenase expressed in pig liver: primary sequence, distribution, and evidence for a single gene. Biochemistry. 1990 Jan 9;29(1):119–124. doi: 10.1021/bi00453a014. [DOI] [PubMed] [Google Scholar]
- Gold M. S., Ziegler D. M. Dimethylaniline N-oxidase and aminopyrine N-demethylase activities of human liver tissue. Xenobiotica. 1973 Mar;3(3):179–189. doi: 10.3109/00498257309151512. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Guan S. H., Falick A. M., Cashman J. R. N-terminus determination: FAD and NADP binding domain mapping of hog liver flavin-containing monooxygenase by tandem mass spectrometry. Biochem Biophys Res Commun. 1990 Jul 31;170(2):937–943. doi: 10.1016/0006-291x(90)92181-x. [DOI] [PubMed] [Google Scholar]
- Guan S. H., Falick A. M., Williams D. E., Cashman J. R. Evidence for complex formation between rabbit lung flavin-containing monooxygenase and calreticulin. Biochemistry. 1991 Oct 15;30(41):9892–9900. doi: 10.1021/bi00105a012. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lawton M. P., Gasser R., Tynes R. E., Hodgson E., Philpot R. M. The flavin-containing monooxygenase enzymes expressed in rabbit liver and lung are products of related but distinctly different genes. J Biol Chem. 1990 Apr 5;265(10):5855–5861. [PubMed] [Google Scholar]
- Lemoine A., Johann M., Cresteil T. Evidence for the presence of distinct flavin-containing monooxygenases in human tissues. Arch Biochem Biophys. 1990 Feb 1;276(2):336–342. doi: 10.1016/0003-9861(90)90729-i. [DOI] [PubMed] [Google Scholar]
- McManus M. E., Stupans I., Burgess W., Koenig J. A., Hall P. M., Birkett D. J. Flavin-containing monooxygenase activity in human liver microsomes. Drug Metab Dispos. 1987 Mar-Apr;15(2):256–261. [PubMed] [Google Scholar]
- Ozols J. Covalent structure of liver microsomal flavin-containing monooxygenase form 1. J Biol Chem. 1990 Jun 25;265(18):10289–10299. [PubMed] [Google Scholar]
- Ozols J. Multiple forms of liver microsomal flavin-containing monooxygenases: complete covalent structure of form 2. Arch Biochem Biophys. 1991 Oct;290(1):103–115. doi: 10.1016/0003-9861(91)90596-b. [DOI] [PubMed] [Google Scholar]
- Rane A. N-oxidation of a tertiary amine (N,N-dimethylaniline) by human fetal liver microsomes. Clin Pharmacol Ther. 1974 Jan;15(1):32–38. doi: 10.1002/cpt197415132. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynes R. E., Sabourin P. J., Hodgson E. Identification of distinct hepatic and pulmonary forms of microsomal flavin-containing monooxygenase in the mouse and rabbit. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1069–1075. doi: 10.1016/0006-291x(85)90294-3. [DOI] [PubMed] [Google Scholar]
- Williams D. E., Ziegler D. M., Nordin D. J., Hale S. E., Masters B. S. Rabbit lung flavin-containing monooxygenase is immunochemically and catalytically distinct from the liver enzyme. Biochem Biophys Res Commun. 1984 Nov 30;125(1):116–122. doi: 10.1016/s0006-291x(84)80342-3. [DOI] [PubMed] [Google Scholar]
- Yamada H., Yuno K., Oguri K., Yoshimura H. Multiplicity of liver microsomal flavin-containing monooxygenase in the guinea pig: its purification and characterization. Arch Biochem Biophys. 1990 Aug 1;280(2):305–312. doi: 10.1016/0003-9861(90)90334-u. [DOI] [PubMed] [Google Scholar]
- Ziegler D. M. Flavin-containing monooxygenases: catalytic mechanism and substrate specificities. Drug Metab Rev. 1988;19(1):1–32. doi: 10.3109/03602538809049617. [DOI] [PubMed] [Google Scholar]