Abstract
Background.
Often considered an inevitable part of male aging, benign prostatic hyperplasia (BPH) is the most common non-life threatening disease to affect men in Western populations. We examine age-related change in prostate size and BPH risk and related serum biomarkers among the Tsimane Amerindians of the Bolivian Amazon who live a traditional lifestyle of hunting and small-scale horticulture. The Tsimane are a critical case study for understanding the etiology of BPH as they have low levels of obesity and metabolic syndrome, as well as lower levels of testosterone than age matched U.S. males, factors associated with BPH in previous research.
Methods.
Ultrasounds were conducted on 348 men aged 28–89 years (median age 56 years). Testosterone, prostate specific antigen, sex hormone binding globulin, and glycosylated hemoglobin were examined in relationship to prostate size and BPH.
Results.
Tsimane have less than half of the BPH prevalence experienced by U.S. men, and prostate volumes 62.6% smaller. While Tsimane have low levels of testosterone and subclinical levels of metabolic syndrome compared to U.S. men, Tsimane with high testosterone were more likely to experience BPH, as were those with higher glycosylated hemoglobin, suggesting targets for clinical interventions to reduce BPH.
Conclusions.
These results have clinical significance for the growing number of men taking testosterone supplementation; even at low levels the additional testosterone exposure could be placing these men at higher risk of BPH. Overall, these data suggest that BPH may not have been an inevitable part of male aging throughout human evolutionary history.
Key Words: Benign prostatic hyperplasia, Prostate specific antigen, Testosterone, Evolutionary medicine, Metabolic syndrome
The prostate is an exocrine gland that produces a portion of the seminal fluid critical for male reproduction. In industrialized societies most men experience prostate enlargement with age. Located surrounding the urethra near the bladder entrance, prostatic hyperplasia can lead to compression of the urethra, dysuria, nocturia, incontinence, and incomplete urination (1). Autopsy data indicate that at least 40% of men in their 50s and 90% of men in their 80s experience anatomical prostate enlargement beyond 20 cc, a condition called benign prostatic hyperplasia (BPH) (1,2). BPH has important health consequences, with approximately 40% of U.S. men requiring medical treatment for BPH in their lifetime (1). In industrialized nations BPH is thought to be an inevitable aspect of aging (1), and is the most common non-life threatening disease of aging experienced by men (3). Despite only affecting the male sex, BPH is the 25th most common contributor to years lived with disability globally in 2010 (4).
High circulating androgen levels are thought to play major contributing roles to BPH etiology. Higher levels of endogenous or exogenous testosterone and dihydrotestosterone (DHT) are associated with larger prostate sizes due to increased stromal and epithelial cell proliferation, as well as inhibition of cell death (2,5–7). Androgen deprivation treatments reduce prostate size and 5-α-reductase inhibitors slow prostate growth (7,8). The prostate is vital for male reproduction, producing one quarter of ejaculate volume (9);thus while there are reproductive benefits to early prostate growth, continued prostate growth at older ages results in negative consequences.
Lifestyle conditions including obesity (10–12), metabolic syndrome (13–15), as well as low levels of physical activity (16) are associated with an increased risk of BPH and other prostatic issues. Traditional subsistence populations show little evidence of diseases associated with sedentary lifestyles (17,18). For the majority of human evolutionary history, people lived in small populations practicing hunting and foraging, with a more recent shift toward horticulture, a lifestyle that requires higher levels of physical activity compared to sedentary Western lifestyles (19). Given that most of human history occurred in environments very different from the cities and sedentary lives in which most people live today, examining prostate size and growth in a subsistence population can give insight into the etiology of the most pervasive benign disease of aging experienced by men (3).
The Tsimane, an indigenous population living in the lowland Amazonian region of Bolivia, rely on hunting, fishing, foraging, and small-scale horticulture for subsistence. The Tsimane live in villages of 30–500 people, with a population approaching 16,000 based on recent census estimates of the Tsimane Health and Life History Project (THLHP). Tsimane face relatively high levels of parasite and pathogen exposure compared to industrial populations as evidenced by high erythrocyte sedimentation rates, leukocyte and immunoglobulin levels, C-reactive protein, and diagnoses of infection (17,20). Although Tsimane experience high prevalence of infectious morbidity, there is little evidence of obesity, hypertension, heart disease, metabolic syndrome, or other diseases associated with aging in industrialized populations (17,18). Thus understanding the prevalence and correlates of BPH in a population without sedentary lifestyle risk factors is of special interest.
The Tsimane and other energy-limited populations experiencing high pathogenic stress face trade-offs between energetic allocations to immune function, and to reproductively beneficial but metabolically costly androgens (21,22). These populations show lower levels of testosterone across all adult ages as compared with men in industrialized nations (23–25), as well as slower and shallower rate of decline with age (23), or no association between testosterone and age (25). Salivary testosterone is approximately 30% lower among Tsimane men compared to age matched U.S. men controlling for BMI (25). Cumulative testosterone exposure is thought to be an important risk factor for prostate growth (26).
Thus we hypothesize that (1) Tsimane men, with significantly lower cumulative androgen exposure, as well as reduced rates of obesity and metabolic syndrome (eg glycosylated hemoglobin, HbA1c) will have smaller prostates than Western men, and (2) lower prevalence of BPH across the lifespan. The majority of circulating testosterone is bound to sex hormone binding globulin (SHBG), a carrier protein that along with albumin may limit the bioavailability of testosterone; though testosterone binding kinetics and the existence of bioavailable testosterone is a debated topic. Prostate specific antigen (PSA) is a hormone secreted by the prostate, and while there is controversy surrounding the predictive power of PSA in prostate cancer, PSA secretion is positively associated with prostate size in population samples, and thus (3) prostate size is hypothesized to positively covary with PSA (10,27–29). While Tsimane are hypothesized to have smaller prostates, reports from industrial populations indicate that men with larger prostates have increased lower urinary tract morbidity, and thus (4) we expect that Tsimane with large prostates will experience greater urinary tract morbidity (1).
Methods
Tsimane
All Tsimane men over the age of 40 years were invited from their home villages to a clinic in the nearby Bolivian market town of San Borja for medical evaluations. Transportation and food were provided by the THLHP and the acceptance rate was 92%. Of the 983 individuals (n = 495 males) who were seen in the clinic during 2009–2010 when prostate ultrasounds were conducted, a subset of 348 males were randomly selected, with 70 separate communities represented out of a possible 79 study communities. All participants provided informed consent, and protocols were approved by Institutional Review Boards at the University of California, Santa Barbara (UCSB) and University of New Mexico.
Prostate Size
A trained physician (ECL) measured prostate size with a Mindray M7 Diagnostic Ultrasound System (Shenzen, China) using a 3C5s convex array probe (3.5 MHz). Prostate volume measured from abdominal ultrasound is highly correlated with both gold-standard trans-rectal ultrasounds (TRUS) (30,31), as well as actual prostate size (32), and is less invasive. Most comparative data from industrial populations (see above) measured prostate volume with TRUS, though there is conflicting evidence as to whether TRUS accurately estimates prostate size for smaller prostates; one study (n = 440) suggests that TRUS underestimates small prostates (<30 cc) (33), while another larger study (n = 2338) finds evidence that TRUS is more accurate for smaller prostate volumes (34). If TRUS was systematically underestimating smaller prostate sizes, that would suggest that the comparative studies actually had larger prostates, and would only strengthen the finding that Tsimane have relatively small prostates. Here, prostate volume in cubic centimeters (cc) was estimated following the formula for an ellipsoid (30). For additional ultrasound details, see Supplemental Material.
Prostate volumes above 20 cc are often considered indicative of anatomical BPH (2,35), though some studies have used cutoffs of 30 cc (36) or 40 cc (11,15); men with prostates >40 cc often report more symptoms and worse outcomes (1,15). Many early studies used autopsy measurements of prostate volume (e.g., (2)), a technique recently validated by MRI studies (37). Thus while several of the population comparison samples in this paper are from older studies, there are relatively few non-pathological studies with comparable quantitative prostate volume data.
Biomarker Measures
Fasting morning testosterone, SHBG, and PSA were measured at the UCSB Human Biodemography Laboratory from serum samples. See Supplemental Material for details.
Statistical Analyses
Hormonal measures were log transformed, with the exception of HbA1c which was normally distributed. Mixed effects linear and logistic regressions were used to analyze covariates of prostate size and BPH, with participant villages coded as a random effect.
Results
The 348 men in this sample ranged from 28 to 89 years of age, with a median age of 56 years. The median BMI in this sample was 23.4 (95% CI 20.2–28.7) kg/m2, and the median body fat 18% (95% CI 13–30%), see Table 1.
Table 1.
Median Descriptive Characteristics for n = 348 Tsimane Men Stratified by Prostate Size
| Prostate <20 cc | Prostate >20 cc | All Men | All Men 95% CI | Min | Max | |
|---|---|---|---|---|---|---|
| Age | 55 | 61 | 56 | 41–79 | 28 | 89 |
| BMI | 23.34 | 23.44 | 23.40 | 20.17–28.70 | 15.43 | 34.87 |
| Body Fat (%) | 18 | 19 | 18 | 13–30 | 8 | 44 |
| Prostate Volume (cc) | 15 | 24 | 16.5 | 10–29 | 4 | 67 |
| HbA1c (%) | 5.5 | 5.6 | 5.6 | 5–6.3 | 4 | 7.3 |
| Systolic BP | 118 | 118 | 118 | 100–140 | 90 | 216 |
| Diastolic BP | 70 | 70 | 70 | 60–84 | 50 | 105 |
Prostate Volume and BPH Prevalence Among Tsimane
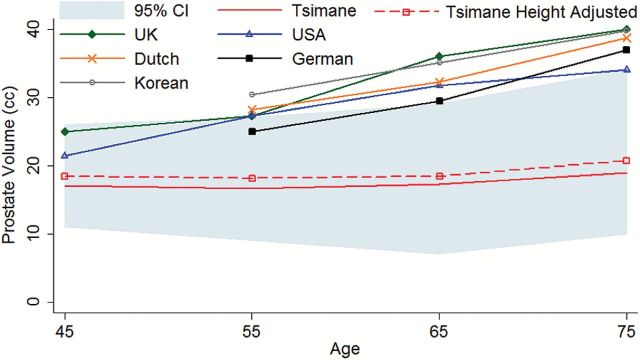
Prostate volumes ranged from 4 to 67 cc, with a median volume of 16.5 (95% CI 7–34) cc. Prostate volume increased with age equivalent to 0.109 cc/y, (p < 0.001) controlling for height (Figure 1). The prevalence of anatomical BPH (defined here as prostate size >20 cc) was positively associated with age, with the odds of BPH increasing each year by approximately 3.4% (95% CI 1.01–1.06, p = 0.002) controlling for height.
Figure 1.
Prostate volume by age. Cross population comparison of prostate volumes with a 95% confidence interval for Tsimane men. The dashed line indicates prostate size for Tsimane men if their height was scaled up to that of adult U.S. males.
Prostate Volume and BPH Prevalence: Comparison to Industrial Populations
Compared to TRUS from multiple industrial populations including 1,240 Caucasian German men (27), 3,924 Caucasian Dutch men (38), 472 Caucasian Scottish men (39), and 631 Caucasian U.S. men (40), the Tsimane have significantly smaller prostate volumes (β = −13.51 cc, p = 0.015) controlling for age and height, and a shallower rate of change with age controlling for height (β = −0.41 cc/y, p = 0.002) (see Figure 1).
The overall age standardized prevalence of BPH among men age 40–80 was 28.4% compared to 60.8% of U.S. men of the same age (2). For Tsimane men aged 60–80, an age standardized 31.7% presented with BPH, compared with 76.0% of U.S. men aged 60–80 (Table 2). Only 0.56% of this sample achieved prostate volumes greater than 40 cc compared to approximately 20% of U.S. males (15).
Table 2.
Tsimane Prevalence of BPH (Prostate>20 cc) by Age and U.S. Prevalence of Histologic BPH from Berry et al. (2)
| Tsimane | USA | |||||||
|---|---|---|---|---|---|---|---|---|
| Age | Sample (n) | Prostate Size (cc) | BPH Cases (>20 CC) | Crude Rate | Sample (n) | Prostate Size (cc) | BPH Cases | Crude Rate |
| <39 | 9 | 14.3 (3.2) | 1 | 11.1% | ||||
| 40–49 | 91 | 16.9 (4.9) | 18 | 19.8% | 94 | 29 (6) | 22 | 23.4% |
| 50–59 | 97 | 16.7 (5.7) | 24 | 24.7% | 191 | 27(8) | 81 | 42.4% |
| 60–69 | 87 | 17.8 (7.3) | 23 | 26.4% | 242 | 35(19) | 171 | 70.7% |
| 70–79 | 48 | 19.0 (6.8) | 18 | 37.5% | 221 | 41(30) | 181 | 81.9% |
| 80+ | 15 | 23.4 (14.0) | 9 | 60.0% | ||||
BMI, Body Fat, and Metabolic Markers
There was no association between body mass index (BMI) and prostate size (p = 0.82), nor were men with a BMI above the median more likely to experience BPH (p = 0.67), controlling for age (see Tables 2 and 3). Likewise, body fat percentage was not associated with prostate volume (p = 0.97), or BPH (p = 0.83), controlling for age and height. Neither systolic nor diastolic blood pressure was associated with prostate volume or BPH (all p>0.29), nor were individuals with high systolic (≥130 mmHg) or high diastolic (≥85mmHg) blood pressure more likely to have larger prostates or BPH (all p>0.43), controlling for age and height. However, men with higher HbA1c had larger prostates (β = 1.88, p = 0.033) and trended towards presentation with BPH (OR = 1.87, 95% CI 0.98–3.58, p = 0.057) controlling for age and height. Men with HbA1c greater than 6% trended toward a higher likelihood of BPH (OR = 1.70, 95% CI 0.883.27, p = 0.115), controlling for age and height.
Table 3.
Linear Regressions Examining Associations Between Covariates and Prostate Size for n = 348 Tsimane Men
| Individual Models | Combined Model | |||||
|---|---|---|---|---|---|---|
| Coefficient (cc) | Std Err | p | Coefficient (cc) | Std Err | P | |
| Age† | 0.109 | 0.030 | <0.001*** | 0.134 | 0.033 | <0.001*** |
| Log PSA | 1.745 | 0.308 | <0.001*** | |||
| HbA1c | 1.867 | 0.876 | 0.033** | 1.841 | 0.896 | 0.04** |
| Body Fat % | 0.002 | 0.068 | 0.975 | |||
| Systolic BP | 0.025 | 0.024 | 0.291 | |||
| Diastolic BP | 0.018 | 0.043 | 0.681 | |||
| Log T | 1.216 | 0.698 | 0.081* | 1.370 | 0.710 | 0.054* |
| Log SHBG | 0.528 | 0.725 | 0.466 | |||
| Height (cm)‡ | 0.065 | 0.068 | 0.342 | 0.078 | 0.076 | 0.303 |
| BMI <20 | Reference | Reference | Reference | |||
| BMI 20–25 | 2.170 | 1.777 | 0.222 | |||
| BMI 25–30 | 1.79 | 1.865 | 0.336 | |||
| BMI >30 | -0.867 | 2.597 | 0.739 | |||
Note: All models represent individual mixed-effects regressions controlling for age and height, with community as a random effect.
*p < 0.1, **p < 0.05, ***p < 0.01.
†Age is controlling for height.
‡Height is controlling for age.
Hormonal Measures and Prostate Size
Men with higher testosterone trended toward larger prostates (β = 1.22, p = 0.081), though greater testosterone is not associated with risk of BPH in a linear fashion (OR = 1.326, 95% CI 0.82–2.15, p = 0.25), controlling for age and height. Individuals in the lowest quintile of testosterone had smaller prostates and significantly less BPH than those in the top two quintiles controlling for height and age (OR = 2.65, 95% CI 1.13–6.20, p = 0.024 and OR = 2.37. 95% CI 1.00–5.60, p = 0.05).
There was a strong positive correlation between PSA and both prostate size (β = 1.74, p < 0.001) and risk of BPH (OR = 1.69, 95% CI 1.27–2.25, p < 0.001) controlling for age and height. The median PSA in this sample was 0.88ng/mL (95% CI 0.11–3.64). Men with PSA greater than 4ng/mL were more likely to present with BPH (OR = 10.43, 95% CI 1.97–55.22, p = 0.006).
SHBG was not associated with prostate size (p = 0.47), or BPH (p = 0.76).
Combined Models
A model with all potential characteristics thought to influence prostate size was also tested (Table 3); age, testosterone, and HbA1c remained in the model while other covariates were eliminated via AIC and log-likelihood ratio tests (height was included to control for individual differences in body size). PSA is a byproduct of prostate size and thus was not included in this model. A similar logistic mixed-effects regression model was also completed with BPH as an outcome; in this model age and HbA1c were the only significant covariates (Table 4).
Table 4.
Impact of Covariates on the Odds Ratio of Benign Prostatic Hyperplasia (prostates >20 cc) for n = 348 Tsimane Men.
| Individual Models | Combined Model | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | Coefficient | 95% CI | P | |
| Age† | 1.034 | (1.01, 1.06) | 0.002*** | 1.034 | (1.01, 1.06) | 0.006*** |
| Log PSA | 1.686 | (1.27, 2.25) | <0.001*** | |||
| HbA1c | 1.874 | (0.98, 3.58) | 0.057* | 1.874 | (0.98, 3.58) | 0.057* |
| Body Fat % | 0.995 | (0.95, 1.04) | 0.832 | |||
| Systolic BP | .00 | (0.98, 1.02) | 0.907 | |||
| Diastolic BP | 0.993 | (0.96, 1.02) | 0.622 | |||
| Log T | 1.326 | (0.82, 2.15) | 0.251 | |||
| Log SHBG | 1.082 | (0.65, 1.80) | 0.759 | |||
| Height (cm)‡ | 1.029 | (0.98, 1.08) | 0.233 | 1.033 | (0.98, 1.09) | 0.237 |
| BMI <20 | Reference | Reference | Reference | |||
| BMI 20–25 | 1.064 | (0.31, 3.65) | 0.922 | |||
| BMI 25–30 | 1.158 | (0.32, 4.21) | 0.824 | |||
| BMI >30 | 0.584 | (0.08, 4.12) | 0.590 | |||
Note: All models represent individual mixed-effects regressions controlling for age and height, with community as a random effect.
*p < 0.1, **p < 0.05, ***p < 0.01.†Age is controlling for height.
‡Height is controlling for age.
Urinary Tract Abnormalities
While Tsimane prostates were significantly smaller than those of men in industrialized populations, Tsimane men with prostates larger than 20 cc trended towards having more bacteria in their urine (β = 0.135, p = 0.096), especially Filamentous bacteria (β = 0.223, p = 0.009) controlling for age. Men with BPH also had more erythrocytes (β = 0.669, p < 0.001) and leukocytes in their urine (β = 1.736, p = 0.045) controlling for age.
Discussion
Tsimane men have significantly smaller prostate volumes, and a reduced rate of prostate growth with age compared to men in industrial populations. The Tsimane prevalence of BPH between the ages of 40–80 is less than half of what is seen in U.S. and British men (2,35), while more advanced cases of BPH (>40 cc) were almost non-existent (< 1% of Tsimane men). PSA is positively associated with prostate size in this population, consistent with previous studies (10,27); men with PSA greater than 4ng/mL were ten times more likely to present with BPH. Despite significantly smaller prostates and a reduced rate of BPH compared to industrial populations, Tsimane men with prostate sizes larger than 20 cc were more likely to experience urinary tract symptoms including higher bacterial loads, and blood in the urine. In this study, it was not possible to dissociate prostate cancer or prostatitis from BPH, and thus some of the larger Tsimane prostates and high PSA values could have been due to factors other than BPH, which could result in an even lower prevalence of BPH in this population.
With a few notable exceptions, research into prostate size is largely conducted in urban European or American populations (36,41,42). Studies of rural populations tend to report fewer Lower Urinary Tract Symptoms (LUTS), as well as smaller anatomical prostate size, and reduced prostate growth with age (36,41). A Korean study found that rural men with prostates larger than 30 cc comprised an age-adjusted 23.3% of their rural sample compared to 40% of an urban Korean sample (36), as measured by TRUS. We find an age-adjusted 6.6% of Tsimane presented with prostates greater than 30 cc in the current study. Only one other population has ever been reported to have a similar prevalence of small prostate size; a relatively uncontrolled study of autopsies conducted on rural Chinese farmers in the 1920s and 1930s reported a 6.6 % prevalence of BPH in men over the age of 40 years (41). Other lines of evidence suggest that reportedly smaller Chinese prostates are due to environmental conditions; Chinese migrants to Australia (median migration time 7.3 years) and Australian born men have similar prostate volumes, both of which are significantly larger than prostates of Chinese men residing in China (43), as measured by TRUS. One study conducted among the Ariaal, nomadic pastoralists practicing a traditional lifestyle in Kenya reported moderate levels of self-reported prostate symptoms (42). While this is one of the only examples of prostate research conducted in a non-industrial population, it is unfortunately difficult to make direct comparisons between the Tsimane and the Ariaal as quantitative measures of prostate size were not collected. Among the Tsimane, self-report measures of urinary issues were not systematically collected, though men with larger prostates do have higher levels of bacteria as well as erythrocytes and leukocytes in their urine. Only two men volunteered complaints related to urination during their clinical consultation; one man presented with anatomical BPH (prostate size of 23 cc), while the second did not (prostate size 16 cc). More studies need to be conducted in non-western, non-industrial populations in order to fully understand the range of variation in prostate size and etiology of BPH.
These results are consistent with previous theoretical and empirical studies suggesting that men with lower levels of testosterone have smaller prostates and are at a lower risk of BPH (6), and also that men with reduced obesity, circulating glucose, and metabolic syndrome face lower levels of BPH (10,11,14). Despite relatively low, subclinical levels of glycosylated hemoglobin, HbA1c was positively associated with prostate size. Each one percentage increase in HbA1c was associated with an increase in prostate size equivalent to nearly two decades of prostate growth. Previous studies report that high circulating glucose and insulin dysregulation are associated with prostate size (14,15,44). There are several potential biological pathways by which metabolic syndrome could impact prostate size; in vitro and in vivo experiments report that insulin is necessary for prostate growth, and that hyperinsulinemia as well as higher levels of IGF-1 and IGF-1 signaling are associated with increased prostate size (15,44). While obesity and hypertension are often associated with metabolic syndrome and prostate size (11,14,15), body fat and blood pressure were not associated with prostate size in this study. That said, Tsimane men have relatively low levels of, and little variation in hypertension and body fat (eg (17,18)). Even with subclinical levels of glycosylated hemoglobin, the strong associations between prostate size and HbA1c suggest that dietary interventions and treatment for metabolic syndrome would be an excellent starting point for reducing the risk of BPH.
Tsimane face reduced food security, high levels of parasite and pathogenic exposure, and physically intensive subsistence strategies (e.g. hunting and slash-and-burn horticulture), and thus Tsimane have a relatively low energy balance compared to industrialized populations. For prostate health, this has two potential effects; first, with reduced energy available, androgens like testosterone are maintained at a lower level (22,25). The relatively lower testosterone levels experienced by subsistence populations were likely the norm throughout the majority of human existence, while men living in industrial populations are not constrained by parasites, pathogens, or food insecurity and thus experience high, perhaps evolutionarily novel levels of testosterone. Indeed, cross-population studies find that men in more advantageous energetic conditions have both higher levels of testosterone in younger ages, as well as greater declines in testosterone at older ages (23). Disparities in cumulative testosterone exposure (26), especially during critical adolescent periods where prostate growth is the most rapid, may set men in industrial populations on a trajectory that results in BPH in late adulthood, while the lower levels of testosterone in subsistence populations may be protective against prostatic disease. Secondly, the relatively constrained energetic balance means that there is less energy and insulin to invest in tissue growth, including the prostate (44). For Tsimane men that maintain a higher energy balance, as evidenced by higher testosterone and HbA1c, there is a trend towards increased prostate size. While the mechanistic etiology linking BPH and obesity needs further research, these results indicate that even for a lean, physically active population with subclinical levels of metabolic syndrome, higher levels of HbA1c were associated with larger prostate sizes. In industrialized populations, baseline obesity and metabolic syndrome are associated with larger prostates and increased prostate growth (11,15), and reports indicate that approximately 80% of variation in treatment response can be attributed to prostate size at baseline (45).
There are two clinical policy recommendations from this line of research. The first is to begin treating both obesity and metabolic syndrome even at sub-clinical levels, before the onset of clinical BPH. The second policy implication is in relation to testosterone supplementation. Prescriptions for testosterone replacement have risen substantially in the last decade, especially for men without clear need of treatment (46). Results presented in the current study suggest that even at low levels of testosterone, men with relatively higher testosterone are at an increased risk of anatomical BPH, thus caution should be observed with any testosterone supplementation, even at relatively low concentrations.
In industrialized populations BPH is thought to be an inevitable consequence of male aging. The data presented here challenge this inevitability, and suggest that throughout much of human evolution, environmental conditions would likely have resulted in lower androgen levels, and limited access to sugar and calories, as well as increased physical activity, all of which would slow prostate growth. While the direct clinical applications of applying an evolutionary framework to a medical condition are not always apparent, BPH is a condition where applying evolutionary logic can provide profound insight into the etiology of the most common disease of male aging (47). BPH may be an example of antagonistic pleiotropy; high androgen levels and prostate growth during puberty could yield fitness benefits at younger ages, but then at older ages continued prostate enlargement can have deleterious consequences (48,49). There is little reason for natural selection to have limited prostate growth as BPH was likely limited throughout most of the human evolutionary past, and individuals that did achieve large prostate sizes would only do so at late ages after completing reproduction, a life stage often considered to be in “selection’s shadow” (48,49). Even then, the detrimental effects of an enlarged prostate are minimal compared to many other potential diseases of senescence.
Conclusion
Men living a traditional forager-horticultural lifestyle have smaller prostates and reduced prevalence of anatomical BPH compared to men living in industrial populations. In this population we see little evidence of enlarged prostates or BPH, suggesting that BPH may not be an inevitable part of male aging throughout human evolutionary history, and that industrialized life with increased risk of metabolic syndrome, sedentary lifestyle, and relatively high levels of testosterone may play a more important role in the etiology of BPH than previously considered.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
National Institutes of Health, National Institute on Aging (NIA R01AG024119-01, R56AG024119-06, and R01AG024119-07).
Supplementary Material
Acknowledgements
We would like to thank Tsimane participants, THLHP personnel, Richard Pelman, Ivan Maldonado, Nohemi Zabala, Ariel Mendez, and Megan Costa.
References
- 1. Oesterling JE. Benign prostatic hyperplasia. Medical and minimally invasive treatment options. N Engl J Med. 1995;332:99–109. doi:10.1056/NEJM199501123320207 [DOI] [PubMed] [Google Scholar]
- 2. Berry SJ, et al. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. [DOI] [PubMed] [Google Scholar]
- 3. Steiner MS, et al. The chimpanzee as a model of human benign prostatic hyperplasia. J Urol. 1999;162:1454–1461. doi:10.1016/S0022-5347(05)68340-1 [PubMed] [Google Scholar]
- 4. Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi:10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Partin AW, et al. Influence of age and endocrine factors on the volume of benign prostatic hyperplasia. J Urol. 1991;145:405–409. [DOI] [PubMed] [Google Scholar]
- 6. Jin B, et al. The effects of chronic high dose androgen or estrogen treatment on the human prostate [corrected]. J Clin Endocrinol Metab. 1996;81:4290–4295. doi:10.1210/jcem.81.12.8954029 [DOI] [PubMed] [Google Scholar]
- 7. Andriole G, et al. Dihydrotestosterone and the prostate: the scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol. 2004;172(4 Pt 1):1399–1403. doi:10.1097/01.ju.0000139539.94828.29 [DOI] [PubMed] [Google Scholar]
- 8. Kaplan SA, D’Alisera PM. Tolerability of alpha-blockade with doxazosin as a therapeutic option for symptomatic benign prostatic hyperplasia in the elderly patient: a pooled analysis of seven double-blind, placebo-controlled studies. J Gerontol A Biol Sci Med Sci. 1998;53:M201–M206. doi:10.1093/gerona/53A.3.M201 [DOI] [PubMed] [Google Scholar]
- 9. Campbell MF, Wein AJ, Kavoussi LR. Campbell-Walsh Urology/Editor-in-Chief, Alan J. Wein ; editors, Louis R. Kavoussi ... [et al.]. 9th ed. Philadelphia: W.B. Saunders; 2012. [Google Scholar]
- 10. Park S-G, Choi H-C, Cho B, Kwon Y-M, Kwon H-T, Park J-h. Effect of central obesity on prostate specific antigen measured by computerized tomography: related markers and prostate volume. J Urol. 2012;187:1589–1593. doi:10.1016/j.juro.2011.12.067 [DOI] [PubMed] [Google Scholar]
- 11. Parsons JK, et al. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol. 2013;189(1 Suppl):S102–S106. doi:10.1016/j.juro.2012.11.029 [DOI] [PubMed] [Google Scholar]
- 12. Wolinsky FD, Krygiel J, Wyrwich KW. Hospitalization for Prostate Cancer Among the Older Men in the Longitudinal Study on Aging, 1984–1991. J Gerontol A Biol Sci Med Sci. 2002;57(2):M115 M121. doi:10.1093/gerona/57.2.M115 [DOI] [PubMed] [Google Scholar]
- 13. Kupelian V, et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston area community health survey. J Urol. 2013;189(1 Suppl):S107–S114; discussion S115. doi:10.1016/j.juro.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 14.De Nunzio C, William A, Stephen JF, Edward G, Parsons JK. The Correlation Between Metabolic Syndrome and Prostatic Diseases. Eur Urol. 2011;61(3):560–570. doi:10.1016/j.eururo.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 15. Parsons JK, Carter HB, Partin AW, et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91:2562–2568. doi:10.1210/jc.2005-2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parsons JK, Kashefi C. Physical activity, benign prostatic hyperplasia, and lower urinary tract symptoms. Eur Urol. 2008;53:1228–1235. doi:10.1016/j.eururo.2008.02.019 [DOI] [PubMed] [Google Scholar]
- 17.Gurven M, et al. Inflammation and infection do not promote arterial aging and cardiovascular disease risk factors among lean horticulturalists. PLoS One. 2009;4:e6590. doi:10.1371/journal.pone.0006590. doi: 10.1371/journal.pone.0006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gurven M, et al. Does blood pressure inevitably rise with age?: longitudinal evidence among forager-horticulturalists. Hypertension. 2012;60:25–33. doi:10.1161/hypertensionaha.111.189100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gurven M, et al. Physical activity and modernization among Bolivian Amerindians. PLoS One. 2013;8:e55679. doi:10.1371/journal.pone.0055679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDade TW, et al. Predictors of C-reactive protein in Tsimane’ 2 to 15 year-olds in lowland Bolivia. Am J Phys Anthropol. 2005;128:906–913. doi:10.1002/ajpa.20222 [DOI] [PubMed] [Google Scholar]
- 21. Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am Nat. 1992;139(3):603–622. [Google Scholar]
- 22. Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. Am J Hum Biol. 2005;17:527–558. doi:10.1002/ajhb.20419 [DOI] [PubMed] [Google Scholar]
- 23. Ellison PT, et al. Population variation in age-related decline in male salivary testosterone. Hum Reprod. 2002;17:3251–3253. doi:10.1093/humrep/17.12.3251 [DOI] [PubMed] [Google Scholar]
- 24. Bribiescas R. Testosterone levels among Aché hunter-gatherer men: A functional interpretation of population variation among adult males. Hum Nat. 1996;7:163–188. doi:10.1007/BF02692109 [DOI] [PubMed] [Google Scholar]
- 25. Trumble BC, Cummings D, von Rueden Cet al. Physical competition increases testosterone among Amazonian forager-horticulturalists: a test of the ‘challenge hypothesis’. Proc R Soc B: Biol Sci. 2012;279(1739):2907–2912. doi:10.1098/rspb.2012.0455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alvarado LC. Population differences in the testosterone levels of young men are associated with prostate cancer disparities in older men. Am J Hum Biol. 2010;22(4):449–455. doi:10.1002/ajhb.21016 [DOI] [PubMed] [Google Scholar]
- 27. Berges R, Oelke M. Age-stratified normal values for prostate volume, PSA, maximum urinary flow rate, IPSS, and other LUTS/BPH indicators in the German male community-dwelling population aged 50 years or older. World J Urol. 2011;29:171–178. doi:10.1007/s00345-010-0638-z [DOI] [PubMed] [Google Scholar]
- 28. Howrey BT, et al. The impact of PSA screening on prostate cancer mortality and overdiagnosis of prostate cancer in the United States. J Gerontol A Biol Sci Med Sci. 2013;68:56–61. doi:10.1093/gerona/gls135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolf AM, Schorling JB. Preferences of elderly men for prostate-specific antigen screening and the impact of informed consent. J Gerontol A Biol Sci Med Sci. 1998;53A:M195–M200. doi:10.1093/gerona/53A.3.M195 [DOI] [PubMed] [Google Scholar]
- 30. Prassopoulos P, et al. Suprapubic versus transrectal ultrasonography in assessing the volume of the prostate and the transition zone in patients with benign prostatic hyperplasia. Abdom Imaging. 1996;21:75–77. doi:10.1007/s002619900017 [DOI] [PubMed] [Google Scholar]
- 31. Huang Foen Chung JWNC, et al. Prostate volume ultrasonography: the influence of transabdominal versus transrectal approach, device type and operator. Eur Urol. 2004;46:352–356. doi:10.1016/j.eururo.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 32. Hricak H, et al. Evaluation of prostate size: a comparison of ultrasound and magnetic resonance imaging. Urol Radiol. 1987;9:1–8. doi:10.1007/bf02932619 [DOI] [PubMed] [Google Scholar]
- 33. Bienz M, et al. Accuracy of transrectal ultrasonography to evaluate pathologic prostate weight: correlation with various prostate size groups. Urology. 2014;84:169–174. doi:10.1016/j.urology.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 34. Loeb S, et al. Accuracy of prostate weight estimation by digital rectal examination versus transrectal ultrasonography. J Urol. 2005;173:63–65. doi:10.1097/01.ju.0000145883.01068.5f [DOI] [PubMed] [Google Scholar]
- 35. Garraway WM, Lee RJ, Collins GN. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469–471. doi:10.1016/0140-6736(91)90543-X [DOI] [PubMed] [Google Scholar]
- 36. Huh JS, Kim YJ, Kim SD. Prevalence of Benign Prostatic Hyperplasia on Jeju Island: Analysis from a Cross-sectional Community-based Survey. World J Mens Health. 2012;30:131–137. doi:10.5534/wjmh.2012.30.2.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turkbey B, et al. Age-related changes in prostate zonal volumes as measured by high-resolution magnetic resonance imaging (MRI): a cross-sectional study in over 500 patients. BJU Int. 2012;110:1642–1647. doi:10.1111/j.1464-410X.2012. 11469.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blanker MH, et al. Strong effects of definition and nonresponse bias on prevalence rates of clinical benign prostatic hyperplasia: the Krimpen study of male urogenital tract problems and general health status. BJU Int. 2000;85:665–671. doi:10.1046/j.1464-410x.2000.00570.x [DOI] [PubMed] [Google Scholar]
- 39. Collins GN, et al. Relationship between prostate specific antigen, prostate volume and age in the benign prostate. Br J Urol. 1993;71:445–450. doi:10.1111/j.1464-410X.1993.tb15990.x [DOI] [PubMed] [Google Scholar]
- 40. Rhodes T, et al. Longitudinal prostate growth rates during 5 years in randomly selected community men 40 to 79 years old. J Urol. 1999;161:1174–1179. doi:10.1016/S0022-5347(01)61621-5 [PubMed] [Google Scholar]
- 41. Gu F. Changes in the prevalence of benign prostatic hyperplasia in China. Chin Med J (Engl). 1997;110:163–166. [PubMed] [Google Scholar]
- 42. Campbell B. High rate of prostate symptoms among Ariaal men from Northern Kenya. Prostate. 2005;62:83–90. doi:10.1002/pros.20120 [DOI] [PubMed] [Google Scholar]
- 43. Jin B, et al. Ethnicity and migration as determinants of human prostate size. J Clin Endocrinol Metab. 1999;84:3613–3619. doi:10.1210/jcem.84.10.6041 [DOI] [PubMed] [Google Scholar]
- 44. Nandeesha H, et al. Hyperinsulinemia and dyslipidemia in non-diabetic benign prostatic hyperplasia. Clin Chim Acta. 2006;370:89–93. doi:10.1016/j.cca.2006.01.019 [DOI] [PubMed] [Google Scholar]
- 45. Boyle P, Lawrence Gould A, Roehrborn CG. Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: meta-analysis of randomized clinical trials. Urology. 1996;48:398–405. doi:10.1016/S0090-4295(96)00353-6 [DOI] [PubMed] [Google Scholar]
- 46. Layton JB, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835–842. doi:10.1210/jc.2013-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nesse RM, Stearns SC, Omenn GS. Medicine needs evolution. Science. 2006;311:1071. doi:10.1126/science.1125956 [DOI] [PubMed] [Google Scholar]
- 48. Kirkwood TB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philos Trans R Soc Lond B Biol Sci. 1991;332:15–24. doi:10.1098/rstb.1991.0028 [DOI] [PubMed] [Google Scholar]
- 49. Williams GC. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution, 1957;11: 398–411. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.