Synopsis
Nanotechnology is an emerging field of medicine with significant potential to become a powerful adjunct to cancer therapy, and in particular, thoracic surgery. Using the unique properties of several different nanometer-sized platforms, therapy can be delivered to tumors in a more targeted fashion, with less of the systemic toxicity associated with traditional chemotherapeutics. In addition to the packaged delivery of chemotherapeutic drugs, nanoparticles show potential to aid in the diagnosis, pre-operative characterization, and intraoperative localization of thoracic tumors and their lymphatics. With increasing interest in their clinical application, there is a rapid expansion of in vitro and in vivo studies being conducted that provide a better understanding of potential toxicities and hopes of broader clinical translation. Focused research into nanotechnology’s ability to deliver both diagnostics and therapeutics has led to the development of a field known as nanotheranostics which promises to improve the treatment of thoracic malignancies through enhanced tumor targeting, controlled drug delivery, and therapeutic monitoring. This article reviews the various types of nanoplatforms, their unique properties, and the potential for clinical application in thoracic surgery.
Keywords: Nanotechnology, nanotheranostics, lung cancer, thoracic surgery
Introduction
Nanotechnology is a rapidly evolving field that offers novel opportunities for the treatment and diagnosis of oncologic disease, specifically in thoracic surgery. The use of nanoparticles, materials generally ranging in size from 1 to 1000 nm, has emerged from the desire to achieve targeted drug delivery that maximizes treatment efficacy, while minimizing toxicity [1–3]. To reach this goal, nanoparticles are being engineered with varying characteristics and increasing complexity. Researchers leverage the chemical and physical characteristics of small molecule drugs, peptides, proteins, nucleic acids, lipids, polymers, and elemental metals to form a number of unique nanoparticles. These nanoparticles can be further enhanced through a variety of approaches, such as surface modification and responsiveness to tumor microenvironment leading to improved pharmacology and active intracellular function [4].
Nanotechnology has broad application in the field of oncology; however, thoracic oncology is uniquely poised to benefit given the relatively poor prognosis of thoracic malignancies, such as lung and esophageal cancer, as well as the intricate thoracic cavity which requires careful navigation. Lung cancer is one of the most common malignancies and is the number one cause of cancer-related death in the United States [5]. With recent guidelines for lung cancer screening from the National Lung Screening Trial, it is anticipated that the incidence of lung cancer will dramatically increase, and thus the number of patients requiring treatment [6]. Nanotechnology will change the way we approach lung cancer in the future, both as an adjunct to thoracic surgery by improving pre-operative diagnosis, intra-operative tumor localization and image-guided lymphatic mapping as well as to thoracic oncology through improved systemic therapy of more advanced disease. For example, traditional chemotherapeutics such as paclitaxel and doxorubicin typically used to treat lung cancer are being packaged within the various nanotechnologies to enhance the pharmacokinetics and biodistribution of drug resulting in improved local concentration. Traditional contrast agents used for computed tomography (CT) and magnetic resonance imaging (MRI) are being delivered via novel nanostructures to provide improved imaging and diagnostic capabilities, while fluorescent nanomarkers are guiding surgeons to the exact tumor location and associated lymphatics [7,8,9]. Clearly, nanotechnology has the potential to impact each stage from the diagnosis to the treatment of thoracic malignancy as researchers are able to tailor composition, structure, and properties to meet the challenges of clinical application.
Material platforms for nanotechnology
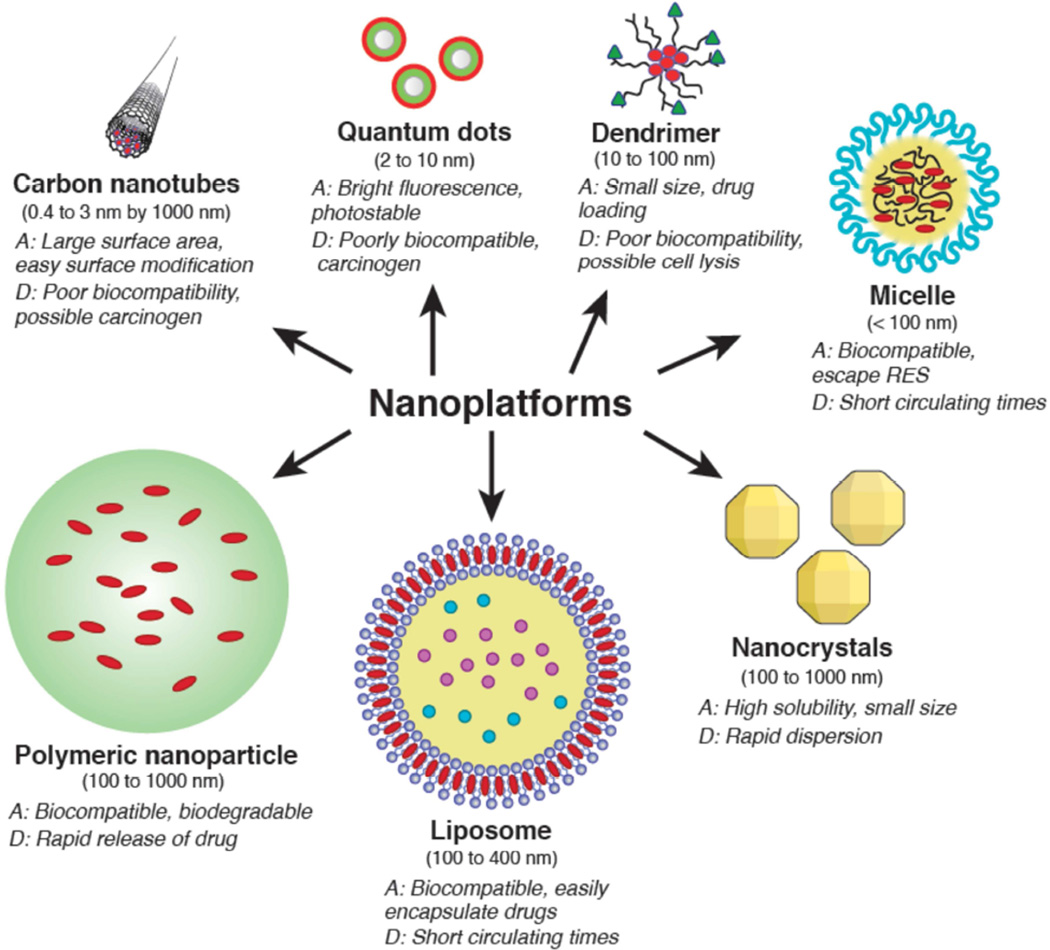
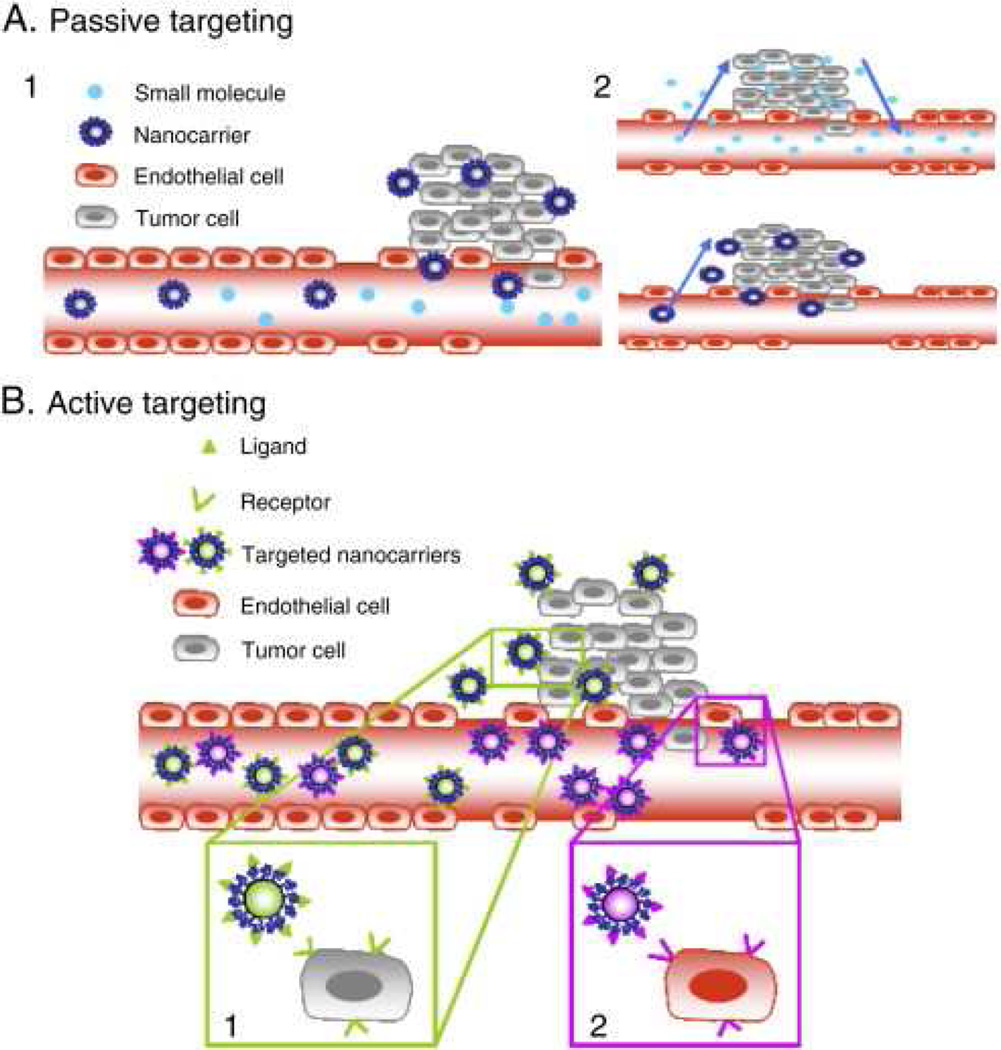
Nanotechnology comprises a variety of platforms including nanocrystals, polymeric nanoparticles, liposomes, quantum dots, micelles, dendrimers, and carbon nanotubes (Figure 1). Each platform possesses distinctive properties such as size, shape, charge, and surface characteristics that can be strategically altered in order to overcome the physiologic challenges faced by traditional chemotherapeutics. Commonly used anti-neoplastic agents in lung cancer such as paclitaxel or docetaxel are often difficult to solubilize with non-specific targeting leading to a vast array of unwanted side effects, however encapsulation of drugs in nanoparticles (NPs) facilitates improved biocompatibility and biodistribution, protects against acidic or enzymatic breakdown, and importantly allows for targeted delivery of drug to tumor-laden tissue [10,11,12]. Tumor neovascularization leads to increased permeability allowing NPs to passively accumulate and remain within tumor tissue through the enhanced permeability and retention effect (EPR), a key mechanism driving NP-mediated tumor targeting (Figure 2) [4]. In addition, NPs can be engineered with a variety of targeting moieties (i.e. ligands, aptamers, or antibodies) that allow active targeting of specific tumors [13]. NPs are modified to provide diagnostic and therapeutic capabilities tailored to specific clinical applications through drug or gene loading, endogenous or exogenous stimuli, contrast or dye loading, or even a combination of these. The distinct advantages and disadvantages of common material platforms are outlined below, with a focus on specific innovations that are certain to impact the field of thoracic oncology.
Figure 1.
Nanoplatforms display distinct advantages and disadvantages.
Figure 2.
Tumor targeting of nanoparticles. A. Permeable vasculature from endothelial cell gaps leads to passive diffusion of small molecules and nanocarriers into the tumor microenvironment. Due to their size, nanocarriers do not diffuse out as easily and hence accumulate within the tumor tissue. B. Specific receptors on nanocarriers target ligands on the tumor tissue. From Danhier F, Oliver F, Veronique P. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. 2010;148(2):135–146; with permission.
Nanocrystals
Nanocrystals are pure drugs that have been processed down to nanometer size, typically 100 to 1000 nm. Drug processing is achieved using a variety of methods including ‘bottom up’ (precipitation) or ‘top down’ (high pressure homogenization) approaches [14,15]. The advantages of nanocyrstal drug delivery stem from high saturation solubility due to a small size and an amorphous state [16]. Nanocrystals can be altered using surface modifiers such as polythylene glycol (PEG) to avoid opsonization and consumption by the reticulendothelial system, allowing for passive tumor accumulation via EPR [14]. Traditional chemotherapeutics such as paclitaxel are being processed into nanocrystal formulations to take advantage of these properties. For example, paclitaxel is traditionally delivered using a Cremophor EL (polyethoxylated castor oil and ethanol mixture) carrier that is responsible for many of the clinical side effects associated with paclitaxel [17]. Promisingly, paclitaxel-loaded nanocrystals demonstrate significantly less toxicity in pre-clinical studies utilizing murine lung cancer models [18]. Nevertheless, further testing is required to better understand the mechanisms of nanocrystal drug delivery as studies show that nanocrystals can rapidly disperse after intravenous administration due to their high intrinsic solubility, dissolution rate, and rapid dilution [14].
Quantum dots
Nanocrystal fluorophores, known as quantum dots (QD), are inorganic fluorescent platforms comprised of a heavy metal within a semiconductor shell, ranging from 2 to 10 nm with emissions ranging from 450 to 850 nm based on the size of the quantum dot. Quantum dots exhibit strong light absorbance, bright fluorescence, and marked photostability facilitating their application as a powerful imaging tool. Pre-clinical studies have successfully used quantum dot technology to visualize tumors and associated lymph nodes following intravenous injection [19,20]. Poor baseline biocompatibility, however, necessitates modification to a hydrophilic state or encapsulation with phospholipids, micelles, polymer beads, etc., which also permits subsequent attachment of targeting moieties [21]. Despite significant interest, clinical translation of quantum dots has been slow due to concerns over heavy metal toxicity. Heavy metals such as cadmium, frequently used in quantum dots, can be toxic particularly if released following exposure to unstable surface coating [22]. Although clinical applicability does remain a possibility as recent studies by Lin et al. showed in a long term murine model no observable toxicity in mice exposed to novel non-cadmium based QD’s [23].
Liposomes
Liposomes, one of the first described nano-sized drug delivery systems, are spherical, bilayer platforms comprised of amphiphilic phospholipids (and cholesterol) ranging in size from 100 to 400 nm [1,2,24]. They are ideal in their ability to encapsulate traditional chemotherapeutics and/or other nanoparticles such as quantum dots to form nanotheranostic vehicles. In addition, they are biocompatible with low toxicity and immunogenicity [25]. Initially, short circulating times due to opsonization inhibited clinical applicability, however, PEGylation led to longer circulating times and the eventual FDA approval of liposomal doxorubicin, known as Doxil [2]. Multiple studies report the liposomal formulation of other traditional chemotherapeutics such as paclitaxel for treatment of lung cancer via intravenous and inhalational therapies [26,27].
Dendrimers
Dendrimers are highly branched, synthetic, nanosize (10 to 100nm) molecules, created from the repetitive addition of branching units to an amine core. Drugs can be encapsulated to improve solubility or attached to the surface for delivery [28]. Newer generations of dendrimers are more hydrophobic, exhibit improved drug stability, and allow specific targeting through increasingly complex branching [24]. For example, Majoros et al. demonstrated enhanced tumor targeting and improved cytotoxicity by conjugating paclitaxel to a poly(amidoamine) dendrimer [29]. In addition to drug delivery, dendrimers have been conjugated to iron oxide particles and used in combination with MRI to deliver magnetic hyperthermia and subsequent cytotoxicty, while dendrimer entrapped gold particles have been utilized to enhance CT imaging of human lung adenocarcinoma [30, 31]. Similar to other platforms, cationic dendrimers are subject to unwanted toxicities due to interaction with cellular membranes that can lead to destabilization and potential cell lysis [32, 33]. In addition, Jones et al. demonstrated certain dendrimers with cationic charge can result in platelet aggregation and alpha granule secretion [34].
Carbon Nanotubes
Carbon nanotubes (CNTs) are graphene sheets rolled into cylinders measuring 0.4 to 3 nm in diameter and up to 1000 nm in length. Cylinders can be single or rolled concentrically to form single, double or multi-walled carbon nanotubes [35]. CNTs are hydrophobic and thus generally not soluble in aqueous solutions. To improve biocompatibility, surface functionalization must occur which also presents an opportunity for ligand binding for tumor-specific targeting. In vitro studies using CNTs, show a dose dependent tumor suppression of human breast cancer cells, and more recently the use of siRNA vectors has demonstrated the eradication of lung cancer xenografts. Although promising, CNT’s have led to systemic toxicity in multiple studies. Large surface area, hydrophobicity, insolubility, a tendency to aggregate, in addition to a serious concern for possible carcinogenesis are all factors that limit the clinical translational of CNTs to date [36, 37]. In a study by Kanno et al. multi-walled carbon nanotubes induced mesothelioma when given to p53 heterozygous mice. Carcinogenesis has been also reported in other studies, and it is postulated that the mechanism is similar to that of asbestos [35].
Polymeric nanoparticles
Polymeric NPs are manufactured using a variety of materials and techniques resulting in biocompatible and biodegradable polymers that can be either natural or synthetic, generally ranging from 100 to 1000 nm. Poly(lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) polymers are the most widely used synthetic polymers as their lactic acid and glycolic acid monomers can be easily hydrolyzed and eliminated [24, 38]. Natural polymers such as chitosan or dextran have also been used in polymeric NP production. Polymeric NPs offer unique advantages for drug delivery given their particularly capacity to encapsulate and protect drugs from degradation as well as the ability to introduce specific functionality via surface modification [39]. Polymeric-drug conjugated with surface modification, such as doxorubicin-loaded PLGA-PEG NPs with conjugated peptide specific for EGFR, demonstrate improved cytotoxity in vitro (a 62.4 reduction in the IC50 when compared to free doxorubicin) as well as in vivo tumor accumulation [40]. In addition, clinical trials investigating polymer conjugated doxorubicin have shown improved anti-tumor cytotoxicty and decreased systemic toxicity compared to drug alone [41]. More recently, a Phase III clinical trial comparing polyglutamic acid conjugated paclitaxel in patients with non-small cell lung cancer revealed significantly fewer severe side effects compared to traditional chemotherapies [42,43]. Although promising, polymeric NPs, specifically PLA and PLGA, are limited by relatively rapid “burst” release of encapsulated drug regardless of whether it is in the tumor or not. Short duration of random drug release has been a driving force behind more targeted structures as well as an interest in environment-responsive polymeric nanoparticles [39].
Micelles
Micelles are colloidal structures, typically less than 100 nm, with a hydrophobic core and a hydrophilic shell that allows for transport of poorly water soluble agents [24, 44]. Clinical trials have been conducted on a micellar formulation containing paclitaxel known as Genexol-PM. In a multicenter phase II trial, Genexol-PM was administered intravenously with cisplatin to patients with advanced NSCLC demonstrating a significant improved median survival time and minimal associated toxicity [45]. Micelles offer a distinct advantage in providing an oral route for some drugs, avoiding renal exclusion, and escaping the reticuloendothelial system. Most toxicity, however, comes from premature release of encapsulated drug [46].
Potential Clinical Application of Nanotechnology in Thoracic Surgery
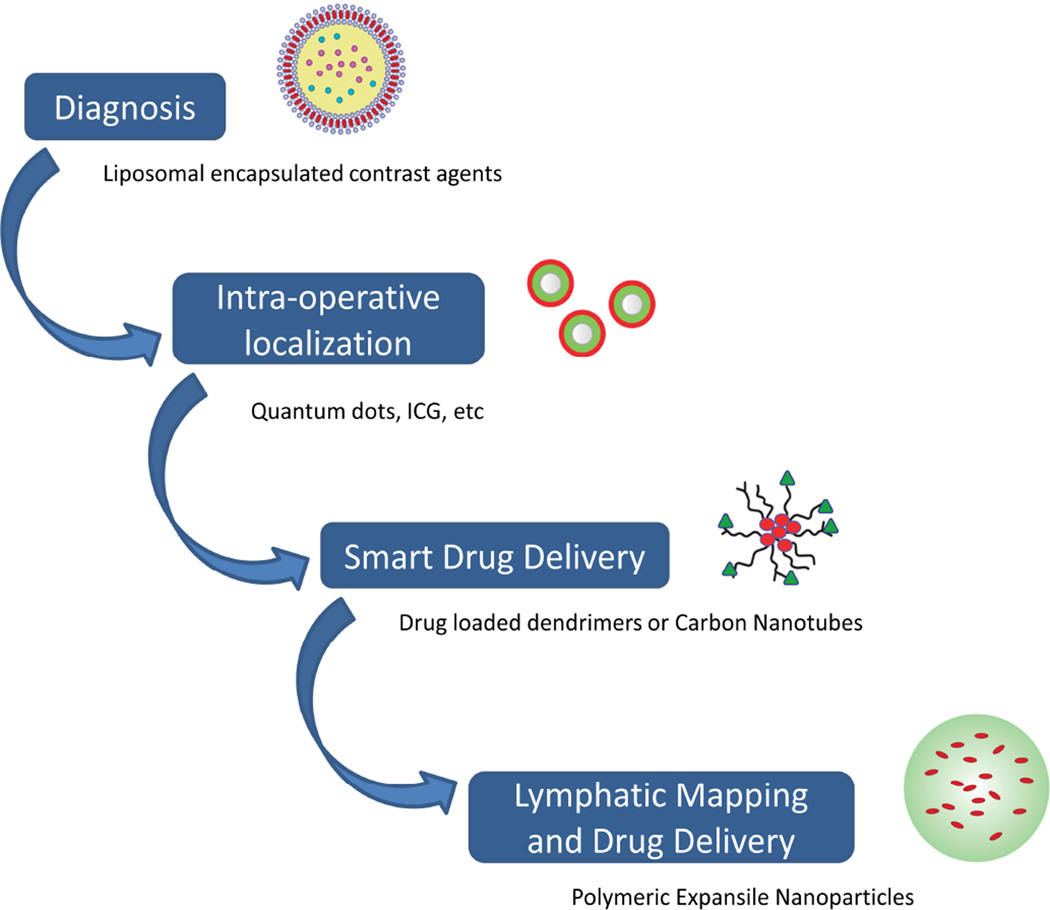
Nanotechnology has the potential to advance the field of thoracic surgery by improving the diagnosis, staging, treatment, and therapeutic monitoring of intra-thoracic malignancies (Figure 3). Through optimization of the material platforms to overcome their limitations, scientists are discovering how to meet the clinical needs of patients. The sensitivity and specificity of imaging is being improved through nanocarrier contrast agents. Surgeons can more accurately localize a lesion intra-operatively while carefully examining the associated lymphatics. Drugs as well as inhibitory genetic material are being delivered in a “smart” fashion through stimuli-responsive platforms. All of these advancements have led to the emergence of “nanotheranostic” multifunctional NPs to meet the challenges of clinical applicability. Some of the exciting new applications are highlighted below.
Figure 3.
Nanoparticles participate in each step from diagnosis of cancer to the various forms of treatment. Nanoparticles can be engineered to function at multiple different steps and in combination with each other.
Nanotechnology in Imaging – Diagnostics and Intraoperative Platforms
Nanoparticles engineered as an adjunct to imaging modalities are rapidly evolving to meet the needs of cancer diagnosis. Specifically, contrast agents are being loaded on the surface or encapsulated within NPs to improve imaging sensitivity and specificity while minimizing systemic toxicity. Contrast agents such as supermagnetic iron oxide (SPIO) nanoparticles that are tagged with tumor specific antibodies (i.e. VEGF) for direct tumor targeting are improving MRI resolution and tumor identification [47,48,49]. Traditional contrast agents formulated onto nanocarriers better facilitate intra-tumoral uptake and minimize nephrotoxicity, as demonstrated by gadolinum nanoparticles for MRI and liposomal iodinated contrast agents for computed tomography [50,51]. These improvements have the potential to greatly improve lung cancer diagnosis and staging, particularly given the suboptimal diagnostic accuracy (79%) and sensitivity (64%) of conventional CT-PET scans as well as the risk for nephrotoxity associated with each intravenous dose of traditional iodinated contrast [52].
Nanotechnology is also a useful tool in identifying and targeting tumors intra-operatively, particularly given the complex and intricate anatomy of the thoracic cavity. With the new lung cancer screening guidelines, an increasing incidence of early stage cancers presents thoracic surgeons with the challenge of finding smaller, non-palpable tumors during surgery [53]. Therefore, a strong need for image guided surgery exists to enable precise tumor resection with maximal parenchymal sparing and avoidance of critical structures. Using murine and rabbit models, one group formulated liposomal encapsulated iodohexal, an iodinated contrast agent, and indocyanine green (ICG), a near-infrared fluorescent dye, for dual pre- and intra-operative tumor localization. Rabbits were inoculated with rabbit lung cancer, VX2, and given the novel liposomal formulation. Four days post-injection tumor deposits correlated with pre-operative CT images and with intra-operative near infrared (NIR) signals [54,55].
A particular area of thoracic surgery to significantly benefit from the field of nanotechnology is that of lymphatic mapping. Lymph node involvement in NSCLC plays an important role in the staging and overall prognosis of patients with lung cancer. For those patients that are clinically node negative, approximately 30 percent of clinical stage 1 patients will be upstaged following surgery [56]. Given the poor prognosis associated with nodal involvement and the variable location, extensive lymphadenectomy has become the standard of care, however it is not without morbidity. This has prompted an exploration into sentinel lymph node (SLN) mapping whereby tumor draining lymph nodes can undergo more in depth immunohistochemical analysis. Early human clinical trials used methylene blue and 99technetium, although neither are ideal for thoracic surgery [57,58]. More recently, near infrared (NIR) technology, which detects light emitted in the 700–900 nm range, and ICG, which migrates from the tumor to the SLN, have been used in combination to improve mapping and biopsy of SLN. In a clinical trial, patients with NSCLC underwent peritumoral ICG injection. Here 26 NIR+ SLNs were identified in 15 patients, with 7 NIR+ SLNs (from six patients) harboring metastatic disease on histologic analysis, which demonstrated 100% SLN identification and 100% correlation of SLN pathology with overall nodal status in lung cancer [59]. Another study leveraged the fluorescent capabilities of quantum dots with direct injection into the lung parenchyma. In this porcine model, a bright fluorescent signal could be visualized in real time migrating to the SLN within minutes [60]. Similar results were noted for lymphatic mapping of the pleural space and esophagus [61,62].
Stimuli-responsive drug release nanoparticles
A major area of nanotechnology application is in smart drug delivery and cancer therapeutics. Paclitaxel, a taxane that stabilizes microtubules and exhibits cytotoxicity against numerous solid tumors, and doxorubicin, an anthracycline that intercalates a host cell’s DNA, are two common chemotherapeutic agents employed [17,63]. Numerous studies have demonstrated superior in vitro and in vivo tumor cytotoxicity when these agents are incorporated into a nanoparticle [64–67]. For example, using a murine xenograft model a paclitaxel chitosan based micellar formulation displayed significant tumor reduction (p<0.01) and less morbidity (approximated by body weight loss) compared to intravenous paclitaxel [68]. These studies represent the improved delivery of chemotherapeutic drug to tumor. In addition, other studies have shown that nanoparticles can function synergistically with known chemotherapies.
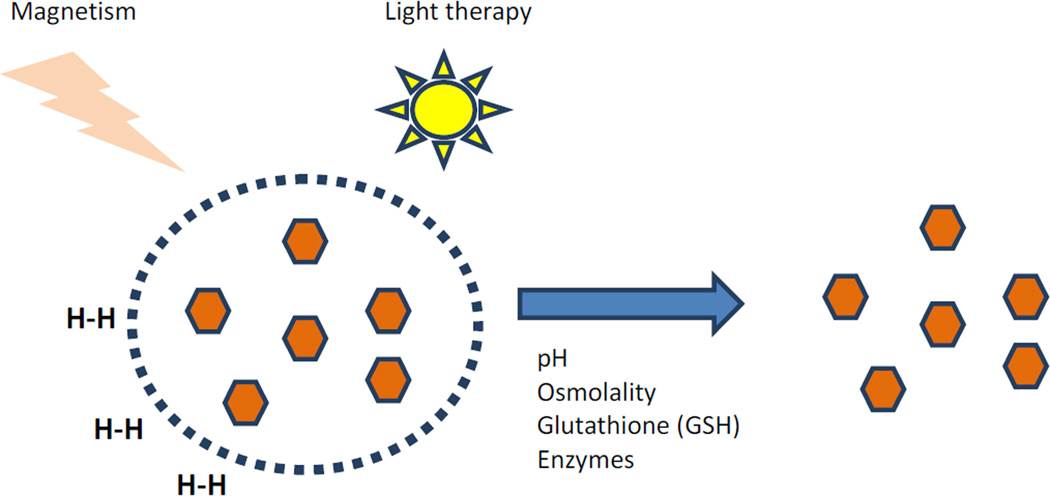
The ultimate goal of nanoparticle drug delivery is to achieve targeted and controlled drug release within tumor. Pursuit of this goal has led to engineering of stimuli-responsive nanoparticles that can be triggered either to act or to release a therapeutic agent based on a specific endogenous microenvironment or an external stimulus. Examples of endogenous and exogenous stimuli being exploited include light, pH or temperature change, applied magnetism, redox environment, or enzymes (Figure 4) [39,69]. Numerous studies are being devised to leverage stimuli unique to cancer cells or other pathologic states, making it clear that the next generation of nanoplatforms will have a “responsive ” functionality to aid in the targeted release of a drug payload as a function of a biologic event or microenvironment.
Figure 4.
Stimuli-responsive nanoparticles. A variety of endogenous and exogenous stimuli can induce a specific action or release of drug.
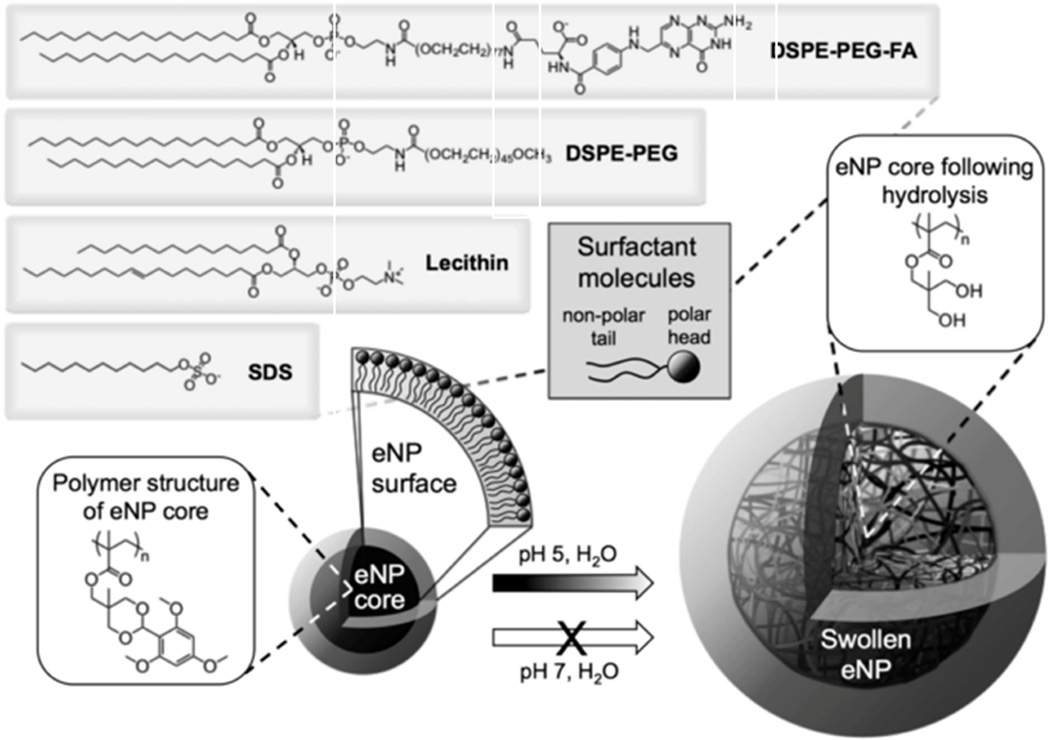
Many polymeric nanoparticles are limited in their clinical translation due to the relatively rapid release of encapsulated drug. As such, stimuli-responsive polymers have been engineered to allow for delayed, tumor-targeted release of drug in response to a tumor micoenvironment. For example, acidic pH within the tumor microenvironment or endosome is well characterized and has been utilized as a “trigger” for chemotherapy release at the site of intracavitary tumors such as mesothelioma [39]. Upon encountering an environmental pH ≤ 5, as occurs in endosomes, cleavage of a protecting group changes the nanoparticle polymer from hydrophobic to hydrophilic with significant expansion of NP size and resultant drug release (Figure 5). The intracellular targeting of chemotherapy release as a function of pH has resulted in markedly superior tumor cytotoxicity and improved survival in in vivo studies [70, 71].
Figure 5.
pH responsive expansile nanoparticles (eNP) expand to release drug when in the presence of a pH of 5. Adapted from Stolzoff M, Ekladious I, Colby AH, et al. Synthesis and Characterization of Hybrid Polymer/Lipid Expansile Nanoparticles: Imparting Surface Functionality for Targeting and Stability. Biomacromolecules. 2015;16(7):1958-66; with permission.
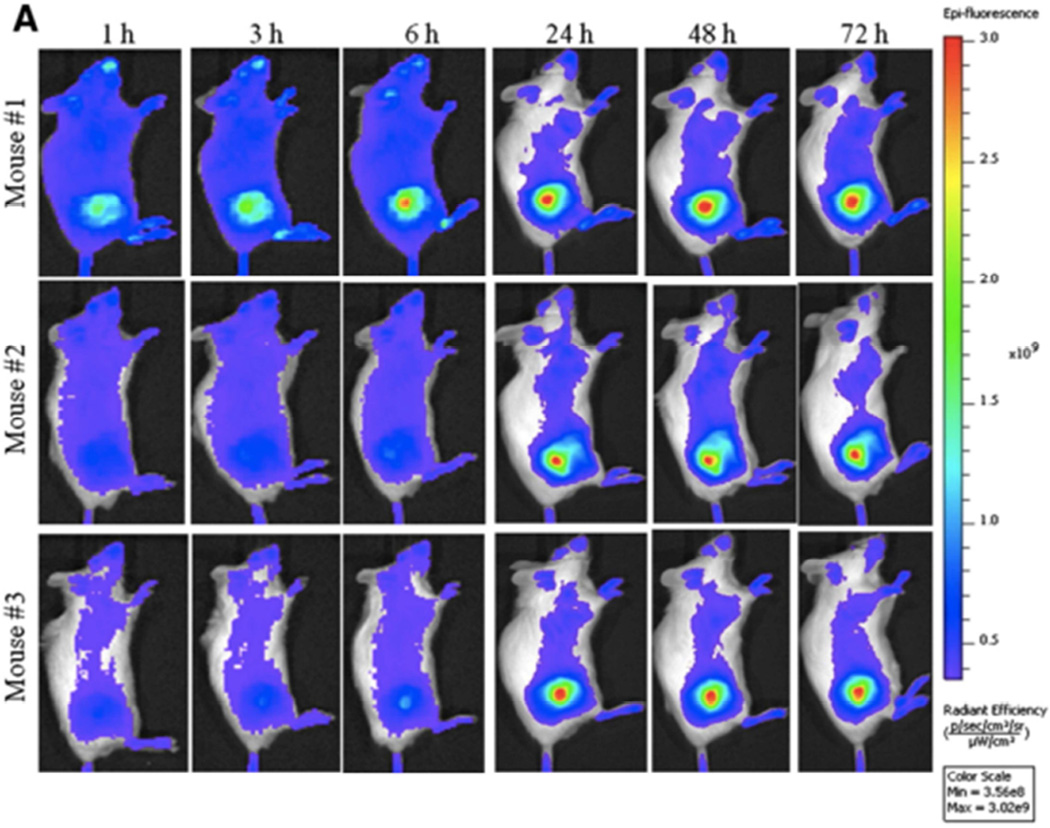
Similarly, redox sensitive nanoparticles have emerged as another popular approach to responsively trigger drug release [72]. Glutathione levels are 2–3 times higher within the cytosol of animal cells and undergo a reduction-oxidative reaction with glutathione disulfide leading to the release of drug. Loaded drug can be further released via redox reaction within the lysosome. Nanoparticles encapsulating doxorubicin were prepared with disulfide bonds to allow for triggered intracellular drug release. Subsequent in vivo fluorescent imaging revealed tumor specific targeting and drug release as can be seen in Figure 6 [73].
Figure 6.
NIR-labeled redox-sensitive nanoparticles reproducibly concentrate in tumor over time. From Nguyen CT, Tran TH, Amiji M, et al. Redox-Sensitive Nanoparticles from amphiphilic cholesterol-based block copolymers for enhanced tumor intracellular release of doxorubicin. Nanomedicine. 2015. [Epub ahead of print]; with permission.
In contrast, photodynamic therapy has been used as an exogenous stimulus to “activate” nanostructures against a variety of malignancies. Using a combination of a photosensitizer and a light source, free radical oxygen species can be generated to induce cell death via localized hyperthermia, apoptosis, or induction of an immune response [74]. Photosensitizers such as porfimer sodium and ICG have been packaged within nanostructures (i.e. porfimer sodium-liposomal system) to limit systemic exposure and decrease side effects such as generalized photosensitivity but permit for direct photodynamic or photothermal cell cytotoxicity [75,76]. Other studies have similarly examined the use of applied near-infrared light to induce the release of drug into the local environment [77].
Lymphatic Drug Delivery
New research has focused on the delivery of chemotherapy specifically to the lymphatics and the treatment of occult lymph node metastases. This work has led to studies investigating chemotherapy drug concentrations within tumor and the draining lymph nodes as a function of the method of drug delivery. Interestingly, drug levels are as much 180–300 times lower in both tumor and lymph nodes following intravenous administration as opposed to peritumoral injection [78]. As a result, local peritumoral nanoparticle delivery is being explored as a unique approach to substantially increase the level of chemotherapeutic agent within both the tumor and lymph nodes. Exploiting the stimuli-dependent release of drug seen in expansile nanoparticles, a murine xenograft model of triple negative breast cancer cells with a propensity for lymph node metastasis was used to assess the ability of paclitaxel loaded eNP (pax-eNP) to control disease. The pax-eNP accumulated in the lymph nodes and were present even after 10 days, a stark contrast to systemic paclitaxel which has a half life of only 7 hours. There was also a significant decrease in the percentage of lymph node metastasis present 6.5 weeks after peritumoral pax-eNP therapy (33% nodal metastasis with pax-eNP and 100% nodal metastasis with unloaded eNP and only, p<0.005) [79]. Prevention of nodal metastasis using nanotechnology to target and enhance drug delivery has the potential to have a major impact on early stage cancers following surgical resection.
Active Tumor-Targeting Platforms
Other investigators have focused on mechanisms for directing nanostructures to a target tumor with enhanced specificity. Epidermal growth factor receptor (EGFR) is expressed in approximately 50–80% of non-small cell lung cancers and typically has a poor prognosis making it an ideal therapeutic target. NPs conjugated to cetuximab, a monoclonal antibody to EGFR, have been tested in a murine model of EGFR-positive NSCLC. Tumor volume was significantly less and there was no overall weight loss in the cetuximab-NP group when compared to cetuximab alone. Tumor migration and EGFR intracellular signaling were also reduced [80]. Further investigation into the mechanism of cetuximab conjugated plasmonic magnetic nanoparticles revealed induced autophagy which was not seen in cetuximab alone indicating an added degree of cytotoxicity when conjugated to a nanoparticle [81].
Nanoparticles for gene delivery
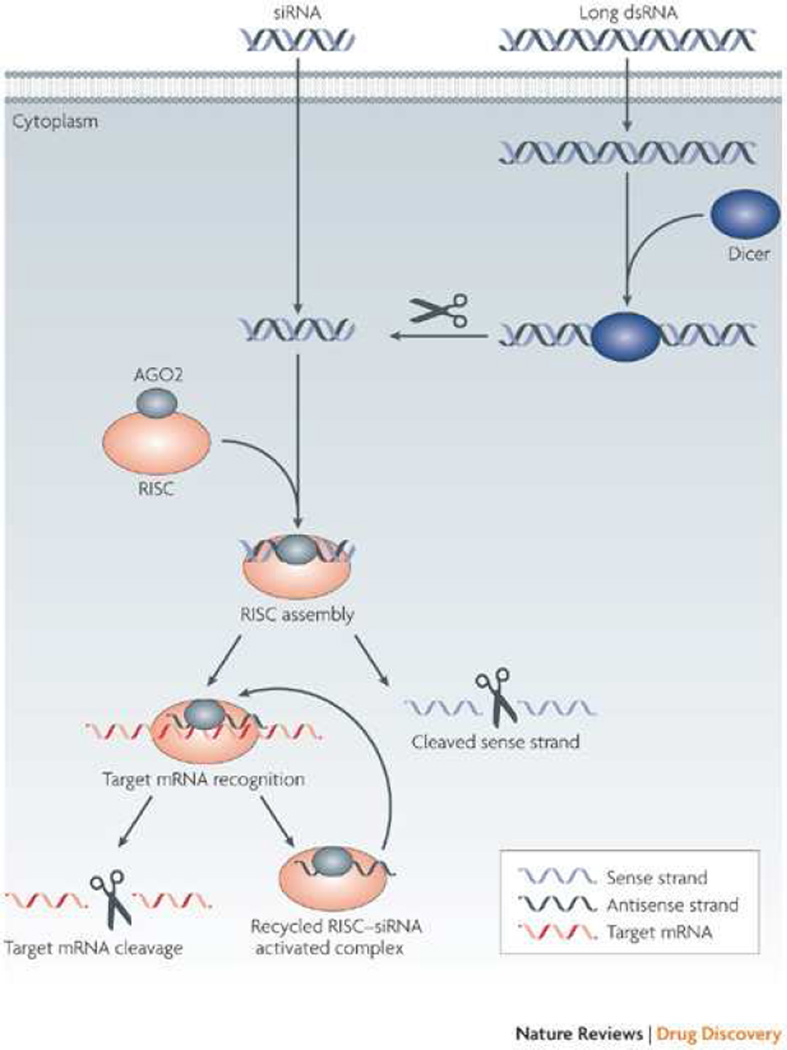
Short intervening RNA (siRNA) segments can target specific mRNA sequences and affect gene translation via the RNA-induced silencing complex (RISC) as in Figure 7 [82,83]. However, clinical translation has been elusive due to poor cellular uptake of nucleic acids, the lack of biocompatible siRNA carriers, rapid degradation by nucleases, and accelerated renal excretion [84]. In an in vivo murine xenograft model of human lung adenocarcinoma, Chen et al. utilized a novel system of magnetic mesoporous silica NPs loaded with vascular endothelial growth factor (VEGF) siRNA, capped with polyethylenimine (PEI), and PEGylated for biocompatibility. Mice subjected to the siRNA NP showed marked tumor suppression, decreased tumor metastasis, and overall improved survival while maintaining favorable tissue biocompatibility and biosafety [85]. In another study, multiwalled carbon nanotubes were loaded with siRNA specific for PLK1. Intra-tumoral injection of human lung cancer xenografts led to drastically reduced tumor growth and increased survival [86]. A major target in siRNA delivery for lung cancer is the KRAS proto-oncogene, one of the most common oncogenes in human cancers which typically portends a poor prognosis. Using a nanoliposomal delivery system for KRAS siRNA, one group found a 90% reduction in KRAS on Western blot analysis, significant tumor reduction (50% vs. 6% in negative control siRNA), and reduced distant metastasis. Numerous other studies are looking at a variety of targets for siRNA (i.e. tissue factor, HDM2, AKT1, etc) incorporated onto an array of basic NP platforms with increasing levels of sophistication [84].
Figure 7.
siRNA can be introduced via nanocarrier or double stranded DNA that is cleaved by the Dicer enzyme within the cell. The siRNA is then incorporated into the RNA-induced silencing complex (RISC) leading to cleavage of target mRNA and thus silencing of the targeted gene. From Whitehead KA, Langer R, Anderson DR. Knocking down barriers: advances in siRNA delivery). Macmillan Publishers Ltd: Nature Reviews Drug Discovery 2009;8(2):129-38; with permission.
Nanotheranostics – A New Frontier in Thoracic Surgery
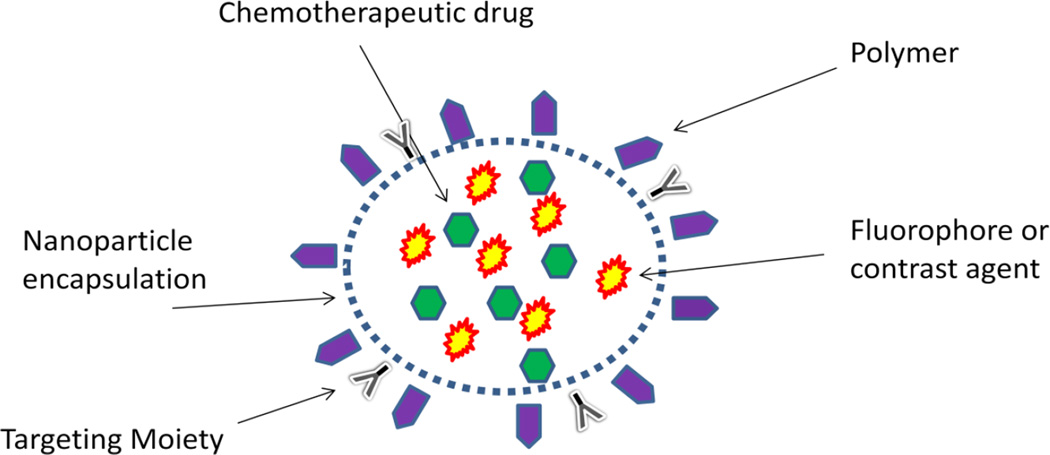
With increasing knowledge of nanoparticles and their capabilities, a new field known as nanotheranostics has evolved in an effort to deliver diagnostic and therapeutic properties on a single, unified platform. For example, multiple nanotechnologies can be combined to take advantage of their distinctive properties. Typical nanotheranostic agents may include a polymer component for stabilization and biocompatibility, an encapsulated therapeutic agent such as a traditional chemotherapeutic drug or siRNA, and an imaging agent such as a fluorophore or contrast agent (Figure 8). Researchers recently encapsulated the MRI contrast agent SPIO with doxorubicin using the copolymer maleimide-PEG-poly(lactic acid) to allow for pre-operative imaging with MRI while delivering chemotherapy all on a single, biocompatible platform [87]. In another study, quantum dots and SPIO were coupled on a chitosan-based nanoparticleplatform with folate conjugates for tumor targeting and then loaded with chemotherapeutic camptothecin [88].
Figure 8.
Nanotheranositics. These agents combine many properties of the basic nanoparticles to form single platforms capable of both diagnostics and therapeutics.
Nanotheranostics are particularly useful in providing surgeons with the opportunity to perform an ideal resection while delivering therapy at the same time. Given the known potential for toxicity associated with quantum dots, some studies have explored the use of small pH-responsive nanoparticles labeled with an NIR fluorophore that also carries paclitaxel to the SLN. Evaluation of this technology in a large animal model demonstrated rapid detection of the SLN upon operative exploration and significant concentration of both the fluorophore and paclitaxel [89,90]. As toxicities of current nanoparticles being engineered to encapsulate both fluorophore and chemotherapeutic drug continue to be delineated, newer nanotheranositic systems have the potential to maximize the benefit of surgery.
Treatment of lung cancer will strongly benefit from the emerging field of nanotheranostics given the poor response to traditional chemotherapies, overall poor prognosis, and difficult surgical anatomy associated with the thoracic cavity. As more early stage lung cancers are discovered, we are at an ideal time to employ nanotheranostics by simultaneously aiding surgeons in finding non-palpable nodules, biopsying sentinel nodes instead of the traditional lymphadenectomy, and delivering chemotherapy to the targeted tumor.
Conclusion
Nanotechonology is an exciting field of medicine that is rapidly evolving from in vitro and in vivo testing to the clinical arena. Through targeted drug delivery, nanoparticles are able to deliver therapeutics at increased local concentrations with minimal systemic toxicity. A variety of material platforms are currently available with specific advantages and disadvantages. With more research into the mechanisms of action and safety profiles, nanoparticles promise to be a major part of medicine and surgery moving forward. Thoracic surgery stands to benefit from the improved diagnostic imaging, advancements in image-guided surgery through tumor localization and lymphatic mapping, and from the enhanced efficacy profile of targeted and stimuli-responsive drug delivery. Most promising is the creation of nanotheranostic systems that integrate each of these advances into a single, unified platform. This new frontier in oncologic therapy promises to transform the care of thoracic surgery patients moving forward.
Key Points.
Nanotechonology is an exciting field of medicine that is rapidly evolving from in vitro and in vivo testing to the clinical arena.
Through targeted drug delivery, nanoparticles are able to deliver therapeutics at increased local concentrations with minimal systemic toxicity.
A variety of material platforms are currently available with specific advantages and disadvantages.
With more research into the mechanisms of action and safety profiles, nanoparticles promise to be a major part of medicine and surgery moving forward.
Thoracic surgery stands to benefit from the improved diagnostic imaging, advancements in image-guided surgery through tumor localization and lymphatic mapping, and from the enhanced efficacy profile of targeted and stimuli-responsive drug delivery.
Acknowledgements
This work was supported in part by Brigham and Women’s Hospital Center for Surgical Innovation, Boston University Center for Integration of Medicine and Innovative Technology, Boston University Nanomedicine Program and Cross-Disciplinary Training in Nanotechnology for Cancer, and Brigham and Women’s Hospital Advanced Training in Surgical Oncology.
This work was supported with funding from the National Science Foundation (DMR-1006601) and the National Institutes of Health (R01CA131044, R01CA149561, R25 CA153955, T32 CA009535).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no disclosures.
References
- 1.Farokhzad OC, Langer R. Impact of Nanotechnology on Drug Delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 2.Heath JR, Davis ME. Nanotechnology and cancer. Annu Rev Med. 2008;59:2251–2265. doi: 10.1146/annurev.med.59.061506.185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 4.Zhu L, Torchilin VP. Stimulus-responsive nanopreparations for tumor targeting. Integr Biol (Camb) 2013;5(1):96–107. doi: 10.1039/c2ib20135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. National Cancer Institute. Bethesda, MD: SEER Cancer Statistics Review, 1975–2012. http://seer.cancer.gov/csr/1975_2012/. [Google Scholar]
- 6.Yanagawa J, Rusch VW. Current Surgical Therapy for Stage IIIA (N2) Non-Small Cell Lung Cancer. Semin Thorac Cardiovasc Surg. 2011;23(4):291–296. doi: 10.1053/j.semtcvs.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Ma P, Mumper RJ. Paclitaxel Nano-Delivery Systems: A Comprehensive Review. J Nanomed Nanotechnol. 2013;4(2):1000164. doi: 10.4172/2157-7439.1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badea CT, Athreya KK, Espinosa G, et al. Computed tomography imaging of primary lung cancer in mice using a liposomal-iodinated contrast agent. PLoS One. 2012;7(4):e34496. doi: 10.1371/journal.pone.0034496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill TK, Abdulahad A, Kelkar SS, et al. Indocyanine green-loaded nanoparticles for image-guided tumor surgery. Bioconjug Chem. 2015;26(2):294–303. doi: 10.1021/bc5005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003;8(24):1112–1120. doi: 10.1016/s1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Zeng X, Li P. Nanomaterials in cancer-therapy drug delivery system. J Biomed Nanotechnol. 2013;9(5):741–750. doi: 10.1166/jbn.2013.1583. [DOI] [PubMed] [Google Scholar]
- 12.Biswas S, Deshpande PP, Perche F, et al. Octa-arginine-modified pegylated liposomal doxorubicin: an effective treatment strategy for non-small cell lung cancer. Cancer Lett. 2013;335(1):191–200. doi: 10.1016/j.canlet.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bazak R, Houri M, El Achy S, et al. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141(5):769–784. doi: 10.1007/s00432-014-1767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao L, Liu G, Ma J, et al. Drug nanocrystals: In vivo performances. J Control Release. 2012;160(3):418–430. doi: 10.1016/j.jconrel.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Pawar VK, Singh Y, Meher JG, et al. Engineered nanocrystal technology: in-vivo fate, targeting and applications in drug delivery. J Control Release. 2014;183:51–66. doi: 10.1016/j.jconrel.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Junghanns JU, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine. 2008;3(3):295–309. doi: 10.2147/ijn.s595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi M, Liu X, Belani CP. Taxanes, past, present, and future impact on non-small cell lung cancer. Anticancer Drugs. 2014;25(5):571–583. doi: 10.1097/CAD.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Park JY, Zhang Y, et al. Targeted cancer therapy with novel high drug-loading nanocrystals. J Pharm Sci. 2010;99(8):3542–3551. doi: 10.1002/jps.22112. [DOI] [PubMed] [Google Scholar]
- 19.Azzazy HM, Mansour MM, Kazmierczak SC. From diagnostics to therapy: prospects of quantum dots. Clin Biochem. 2007;40(13–14):917–927. doi: 10.1016/j.clinbiochem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Schulz MD, Khullar O, Frangioni JV, et al. Nanotechnology in thoracic surgery. Ann Thorac Surg. 2010;89(6):S2188–S2190. doi: 10.1016/j.athoracsur.2010.02.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akerman ME, Chan WC, Laakkonen P, et al. Nanocrystal targeting in vivo. Proc Natl Acad Sci U S A. 2002;99(20):12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottrill M, Green M. Some aspects of quantum dot toxicity. Chem Commun (Camb) 2011;47(25):7039–7050. doi: 10.1039/c1cc10692a. [DOI] [PubMed] [Google Scholar]
- 23.Lin G, Ouyang Q, Hu R, et al. In vivo toxicity assessment of non-cadmium quantum dots in BALB/c mice. Nanomedicine. 2015;11(2):341–350. doi: 10.1016/j.nano.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Muthu MS, Leong DT, Mei L, et al. Nanotheranostics - application and further development of nanomedicine strategies for advanced theranostics. Theranostics. 2014;4(6):660–677. doi: 10.7150/thno.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraft JC, Freeling JP, Wang Z, et al. Emerging Research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci. 2014;103(1):29–52. doi: 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Zhou J, Wang Y, et al. A phase I clinical and pharmacokinetic study of paclitaxel liposome infused in non-small cell lung cancer patients with malignant pleural effusions. Eur J Cancer. 2010;46(8):1474–1480. doi: 10.1016/j.ejca.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Wang L, Lv P, et al. Transferrin-conjugated doxorubicin-loaded lipid coated nanoparticles for the targeting and therapy of lung cancer. Oncol Lett. 2015;9(3):1065–1072. doi: 10.3892/ol.2014.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48(3):416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majoros IJ, Myc A, Thomas T, et al. PAMAM dendrimer-based multifunctional conjugate for cancer therapy: synthesis, characterization, and functionality. Biomacromolecules. 2006;7(2):572–579. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]
- 30.Walter A, Billotey C, Garofalo A, et al. Mastering the shape and composition of dendronized iron oxide nanoparticles to tailor magnetic resonance imaging and hyperthermia. Chem Mater. 2014;26(18):5252–5264. [Google Scholar]
- 31.Wang H, Zheng L, Peng C, et al. Folic acid-modified dendrimer-entrapped gold nanoparticles as nanoprobes for targeted CT imaging of human lung adencarcinoma. Biomaterials. 2013;34(2):470–480. doi: 10.1016/j.biomaterials.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 32.Fischer D, Li Y, Ahlemeyer B, et al. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24(7):1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 33.Madaan K, Kumar S, Poonia N, et al. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6(3):139–150. doi: 10.4103/0975-7406.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones CF, Campbell RA, Franks Z, et al. Cationic PAMAM dendrimers disrupt key platelet functions. Mol Pharm. 2012;9(6):1599–1611. doi: 10.1021/mp2006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rastogi V, Yadav P, Bhattacharya SS, et al. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells. J Drug Deliv. 2014;2014:670815. doi: 10.1155/2014/670815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Yanez Y, Munoz B, Albores A. Mechanisms of toxicity by carbon nanotubes. Toxicol Mech Methods. 2013;23(3):178–195. doi: 10.3109/15376516.2012.754534. [DOI] [PubMed] [Google Scholar]
- 37.Takagi A, Hirose A, Nishimura T, et al. Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J Tox Sci. 2008;33(1):105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- 38.Wang AZ, Langer R, Farokhzad OC. Nanoparticle Delivery of Cancer Drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 39.Colson YL, Grinstaff MW. Biologically responsive polymeric nanoparticles for drug delivery. Adv Mater. 2012;24(28):3878–3886. doi: 10.1002/adma.201200420. [DOI] [PubMed] [Google Scholar]
- 40.Liu CW, Lin WJ. Polymeric nanoparticles conjugate a novel heptapeptide as an epidermal growth factor receptor-active targeting ligand for doxorubicin. Int J Nanomedicine. 2012;7:4749–4767. doi: 10.2147/IJN.S32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasey PA, Kaye SB, Morrison R, et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Clin Cancer Res. 1999;5(1):83–94. [PubMed] [Google Scholar]
- 42.Langer CJ. CT-2103: A novel macromolecular taxane with potential advantages compared with conventional taxanes. Clin Lung Cancer. 2004;6(Suppl 2):S85–S88. doi: 10.3816/clc.2004.s.020. [DOI] [PubMed] [Google Scholar]
- 43.Langer CJ, O’byrne KJ, Socinski MA. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J Thorac Oncol. 2008;3(6):623–630. doi: 10.1097/JTO.0b013e3181753b4b. [DOI] [PubMed] [Google Scholar]
- 44.Frank D, Tyagi C, Tomar L, et al. Overview of the role of nanotechnological innovations in the detection and treatment of solid tumors. Int J Nanomedicine. 2014;9:589–613. doi: 10.2147/IJN.S50941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim DW, Kim SY, Kim HK, et al. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007;18(12):2009–2014. doi: 10.1093/annonc/mdm374. [DOI] [PubMed] [Google Scholar]
- 46.Moghimi SM, Hunter AC, Murray JC, et al. Cellular distribution of nonionic micelles. Science. 2004;303(5658):626–628. doi: 10.1126/science.303.5658.626. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh WJ, Liang CJ, Chieh JJ, et al. In vivo tumor targeting and imaging with anti-vascular endothelial growth factor antibody-conjugated dextran-coated iron oxide nanoparticles. Int J Nanomedicine. 2012;7:2833–2842. doi: 10.2147/IJN.S32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mallia RJ, McVeigh PZ, Fisher CJ. Wide-field multiplexed imaging of EGFR- targeted cancers using topical application of NIR SERS nanoprobes. Nanomedicine (Lond) 2015;10(1):89–101. doi: 10.2217/nnm.14.80. [DOI] [PubMed] [Google Scholar]
- 49.Koyama T, Shimura M, Minemoto Y, et al. Evaluation of selective tumor detection by clinical magnetic resonance imaging using antibody-conjugated superparamagnetic iron oxide. J Control Release. 2012;159(3):413–418. doi: 10.1016/j.jconrel.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Key J, Leary JF. Nanoparticles for multimodal in vivo imaging in nanomedicine. Int J Nanomedicine. 2014;9:711–726. doi: 10.2147/IJN.S53717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kong WH, Lee WJ, Cui ZY, et al. Nanoparticulate carrier containing water-insoluble iodinated oil as a multifunctional contrast agent for computed tomography imaging. Biomaterials. 2007;28(36):5555–5561. doi: 10.1016/j.biomaterials.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 52.Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009;361(1):32–39. doi: 10.1056/NEJMoa0900043. [DOI] [PubMed] [Google Scholar]
- 53.Chen VW, Ruiz BA, Hsieh MC, et al. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer. 2014;120(Suppl 23):3781–3792. doi: 10.1002/cncr.29045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng J, Muhanna N, De Souza R, et al. A multimodal nano agent for image-guided cancer surgery. Biomaterials. 2015;67:160–168. doi: 10.1016/j.biomaterials.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Anayama T, Nakajima T, Dunne M, et al. A novel minimally invasive technique to create a rabbit VX2 lung tumor model for nano-sized image contrast and interventional studies. PLoS One. 2013;8(6):e67355. doi: 10.1371/journal.pone.0067355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Cunha J, Herndon JEHerzan DL, et al. Poor correspondence between clinical and pathologic staging in stage 1 non-small cell lung cancer: results from CALGB 9761, a prospective trial. Lung Cancer. 2005;48(2):241–246. doi: 10.1016/j.lungcan.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Liptay MJ, D’amico TA, Nwogu C, et al. Intraoperative sentinel node mapping with technitium-99 in lung cancer: results of CALGB 140203 multicenter phase II trial. J Thorac Oncol. 2009;4(2):198–202. doi: 10.1097/JTO.0b013e318194a2c3. [DOI] [PubMed] [Google Scholar]
- 58.Rzyman W, Hagen OM, Dziadziuszko R, et al. Blue-dye intraoperative sentinel lymph node mapping in early non-small lung cancer. Eur J Surg Oncol. 2006;32(4):462–465. doi: 10.1016/j.ejso.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Gilmore DM, Khullar OV, Jaklitsch MT, et al. Identification of metastatic nodal disease in a phase 1 dose-escalation trial of intraoperative sentinel lymph node mapping in non-small cell lung cancer using near-infrared imaging. J Thorac Cardiovasc Surg. 2013;146(3):562–570. doi: 10.1016/j.jtcvs.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soltesz EG, Kim S, Laurence RG, et al. Intraoperative sentinel lymph node mapping of the lung using near-infrared fluorescent quantum dots. Ann Thorac Surg. 79(1):269–277. doi: 10.1016/j.athoracsur.2004.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parungo CP, Colson YL, Kim SW, et al. Sentinel lymph node mapping of the pleural space. Chest. 2005;127(5):1799–1804. doi: 10.1378/chest.127.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parungo CP, Ohnishi S, Kim SW, et al. Intraoperative identification of esophageal sentinel lymph nodes with near-infrared fluorescence imaging. J Thorac Cardiovasc Surg. 2005;129(4):844–850. doi: 10.1016/j.jtcvs.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young RC, Ozols RF, Myers CE. The anthracycline antineoplastic drugs. N Engl J Med. 1981;305(3):139–153. doi: 10.1056/NEJM198107163050305. [DOI] [PubMed] [Google Scholar]
- 64.Majoros IJ, Myc A, Thomas T, et al. PAMAM dendrimer-based multifunctional conjugate for cancer therapy: Synthesis, characterization, and functionality. Biomacromolecules. 2006;7(2):572–579. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]
- 65.Guo Y, Wang L, Lv P, et al. Transferrin-conjugated doxorubicin-loaded lipid-coated nanoparticles for the targeting and therapy of lung cancer. Oncol Lett. 2015;9(3):1065–1072. doi: 10.3892/ol.2014.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollis CP, Weiss HL, Evers BM, et al. In vivo investigation of hybrid Paclitaxel nanocrystals with dual fluorescent probes for cancer theranostics. Pharm Res. 2014;31(6):1450–1459. doi: 10.1007/s11095-013-1048-x. [DOI] [PubMed] [Google Scholar]
- 67.Zhao T, Chen H, Dong Y, et al. Paclitaxel-loaded poly(glycolide-co- ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate nanoparticles for lung cancer therapy. Int J Nanomedicine. 2013;8:1947–1957. doi: 10.2147/IJN.S44220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang C, Qu G, Sun Y, et al. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials. 2008;29(9):1233–1241. doi: 10.1016/j.biomaterials.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 69.Cheng R, Meng F, Deng C, et al. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34(14):3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 70.Stolzoff M, Ekladious I, Colby AH, et al. Synthesis and Characterization of Hybrid Polymer/Lipid Expansile Nanoparticles: Imparting Surface Functionality for Targeting and Stability. Biomacromolecules. 2015;16(7):1958–1966. doi: 10.1021/acs.biomac.5b00336. [DOI] [PubMed] [Google Scholar]
- 71.Liu R, Gilmore DM, Zubris KA, et al. Prevention of nodal metastases in breast cancer following the lymphatic migration of paclitaxel-loaded expansile nanoparticles. Biomaterials. 2013;34(7):1810–1819. doi: 10.1016/j.biomaterials.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng R, Feng F, Meng F, et al. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release. 2011;152(1):2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen CT, Tran TH, Amiji M, et al. Redox-Sensitive Nanoparticles from amphiphilic cholesterol-based block copolymers for enhanced tumor intracellular release of doxorubicin. Nanomedicine. 2015 doi: 10.1016/j.nano.2015.06.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voon SH, Kiew LV, Lee HB, et al. In vivo studies of nanostructure-based photosensitizers for photodynamic cancer therapy. Small. 2014;(24):4993–5013. doi: 10.1002/smll.201401416. [DOI] [PubMed] [Google Scholar]
- 75.Jiang F, Lilge L, Grenier J, et al. Photodynamic therapy of U87 human glioma in nude rat using liposome-delivered photofrin. Lasers Surg Med. 1998;22(2):74–80. doi: 10.1002/(sici)1096-9101(1998)22:2<74::aid-lsm2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 76.Jian WH, Yu TW, Chen CJ, et al. Indocyanine Green-Encapsulated Hybrid Polymeric Nanomicelles for Photothermal Cancer Therapy. Langmuir. 2015;31(22):6202–6210. doi: 10.1021/acs.langmuir.5b00963. [DOI] [PubMed] [Google Scholar]
- 77.Yang S, Li N, Liu Z, et al. Amphiphilic copolymer coated upconversion nanoparticles for near-infrared light triggered dual anticancer treatment. Nanoscale. 2014;6(24):14903–14910. doi: 10.1039/c4nr05305b. [DOI] [PubMed] [Google Scholar]
- 78.Chen J, Wang L, Yao Q, et al. Drug concentrations in axillary lymph nodes after lymphatic chemotherapy on patients with breast cancer. Breast Cancer Res. 2004;6(4):R474–R477. doi: 10.1186/bcr819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu R, Gilmore DM, Zubris KA, et al. Prevention of nodal metastases in breast cancer following the lymphatic migration of paclitaxel-loaded expansile nanoparticles. Biomaterials. 2013;34(7):1810–1819. doi: 10.1016/j.biomaterials.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qian Y, Qiu M, Wu Q, et al. Enhanced cytotoxic activity of cetuximab in EGFR-positive lung cancer by conjugating with gold nanoparticles. Sci Rep. 2014;4:7490. doi: 10.1038/srep07490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuroda S, Tam J, Roth JA, et al. EGFR-targeted plasmonic magnetic nanoparticles suppress lung tumor growth by abrogating G2/M cell-cycle arrest and inducing DNA damage. 2014;9:3825–3839. doi: 10.2147/IJN.S65990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Draz MS, Fang BA, Zhang P, et al. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics. 2014;4(9):872–892. doi: 10.7150/thno.9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. 2009;8(2):129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merkel OM, Rubinstein I, Kissel T. siRNA Delivery to the lung: What’s new? Adv Drug Deliv Rev. 2014;75:112–128. doi: 10.1016/j.addr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y, Gu H, Zhang DS, et al. Highly effective inhibition of lung cancer growth and metastasis by systemic delivery of siRNA via multimodal mesoporous silica-based nanocarrier. Biomaterials. 2014;35(38):10058–10069. doi: 10.1016/j.biomaterials.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 86.Guo C, Al-Jamal WT, Toma FM, et al. Design of Cationic Multiwalled Carbon Nanotubes as Efficient siRNA Vectors for Lung Cancer Xenograft Eradication. Bioconjug Chem. 2015;26(7):1370–1379. doi: 10.1021/acs.bioconjchem.5b00249. [DOI] [PubMed] [Google Scholar]
- 87.Luk BT, Zhang L. Current advances in polymer-based nanotheranostics for cancer treatment and diagnosis. ACS Appl Mater Interfaces. 2014;6(24):21859–21873. doi: 10.1021/am5036225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang C, Ravi S, Garapati US, et al. Multifunctional Chitosan Magnetic-Graphene (CMG) Nanoparticles: a Theranostic Platform for Tumor-targeted Co- delivery of Drugs, Genes and MRI Contrast Agents. J Mater Chem B Mater Biol Med. 2013;1(35):4396–4405. doi: 10.1039/C3TB20452A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khullar OV, Griset AP, Gibbs-Strauss SL, et al. Nanoparticle migration and delivery of Paclitaxel to regional lymph nodes in a large animal model. J Am Coll Surg. 2012;214(3):328–337. doi: 10.1016/j.jamcollsurg.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khullar O, Frangioni JV, Grinstaff M, et al. Image-guided sentinel lymph node mapping and nanotechnology-based nodal treatment in lung cancer using invisible near-infrared fluorescent light. Semin Thorac Cardiovasc Surg. 2009;21(4):309–315. doi: 10.1053/j.semtcvs.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]