Abstract
Objective
To investigate the impact of gestational exposure to selective serotonin reuptake inhibitors (SSRIs) on offspring neurodevelopment.
Method
This is a cohort study using national register data in Finland between the years 1996-2010. Pregnant women and their offspring were categorized into four groups: SSRI exposed (n=15,729); exposed to psychiatric disorder, no antidepressants (n=9,651); exposed to SSRIs only before pregnancy (n=7,980); and unexposed to antidepressants and psychiatric disorders (n=31,394). We investigated the cumulative incidence of offspring diagnoses of depression, anxiety, autism spectrum disorders (ASDs), and attention-deficit/hyperactivity disorders (ADHD) for the four groups from birth to 14 years, adjusting for confounders.
Results
The cumulative incidence of depression among offspring exposed prenatally to SSRIs was 8.2% (95% CI, 3.1-13.3%) by age 14.9, compared to 1.9% (95% CI, 0.9-2.9%) in the psychiatric disorder, no medication group (adjusted hazard ratio [HR], 1.78; 95% CI, 1.12-2.82; p=.02) and to 2.8% (95% CI, 1.4-4.3%) in the SSRI discontinued group (HR 1.84; 95% CI, 1.14-2.97; p=.01). Rates of anxiety, ASD, and ADHD diagnoses were comparable to rates in offspring of mothers with a psychiatric disorder but no medication during pregnancy. Comparing SSRI exposed to unexposed, the HRs were significantly elevated for each outcome.
Conclusion
Prenatal SSRI exposure was associated with increased rates of depression diagnoses in early adolescence but not with ASD or ADHD. Until confirmed, these findings must be balanced against the substantial adverse consequences of untreated maternal depression.
Keywords: SSRI, pregnancy, offspring, depression, ASD, ADHD
Introduction
Exposure to selective serotonin reuptake inhibitors (SSRIs) during sensitive developmental periods in rodents produces long-lasting effects on behavior, neural circuitry, morphology, and physiology.1-3 Importantly, the developmental period during which rodents are sensitive to the effects of SSRI exposure overlaps extensively with human brain development during the fetal period. Because the serotonin (5HT) system is highly conserved across phylogeny, we suspected that similar effects might be seen in humans as SSRIs pass readily through the placenta and enter fetal circulation.4 SSRIs have been prescribed increasingly to pregnant women since their introduction 30 years ago.5,6 Yet no studies have been undertaken that followed children beyond 6 years of age for depressive disorders, which typically emerge after the onset of puberty.7, 8 Research on associations between SSRI exposure and other neurodevelopmental disorders, including autism spectrum disorders (ASDs) and attention-deficit/hyperactivity disorder (ADHD), has shown inconsistent results.9-14
The potential effect of prenatal SSRI exposure on vulnerability to later life disorders is confounded by maternal and paternal mental illness that would increase the risk of neuropsychiatric diagnoses in these children. To address these potential confounds and to better address the long-term safety of SSRI use during pregnancy for the offspring, we performed a large population-based study to investigate the effect of prenatal exposure to SSRIs, or maternal depression-related psychiatric disorders without medication, on offspring neurodevelopment through 14 years of age. Because of the steadily increasing use of SSRIs during pregnancy and the potential long-term burden of offspring depression and anxiety, this question carries substantial public health importance.
Method
Data Sources and Study Population
We used a population-based, prospective cohort study design. All data were collected from national registers linked by the unique personal identification number assigned to all citizens and permanent residents of Finland. In Finland, all children regularly attend child welfare clinics where trained public health nurses or physicians perform medical examinations; this is offered to all and is free of charge. These services are particularly designed to identify psychiatric and neurodevelopmental disorders. Annual examinations are performed for all school children and, when indicated, a referral is made to specialized health care; these conditions are then recorded in the inpatient and outpatient registries. Specialized care includes hospital outpatient clinics and inpatient hospital units that are run by specialists. Psychiatric services are run by psychiatrists, who are required to have a six-year postdoctoral specialist education. A detailed description of the registers and the study design has been published previously.5
The total sampling frame includes 845,345 singleton live births in Finland between January 1, 1996 and December 31, 2010. Accordingly, the age range of children in the study cohort is from birth to age 14. The mother–child dyads were identified from the national Medical Birth Register (MBR). The register collects data on maternal demographics, reproductive and medical history, health-related behaviors, diagnoses during pregnancy and delivery, and neonatal outcome since 1987, using the International Classification of Diseases, 10th Revision (ICD-10) coding since 1996.
The Drug Reimbursement Register, maintained since 1995, collects data on prescription drug purchases and was used to identify the study groups. Drug purchases are recorded concomitantly with the purchase at pharmacies using the International Anatomic-Therapeutic-Chemical (ATC) classification, and drugs are supplied for a maximum of three months at a time. The Special Reimbursement Register contains data on chronic illnesses requiring continuous medication since 1964.
The Hospital Discharge Register (HDR) includes inpatient diagnoses covering all somatic and psychiatric hospitals in Finland since 1969, and outpatient diagnoses in public hospitals since 1998. The diagnoses are coded using the ICD-8 (1969–1986), ICD-9 (1987–1995), and ICD-10 since 1996. Information on parental psychiatric diagnoses (ICD-8, ICD-9 and ICD-10), and diagnoses of neuropsychiatric disorders in offspring (ICD-10) were derived from this register. Patients treated solely in public primary or in private care are not included in this register.
The national population register contains basic information, including country of birth, marital status, marriages and divorces, and deaths of all Finnish citizens and other citizens residing permanently in Finland.
The register administrators and the data protection authority approved the utilization of sensitive health registry data for scientific research and the data linkages. The Institutional Ethical Review Board at the National Institute for Health and Welfare, the Social Insurance Institution in Finland, and the Institutional Review Board of the New York State Psychiatric Institute approved the study protocol. All data were anonymized and de-identified prior to analysis. The study participants were not contacted and, according to Finnish legislation, informed consent was therefore not required.
Exposure Groups, Mother–Child Dyads
SSRI Exposed, n=15,729
Mothers in this group had one or more purchases of SSRIs (fluoxetine, citalopram, paroxetine, sertraline, fluvoxamine, escitalopram) during the period from 30 days before pregnancy until the end of pregnancy; the date of purchase indicated the beginning date for each exposure. The beginning of gestation corresponding to the last menstrual period was calculated from the best clinical estimation of gestational age at birth, primarily based on ultrasound and MBR registration. Information on diagnosis of depression or depression-related diagnoses was available for 4,811 (30.6%) mothers in this group; that is mothers, who had been treated in inpatient care or outpatient specialized care, and accordingly had a diagnosis in the HDR.
Among the mothers with diagnoses available in the register, 4,713 (98.0%) had a diagnosis related to affective disorders (depression, anxiety, bipolar disorder), and 265 (5.5%) had a diagnosis of non-affective or undefined psychosis.
The mutually exclusive comparison cohorts included:
Psychiatric disorder, no medication group (n=9,651)
This group was exposed to maternal psychiatric diagnosis but no SSRI during pregnancy. This group included all mothers who had a diagnosis of depression or other psychiatric disorder related to depression or SSRI use, obtained from the HDR (ICD-10 F20-F48; ICD-9 295-298, 300) from one year before pregnancy until discharge (≤ 3 weeks) from hospital after delivery, and no purchases of antidepressants (ATC codes N06A, N06CA) or antipsychotics (N05A) from three months before until the end of pregnancy. A total of 9,407 (97.5%) of mothers had a diagnosis related to affective disorders, and 424 (4.4%) had a diagnosis of non-affective or undefined psychosis.
SSRI Discontinued Group (n=7,980)
This group was exposed to SSRIs only prior to pregnancy. Mothers had one or more purchases of SSRIs during one year before pregnancy until three months before pregnancy but no purchases of antidepressant or antipsychotic drugs during three months before pregnancy until delivery.
Unexposed (n=31,394)
This group was unexposed to SSRIs and had no diagnosis of depression or other psychiatric disorder related to depression or SSRI use. This group included mothers with neither purchases of antidepressants nor antipsychotics, and no depression or related psychiatric disorder at any time prior to or during pregnancy. Two unexposed per 1 participant exposed to SSRI were matched for offspring date of birth within +/-6 months.
Outcome Variables
The outcome variables included: 1) depression, including depressive disorders and unspecified affective disorders (ICD-10 F32-39); 2) anxiety, including anxiety disorder (F40-41); 3) autism spectrum disorder, (ASD; F84, but excluding Rett's syndrome, F84.2); 4) attention-deficit/hyperactivity disorder (ADHD; F90). Only ICD codes used after the diagnosis was established (ICD-10 F-codes for psychiatric disorders) were included; codes used in the evaluation process (ICD-10 Z-codes) were excluded. We excluded those with a depression diagnosis only during the first two years of life if the diagnosis was not recorded at later stages.
Covariates
Data on covariates were derived from the registers described above and are delineated in Table 1. While the register data have high coverage for most covariates, data on socioeconomic status (SES) are recorded less accurately. The SES classification is based on maternal occupation, and nearly one third of participants were classified as “others,” including students, housewives, entrepreneurs, and those who were unemployed.
Table 1. Maternal, Neonatal, and Family Characteristics Tested as Covariates by the 4-Class Exposure Status.
| Characteristics | Exposure | Association of covariate with exposure status | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SSRI exposed | Psychiatric disorder, no medication | SSRI discontinued group | Unexposed | p-value | |||||
| n=15,729 | n=9,651 | n=7,980 | n=31,394 | ||||||
| n | % | n | % | n | % | n | % | ||
| Maternal age | <.0001 | ||||||||
| ≤19 | 524 | 3.3 | 954 | 9.9 | 217 | 2.7 | 775 | 2.5 | |
| 20-29 | 7,426 | 47.2 | 4,530 | 46.9 | 3,632 | 45.5 | 15,523 | 49.5 | |
| 30-39 | 7,023 | 44.7 | 3,768 | 39.0 | 3,826 | 47.9 | 13,959 | 44.5 | |
| ≥40 | 756 | 4.8 | 399 | 4.1 | 305 | 3.8 | 1,137 | 3.6 | |
| Place of residence | <.0001 | ||||||||
| urban | 10,687 | 68.0 | 6,706 | 69.5 | 5,608 | 70.3 | 21,233 | 67.8 | |
| semiurban | 2,552 | 16.2 | 1,512 | 15.7 | 1,161 | 14.6 | 5,050 | 16.1 | |
| rural | 2,489 | 15.8 | 1,427 | 14.8 | 1,209 | 15.2 | 5,054 | 16.1 | |
| Marital status at birth | <.0001 | ||||||||
| married or in relationship/divorced | 13,364 | 89.6 | 7,918 | 88.1 | 6,940 | 92.4 | 28,832 | 95.5 | |
| Socioeconomic status | <.0001 | ||||||||
| upper white collar worker | 1,857 | 11.8 | 1,219 | 12.6 | 1,207 | 15.1 | 5,360 | 17.1 | |
| lower white collar worker | 4,954 | 31.5 | 2,824 | 29.3 | 2,683 | 33.6 | 10,479 | 33.4 | |
| blue collar worker | 2,493 | 15.9 | 1,435 | 14.9 | 1,263 | 15.8 | 4,271 | 13.6 | |
| other a | 3,669 | 23.3 | 2,365 | 24.5 | 1,499 | 18.8 | 5,728 | 18.3 | |
| unknown | 2,756 | 17.5 | 1,808 | 18.7 | 1,328 | 16.6 | 5,556 | 17.7 | |
| Parity | <.0001 | ||||||||
| no previous births | 6,534 | 41.6 | 4,455 | 46.2 | 3,665 | 45.9 | 12,817 | 40.8 | |
| Smoking during pregnancy | 4,575 | 29.9 | 2,737 | 29.1 | 1,855 | 23.8 | 3,947 | 12.9 | <.0001 |
| Exposure to teratogenicb drugs | 18 | 0.1 | 10 | 0.1 | 13 | 0.2 | 30 | 0.1 | .44 |
| Exposure to anxiolyticsc and sedatives | 2,933 | 18.7 | 426 | 4.4 | 329 | 4.1 | 147 | 0.5 | <.0001 |
| Exposure to antiepilepticd drugs | 500 | 3.2 | 162 | 1.7 | 77 | 1.0 | 136 | 0.4 | <.0001 |
| Entitlement to special reimbursement for any chronic disease ever | 179 | 1.1 | 126 | 1.3 | 94 | 1.2 | 230 | 0.7 | <.0001 |
| Preterm birth <37wk | 821 | 5.2 | 608 | 6.3 | 379 | 4.8 | 1,379 | 4.4 | <.0001 |
| Birth weight <2,500g | 572 | 3.6 | 430 | 4.5 | 280 | 3.5 | 989 | 3.2 | <.0001 |
| Neonatal care unit | 2,405 | 15.3 | 1,160 | 12.0 | 857 | 10.7 | 3,032 | 9.7 | <.0001 |
| Maternal history of other psychiatric diagnosese | 3,589 | 22.8 | 2,532 | 26.2 | 997 | 12.5 | 612 | 2.0 | <.0001 |
| Maternal history of substance abusef | 1,815 | 11.5 | 1.096 | 11.4 | 525 | 6.6 | 240 | 0.8 | <.0001 |
| Paternal history of psychiatric diagnosesg | 3,393 | 21.6 | 2,251 | 23.3 | 1,356 | 17.0 | 2,885 | 9.2 | <.0001 |
| Mother's country of birth | <.0001 | ||||||||
| other than Finland | 706 | 4.5 | 591 | 6.1 | 367 | 4.6 | 4,280 | 13.6 | |
| Parental death | 422 | 2.7 | 243 | 2.5 | 162 | 2.0 | 261 | 0.8 | <.0001 |
Note: Differences between the groups analyzed by χ2 test and when not valid, by Fischer's exact test. Percentages were calculated from non-missing data. Missing values, n (%): place of residence, 66 (0.1); marital status, 3,158 (4.9); previous births, 20 (0.0); smoking during pregnancy, 1,629 (2.5); mother's country of birth, 1 (0.0). SSRI = selective serotonin reuptake inhibitor.
E.g. students, housewives, entrepreneurs, unemployed
Teratogenic drugs: purchases of isotretinoin (Anatomic-Therapeutic-Chemical [ATC], code D10BA), acitretin (D05BB02), antineoplastic agents (L01), methotrexate (L04AX03), mychophenolate (L04AA06), misoprostol (A02BB01 and M01AB55), carbimazole (H03BB01) any time during pregnancy or one month prior to pregnancy.
Anxiolytics and sedatives: purchases (ATC codes N05B, N05C) any time during pregnancy or one month prior to pregnancy.
Antiepileptic drugs: purchases (ATC code N03) any time during pregnancy or one month prior to pregnancy.
Maternal history of other psychiatric diagnoses: any other psychiatric diagnosis than depression-related disorders (F20-F48 used as inclusion/exclusion criteria) or substance abuse (International Classification of Diseases 10th Revision [ICD-10] F50-F99; 9th Revision [ICD-9] 299, 301-302, 307, 309, 312-319; 8th Revision [ICD-8] 301, 302.10-302.99, 305-308, 310-315), recorded ever before delivery.
Maternal history of substance abuse: (ICD-10) F10-F19; (ICD-9) 291, 292, 303, 304, 305; (ICD-8) 291, 294.30, 303, 304, ever recorded.
Paternal history of psychiatric diagnoses: (ICD-10) F10-F99; (ICD-9) 291-292, 295-309, 312-319; (ICD-8) 291, 294.30, 295-301, 302.10-308, 310-315, ever recorded.
Statistical Analyses
Clinically relevant and plausible covariates were first tested (Table 1). Sex was included in all adjusted models. Because SES and maternal psychiatric disorders are associated with psychiatric drug use and child neurodevelopment, SES and maternal history of other psychiatric diagnoses (excluding depression-related disorders, which were used in defining one comparison group, and substance abuse; see Table 1 for ICD codes) were further included in all adjusted analyses. Other covariates were included in the models if they were associated with both exposure and outcome at p<.1.
To take into account the fact that children born from 1996-2010 were aged 0-14 years at the end of the follow-up in 2010, we used survival methods. Events in the survival analysis were defined as age at the first diagnosis of the studied outcome. Separate survival analyses were conducted for each of the four outcomes. Time observations after migrating, dying, and the end of the follow-up on December 31, 2010 were censored. We plotted the cumulative incidence of offspring diagnoses among the SSRI exposed, the psychiatric disorder, no medication group, the SSRI discontinued group, and the unexposed group using the Kaplan-Meier method. To compare offspring psychiatric diagnoses between these four groups, Cox proportional hazards models were used. Data from siblings in the same family were treated as clusters because outcomes from the same family are likely to be related. Robust sandwich estimates of the variance of the estimated Cox regression parameters were used for this purpose. Each outcome was analyzed separately by fitting two models: a crude model adjusted only for sex, and a model adjusted for additional covariates associated with the exposure and each specific outcome. The “unknown” category for SES was included, as the missing observations occurred completely at random. The age-specific depression and anxiety rates in offspring by different exposure groups were estimated with a Poisson-regression model, assuming a Poisson error distribution. All analyses were performed in SAS (SAS 9.4, SAS Institute, Cary, NC, USA).
Results
Maternal and family characteristics tested as covariates by exposure status are shown in Table 1, tested by offspring outcome diagnosis in Table S1 (available online), and specifically for offspring depression in Table S2 (available online). The distribution of offspring by birth year demonstrates that in the SSRI-exposed group, fewer offspring were in the oldest age cohort (1.6%) compared to offspring in the psychiatric disorder, no medication group (2.3%) or in the SSRI-discontinued group (3.4%) (Table S3, available online). The person years under observation in each of the offspring groups are presented in Table S4 (available online). The number and percentage of offspring who migrated or died according to exposure group in different age groups is shown in Table S5 (available online).
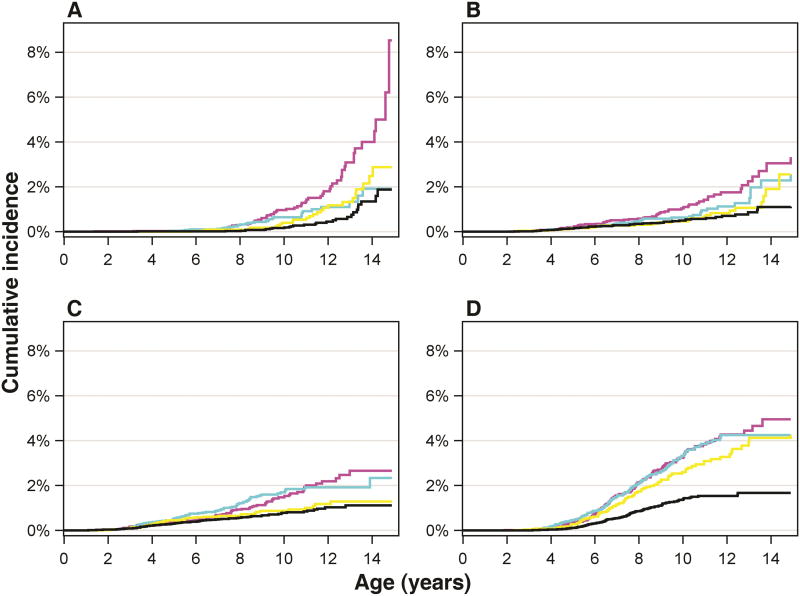
Children exposed to SSRIs during gestation were diagnosed with depression increasingly from age 12 onwards compared to the control groups that were not exposed, reaching a cumulative incidence of 8.2% by age 14.9, compared to 1.9% in the psychiatric disorder, no medication group, 2.8% in the SSRI discontinued group, and 1.6% in the unexposed group (Figure 1A, Table 2; Figure S2, available online). The adjusted hazard ratio (HR) for depression in offspring exposed to SSRIs was 1.78 (95% CI, 1.12-2.82; p= .02) compared to the psychiatric disorder, no medication group and 1.84 (95% CI, 1.14-2.97; p=.01) compared to the SSRI-discontinued group (Figure 2A and B; Table S6, available online). The age-specific incidence rate per 10,000 person-years of offspring depression in the oldest age group, 12-14 years, was 180.7 in the SSRI-exposed group compared to 35.1 in the psychiatric disorder, no medication group, 84.8 in the SSRI-discontinued group, and 58.4 in the unexposed group (Table S7, available online). We tested for sex interaction, and there was no significant interaction in comparisons between the main exposure groups (SSRI-exposed group; psychiatric diagnosis, no medication group; SSRI discontinued group) and depression.
Fig 1.
The cumulative incidence (%) of offspring diagnoses by age. Note: The cumulative incidence (%) of offspring diagnoses by age among offspring born between January 1, 1996, and December 31, 2010, and followed up to December 31, 2010 (age range from 0.0 to 14.9 years at the end of follow-up). Participants were censored from the age-defined analysis after December 31, 2010. Offspring exposed prenatally to selective serotonin reuptake inhibitors (SSRIs) are represented by magenta line, offspring exposed to maternal psychiatric disorder but no medication by cyan line, offspring of mothers discontinuing SSRI use prior to pregnancy by yellow line, and offspring unexposed to maternal psychiatric disorder and medication by black line. Panels A-D illustrate separately offspring depression (A), anxiety (B), autism spectrum disorder (ASD; C) and attention-deficit/hyperactivity disorder (ADHD; D).
Table 2.
Cumulative Incidence and 95% CIs by Age 14 for Depression, Anxiety, Autism Spectrum Disorders (ASDs) and Attention- Deficit/Hyperactivity Disorders (ADHD) in Offspring of Mothers Using Selective Serotonin Reuptake Inhibitors (SSRIs) During Pregnancy, Offspring of Mothers With Psychiatric Disorder but no Antidepressant Use, Offspring of Mothers Discontinuing SSRI Use Prior to Pregnancy, and in Unexposed Offspring
| SSRI Exposed n=15,729 | Psychiatric Disorder, no medication exposed n=9,651 | SSRI Discontinued Group n=7,980 | Unexposed n=31,394 | |||||
|---|---|---|---|---|---|---|---|---|
| Offspring diagnosis | n | Cumulative incidence,a % (95% CI) | n | Cumulative incidence,a % (95% CI) | n | Cumulative incidence,a % (95% CI) | n | Cumulative incidence,a % (95% CI) |
| Depression | 60 | 8.2 (3.1-13.3) | 30 | 1.9 (0.9-2.9) | 25 | 2.8 (1.4-4.3) | 30 | 1.6 (0.8-2.4) |
| Anxiety | 65 | 3.1 (1.8-4.3) | 39 | 2.3 (1.3-3.2) | 27 | 2.6 (0.9-4.2) | 62 | 1.1 (0.6-1.6) |
| ASD | 88 | 2.7 (1.9-3.5) | 79 | 2.3 (1.9-2.8) | 40 | 1.3 (0.8-1.8) | 100 | 1.1 (0.8-1.4) |
| ADHD | 160 | 4.9 (3.8-6.0) | 137 | 4.2 (3.5-5.0) | 93 | 4.1 (3.1-5.1) | 124 | 1.7 (1.3-2.0) |
Note: Depression included depressive disorders and unspecified affective disorders (International Classification of Diseases 10th Revision [ICD-10] code: F32-39). ICD-10 code for anxiety: F40-41. ICD-10 code for ASD: F84 (excluding Rett's syndrome, F84.2). ADHD (ICD-10 F90).
Kaplan-Meier method.
Fig 2.
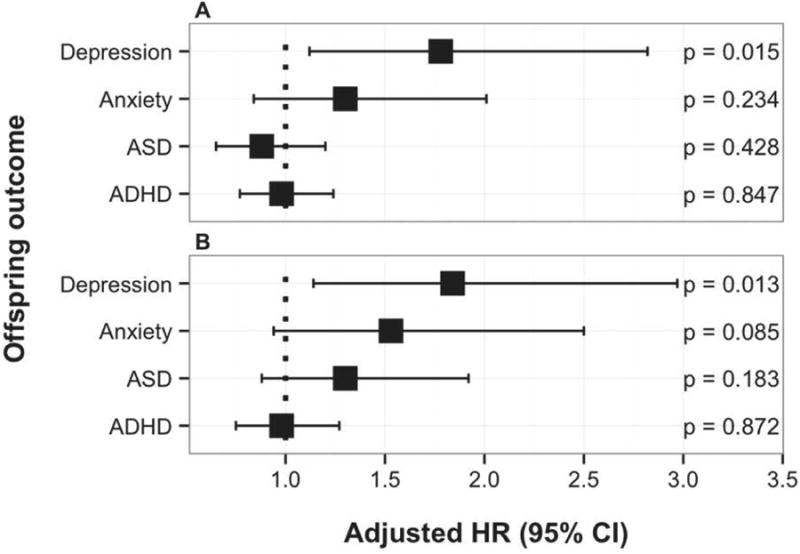
Adjusted hazard ratios (HR) and 95% CIs for offspring diagnoses. Note: Adjusted HR and 95% CIs of offspring diagnoses when comparing offspring exposed prenatally to selective serotonin reuptake inhibitors (SSRIs) to offspring exposed to maternal psychiatric disorder but no medication (panel A), and to offspring born to mothers who discontinued SSRI use prior to pregnancy (panel B). ADHD = attention-deficit/hyperactivity disorder; ASD = autism spectrum disorder.
Although gestational SSRI exposure was associated with higher rates of depression in adolescent offspring, there was no increase in diagnosed anxiety disorders (Figure 1B, Figure 2, Table S6, available online). Likewise, the age-specific incidence rate of anxiety in the oldest age group differed only marginally between the SSRI-exposed, the psychiatric disorder, no medication, and the SSRI-discontinued groups (Figure 1B; Table S8, available online). Rates of ASD and ADHD diagnoses in the SSRI-exposed group were comparable to rates in offspring of mothers with a psychiatric disorder who did not use SSRIs during pregnancy, and to rates in offspring of mothers who discontinued SSRIs prior to pregnancy (Figure 1C, 1D; Figure 2; Table S6, available online). The age-specific incidence rates of ASD and ADHD are presented in Tables S9 and S10 (available online). Comparing SSRI exposed to unexposed, the HRs were significantly elevated for each outcome (Table S6, available online). While there was an increasing trend for ASD, no significant HRs were observed for any of the outcomes when comparing the psychiatric disorder, no medication group to the SSRI-discontinued group, whereas comparing the psychiatric disorder, no medication group to the unexposed group, the HRs were increased for ASD and ADHD (Table S11, available online).
Restricting exposure to SSRI monotherapy (n=12,121) still yielded increased HRs for offspring depression when compared to the psychiatric disorder, no medication group (HR, 1.85; 95% CI, 1.15-2.98; p=.01) and to the SSRI-discontinued group (HR, 2.12; 95% CI, 1.29-3.48; p=.003). Following adjustment for other possible indicators of maternal illness severity including diagnoses related to suicidal behavior, the HR for offspring depression remained significantly elevated in the SSRI-exposed compared to all comparison groups (Table S12, available online).
Discussion
Statement of Principal Findings
Using national register data, we observed increased rates of depression emerging at 12-14 years in offspring exposed prenatally to SSRIs. Taking into account maternal underlying psychiatric disorder. SSRI exposure was not associated with an increased risk of ASDs, ADHD, or anxiety. This study, like all studies attempting to answer questions about the long-term effects of in utero exposure to SSRIs, was observational, as it would not be possible to carry out a clinical trial of different exposures specially looking for effects manifesting earliest at 12-14 years of age, such as depression.
Strengths and Weaknesses
A major strength is that we took into account maternal underlying psychopathology. Antenatal depression itself is associated with an increased risk of emotional disorders in offspring,15 suggesting by extension that maternal depression severity impacts offspring risk. Hence, we controlled for potential depression severity differences between the SSRI-exposed and the psychiatric diagnosis, no medication groups. Two prior studies have shown that, contrary to recommendations, the severity of maternal psychiatric symptoms is not related to the choice to continue or discontinue medication use during pregnancy,16,17 allowing us to use the SSRI-discontinued group as a depression severity control. Furthermore, we excluded women using multiple psychotropic medications (a proxy for severity) and restricted our exposure to SSRI monotherapy. This refined SSRI exposure group still yielded increased HRs for offspring depression when compared to the psychiatric disorder, no medication group and the SSRI-discontinued group. Following adjustment for other possible indicators of maternal illness severity, including previous diagnoses related to suicidal behavior, the HR for offspring depression remained significantly elevated in the SSRI-exposed compared to all comparison groups. Hence, when both main comparison groups were adjusted for these “severity proxies,” the significance of SSRI exposure as the principal variable did not diminish.
Recent studies found that increased rates of ASD associated with in utero exposure to SSRIs became non-significant when controlled for maternal psychiatric illness.9, 10 Our findings are in line with those results and show that, similar to ASD, SSRI exposure added no additional risk above mothers' psychiatric illness to offspring ADHD and anxiety. Yet offspring of both groups (SSRI-exposed and psychiatric disorder, no medication groups) had higher risks of ASD and ADHD than offspring of mothers with no diagnosis and no psychotropic medication use. Likewise, the use of SSRI medications before pregnancy did not increase the rates of these disorders over and above the rates seen with maternal illness alone and no gestational SSRI use. We acknowledge that in this register-based setting, we cannot fully confirm the comparability of the exposure groups, but these observations support the notion that our main comparison groups (SSRI-exposed, psychiatric disorder, no medication group, and SSRI-discontinued group) were not biased by increased severity of illness in mothers using SSRIs during pregnancy.
A further strength is the high quality of the register data included in the study. The quality and coverage of the MBR have been validated and are considered good,18 and the Population Register covers all Finnish citizens and permanent residents in Finland.19 The quality of the HDR has been validated and is good for psychiatric diagnoses.20 Approximately 1% of children in Finland have been diagnosed in specialized services with ASD until age 14,8 close to population-based estimates in Western countries.21 Therefore, it is likely that most children with ASD in the community are identified in the register. Further, the diagnostic validity of Finnish register-based diagnoses of ASD is high.22
As we have described in detail in previous work,5,8 children under 16 years have yearly medical check-ups in Finland; those who have serious psychiatric disorders are referred to specialized services for assessment. Therefore, it is likely that children with severe and moderate problems are found in registers. However, those with less serious problems might not be identified in medical check-ups or may not be referred to specialized services, potentially reducing the generalizability of our findings. Differential treatment seeking is also a potential limitation if children with depression in the community have different probabilities of being referred to specialized services depending on mother's medication use. It is unlikely, though, that offspring referral to specialized services would depend on the timing of mother's SSRI use (before or during pregnancy). The specific impact of SSRI exposure on offspring depression but not on other neuropsychiatric diagnoses also mitigates the possible confound of surveillance bias.
A limitation is that even in this large population-based sample, the numbers were too small to allow trimester-specific analyses. As in all register-based studies, a major limitation is that we cannot confirm adherence to the purchased drugs. However, noncompliance would result in overestimating exposure, likely biasing the observed associations towards null. Previous research has also indicated a good correlation between prescription register data and self-reported antidepressant use.23 We also consider the risk of false negatives, unexposed offspring who in fact were exposed, as minimal, because the drug imbursement register covers over 98% of all drug purchases in Finland.24 We had no direct information on alcohol or illicit drug use, but we controlled for substance abuse for both parents by identifying related diagnoses in the HDR. A further limitation is that we had no information about postnatal environment. Although the findings on maternal SSRI use and depression persisted adjusting for previous diagnoses related to suicidal behavior, it is possible that postnatal experiences associated with maternal SSRI use and depression may have influenced the results.
Comparison to Previous Studies
Previous studies investigating association between prenatal exposure to SSRIs and child internalizing behavior outcomes have shown inconsistent results.25,26,27 The sample sizes in those clinical studies were small, ranging from 22 to 62 children, and the follow-up time extended only until three to six years of age. In the largest sample, children exposed prenatally to SSRIs were more likely to present with internalizing problems by age six than those exposed to maternal depression only, but the severity of maternal depression rather than antidepressant use predicted child behavior.25 In the other studies, maternal anxiety and SSRI use26 and maternal depression and anxiety only27 were associated with an increased risk of offspring internalizing behavior. Two of the studies25,26 assessed child behavior at only one time point and by maternal self-report, which may have caused misclassification bias. Prior studies of prenatal SSRI exposure in relation to offspring ASD and ADHD have revealed conflicting results. In line with our results, two register-based studies9,10 and a study utilizing electronic health records11 did not show an association between SSRIs and offspring ASD. Further, a register-based study found no independent association with SSRI exposure during pregnancy and offspring ADHD after controlling for confounders.12 Our study provides additional support for these results by showing that there is no association for ASD or ADHD when offspring exposed to SSRIs are directly compared to offspring whose mothers were in treatment for a depression-related disorder during pregnancy. However, our results are contrary to a study including nearly 300 children with ASD in the Kaiser Permanente health system,13 and two other population-based studies, 14,29 both suggesting an association between SSRI use during pregnancy and ASD, the latter observing an association specifically with second and third trimester exposure in offspring of women predominantly of lower socioeconomic status.29 Further, a case-control study including 2,243 children with ADHD showed an association between antidepressant exposure during pregnancy and ADHD after controlling for maternal depression.11 The discrepancy between that study and our negative finding might be explained by the fact that the prior study analyzed maternal depression regardless of whether it occurred before or during pregnancy, while we focused on maternal depression and related disorders close to and during pregnancy, i.e. the time period with a particularly high risk for offspring adversity.
Taken together, our results related to offspring risk of ASD or ADHD after gestational exposure to SSRIs are reassuring. However, if SSRI exposure during gestation has delayed but significant effects on offspring risk for depression, research regarding timing of exposure, and considerations regarding antidepressant mechanism of action and increased use of validated psychotherapies28 may be needed to maximize maternal benefits while minimizing risk to the long-term health of the developing fetus. Clearly, further research is needed to follow offspring through adolescence; given the typical age of onset of depression, this would substantially increase the sample size as well as the generalizability of the findings. Meanwhile, until either confirmed or refuted, these findings must be balanced against the substantial adverse consequences of untreated maternal depression.
Supplementary Material
Clinical Guidance.
Our study suggests that gestational SSRI exposure increases the risk of depression in early adolescent offspring, taking into account maternal psychiatric illness.
We found no increased risk of anxiety, autism spectrum disorders or ADHD in children exposed prenatally to SSRIs
The oldest study cohort was only reaching the age of risk for depression, and further research is urgently needed to follow offspring through adolescence.
Meanwhile, until either confirmed or refuted, these findings must be balanced against the substantial adverse consequences of untreated maternal depression.
Acknowledgments
The research was supported by NIH Grant P50MH090966 (all authors), the Sackler Institute for Developmental Psychobiology of Columbia University (M.W., J.G.), grants from the Sigrid Juselius Foundation, the Foundation for Pediatric Research in Finland, and the Finnish Medical Foundation (D.G.).
Ms. Hinkka-Yli-Salomäki and Drs. McKeague and Wickramaratne served as the statistical experts for this research.
The authors wish to thank Juha-Pekka Virtanen, BSc, for data management, and Jukka Huttunen, Project Coordinator, both of the Department of Child Psychiatry, University of Turku, Turku, Finland, for administrative support.
Dr. Weissman has received funding from the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), the National Alliance for Research on Schizophrenia and Depression (NARSAD), the Sackler Foundation, and the Templeton Foundation; and has received royalties from Oxford University Press, Perseus Press, the American Psychiatric Association Press, and MultiHealth Systems, in the past three years.
Footnotes
Disclosure: None of these disclosures pose conflicts of interest.
Drs. Malm, Brown, Gissler, Gyllenberg, McKeague, Wickramaratne, Artama, Gingrich, Sourander, and Ms. Hinkka-Yli-Salomäki report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 2.Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rampono J, Simmer K, Ilett KF, et al. Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry. 2009;42:95–100. doi: 10.1055/s-0028-1103296. [DOI] [PubMed] [Google Scholar]
- 5.Malm H, Artama M, Brown AS, et al. Infant and childhood neurodevelopmental outcomes following prenatal exposure to selective serotonin reuptake inhibitors: overview and design of a Finnish Register-Based Study (FinESSI) BMC Psychiatry. 2012;12:217. doi: 10.1186/1471-244X-12-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huybrechts KF, Palmsten K, Mogun H, et al. National trends in antidepressant medication treatment among publicly insured pregnant women. Gen Hosp Psychiatry. 2013;35:265–71. doi: 10.1016/j.genhosppsych.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. Am J Psychiatry. 2006;163:1001–1008. doi: 10.1176/ajp.2006.163.6.1001. [DOI] [PubMed] [Google Scholar]
- 8.Gyllenberg D, Gissler M, Malm H, et al. Specialized service use for psychiatric and neurodevelopmental disorders by age 14 in Finland. Psychiatr Serv. 2014;65:367–373. doi: 10.1176/appi.ps.201200544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Eng J Med. 2013;369:2406–2415. doi: 10.1056/NEJMoa1301449. [DOI] [PubMed] [Google Scholar]
- 10.Sørensen MJ, Grønborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–459. doi: 10.2147/CLEP.S53009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry. 2015;20:727–34. doi: 10.1038/mp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laugesen K, Olsen MS, Telén Andersen AB, Frøslev T, Sørensen HT. In utero exposure to antidepressant drugs and risk of attention deficit hyperactivity disorder: a nationwide Danish cohort study. BMJ Open. 2013;3:e003507. doi: 10.1136/bmjopen-2013-003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–12. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- 14.Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ. 2013;346:f2059. doi: 10.1136/bmj.f2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson RM, Evans J, Kounali D, et al. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry. 2013;70:1312–19. doi: 10.1001/jamapsychiatry.2013.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 17.Yonkers KA, Gotman N, Smith MV, et al. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology. 2011;22:848–854. doi: 10.1097/EDE.0b013e3182306847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gissler M, Schelley J. Quality of data on subsequent events in a routine Medical Birth Register. Med Inform Internet Med. 2002;27:33–38. doi: 10.1080/14639230110119234. [DOI] [PubMed] [Google Scholar]
- 19. [Accessed September 30, 2015];Population Register Centre. Available: http://www.vrk.fi/
- 20.Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–515. doi: 10.1177/1403494812456637. [DOI] [PubMed] [Google Scholar]
- 21.Elsabbagh M, Divan G, Koh YJ, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5:160–79. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lampi KM, Sourander A, Gissler M, et al. Brief report: validity of Finnish registry-based diagnoses of autism with the ADI-R. Acta Paediatr. 2010;99:1425–1428. doi: 10.1111/j.1651-2227.2010.01835.x. [DOI] [PubMed] [Google Scholar]
- 23.Stephansson O, Granath F, Svensson T, Haglund B, Ekbom A, Kieler H. Drug use during pregnancy in Sweden - assessed by the Prescribed Drug Register and the Medical Birth Register. Clin Epidemiol. 2011;3:43–50. doi: 10.2147/CLEP.S16305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martikainen J, Pajunen R, Saastamoinen L, Voipio T. Finnish Medicines Agency and the Social Insurance Institution in Finland (Tampere) Juvenes Print and Tampereen; Yliopistopaino Oy: 2010. Finnish Statistics on Medicines 2009. [Google Scholar]
- 25.Nulman I, Koren G, Rovet J, Barrera M, Pulver A, Streiner D. Neurodevelopment of children following prenatal exposure to venlafaxine, selective serotonin reuptake inhibitors, or untreated maternal depression. Am J Psychiatry. 2012;169:1165–1174. doi: 10.1176/appi.ajp.2012.11111721. [DOI] [PubMed] [Google Scholar]
- 26.Oberlander TF, Papsdorf M, Brain UM, Misri S, Ross C, Grunau RE. Prenatal effects of selective serotonin reuptake inhibitor antidepressants, serotonin transporter promoter genotype (SLC6A4), and maternal mood on child behavior at 3 years of age. Arch Pediatr Adolesc Med. 2010;164:444–451. doi: 10.1001/archpediatrics.2010.51. [DOI] [PubMed] [Google Scholar]
- 27.Misri S, Reebye B, Kendrick K, Carter D, Ryan D, Grunau RE. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry. 2006;163:1026–32. doi: 10.1176/ajp.2006.163.6.1026. [DOI] [PubMed] [Google Scholar]
- 28.Huhn M, Tardy M, Spineli LM, et al. Efficacy of Pharmacotherapy and Psychotherapy for Adult Psychiatric Disorders: A Systematic Overview of Meta-analysis. JAMA Psychiatry. 2014;71:706–715. doi: 10.1001/jamapsychiatry.2014.112. [DOI] [PubMed] [Google Scholar]
- 29.Boukhris T, Sheehy O, Mottron L, Bérard A. Antidepressant Use During Pregnancy and the Risk of Autism Spectrum Disorder in Children. JAMA Pediatr. 2016;170:117–24. doi: 10.1001/jamapediatrics.2015.3356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.