Abstract
Objective
This study investigated genetic and environmental influences on behavior in a cohort of 600 children followed prenatally to 18 years.
Method
A randomized controlled trial of prenatal/infancy nurse home visits (NHV) was conducted in 600 predominantly African American mothers and their firstborn children from Memphis, TN. Mothers were assessed in pregnancy for mental health (MH), self-efficacy, and mastery. Mothers reported longitudinally on smoking and alcohol/drug use. The functional polymorphisms SLC6A4 5-HTTLPR, FKBP5 rs1360780 and DRD2/ANKK1 rs1800497 were genotyped together with 186 ancestry informative markers. Composite externalizing disorders (ED) continuous total scores from the mother-report Achenbach Child Behavior Checklist were included as dependent variables in regression analyses for time points 2, 6, 12, and 18 years.
Results
Behaviors at younger ages strongly predicted later behaviors (p<.0001). Children whose mothers had high self-efficacy and had received NHV were better behaved at age 2. Poorer maternal MH adversely influenced ED up to 12 years, but at age 18, maternal mastery exerted a strong, positive effect (p=.0001). Maternal smoking was associated with worse ED at 6 and 18. Main and interactive effects of genetic polymorphisms varied across childhood: FKBP5 rs1360780 up to age 6, 5-HTTLPR from 6 to 12, and DRD2/ANKK1 rs1800497 from 2 to 18.
Conclusion
Our study suggests that maternal MH and resilience measured in pregnancy have long-lasting effects on child behavior. Maternal smoking across childhood and genetic factors also play a role. NHV had a positive effect on early behavior. Our findings have implications for prevention of pathological behaviors in adulthood.
Clinical trial registration information
Age-17 Follow-Up of Home Visiting Intervention; http://clinicaltrials.gov/; NCT00708695.
Keywords: Achenbach Child Behavior Checklist, nurse home visiting, 5-HTTLPR, FKBP5, DRD2/ANKK1
INTRODUCTION
Both genetic and environmental factors influence the development of externalizing behaviors, such as aggression and antisocial behavior, across childhood and adolescence. Externalizing behaviors in children as young as age 3 persist and have been associated with increased risk for psychopathology, including alcoholism and drug addiction, in adulthood.1,2 Studies have shown that externalizing behaviors are highly heritable (55–65%).3–5 Variation in genes implicated in dopaminergic, serotonergic, and neuroendocrine systems appears to increase risk for development of externalizing behaviors.3
The environment in which a child is reared, particularly that provided by the primary caregiver, most often the mother, also has implications for behavioral development. For example, maternal stress during pregnancy is associated with emotional problems in childhood,6 and early life maternal psychopathology can result in long-lasting behavioral problems.2,7 Many studies have shown that children of mothers with depression have increased rates of antisocial behavior, depression, and high emotionality8–10 and are often exposed to harsh discipline and poor nurturing.11 Results of a few studies suggest that the psychopathology trajectory can be altered by means of prenatal/early childhood educational home-visiting interventions; for example, a paraprofessional-delivered home visitation intervention program for American Indian teenage mothers resulted in improved parenting skills and fewer externalizing and internalizing child behavior problems at 3 years.12 A longitudinal nurse home visitation (NHV) study in predominantly Caucasian, impoverished mothers in Elmira, NY showed that adolescents in the NHV group had fewer arrests and convictions, consumed less alcohol, and smoked less than controls, but there were no differences in other behavioral problems.13,14
The current study was undertaken within a predominantly African American (AA) sample of primarily low income, unmarried women living in Memphis, TN who, together with their firstborn children, had been followed from pregnancy through their child’s 18th birthday.15 During pregnancy, mothers had been randomly assigned to a control group or to prenatal and infancy NHV, the aim of which was to improve pregnancy outcomes, children’s health and development, and mothers’ health and life-course. The current study was limited to the 600 firstborn children who were interviewed at the 18-year assessment and provided DNA, together with their mothers, who had been assessed during pregnancy for mental health, measures of resilience (self-efficacy and mastery), and longitudinally for smoking, alcohol and drug use. All these maternal measures are hypothesized to influence externalizing behaviors in children. Mothers also provided DNA. Three common, functional polymorphisms in genes implicated in the dopaminergic, serotonergic, and neuroendocrine systems were genotyped in the children and their mothers. All three polymorphisms predict emotional dysregulation and behavioral disorders. The DRD2/ANKK1 Glu713Lys rs1800497 polymorphism has been associated with increased ventral striatal activation during reward processing16; 5-HTTLPR, the promoter polymorphism in the serotonin transporter gene SLC6A4, has been widely implicated in stress sensitivity;17 and the rs1360780 SNP in the FKBP5 HPA-axis gene has been associated with stress-related disorders and aggression.18–20
The purpose of the current study was to investigate the impact of maternal and genetic influences together with an NHV program, on the development of externalizing behaviors in children aged 2 to 18 years, including the externalizing behaviors of alcohol and drug use disorder (AUD, DUD) and smoking behavior at age 18 years.
METHOD
Full details of the study, including participant retention rates and visit attendance patterns, have been published.15,21,22 A total of 1,289 eligible women, living in highly disadvantaged urban neighborhoods, was consecutively recruited from a public system for obstetric and pediatric care between 1990–1991. Of these, 151 declined to participate. In all, 1,138 primarily self-described African American women at less than 29 weeks gestation with no previous live births and with at least 2 of 3 risk factors (unmarried, < 12 years education, unemployed) were randomly assigned to four treatment groups.15 Two groups were used to examine birth outcomes only and were not followed after childbirth. The focus of the current study is on the other two groups (N = 742): (a) a control group (n = 514) in which women were provided free transportation for scheduled prenatal care plus developmental screening and referral services for the child at ages 2, 6, and 12 years, and (b) a treatment group (n = 228) that in addition had intensive NHV during pregnancy (mean [SD] visits = 7.5 [3.8]) and through the child’s second birthday (mean [SD] visits = 27.9 [13.8]). There was no difference in characteristics of mothers between the two groups at recruitment.15
When children were 18 years old, a total of 657 mothers and 669 children were eligible for follow-up. The attrition in numbers was predominantly due to maternal and child deaths. Only 27 mothers/children chose to drop out before the 18-year follow-up. Of the eligible participants, 94% of mothers and children were interviewed. Saliva for DNA extraction was collected from 96% of these children, and 94% of the mothers and genotypes were available for 600 children (NHV group n = 186, controls n = 414) and 561 mothers. There was no difference in baseline characteristics of mothers in the NHV and control groups (Table S1, available online). Mothers lost to the study did not differ from mothers retained in the study at 18 years in baseline mental health or Pearlin Mastery scores. In the control group only, mothers lost to the study had significantly higher baseline self-efficacy scores than study-retention mothers.
The study was approved by the University of Rochester institutional review board (secondary approval by the University of Colorado). The mothers provided informed consent before randomization and were re-consented at each follow-up assessment. Mothers provided consent for their children under age 18, and the children were assented. Youth age 18 years and older provided informed consent.
Mothers’ Assessments at Baseline During Pregnancy
Mental Health
A mental health composite score (MH), based on measures of depression, anxiety, and emotional dysregulation, was derived from the 38-item Rand mental health battery.23
Self-Efficacy
The self-efficacy scale was created specifically for the NHV program based on Bandura’s work.24 Mothers were asked a series of 10 questions focusing on their confidence in their ability to perform parenting-specific tasks such as understanding the baby’s needs and feelings, talking, reading and playing with the baby, and taking the baby for regular clinic/doctor check-ups.25 Scores per question ranged from 1 (low confidence) to 5 (high confidence). Cronbach’s alpha was 0.68.25
Pearlin Mastery
The Pearlin Mastery score (PM), a measure of an individual’s personal sense of mastery and control over one’s own behavior and life opportunities, was derived from the 7-item Pearlin Mastery Scale that has been widely used since 1978.26
The MH, self-efficacy, and PM scores were standardized to a mean of 100 and a standard deviation (SD) of 10. Higher scores denote better functioning.
Maternal Smoking, Alcohol and Drug Use
Mothers were questioned about cigarette use (Table S1, available online) and alcohol and drug use at baseline during pregnancy and when their children were aged 6 and 12 years. Since we were primarily interested in the influence of maternal smoking/non-smoking on the child, the smoking phenotype used in analyses was average cigarettes (cigs)/day over the past 30 days in the total sample that included smokers (at least one cig/day) and non-smokers. The proportion of mothers with any alcohol/drug use was very low (see Supplement 1, available online) and therefore these data were not included in any analyses.
Behavioral Measures in Children
Achenbach Child Behavior Checklist (CBCL)
Mothers completed the CBCL for preschoolers27 at child age 2 years and the CBCL for school-age children28 at child ages 6, 12, and 18 years. The composite externalizing disorders continuous total scores (ED) (rule-breaking behavior, aggressive behavior) were derived. Numerically higher ED scores signify more disturbed behavior.
Assessment of Alcohol/Drug Use Disorders and Smoking at Age 18
DSM-IV diagnoses of AUD and DUD were derived from the Substance Abuse Module (SAM) of the composite international diagnostic interview (CIDI) that was completed by youth at age 18 years. The smoking history assessment included average cigs/day over the past 30 days.
Genotyping
Genomic DNA was isolated from saliva from mothers and children using standard protocols. Full genotyping methods are provided in Supplement 1, available online. The children’s minor allele frequencies for two functional SNPs were: FKBP5 rs1360780 (0.41) and DRD2/ANKK1 Glu713Lys rs1800497 (0.34). The children’s allele frequencies for the triallelic 5-HTTLPR polymorphism were: S = 0.26, LA = 0.48, LG = 0.26. Alleles were grouped as low activity (SS, SLG, LGLG) (0.27), medium activity (SLA, LALG ) (0.49), and high activity (LALA) (0.24) variants.
Assessment of Population Stratification Using Ancestry Informative Markers
Full details are provided in Supplement 1, available online. The mean (standard error [SE]) African ancestry score of the total sample was 0.76 (0.01) (median, 0.83) for mothers and children. A subset of 34 children had > 0.54 European ancestry (median = 0.93).
Statistical Analyses
Since 97% of children had complete or near-complete CBCL data, all children’s data were included in the analyses. Table S2 (available online) shows the sample size of all variables used in the analyses.
Regression analyses with the continuous total ED scores at ages 2, 6, 12, and 18 years as the dependent variables and likewise with youth smoking at age 18 as the dependent variable were undertaken using JMP 11 software. Backward stepwise regression was performed with predictor variables and predicted interaction terms being eliminated from the model in an iterative process if the level of significance was p > .1. Predictor variables included earlier behavioral measures, sex, NHV, maternal measures in pregnancy (MH, PM, and self-efficacy), maternal smoking (pregnancy, child-age 6 and 12 years), mother and child genotypes, and mother and child African ancestry. Interaction terms included NHV × MH, NHV × PM, NHV × self-efficacy, and the three functional variants × MH, × MP and × self-efficacy. The three genotypes were entered (e.g. AA, AC, CC) in the model rather than as coded 0, 1, 2 since the former is a more conservative approach. A logistic regression analysis with any DSM-IV addiction in youth age 18 as the dependent variable was similarly undertaken.
RESULTS
The mothers’ demographics are presented in Table S1 (available online). CBCL ED continuous total scores were correlated over time: r = 0.57–0.23, p < .0001 (Figure S1, available online).
Independent Predictors of ED in Childhood, Ages 2 Years Through 12 Years
The results of the regression analyses are presented in Table 1 and illustrated in Figure 1. The whole model accounted for 14% of variance in behavior at age 2 and 33–34% at ages 6 and 12 years.
TABLE 1.
Independent Effects on the Composite Externalizing Disorder Continuous Scores of the Total Group of Children at Ages 2, 6, and 12 Years
| Independent Variables, Age 2 Years | F Value | p Value |
|---|---|---|
| FB sex | 11.2 | .001 |
| FB NHV | 4.6 | .032 |
| FB DRD2/ANKK1 rs1800497 | 1.7 | .178 |
| FB FKBP5 rs1360780 | 3.8 | .024 |
| M in pregnancy | ||
| Mental health | 13.9 | .0002 |
| Pearlin mastery | 0.5 | .460 |
| Self-efficacy | 0.1 | .841 |
| NHV group × self-efficacy | 6.2 | .013 |
| FB DRD2/ANKK1 rs1800497 × M Pearlin mastery | 3.8 | .024 |
| FB FKBP5 rs1360780 × M Pearlin mastery | 4.1 | .017 |
| Independent Variables, Age 6 Yearsb | F Value | p Value |
| FB age 2 externalizing disorders | 109.3 | <.0001 |
| FB sex | 14.3 | .0002 |
| FB DRD2/ANKK1 rs1800497 | 3.5 | .030 |
| FB FKBP5 rs1360780 | 1.5 | .229 |
| FB 5-HTTLPR | 3.4 | .033 |
| M in pregnancy | ||
| Mental health | 6.8 | .010 |
| Pearlin mastery | 1.9 | .166 |
| 6yrs cigs/day | 12.8 | .0004 |
| FB DRD2/ANKK1 rs1800497 × M Pearlin mastery | 3.4 | .033 |
| FB FKBP5 rs1360780 × M Pearlin mastery | 4.6 | .011 |
| Independent Variables, Age 12 Yearsc | F Value | p Value |
| FB age 6 externalizing disorders | 223.3 | <.0001 |
| FB sex | 2.2 | .135 |
| FB 5-HTTLPR | 2.5 | .083 |
| M in pregnancy | ||
| Mental health | 5.5 | .019 |
Note: Boldface data highlights significant results. Independent variables are included in the model at each time point if they have a main or interactive effect at p ≤ .1. FB = firstborn children; M = mother; NHV = nurse home-visiting group vs. controls.
Whole model: n = 521, F = 6.0, 14 df, p < .0001, variance = 0.14.
Whole model: n = 492, F = 15.6, 15 df, p < .0001, variance = 0.33.
Whole model: n = 534, F = 54.1, 5 df, p < .0001, variance = 0.34.
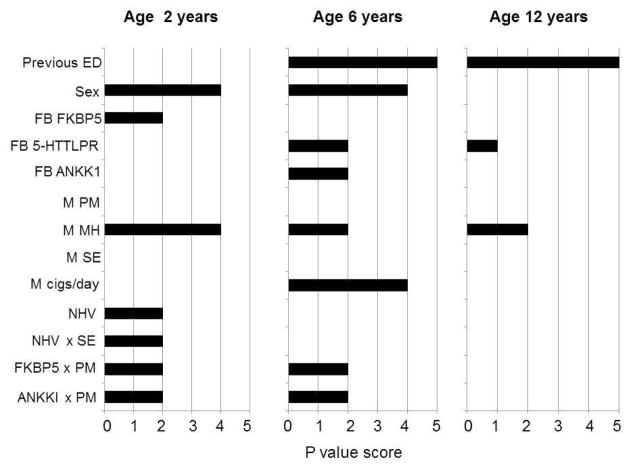
FIGURE 1.
Independent effects on firstborn children’s composite externalizing disorders (ED) scores at three time points. Note: p value scores: 0: p > .1; 1: p = .1–.05; 2: p < .05–.01; 3: p < .01–.005; 4: p < .005–.0001; 5: p < .0001. FB = firstborn child; FB ANKK1 = child DRD2/ANKK1 rs1800497 genotype; FB FKBP5 = child FKBP5 rs1360780 genotype; M = mother; M cigs/day = maternal smoking at child age 6 yrs; M PM = maternal Pearlin mastery score; M SE = maternal self-efficacy score; NHV = nurse home visiting group vs. controls; previous ED = ED score at previous time point. Interaction terms: NHV × M SE; child FKBP5 rs1360780 genotype × M PM; child DRD2/ANKK1 rs1800497 × M PM. Full details of the regression analyses are provided in Table 1.
The strongest predictors of ED at ages 6 and 12 were measures of behavior at earlier ages (p < .0001). ED scores were generally worse in boys. Within the regression models, there were no independent effects of child or maternal African ancestry on child behavior: age 2, p >.63; age 6, p>.37; age 12, p>.13. Maternal genotypes did not predict child behavior at any age.
NHV
There was a positive effect of NHV on ED at age 2 years and an interactive effect with maternal self-efficacy: children of NHV mothers with high self-efficacy (defined by a median split) had significantly better scores compared with the other three groups. The predictive effect of NHV on behavior did not alter when regression analyses were repeated without post-baseline predictors (earlier child behavior, maternal smoking) that might themselves have been influenced by NHV.
Maternal Measures
MH measured in pregnancy exerted a persistent effect on ED. Poorer maternal MH was associated with worse childhood behaviors. PM measured in pregnancy did not have a main effect on ED. Mothers’ smoking at child age 6 years was linked with poorer ED at age 6 years.
Child Genotype Effects
FKBP5: at age 2 there were main effects for rs1360780 on ED and interactive effects with PM: the minor TT homozygote was associated with better ED scores but only in children of mothers with high PM. At age 6 the interactive effect remained: low PM mothers’ children who were not minor homozygote carriers had the worst behavior.
DRD2/ANKK1: at ages 2 and 6 there was an interactive effect of rs1800497 with PM: the major CC homozygote/C allele respectively was associated with the worst ED scores only in children of mothers with low PM.
SLC6A4: 5-HTTLPR had a main effect on ED at age 6 and a trend effect at age 12. The high activity variant was associated with worse ED scores relative to the low/medium activity variants.
Behavioral Disorders at Age 18 Years
Effects of African Ancestry
The regression analysis in the total sample of children aged 18 showed that there were highly significant effects of maternal (p = .0024) and child (p = .0080) African ancestry on ED, indicating that analyses in the total group were likely to be influenced by population stratification. Therefore, the subgroup of 34 children with European ancestry > 0.54 (median = 0.93) was excluded, and all analyses at age 18 were carried out in the AA sample: African ancestry = mean (SD) 0.82 (0.08), median 0.83.
Independent Predictors of ED in African American Youth at Age 18 Years
As seen in Table 2, ED at age 12 was the strongest predictor of behavior at age 18 (p < .0001). PM, measured in pregnancy, had a strong effect on ED (p = .0001); 18-year-old children of higher mastery mothers had better ED outcomes. In contrast, maternal smoking recorded at child age 12 was associated with poorer ED outcomes at age 18. There was a genetic influence on ED: the DRD2/ANKK1 rs1800497 minor allele was associated with poorer ED outcomes, but there was no interactive effect with PM.
TABLE 2.
Independent Effects on the Composite Externalizing Disorder Continuous Scores of the African American Firstborn Children at Age 18 Years
| Independent Variables, Age 18 Yearsa | F value | p Value |
|---|---|---|
| FB age 12 externalizing disorders | 137.0 | <.0001 |
| FB sex | 3.4 | .065 |
| FB DRD2/ANKK1 rs1800497 | 3.8 | .023 |
| FB African ancestry | 3.2 | .076 |
| M in pregnancy | ||
| Pearlin mastery | 14.9 | .0001 |
| 12 yrs cigs/day | 5.8 | .017 |
| African ancestry | 5.8 | .017 |
Note: Boldface data highlights significant results. Independent variables are included in the model at each time point if they have a main or interactive effect at p ≤ .1. FB = firstborn children; M = mother.
For the whole model, n = 404, F = 24.8, 8 df, p < .0001, variance = 0.33.
DSM-IV Substance Dependence Diagnoses and Smoking in African American Youth at Age 18 Years
A total of 72 (13%) of the 563 AA youths had a DSM-IV diagnosis of any addiction (AUD and/or DUD). Of these addicted individuals, 22% had AUD only, 21% had cannabis use disorders only, and 57% had comorbid AUD and cannabis use disorders. The results of a logistic regression analysis for any addiction are shown in Table S3 (available online). Genotypes were not included since the sample size of addicted individuals was too small. The strongest predictors were sex (the addicted youth were predominantly male [68%; p = .0003]) and youth smoking (p < .0001).
The median age for smoking a whole cigarette was 16 years. A total of 75/564 (13%) AA youth admitted smoking at least one cigarette (cig)/day in the past 30 days. Of these, only 8 individuals smoked ≥ 10 cigs/day (max 25 cigs/day). The results of a regression analysis of the sample of smokers and non-smokers are provided in Table S4 (available online). Genotypes were not included due to the small sample size of smokers. The strongest predictors of increased youth smoking were any addiction (p < .0001) and mothers’ smoking during pregnancy (p = .005).
Secondary Analysis: Qualitative Assessment of European Ancestry Subgroup of Youth Age 18 Years
Within the total sample, there was a subgroup of 34 children with 0.93 median European ancestry. Of these, 42% had a DSM-IV diagnosis of AUD/DUD, compared with 13% of AA youth, and 51.5% smoked at least one cig/day (mean[SE)], median = 11.9 [3.1], 10) compared with 13% of AA youth. The majority of the European-ancestry mothers smoked at least one cig/day at the three time points from recruitment to age 12 years: 79%, 78%, and 73%, respectively. In contrast, the percentage of AA mothers who smoked was much lower at the respective three time points: 8%, 20%, and 28%.
DISCUSSION
This is a sub-study using data derived from a longitudinal study of the effects of prenatal and infancy NHV on health outcomes in a group of urban, predominantly African American mothers and their firstborn children in Memphis, TN. Previous publications based on this dataset (see later) have focused on outcomes in children at time points 2, 6, 9, and 12 years, but this is the first longitudinal analysis. This paper is the first to include genetic analyses.
Our study investigated maternal influences, genetic influences, and the NHV program on the development of externalizing behaviors in a cohort of 600 firstborn children followed from pregnancy, through toddlerhood, early childhood, and adolescence to age 18 years. We chose the CBCL composite ED continuous score as the main outcome measure because we wanted to look at behaviors that are normally distributed in the general population.
A notable finding of this study was that poorer maternal MH in pregnancy had a strong, adverse effect on ED up to age 12 years. This result is in agreement with numerous studies showing that maternal stress during pregnancy is associated with emotional problems, including hyperactivity and anxiety, in childhood.6 Other studies have shown that maternal depression during the first year of life has a significant impact on child behavior.2,29 The fact that the maternal MH measure in pregnancy (depression, anxiety, and emotional dysregulation) had a significant effect on child behavior over a period of 12 years suggests that this may be a trait measure that is stable over time. Indeed, depression and anxiety symptoms are moderately (40%) heritable in women and share a common genetic origin.30,31 It should also be noted that our measure of ED is the perception of the mother, and the more stressed/depressed a mother feels, the more likely she is to rate her child’s behavior as difficult.32 In line with this, it is noteworthy that MH had the strongest impact on ED in toddlerhood when child behavior is more likely to be volatile. Mastery is also moderately (40%) heritable and can be considered a trait.33 PM, measured in pregnancy, had no effect on ED in childhood; however, it had a very strong effect at age 18; the children of more masterful mothers had better ED outcomes. Thus the results of our longitudinal study suggest that maternal influences on child behavior may differ in childhood and adolescence/early adulthood.
Maternal smoking, defined as smoking at least one cigarette/day/past 30 days, had a negative impact on child behavior at different time points. Maternal smoking at child age 6 was strongly associated with poorer ED at that age whereas maternal smoking at child age 12 predicted poorer ED at age 18. It is well known that smoking is often comorbid with externalizing disorders; hence mothers who smoked would be more likely to exhibit externalizing disorders. Therefore one possibility to be considered is that it was the mothers’ behavior rather than smoking per se that influenced their children’s behavior. Our results showing adverse effects of maternal smoking on children’s behavior are supported by similar findings from a national probability sample of nearly 13,000 individuals.34 Interestingly, in our study, the mother’s smoking during pregnancy, but at no other time point, was associated with her children smoking at age 18. One explanation for this finding is that numerous studies have shown that prenatal smoking can damage the developing fetal brain, increasing the risk of behavioral disinhibition. This finding could bolster existing preventive strategies aimed at pregnant women.
There was no effect of ancestry on child behavior from ages 2 through to 12 years, and therefore analyses were performed in the total sample. However, at 18 years, there was a highly significant effect of ancestry within the ED regression models, AUD/DUD, and smoking indicating population stratification. Further investigation showed that a European ancestry subgroup of 34 youth had three times the prevalence of AUD/DUD and four times the prevalence of smoking compared with AA youth. Moreover, their mothers were much heavier smokers than the AA mothers. Indeed, the low proportion of AA youth at age 18 with DSM-IV AUD/DUD and the small proportion of heavy smokers resulted in low power for these analyses. One possibility is that this is due to under-reporting of what might be considered “socially undesirable” behavior. Indeed, another community-based study has also shown that AA women report lower rates of smoking in pregnancy than EA women.34 However, the protective effect of African ancestry on substance use disorders has been definitively noted in a sample of inpatients dependent on alcohol/drugs.35
In the current study, we found that NHV had a beneficial effect on ED, but only in the group of toddlers whose mothers had high self-efficacy. There was no effect at later ages. However, this NHV program has had a significant impact on children and their mothers in other domains. In children, NHV was associated with reductions in injuries within the first 2 years of life,15 higher intellectual functioning, and fewer problems in the borderline/clinical range of the CBCL Total Problems scale at age 6 years36 and improved school performance at age 9 and 12 years.37,38 In mothers, the NHV program is linked to reduced rates of pregnancy-induced hypertension,15 subsequent pregnancies/births, reductions in welfare use,36,39 and all-cause mortality 20 years later.22
We also found differing influences of children’s genetic variation across development: FKBP5 rs1360780 predicted behavior up to the age of 6; 5-HTTLPR from 6 to 12, and DRD2/ANKK1 rs1800497 from 6 to 18 years. Our results are in agreement with earlier findings that genetic risk for externalizing disorders such as addiction unfolds across development from childhood to adulthood.40–42 For FKBP5 rs1360780, the minor homozygote was associated with better ED scores, but only in children of masterful mothers. However, studies in adults have shown that the minor allele is associated with risk for stress-related disorders.18,19 One possible interpretation of our results in children is that the protective aspect of a masterful mother may override the deleterious influence of a single genetic variant on behavior. The high-activity 5-HTTLPR variant was associated with worse ED scores in children. This finding is in line with a recent study in young adults,43 although in most adult studies the low activity variant has been implicated.17 Finally, the DRD2/ANKK1 rs1800497 minor allele was associated with poorer ED outcomes in 18-year-old youth. This result is in agreement with findings in adults that the minor allele is associated with negative emotionality.16,44
This study has numerous strengths, including the large sample size of AA children and mothers, a population that has been somewhat neglected in behavioral/genetic research studies, the longitudinal behavioral measures in children, and the use of AIMs that successfully detected population stratification in this admixed samples. Limitations include that there was a degree of attrition from baseline, largely due to maternal and child deaths; however, the sample was not biased by the inclusion of incomplete data from children whose mothers were only interviewed at earlier ages. Child behavior was assessed only by mothers and, other than smoking and alcohol/drug use, the maternal measures were obtained only during pregnancy. However, MH and PM are moderately heritable30,31,33 and are therefore likely to be traits that are stable over time. We do not know about influences of other family members on child behavior; only 2% of mothers lived alone at baseline, and many children gained siblings during the study. Nevertheless, our findings from the regression analyses appear to be robust over time.
In conclusion, our study has shown that there are long-lasting maternal influences on behavior from early childhood to adolescence/early adulthood and that genetic factors play a role, possibly exerting different effects across development. The NHV intervention program had a positive effect on early childhood behavior.
Supplementary Material
Acknowledgments
The research was supported by the Robert Wood Johnson Foundation (017934, 11084, 027901); the Carnegie Corporation of New York (B 5492); the Pew Charitable Trusts (88-0211-000, 93-02363-000); the William T. Grant Foundation (88-1246-88, 91-1246-88); and a Senior Research Scientist Award (1-K05-MH01382-01) to David Olds. Further grants: NIMH: 1R01MH68790-01, R01-MH61428-01; NICHD: R01-HD-043492; NINR: NR01-01691-05; DHHS and the National Center for Child Abuse and Neglect, through a transfer of funds to the NINR; OJJDP Grant 2004-52854-CO-JS0; the Administration for Children and Families, DHHS, 90PD0215/01, 90PJ0003; the Bureau of Maternal and Child Health MCJ 360579; and the Hearst Foundation. This research was funded in part by the intramural program of the National Institute on Alcohol Abuse and Alcoholism, NIH.
Footnotes
Supplemental material cited in this article is available online.
Disclosure: The Prevention Research Center for Family and Child Health, directed by Dr. David Olds of the University of Colorado School of Medicine, has a contract with the Nurse-Family Partnership (NFP) to conduct research to improve the NFP program and its implementation. Dr. Olds was employed by this center at the time the study was conducted. Dr. Olds is the founder of the Nurse-Family Partnership. Drs. Enoch, Kitzman, Smith, Hodgkinson, Goldman, Olds, and Ms. Anson report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Mary-Anne Enoch, Laboratory of Neurogenetics, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health (NIH), Bethesda, MD.
Dr. Harriet Kitzman, University of Rochester School of Nursing, Rochester, NY.
Dr. Joyce A. Smith, University of Rochester School of Nursing, Rochester, NY.
Dr. Elizabeth Anson, University of Rochester School of Nursing, Rochester, NY.
Dr. Colin A. Hodgkinson, Laboratory of Neurogenetics, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health (NIH), Bethesda, MD.
Dr. David Goldman, Laboratory of Neurogenetics, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health (NIH), Bethesda, MD.
Dr. David L. Olds, University of Colorado, Aurora.
References
- 1.Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Arch Gen Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- 2.Enoch MA, Steer CD, Newman TK, Gibson N, Goldman D. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes Brain Behav. 2010;9:65–74. doi: 10.1111/j.1601-183X.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waltes R, Chiocchetti AG, Freitag CM. The neurobiological basis of human aggression: A review on genetic and epigenetic mechanisms. Am J Med Genet B Neuropsychiatr Genet. 2015 Oct 22; doi: 10.1002/ajmg.b.32388. [Epub ahead of print] Review. [DOI] [PubMed] [Google Scholar]
- 4.Korhonen T, Latvala A, Dick DM, et al. Genetic and environmental influences underlying externalizing behaviors, cigarette smoking and illicit drug use across adolescence. Behav Genet. 2012;42:614–625. doi: 10.1007/s10519-012-9528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bornovalova MA, Hicks BM, Iacono WG, McGue M. Familial transmission and heritability of childhood disruptive disorders. Am J Psychiatry. 2010;167:1066–1074. doi: 10.1176/appi.ajp.2010.09091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talge NM, Neal C, Glover V Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagner DM, Pettit JW, Lewinsohn PM, Seeley JR, Jaccard J. Disentangling the temporal relationship between parental depressive symptoms and early child behavior problems: a transactional framework. J Clin Child Adolesc Psychol. 2013;42:78–90. doi: 10.1080/15374416.2012.715368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke L. The impact of maternal depression on familial relationships. Int Rev Psychiatry. 2003;15:243–255. doi: 10.1080/0954026031000136866. [DOI] [PubMed] [Google Scholar]
- 9.Evans J, Xu K, Heron J, et al. Emotional symptoms in children: The effect of maternal depression, life events, and COMT genotype. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:209–218. doi: 10.1002/ajmg.b.30789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim-Cohen J, Moffitt TE, Taylor A, Pawlby SJ, Caspi A. Maternal depression and children’s antisocial behavior: nature and nurture effects. Arch Gen Psychiatry. 2005;62:173–181. doi: 10.1001/archpsyc.62.2.173. [DOI] [PubMed] [Google Scholar]
- 11.McLearn KT, Minkovitz CS, Strobino DM, Marks E, Hou W. The timing of maternal depressive symptoms and mothers’ parenting practices with young children: implications for pediatric practice. Pediatrics. 2006;118:174–182. doi: 10.1542/peds.2005-1551. [DOI] [PubMed] [Google Scholar]
- 12.Barlow A, Mullany B, Neault N, et al. Paraprofessional-delivered home-visiting intervention for American Indian teen mothers and children: 3-year outcomes from a randomized controlled trial. Am J Psychiatry. 2015;172:154–162. doi: 10.1176/appi.ajp.2014.14030332. [DOI] [PubMed] [Google Scholar]
- 13.Olds D, Henderson CR, Jr, Cole R, et al. Long-term effects of nurse home visitation on children’s criminal and antisocial behavior: 15-year follow-up of a randomized controlled trial. JAMA. 1998;280:1238–1244. doi: 10.1001/jama.280.14.1238. [DOI] [PubMed] [Google Scholar]
- 14.Eckenrode J, Campa M, Luckey DW, et al. Long-term effects of prenatal and infancy nurse home visitation on the life course of youths: 19-year follow-up of a randomized trial. Arch Pediatr Adolesc Med. 2010;164:9–15. doi: 10.1001/archpediatrics.2009.240. [DOI] [PubMed] [Google Scholar]
- 15.Kitzman H, Olds DL, Henderson CR, Jr, et al. Effect of prenatal and infancy home visitation by nurses on pregnancy outcomes, childhood injuries, and repeated childbearing. A randomized controlled trial. JAMA. 1997;278:644–652. [PubMed] [Google Scholar]
- 16.Nymberg C, Banaschewski T, Bokde AL, et al. IMAGEN consortium. DRD2/ANKK1 polymorphism modulates the effect of ventral striatal activation on working memory performance. Neuropsychopharmacology. 2014;39:2357–2365. doi: 10.1038/npp.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holz NE, Buchmann AF, Boecker R, et al. Role of FKBP5 in emotion processing: results on amygdala activity, connectivity and volume. Brain Struct Funct. 2015;220:1355–1368. doi: 10.1007/s00429-014-0729-5. [DOI] [PubMed] [Google Scholar]
- 19.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34:S186–95. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Bevilacqua L, Carli V, Sarchiapone M, et al. Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Arch Gen Psychiatry. 2012;69:62–70. doi: 10.1001/archgenpsychiatry.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland ML, Xia Y, Kitzman HJ, Dozier AM, Olds DL. Patterns of visit attendance in the nurse-family partnership program. Am J Public Health. 2014;104:e58–65. doi: 10.2105/AJPH.2014.302115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olds DL, Kitzman H, Knudtson MD, Anson E, Smith JA, Cole R. Effect of home visiting by nurses on maternal and child mortality: results of a 2-decade follow-up of a randomized clinical trial. JAMA Pediatr. 2014;168:800–806. doi: 10.1001/jamapediatrics.2014.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ware JE, Veit CT, Donald CA. Refinements in the measurement of mental health for adults in the Health Insurance Study. Santa Monica, CA: Rand Corp; 1985. [Google Scholar]
- 24.Bandura A. Self efficacy: The exercise of control. New York: W.H. Freeman and Co; 1997. [Google Scholar]
- 25.Holland ML, Yoo BK, Kitzman H, et al. Self-efficacy as a mediator between maternal depression and child hospitalizations in low-income urban families. Matern Child Health J. 2011;15:1011–1019. doi: 10.1007/s10995-010-0662-z. [DOI] [PubMed] [Google Scholar]
- 26.Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav. 1978;19:2–21. [PubMed] [Google Scholar]
- 27.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool forms and Profiles. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 28.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 29.Bagner DM, Pettit JW, Lewinsohn PM, Seeley JR. Effect of maternal depression on child behavior: a sensitive period? J Am Acad Child Adolesc Psychiatry. 2010;49:699–707. doi: 10.1016/j.jaac.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burton KL, Williams LM, Richard Clark C, et al. Sex differences in the shared genetics of dimensions of self-reported depression and anxiety. J Affect Disord. 2015;188:35–42. doi: 10.1016/j.jad.2015.08.053. [DOI] [PubMed] [Google Scholar]
- 31.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 32.Gross D, Conrad B, Fogg L, Wothke W. A longitudinal model of maternal self-efficacy, depression, and difficult temperament during toddlerhood. Res Nurs Health. 1994;17:207–215. doi: 10.1002/nur.4770170308. [DOI] [PubMed] [Google Scholar]
- 33.Gatt JM, Burton KL, Schofield PR, Bryant RA, Williams LM. The heritability of mental health and wellbeing defined using COMPAS-W, a new composite measure of wellbeing. Psychiatry Res. 2014;219:204–213. doi: 10.1016/j.psychres.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Weitzman M, Gortmaker S, Sobol A. Maternal smoking and behavior problems of children. Pediatrics. 1992;90:342–349. [PubMed] [Google Scholar]
- 35.Ducci F, Roy A, Shen PH, et al. Association of substance use disorders with childhood trauma but not African genetic heritage in an African American cohort. Am J Psychiatry. 2009;166:1031–1040. doi: 10.1176/appi.ajp.2009.08071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olds DL, Kitzman H, Cole R, et al. Effects of nurse home-visiting on maternal life course and child development: age 6 follow-up results of a randomized trial. Pediatrics. 2004;114:1550–9. doi: 10.1542/peds.2004-0962. [DOI] [PubMed] [Google Scholar]
- 37.Olds DL, Kitzman H, Hanks C, et al. Effects of nurse home visiting on maternal and child functioning: age-9 follow-up of a randomized trial. Pediatrics. 2007;120:e832–845. doi: 10.1542/peds.2006-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitzman HJ, Olds DL, Cole RE, et al. Enduring effects of prenatal and infancy home visiting by nurses on children: follow-up of a randomized trial among children at age 12 years. Arch Pediatr Adolesc Med. 2010;164:412–418. doi: 10.1001/archpediatrics.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olds DL, Kitzman HJ, Cole RE, et al. Enduring effects of prenatal and infancy home visiting by nurses on maternal life course and government spending: follow-up of a randomized trial among children at age 12 years. Arch Pediatr Adolesc Med. 2010;164:419–424. doi: 10.1001/archpediatrics.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards AC, Kendler KS. Alcohol consumption in men is influenced by qualitatively different genetic factors in adolescence and adulthood. Psychol Med. 2013;43:1857–1868. doi: 10.1017/S0033291712002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heitzeg MM, Villafuerte S, Weiland BJ, et al. Effect of GABRA2 genotype on development of incentive-motivation circuitry in a sample enriched for alcoholism risk. Neuropsychopharmacology. 2014;39:3077–3086. doi: 10.1038/npp.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Beek JH, Kendler KS, de Moor MH, et al. Stable genetic effects on symptoms of alcohol abuse and dependence from adolescence into early adulthood. Behav Genet. 2012;42:40–56. doi: 10.1007/s10519-011-9488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovallo WR, Enoch MA, Yechiam E, et al. Differential impact of serotonin transporter activity on temperament and behavior in persons with a family history of alcoholism in the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res. 2014;38:1575–1581. doi: 10.1111/acer.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savitz J, Hodgkinson CA, Martin-Soelch C, et al. DRD2/ANKK1 Taq1A polymorphism (rs1800497) has opposing effects on D2/3 receptor binding in healthy controls and patients with major depressive disorder. Int J Neuropsychopharmacol. 2013;16:2095–2101. doi: 10.1017/S146114571300045X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.