Abstract
Mendelian susceptibility to mycobacterial diseases (MSMD) is a rare inheritance syndrome, characterized by a disseminated infection with mycobacterium in children following BCG vaccination at birth. Regarding the vaccination program in Iran, it may consider as a public health problem. The pathogenesis of MSMD is dependent on either insufficient production of IFN-gamma (γ) or inadequate response to it. Here, we want to introduce three cases including two siblings and one girl from two unrelated families with severe mycobacterial infections referred to Immunology, Asthma and Allergy Research Institute (IAARI), from 2013 to 2015; their MSMD was confirmed by both cytokine assessment and genetic analysis. Regarding the clinical features of the patients, cell proliferation against a mitogen and BCG antigen was ordered in a lymphocyte transformation test (LTT) setting. ELISA was performed for the measurement of IL-12p70 and IFN-γ in whole blood samples activated by BCG + recombinant human IFN-γ and BCG + recombinant human IL-12, respectively. In contrast to mitogen, the antigen-dependent proliferation activity of the patients’ leukocytes was significantly lower than that in normal range. We identified a homozygous mutation in IL12RB1 gene for two kindred who had a homozygous mutation affecting an essential splice site. For the third patient, a novel frameshift deletion in IL12RB1 gene was found. The genetic study results confirmed the impaired function of stimulated lymphocytes to release IFN-γ following stimulation with BCG+IL-12 while the response to rhIFN-γ for IL-12p70 production was relatively intact. Our findings show that cellular and molecular assessments are needed for precise identification of immunodeficiency disorders especially those without clear-cut diagnostic criteria.
Keywords: Mendelian, IL-12Rβ1 Deficiency, Interfron-gamma, Interleukin 12, Mycobacterium
Introduction
Mendelian susceptibility to mycobacterial disease (MSMD) is a rare primary immunodeficiency resulting in a selective bias to infectious disease caused by poorly pathogenic mycobacteria, such as Mycobacterium bovis Bacille Calmette-Guérin (BCG) vaccines and environmental mycobacteria (EM), and more virulent M. tuberculosis. Susceptibility to other infectious agents like Salmonella, Candida and even other intra-macrophagic bacteria, fungi and parasites has also been observed in rare patients (1). “BCG-osis” due to disseminated infection following BCG (bacille Calmette-Guérin) vaccination, practiced worldwide, is a typical presentation of MSMD. The pathogenesis of MSMD is dependent on either insufficient production of IFN-gamma (γ) or inadequate response to it. Since the first genetic etiology of MSMD was introduced in an infant with fatal BCG infection (2, 3), the scientists found nine genes whose germline mutations underlie MSMD including IL12B, IL12RB1, IFNGR1, IFNGR2, STAT1, IKBKG, CYBB, IRF8 and ISG15(1). Accordingly, some inborn errors like IL12B and IL12RB1 deficiencies are related to impaired IL-12/IL-23-dependent IFN-γ production, while others such as IFNGR1, IFNGR2, and STAT1 deficiencies are associated with impaired cellular responses to IFN-γ (4). Patients with impaired IFN-γ production can be treated by antibiotics and recombinant human IFN- γ; patients suffering from complete defects in IFN-γR signaling the hematopoietic stem cell transplantation (HSCT) is the only medicinal option to date (5).
These individual genetic defects may lead to the heterogeneity of clinical manifestations and their susceptibility to mycobacterial infections. Therefore, we want to introduce three cases including two siblings and one girl from two unrelated kindred with severe mycobacterial infections; their MSMD was confirmed by both cytokine assessment and genetic analysis.
Case Reports
The cases are two brothers born to unrelated and non-consanguineous parents and one girl born to consanguineous parents referred to Immunology, Asthma and Allergy Research Institute (IAARI), Tehran University of Medical Sciences, from 2013 to 2015. Demographic information and laboratory findings of patients are shown in Table 1 and Table 2, respectively.
Table 1:
Patients’ characteristics
| Cases | Age (yr) | Age of onset (yr) | Onset of BCG-osis | Clinical manifestations related to BCG-osis |
|---|---|---|---|---|
| Patient 1 | 10.5 | 8 | 3 months | Lymphadenopathy in vaccine injection site, oral candidiasis, bloody diarrhea (at 3.5yr) |
| Patient 2 | 5 | 2.5 | 3 months | Lymphadenopathy in vaccine injection site, petechiae, large fistules with exudate containing mycobacterial particles |
| Patient 3 | 5 | 2.5 | 4 months | Axillary, cervical and mesenteric lymphadenopathy following BCG vaccination |
Table 2:
Laboratory findings of siblings
| Lab tests | Patient 1 | Patient 2 | Patient 3 | Normal range* | |
|---|---|---|---|---|---|
| WBC (per μl) | 9100 | 12000 | 9100 | 5000–20000 | |
| T cell subsets | CD3 (%) | 65 | 58 | 61 | 60–76 |
| CD4 (%) | 32 | 39 | 37 | 31–47 | |
| CD8 (%) | 25 | 23 | 18 | 18–35 | |
| B cell subsets (%) | CD19 (%) | 9 | 10 | 22 | 14–76 |
| NK cell subsets (%) | CD16/56 (%) | 6 | 1.6 | ND | 3–17 |
| Immunoglobulins (mg/dl) | IgM | 180 | 28 | ND | 43–196 |
| IgG | 1721 | 799 | ND | 463–1236 | |
| IgA | 114 | 11 | ND | 25–154 | |
| Neutrophil function tests | NBT | 100% | 100% | 100% | 90–100% |
| DHR | 129 | 107.4 | 106.6 | 50–200 | |
| Lymphocyte transforming test (LTT)* | PHA | 3.3 | 3.13 | 3.35 | 3.57 |
| BCG | 2.3 | 1.7 | 1.036 | 3.53 |
Stimulation index, ND: not determinated
The patients had mycobacterial infections and MSMD was introduced after ruling out of other immunodeficiency disorders such as HIV infection, severe combined immunodeficiency, and chronic granulomatous disease.
Informed consents were obtained from the patients’ parents. This study has been approved by Ethics Committee of IAARI (approval number: 412/p/171). Polyclonal and specific proliferation functions of T cells were evaluated in a LTT setting. Peripheral blood mononuclear cells (PBMCs) were isolated from 2–3 ml heparinized blood by density-gradient centrifugation on Ficoll-Histoprep. Then, 100000 cells were cultured in each well including the control well (without stimulation), Phytohemagglutinin (PHA, as a mitogen)-treated well and BCG (as a specific antigen)-treated well at 37 °C, 5% CO2 incubator. For each test, a healthy control was evaluated in the same culture and stimulation conditions concomitantly. Each sample was assayed in triplicate. Cell proliferation was assessed by using a colorimetric Bromodeoxyuridine (5-bromo-2'-deoxyuridine) [BrdU] proliferation kit according to the manufacturer's instructions (Roche). In brief, BrdU was added to the wells on day 4 as an overnight incubation manner. Thereafter, DNA-incorporation of BrdU was measured following the fixation of DNA and adding HRP-conjugated anti-Brdu antibody. Enzyme-substrate interaction was detected as the change of colorless to a blue color of the substrate and stopped using 2% sulfuric acid. The absorbance was read at 450 nm within maximum 30 min of adding stop solution by a microplate spectrophotometer (Powerwave, Biotek, USA). Final results were calculated as a stimulation index (SI):
IL-12p70 and IFN-γ were measured in an activated whole blood sample setting as described previously (6). In brief, heparinized whole blood samples were diluted 1:2 in RPMI 1640 supplemented with 100 U/ml penicillin and 100μ g/ml streptomycin (GibcoBRL) and dispensed in a final volume of 1 ml into four separate wells of a 24-well plate. Then, cells were activated in a setting of 12–18 h with BCG + recombinant human IFN-γ (rhIFN-γ, 5000 IU/ml; Imukin, Boehringer Ingelheim) and in a setting of 48 h with BCG + recombinant human IL-12 (rhIL-12, 20 ng/ml; R&D Systems) for IL-12p70 and IFN-γ assessments respectively. Two wells were also considered for medium alone and live BCG as controls. Cytokine levels were evaluated in supernatant using an ELISA kit (ebioscience) according to the manufactor’s instruction. The final results were standardized by expressing them per million PBMC, as the unit pg/ml/106 PBMC. The number of PBMC was determined using the blood cell counts carried out on day 0.
Primers and conditions used for PCR amplification of the coding exons, including the flanking intronic sequences are available on request. Amplified PCR products were controlled by gel electrophoresis in a 1% agarose gel and were purified by centrifugation through Sephadex G-50 Superfine resin (Amersham GE). PCR products were sequenced by dideoxynucleotide termination with the BigDye terminator kit v3.1 (Applied Biosystems) and the PCR primers. Sequencing products were purified by centrifugation through Sephadex G-50 Superfine resin and analysed on an ABI Prism 3130xl apparatus (Applied Biosystems). Sequences files and chromatograms were analyzed with GENALYS Software from CNG, France.
According to the results of lymphocyte transforming test (LTT), both patients showed a normal response to the mitogen (PHA), but impaired proliferation against BCG. BCG-treated cells of younger patient showed lower proliferating activity than the older one. These results were also in parallel to their clinical manifestations regarding the worse general condition of younger brother.
Production of IL-12 or IFN-γ was assessed following stimulation of diluted whole blood with BCG alone, BCG plus IFN-γ, and BCG plus IL-12. We added IL-12 or IFN-γ to BCG as they are well-known to be potent inducers of IFN-γ and IL-12 respectively. The levels of IL-12p70 at 12–18 h and IFN-γ at 48 h after stimulation have been shown in Table 3. Cells without treatments were considered as control.
Table 3:
In vitro production of IFN-γ and IL-12p70 levels following stimulation in two brothers and one girl with MSMD and healthy control subjects
| Cytokine | Stimulators | Patient 1 | Patient 2 | Patient 3 | Healthy control-1 | Healthy control-2 |
|---|---|---|---|---|---|---|
| IFN-γ (pg/ml) | Medium | 0 | 21.5 | 54 | 0 | 26 |
| BCG | 470.7 | 261 | 39 | 373 | 503 | |
| BCG +rhIL-12 | 1126.6 | 736 | 68 | 4490 | 3675 | |
| SI* | 2.4 | 2.8 | 1.74 | 12 | 7.3 | |
| IL-12 (p70) (pg/ml) | Medium | 16.3 | 50.8 | 0 | 55.4 | 92.4 |
| BCG | 237.9 | 95.9 | 161.7 | 178 | 177.8 | |
| BCG + rhIFN-γ | 2960 | 1186 | 647.4 | 2212 | 3247 | |
| SI* | 12.4 | 12.3 | 4 | 12.4 | 18.2 |
Stimulation index (BCG+cytokine/BCG)
According to the stimulation index results, both patients showed lower level of IFN-γ following stimulation with BCG+IL-12 compared with healthy controls (3.5–4 times less). In contrast, no considerable difference in the level of IL-12p70 was found between the patients and two healthy controls.
We sequenced the exons 1 to 17 of IL12RB1 using genomic DNA for patients and their parents and compared them with a healthy control. We identified a homozygous mutation in IL12RB1 gene for two kindred. Patients 1 and 2 had a homozygous mutation affecting an essential splice site, c.1791+2T>G, (Fig. 1A).
Fig. 1:
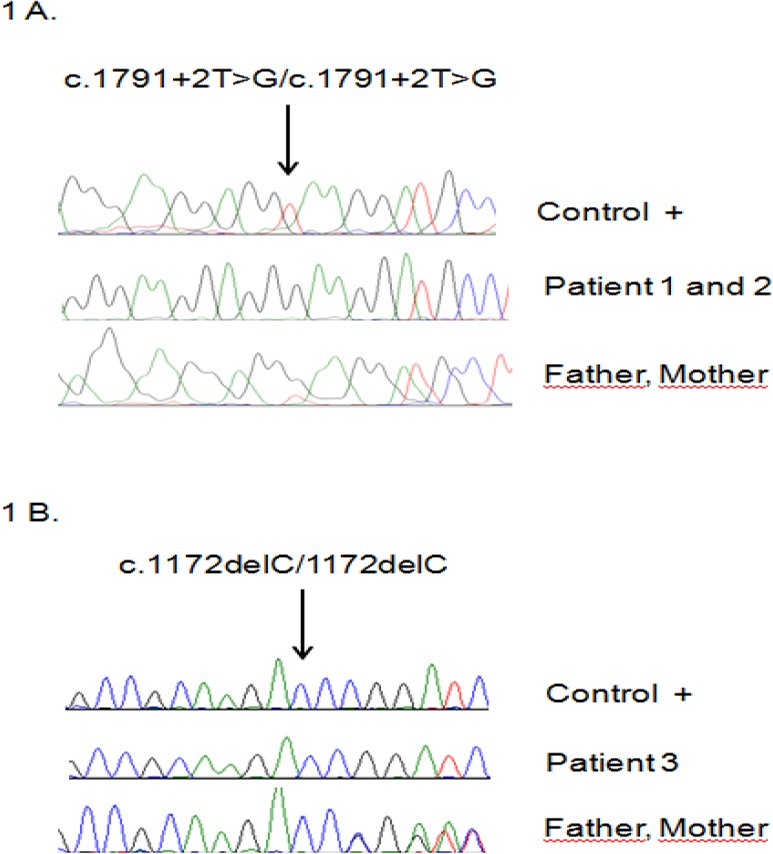
Sequencing analysis by Sanger method. The mutation in exon 15 of IL12RB1 gene was found in three MSMD patients: two kindreds (patient 1 and 2) (1A) and their parents. The results related to the girl patient and her parents has also been shown (patient #3) (1B)
This mutation is one of the most frequent reported in IL-12RB1 deficiency (7). Both parents are heterozygous for this mutation. The sequencing analysis of the patient 3 as a MSMD girl has been shown in Figure 1B indicating a homozygous mutation in IL12RB1 gene with a novel frameshift deletion c.1172delC. Both parents are heterozygous for this mutation.
Noticeably, the genetic study results confirmed the impaired function of stimulated lymphocytes to release IFN-γ following stimulation with BCG+IL-12 while the response to rhIFN-γ for IL-12p70 production was relatively intact. Thus, these mutations confer complete deficiency in three patients.
Discussion
Live BCG vaccination has been developed since 1921 for immunization of newborns to prevent the occurrence of tuberculosis all over the world especially in endemic regions (8). Vaccination against tuberculosis is done for all newborns at birth in Iran (9). It remains the most commonly used vaccine worldwide. Although BCG vaccine is considered to be safe, some complications have been reported like cellulitis, abscesses at the site of inoculation, regional lymphadenitis (BCGitis) and disseminated BCG infection (BCG-osis) (10). Immunocompromised children are more susceptible to BCG-osis as a rare but most serious complication following vaccination (11). According to the main role of IFN-γ axis in immunity against intracellular microorganisms like mycobacteria, any alteration in related genes may result to impair the immune response.
Since the first report on disseminated mycobacterial infection due to the IFN-γ receptor gene defect, some other mutations in autosomal or X-linked genes have been indicated to be involved in susceptibility to mycobacterial infections (1, 3, 12). Of them, it seems to be more common genetic etiology of MSMD, mutations in the exons of IL12RB1 gene leading to impaired production of IFN-γ in response to IL-12. The clinical phenotype is the same from that of patients with IL12B gene mutations related to defective IL-12/IL-23 secretion (4, 13).
The right and timely diagnosis of BCG-osis are of essential factors for treating the patients with disseminated infection. To help the patients referred to our center, we set up the in vitro diagnostic tests for evaluating the proliferation capacity of T cells (LTT) and production of IL-12 and IFN-γ following stimulation to determine the probable defects in IFN-γ axis. Sanger method and whole exome-sequencing can also help us to confirm the disease and to find the mutations. Here, we reported three MSMD cases with BCG-osis that were diagnosed with impaired proliferation and function of lymphocytes and confirmed by genetic analysis. IL12RB1 gene defect was found including a homozygous nucleotide change for two siblings (c.1791+2T>G) and a homozygous deletion for the girl (c.1172delC) conferring a complete deficiency in them (Fig. 1). Therefore, these mutations are likely to be translated to a functional defect regarding the impaired IFN-γ production following stimulation with BCG+IL-12 in patients’ PBMCs compared with healthy controls. Noticeably, the worse clinical manifestation of younger brother (including the large fistules with exudate containing mycobacterial particles) was in accordance with the lower IFN-γ production than that of older brother. Our findings show that cellular and molecular assessments are needed for precise identification of immunodeficiency disorders especially those without clear-cut diagnostic criteria. We should mention that there is a minor limitation in the methods applied in our study; we considered whole exome sequencing (instead of whole genome sequencing) which is capable to recognize only those variants found in the coding region of genes that impair protein function. Therefore, we could not identify the structural and non-coding variants associated with the disease with applying this method.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
We would like to thank Dr. Soheila Ajdari from Immunology Department of Pasteur Institute for her kind collaboration. The authors declare that there is no conflict of interests.
References
- 1. Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. ( 2014). Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol, 26: 454– 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile J-F, Newport M, Levin M, Blanche S, Seboun E, Fischer A. ( 1996). Interferon-γ–receptor deficiency in an infant with fatal bacille Calmette–Guérin infection. N Engl J Med, 335: 1956– 61. [DOI] [PubMed] [Google Scholar]
- 3. Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. ( 1996). A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med, 335: 1941– 9. [DOI] [PubMed] [Google Scholar]
- 4. Haverkamp MH, van de Vosse E, van Dissel JT. ( 2014). Nontuberculous mycobacterial infections in children with inborn errors of the immune system. J Infect, 68 Suppl 1: S134– 50. [DOI] [PubMed] [Google Scholar]
- 5. Roesler J, Horwitz ME, Picard C, Bordigoni P, Davies G, Koscielniak E, Levin M, Veys P, Reuter U, Schulz A, Thiede C, Klingebiel T, Fischer A, Holland SM, Casanova JL, Friedrich W. ( 2004). Hematopoietic stem cell transplantation for complete IFN-gamma receptor 1 deficiency: a multi-institutional survey. J Pediatr, 145: 806– 12. [DOI] [PubMed] [Google Scholar]
- 6. Feinberg J, Fieschi C, Doffinger R. ( 2004). Bacillus Calmette Guérin triggers the IL-12/IFN-γ axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur J Immunol, 34: 3276– 84. [DOI] [PubMed] [Google Scholar]
- 7. van de Vosse E, Haverkamp MH, Ramirez-Alejo N, Martinez-Gallo M, Blancas-Galicia L, Metin A, Garty BZ, Sun-Tan C, Broides A, de Paus RA, Keskin O, Cagdas D, Tezcan I, Lopez-Ruzafa E, Arostegui JI, Levy J, Espinosa-Rosales FJ, Sanal O, Santos-Argumedo L, Casanova JL, Boisson-Dupuis S, van Dissel JT, Bustamante J. ( 2013). IL-12Rbeta1 deficiency: mutation update and description of the IL12RB1 variation database. Hum Mutat, 34: 1329– 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seyfhashemi M, Hemati A, Mazaheri M, Azarbarin A, Hamedi P. ( 2005). Complications of BCG vaccination in children. Iran J Pediatr, 15: 209– 14. [Google Scholar]
- 9. Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. ( 2011). The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med, 8:e1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rezai MS, Khotaei G, Mamishi S, Kheirkhah M, Parvaneh N. ( 2008). Disseminated Bacillus Calmette-Guerin infection after BCG vaccination. J Trop Pediatr, 54: 413– 6. [DOI] [PubMed] [Google Scholar]
- 11. Shahmohammadi S, Saffar MJ, Rezai MS. ( 2014). BCG-osis after BCG vaccination in immunocompromised children: Case series and review. J Pediatr Rev, 2: 47– 54. [Google Scholar]
- 12. Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova JL. ( 1996). Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med, 335: 1956– 61. [DOI] [PubMed] [Google Scholar]
- 13. Prando C, Samarina A, Bustamante J, et al. ( 2013). Inherited IL-12p40 deficiency: genetic, immunologic, and clinical features of 49 patients from 30 kindreds. Medicine (Baltimore), 92: 109– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]


