Abstract
Background
Osteoarthritis is the most common reason for pain in older adults, and the individual and economic burden of this disease is immense. The chronic character of osteoarthritis requires a long-term therapeutic treatment. In this regard life-style interventions such as physical exercises that can be carried out by the patient himself are recommended as first line treatment. There is evidence for the short-term benefit of exercise therapy in terms of pain reduction and physical functioning. Nonetheless research agendas highlight the need for multifaceted interventions that incorporate exercise strategies into patient care. Studies should be conducted with appropriate sample sizes and should allow statements on long-term effects as well as cost-utility and safety. These open questions are under the scope of this study.
Methods/design
This is a controlled study in the context of health services research. The study population consists of n = 1400 subjects with hip or knee osteoarthritis. The intervention group will be recruited from participants of a country-wide health insurance offer for people with hip or knee osteoarthritis. Potential participants for the control group (ratio 10:1 (control vs. intervention) will be filtered out from the insurance data base according to pre-defined matching criteria and asked by letter for their participation. The final statistical twins from the responders (1:1) will be determined via propensity score matching. The progressive training intervention comprises 8 supervised group sessions, supplemented by home exercises (2/week over 11 weeks). Exercises include mobilization, strengthening and training of postural control. Primary outcomes are pain and function measured with the WOMAC Index immediately after the intervention period. Among other things, health related quality of life, self-efficacy, cost utility and safety will be evaluated as secondary outcomes. Participants will be followed up 6, 12 and 24 month after baseline.
Discussion
Results of this trial will document the effects of clinical as well as economic outcomes in a regular health care setting on the basis of a large sample size. As such, results of this trial might have great impact on future implementations of group- and home-based exercises in hip or knee osteoarthritis.
Trail registration
German Clinical Trial Register DRKS00009251. Registered 10 September 2015.
Electronic supplementary material
The online version of this article (doi:10.1186/s12889-016-3030-0) contains supplementary material, which is available to authorized users.
Keywords: Hip osteoarthritis, Knee osteoarthritis, Group training, Home exercise, Propensity score matching, Health care research, WOMAC, Long-term effects, Cost-analysis, Safety
Background
Epidemiology
Osteoarthritis (OA) is the most common reason for pain in older adults. OA is further associated with joint stiffness, crepitus, occasional effusion, variable degrees of inflammation, functional impairment, worsening of the general health status and a decrease in health related quality of life [1–3]. Several intrinsic and extrinsic risk factors are related to OA such as age (older), overweight, gender (women), genetic factors, occupational joint loads etc. In the German health reporting system 2010, approximately 27 % of the women and 18 % of the men reported a previous OA diagnosis [2]. The true value may even be higher as not every person suffering from typical OA symptoms will consult a doctor [2]. The knee and hip joints are the most commonly affected weight bearing joints. According to disease related symptoms and its prevalence, OA has a relevant impact on the individual person and the need for health services and consequently increases direct and indirect health care costs [2]. Cumulative cost of absence from work, medical costs and community and social services in Europe is currently estimated at 0.5 % of gross national product [4]. These costs will further increase in due consideration of the demographic change with a growing number of older adults and the increasing incidence of obesity as a relevant risk factor for knee OA [1, 5]. Research priorities should therefore be placed on the development and evaluation of effective and cost-efficient treatment strategies to counteract the aforementioned increase of personal as well as economic burden of OA.
Treatment of lower limb osteoarthritis
To date joint replacement may be the only option for severe symptoms in hip or knee OA. In earlier stages, conservative therapeutic interventions are important to reduce pain and increase function and health-related quality of life [6]. Pharmacological therapies have significant toxicities limiting their permanent use in chronic diseases [4]. Non-pharmaceutical interventions such as physical exercise programs are therefore important therapeutic options. Treatment effects of these programs are similar to simple analgesics and oral non-steroidal anti-inflammatory drugs. However reports on known side-effects such as pain increase or falls are rare, thus exercise appears to be a safe intervention [7–9]. It is therefore hardly surprising that various international guidelines, expert committees and health institutions recommend a regular exercise regimen as a first line treatment for OA. Regimes should account for strengthening and/or low impact aerobic exercises. Further recommendations include aquatic exercises, Tai Chi, range of motion exercises and neuromuscular education [10–14].
Evidence is given for the short-term efficacy of joint specific exercise programs with respect to pain reduction and increase of function in subjects with knee and hip OA [8, 15, 16].
While the evidence suggests that exercise is an effective intervention further studies are needed:
to test multi-faceted interventions that incorporate the exercise strategies into patient care [15].
to assess effectiveness and safety of non-pharmacological management strategies [11].
to assess the long-term effectiveness in terms of symptom relief, disease progression and exercise adherence [8, 11, 15].
to assess efficiency of the intervention from an economic perspective [11].
to assess which professional can best deliver the intervention [11].
to conduct studies with appropriate sample sizes [11].
The above mentioned research topics will be addressed in this study on exercise intervention in subjects with hip and/or knee OA.
Study purpose
This study will be conducted in the context of health services research. The exercise program is based on an intervention that was previously evaluated in a randomized placebo-controlled trial [17].
The primary aim of this study is to determine whether an 11-week progressive training program comprised of group sessions and home-based exercises decreases symptom related pain and increases physical function in subjects with hip and/or knee osteoarthritis in comparison to a matched control group in the short term. Effectiveness is quantified by the subscales pain and physical function of the WOMAC Index 3.1 NRS.
Secondary outcomes will be differentiated into a short-term perspective (3 months), mid-term perspective (6 months) and a long-term perspective (follow-up data 12 and 24 months after initiation of the intervention).
The secondary aims are to determine whether an 11-week progressive training program comprising group sessions and home-based exercises…
-
(I)
…improves health-related quality of life and/or global rating of health.
-
(II)
…decreases pain (mid-term, long-term only, as short-term is the primary study outcome)
-
(III)
…improves physical function (mid-term, long-term only, as short-term is the primary study outcome)
-
(IV)
…improves self-efficacy and physical activity levels
-
(V)
…delays time to surgery
-
(VI)
…is efficient with regard to personal and/or social costs
…in comparison to a matched control group (CO).
It is of further interest if group sessions and home-based exercises are feasible in a general health care setting in terms of exercise adherence and safety. Both will be monitored throughout the intervention period in the intervention group only.
Methods/design
Design (Fig. 1)
Fig. 1.
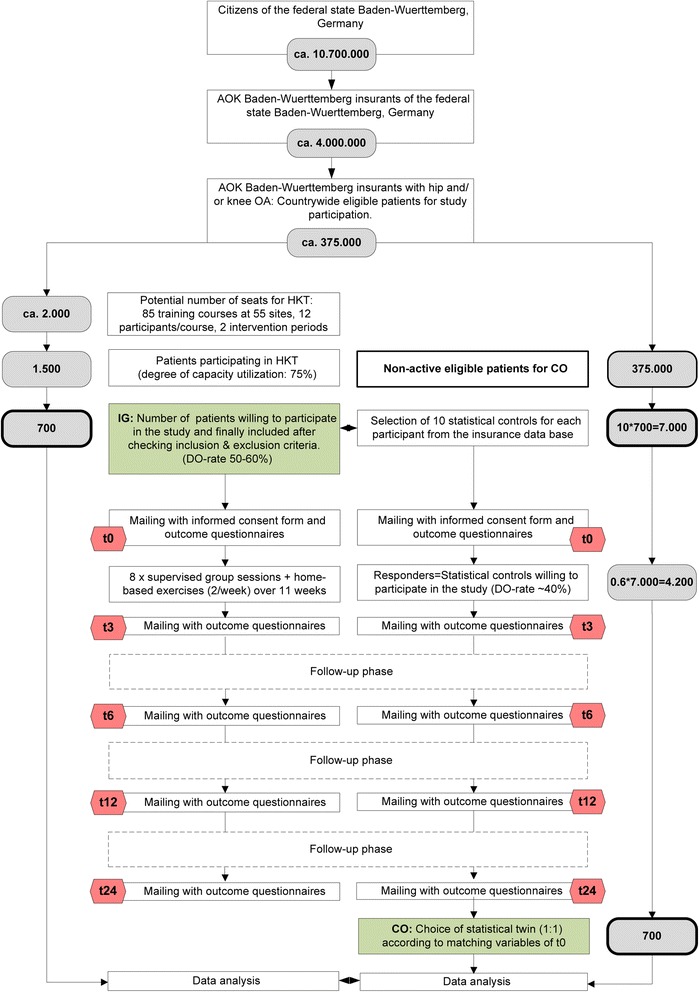
Study flow chart
This is a multi-center non-randomized controlled trial of an 11-week hip and knee training (HKT) with a 24 months follow-up period in the context of health services research. The exercise intervention comprises 8 supervised group sessions (1× / week for 8 weeks) and a home exercise program (2× / week for 11 weeks). All participants are insurance holders of a large statutory health insurance company covering approx. 40 % of the total population of the state Baden-Wuerttemberg. Participants of the intervention group (IG) will be compared to “statistical twins” (matched pairs) to investigate the effects of an exercise intervention on clinical and economic outcomes. Measurements will be taken at baseline (t0), 3 months (t3), 6 months (t6), 12 months (t12) and 24 months (t24). Economic data will further be evaluated retrospectively for two years prior to baseline.
- Recruitment of the intervention group (IG)
- IG is recruited out of eligible participants of a countrywide health care offer of the above mentioned insurance company. Prerequisites for exercise participation are (1) membership in the insurance company, (2) referral from an orthopaedic surgeon or general practitioner because of complaints at the hip and/or knee. To be included into the accompanying study, exercise participants further have to confirm a self-reported lifetime prevalence of hip and/or knee OA. They have to affirm the following two questions according to the German Federal State health reporting system criteria [2]:
- 1. “Have you ever visited a doctor because of complaints in the hip and/or knee joint?”
- 2. “If yes, did a medical doctor ever ascertain OA or a degenerative disorder at your knee and/or hip joint?”
- Further inclusion- and exclusion criteria for study participation are listed in Table 1.
- The exercise intervention is accessible at 55 locations (study sites) of the country, offering 85 exercise courses each half year. The number of participants per exercise course is restricted to twelve persons. The recruitment period covers two course terms. Assuming twelve participants in each course, the intervention can be provided to approximately 2000 insurants. Eligible patients can participate on condition that their physicians prescribe the health care offer. Interested patients register for the intervention program at the location nearby and will be informed about the accompanying scientific evaluation of the exercise program. In this context, exclusion criteria are requested and only eligible patients will finally be allowed for the exercise intervention. Further details including the study information sheet for potential study participants, the informed consent and a check-list for all inclusion and exclusion criteria will be delivered by mail. This mail will be sent to each participant prior to the first training session. The mail further includes the self-administered questionnaires. In the context of all aforementioned stages it will be pointed out that study participation is not a presupposition for exercise participation. Eligibility of the patients to enter the exercise program and to participate in the study will finally be determined by checking the inclusion- and exclusion criteria for the exercise intervention 1st in the context of the first visit and 2nd by information recorded via the self-administered questionnaire at t0 (Table 1). Patients refraining from the study still are eligible to receive the exercise program.
- Feasibility of recruitment
- We estimate that the offered exercise courses will be booked by 1500 patients (corresponding to 75 % occupancy rate). We further estimate a pre-study drop-out rate of 50–60 % due to lack of interest in study participation or exclusion according to study criteria. Thus approximately 700 patients will be allocated to the trial.
- Recruiting and selection of the control group (CO)
- (1) In a first step the insurance ID of study participants will be forwarded to a specific department of the health insurance company. This department is responsible for the extraction of ten statistical controls for each study participant of the intervention group according to the procedure described in the statistical part of this manuscript. This pool of controls shall ensure the proper selection of one matched statistical twin for each participant in the second matching step according to number (5). The factor ten has already been proven successful in a previous study of the insurance company and was therefore adopted for the present study [18].
- (2) The address and insurance number of the selected statistical controls according to (1) will be forwarded to another department of the company. This department will then forward study details (study information sheet for participants, informed consent form, questionnaires) by mail in order to ask if they are willing to participate in the control group of the study.
- (3) Subsequently all duly completed questionnaires at t0 will be checked for in- and exclusion criteria which are part of the initial self-administered questionnaire (Table 1).
- (4) Included study participants will then be recontacted to ask for follow-up data at t3, t6 and t12 (follow-up data will be gathered from all controls as the availability of economic data at t0 is temporally delayed for 12 month for organizational reasons).
- (5) The final statistical twins (1:1 matching) will be selected using Propensity Score Matching (PSM). According to Austin, the propensity score can be explained as “the probability of treatment assignment conditional on observed baseline characteristics”. Matched-pairs are generated with nearest neighbor matching. “The nearest neighbor matching selects for matching to a given treated subject that untreated subject, whose propensity score is closest to that of the treated subject” [19]. Input variables for PSM are derived from the baseline data of the self-administered questionnaires (SAQ) as well as economic data from the insurance data base (IDB). The detailed matching procedure is described in the statistical part of this manuscript.
- Feasibility of recruitment
- The selection of statistical twins is designed according to a successful procedure of a previous study of the insurance company. In this study on the efficacy of an exercise program for patients with back pain, a responder rate of the controls of 60 % could be achieved [18].
Table 1.
Inclusion and exclusion criteria for study participation (t0 = baseline)
| Inclusion criteria (all criteria relevant for study eligibility) |
| • Insurance holder of the insurance company offering the exercise program since two or more years prior t0 |
| • Referral from an orthopaedic surgeon or general practitioner |
| • Self-reported lifetime prevalence of hip and/or knee OA that was previously diagnosed by a medical practitioner • Physical and mental ability to participate in the interventional program and to answer self-administered questionnaires at t0 |
| Exclusion criteria (one or more positive answers lead to non-eligibility) |
| • Significant established osteoporosis requiring treatment, previous spontaneous or low impact fracture prior t0 |
| • Co-morbidities leading to major impairments in everyday life and representing contra-indications for physical activities at t0 |
| • Artificial joint replacement at the knee and/or hip joint within the last 6 months prior t0 |
| • Artificial joint replacement at the knee and/or hip joint with instable anchoring at t0 |
| • Artificial joint replacement at the knee and/or hip joint with rradiologic signs of implant loosening at t0 |
| • Artificial joint replacement at the knee and/or hip joint accompanied with acute joint inflammation at t0 |
| • Current pain at rest or with activity due to artificial joint replacement at the knee and/or hip joint |
| • Luxation as an adverse event of artificial hip replacement prior t0 |
| • Surgery at the lower extremity within the last 3 months prior t0 |
| • Regular use of gait aids |
| • Self-reported acute illness at t0 |
| • < 15 pointsa on the WOMAC Index subscale pain (0–100) and < 15 points on the WOMAC Index subscale physical function (0–100) at t0 |
| • Insufficient German language ability for self-administered questionnaires (IG) at t0 |
| • Current employment in the health care insurance at t0 |
aExclusion criteria is valid for the primary data analysis only. Low values indicate less pain and improved function: 0 points = no pain and maximum physical functioning respectively
Randomization process and allocation concealment
Not applicable.
Blinding
The blinding of subjects to treatment is not possible as treatment exposure is evident.
The blinding of assessors of questionnaires is not applicable, as outcome measures are self-administered by the patients.
The blinding of the statistician who performs propensity score matching of controls is warranted with regard to any follow-up data (t3, t6, t12, t24) until all subjects of the intervention groups have their statistical twin.
The blinding for analysis of primary outcomes is warranted as group allocation is blinded for the statistician from t0 to t3.
The blinding for further outcomes is not warranted as interim reports will unblind results of the primary analysis.
Interventions
All participants of the intervention group are requested to refrain from seeking other forms of treatment during the 11 week-intervention period from t0 to t3.
Hip and knee training (HKT)
The HKT is a comprehensive training program based on a previously evaluated 12-week exercise program specifically designed for patients with hip OA (17;20). The HKT was modified to allow its application for knee patients and reduced to 11 weeks with 8 supervised training sessions.
The HKT contains 8 weeks of group (1× / week, 60–90 min) and home based exercises (2× / week, 30–40 min) followed by 3 weeks of home-based exercises only (2× / week, 30–40 min). The 8-week group sessions include theoretical education and practical contents and enhance social contacts. The educational part improves the accomplishment of exercises. In this regard, participants are introduced into anatomical basics. They learn how to find and feel anatomical landmarks such as the spina iliaca anterior superior or the junction from the pelvis to the lower spine. Anatomical landmarks are important reference points to control a proper exercise execution. Participants are further introduced into training principles. They learn how to rate their subjective physical strain, how to deal with pain and how to report their home training sessions and potential adverse events in the training log. The theoretical contents are imparted in the supervised training sessions and can also be read up in the exercise manual that every participant receives. The supervised sessions enhance social contacts by having group-based introductions and feedback before and after the exercises, and by employing partner and group exercises. In the group sessions, subjects are introduced to the exercises they have to do at home.
Both, group and home training sessions include hip and knee exercises for motor learning and mobilization (MM), strength training (S) and exercises to improve postural control (PC). Elastic rubber bands, stability trainers (pads), exercise balls and exercise mats are used as training devices. Intensity and structure of the exercises follow a progressive concept. A more detailed description of group and home-based training modalities is given in the classification of the training phases and the exercise dosages of the different exercise tasks (Tables 2 and 3).
Table 2.
Phased exercise program “Hip and knee training (HKT)”
| Phase | Week | Home-based training | Group sessions Theory / Training | Objective |
|---|---|---|---|---|
| 1 | 1-3 | ✔ | 60 min / 30 min 40 min / 50 min 30 min / 60 min |
Mobilization, Motor learning |
| 2 | 4-7 | ✔ | 30 min / 60 min - / 60 min - / 60 min - / 60 min |
Balance training for postural control (static conditions), Strength endurance |
| 3 | 8 | ✔ | - / 60 min | Balance training for postural control (dynamic conditions), Endurance & maximum strength |
| 9-11 | ✔ | - / - | Balance training for postural control (dynamic conditions), Endurance & maximum strength |
Table 3.
Exercise dosage
| Objective | Sets/Repetitions(reps)/Intensity | Rest |
|---|---|---|
| Motor learning Mobilization Stretching |
1 sets of 10 reps at <30 % MVC 1 sets of 30 reps at <30 % MVC 2 sets of 20 sec |
A few sec <1 min A few sec |
| Strength endurance | 2 sets of 20–25 reps at 30-40 % MVC 3 sets of 20–25 reps at 30-40 % MVC |
1 min 1 min |
| Maximum strength | 3 sets of 10–15 reps at 70 % MVC 4 sets of 10–15 reps at 70 % MVC |
1-2 min 1–2 min |
| Postural control (static) Postural control (dynamic) |
1 set of 6 reps of 15 sec 1 set of 6 reps of 15 sec |
30-60 sec 30–60 sec |
MVC Maximum voluntary contraction
The home training consists of 30 exercises for MM and 20 exercises for S. All subjects receive the exercise manual “Das Tübinger Hüftkonzept” (“The Tübingen Hip Concept”) [21]. Aside of its theoretical contents as outlined above, the exercise manual includes written instructions and pictures for the home training exercises. Patients with knee OA further receive a complementary leaflet where twelve MM hip exercises and ten S hip exercises are substituted by corresponding knee specific exercises. A brief description of the exercises for hip and knee patients (Additional file 1, Additional file 2) and an excerpt of the German-language exercise manual (Additional file 3) are depicted in the additional files.
Phase one (week 1–3) includes mobility exercises to increase flexibility of the pelvis, hip and knee joint and to enhance motor control to allow proper exercise execution. Examples of exercises for the hip and the knee joint are given in Fig. 2. Additional muscle stretching exercises address hip and knee flexors and extensors as well as hip adductors. Exercises in phase one are predominantly executed in a supine or seated position.
Fig. 2.
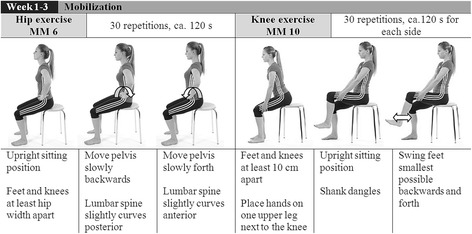
Example of an exercise for hip and knee motor learning and mobilization (MM)
The second phase (week 4–7) is divided into static balance exercises for postural control at the beginning of the training session followed by strengthening exercises. The difficulty of the static balance exercises can be adjusted according to the individual skills of the patients (difficulty level A–D, Fig. 3). Subsequent to the balance exercises the training program continues with open and closed kinetic chain exercises to improve muscular endurance. As displayed in Fig. 4, subjects have the possibility to match the strengthening exercises with the individual performance level by selecting exercise “a” or ”b”.
Fig. 3.
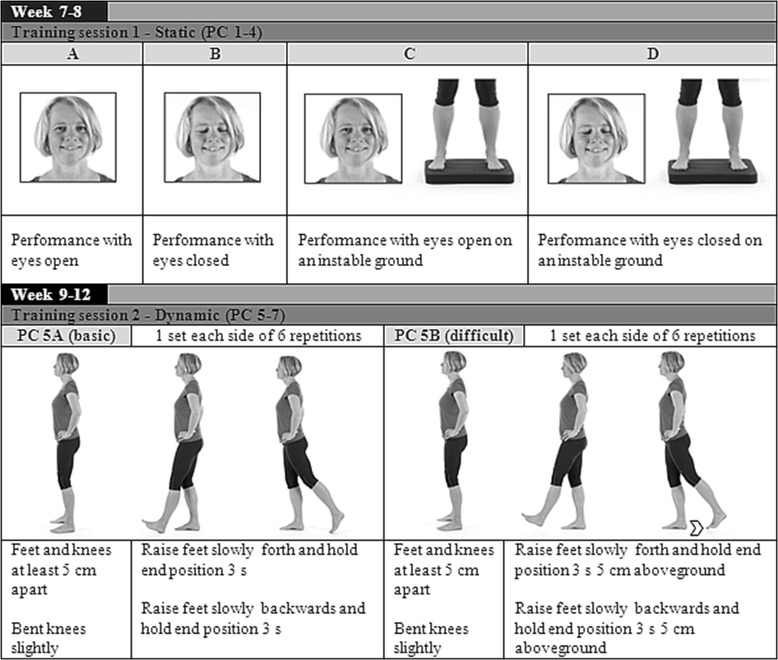
Example of static and dynamic exercises for postural control (PC)
Fig. 4.
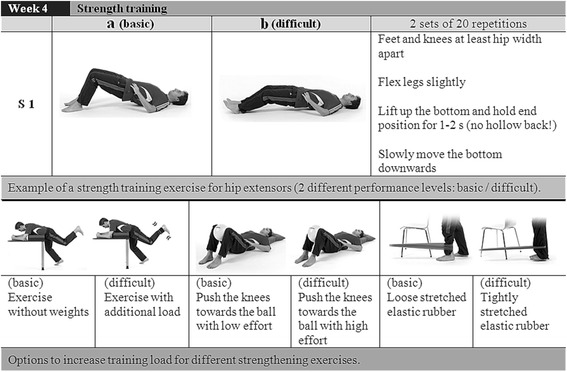
Examples for progressive strength training exercises (S)
Demands of the exercises increase in phase three (week 8 to 11) to account for the progressive character of the exercise intervention. The balance exercises for postural control are expanded with three additional dynamic exercises. The difficulty of the dynamic balance training can be adjusted according to the individual skills of the patients (difficulty level PC-A or PC-B, Fig. 3). The strengthening exercises of this phase focus on the improvement of maximum strength.
The intensity of exercises in the different phases is controlled and quantified by the subjects’ subjective rating in dependence of the Borg perceived exertion scale [22]. While subjects should practice with low intensity during the first phase, the pre-settings of exercise intensity during the second and third phases are high. Subjects are advised to work out with a “hard” to “very hard” exercise intensity that would result in an exertion level of at least 14 on the Borg-Scale at the end of each set. The strength endurance phase (phase two) is characterized by subjects performing exercises with 2–3 series and 20 to 25 repetitions, corresponding to ~30–40 % maximum voluntary contraction (MVC) [23]. The hypertrophy phase includes exercises with 3–4 series and 10 to 15 repetitions, corresponding to ~70 % MVC [23]. The percentage MVC is estimated by the number of repetitions that can be conducted appropriately but with the subjective feeling of a hard workout. An overview of the exercise dosage is depicted in Table 3.
Group sessions will be conducted at the 55 country-wide training centres of the insurance company. They will be administered by health professionals of the company (i.e. physiotherapists, exercise scientists, trainers). All health professionals were additionally introduced into the hip and knee training in the context of a 2 days training program. They received a curriculum for the eight group sessions as well as detailed information on each particular exercise for the home training program [21], and they were introduced in how to teach participants accordingly. The educational program was held by the health professionals who designed the standardized intervention program.
Control group (CO)
CO receives general care. Participants of the control group may attend other offers of the health insurance company. Interventions are not restricted. However physical activity along the study period is monitored via self-administered questionnaires.
Collection points (Fig. 5)
Fig. 5.
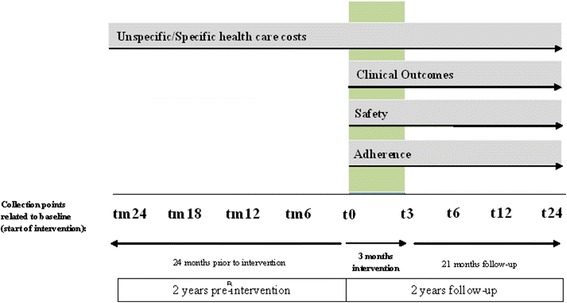
Collection points and outcome measures. Figure legend: t = collection point, m = minus. 0, 3, 6, 12, 24 = month of collection point related to baseline (t0)
Participants will be assessed at baseline (t0) and after (t3) the 11-week intervention period. Three follow-ups are conducted by mail 3 (t6), 6 (t12) and 21 (t24) months after the end of the intervention period. Economic evaluation will further compare data 6 (tm6), 12 (tm12), 18 (tm18) and 24 (tm24) months retrospectively (m = minus) prior to t0.
Outcome measures (Table 4, Fig. 5)
Table 4.
Outcome measures
| Characteristics & confounders | Description and instrument | Data source | Sample | Collection points |
|---|---|---|---|---|
| Patient’s characteristics | Date of birth, gender, ethnicity, BMI (height, weight), site (s) of OA diagnosis, date of first OA diagnosis, labour situation (working, retired, unemployed, in rehabilitation status) | SAQ, IDB | IG, CO | t0, t3, t6, t12, t24 |
| Primary outcome measure | Clinical Outcomes | |||
| Pain | WOMAC Index 3.1 German (11-box NRS): subscale pain | SAQ | IG, CO | t0, t3 |
| Function | WOMAC Index 3.1 German (11-box NRS): subscale physical functioning | SAQ | IG, CO | t0, t3 |
| Secondary outcome measures | Clinical Outcomes | IG, CO | ||
| Stiffness, disease specific impairment | WOMAC Index 3.1 German (11-box NRS): subscale stiffness, overall score | SAQ | IG, CO | t0, t3, t6, t12, t24 |
| Pain, physical function | WOMAC Index 3.1 German (11-box NRS): subscale pain, subscale physical function | SAQ | IG, CO | t6, t12, t24 |
| Health related quality of life | VR-12 incl. VR-6D utility index (4-week-time-slot) | SAQ | IG, CO | t0, t3, t6, t12, t24 |
| General Self-Efficacy | General self-efficacy scale (GSE) | SAQ | IG, CO | t0, t3, t6, t12, t24 |
| Response to exercise | Omeract-OARSI Set of Responder Criteria: composite score with minimum absolute and relative change levels for pain or pain & function | SAQ | IG, CO | t0, t3, t6, t12, t24 |
| Physical activity status | Habitual physical and sports activity status | SAQ | IG, CO | t0, t3, t6, t12, t24 |
| Time to surgery | Endpoint “elective joint replacement” | IDB | IG, CO | t0, t3, t6, t12, t24 |
| Patient satisfaction | ||||
| Modified version of the ZUF-8 Questionnaire to assess patient satisfaction | SAQ | IG | t3 | |
| Economic data | ||||
| Unspecific and specific (OA-related) health care costs | Out-patient costs, hospital costs, costs related to drugs, adjuvants and physical modalities, rehabilitation costs, sick-pay | IDB | IG, CO | tm24, tm18, tm12, tm6 t0, t3, t6, t12, t24 |
| Unspecific/specific (OA related) periods of disability | Days of disability (overall and related to OA) | IDB | IG, CO | tm24, tm18, tm12, tm6 t0, t3, t6, t12, t24 |
| Intervention related costs | Costs for human and physical resources/session | IDB | IG | t3, t6, t12, t24 |
| Adherence to exercise | ||||
| Training adherence | Summarized number of attended training sessions according to training log | SAQ | IG | t3 |
| Safety evaluation | ||||
| Adverse events and side-effects | Summarized number and details of adverse events and side-effects according to training log | SAQ | IG | t3 |
Data Source: Self-administered questionnaire via postal mailing (SAQ), insurance data base (IDB). Sample: Participants of the intervention group (IG), matched pairs control group (CO)
Annotation: Unless otherwise stated, measures are conducted at baseline and at all follow-ups.
The Western Ontario McMasters Universities Osteoarthritis Index (WOMAC® NRS 3.1 German Index) is a disease-specific instrument used to evaluate self-reported pain, stiffness and functional impairment. It is a valid, reliable and responsive score, easy to complete, simple to score and available in multiple language forms and scaling formats [24]. The scales will be transformed into values from zero (no limitation) to 100 (maximum limitation).
The OMERACT-OARSI Set of Responder Criteria is a composite score with minimum absolute and relative change levels for pain or pain & function. Input variables for pain and function will be derived from the WOMAC Subscales pain and functional impairment [25].
The VR-12 is an open access version of the SF-12 [26]. It comprises 8 different scales, four of them related to physical health (physical functioning, role-physical, bodily pain, general health), and four of them related to mental health (vitality, social functioning, role-emotional, mental health). The VR-6D is a utility measure derived from the VR-12. The 4 week time-slot will be used for analysis.
General self-efficacy will be measured with the German version of the General Self-Efficacy scale (GSE) with a four-point Likert scale (“not right” versus “definitely right”). Ten items are designed to tap this construct. Each item refers to successful coping and implies an internal-stable attribution of success. The scale ranges from 10 to 40 points with 40 points indicating maximum self-efficacy [27].
Habitual physical activity status will be quantified with regard to sports activities as well as physical activities in everyday life. Questions are similar to those being used in the German Health Update 2012 [28, 29].
Patient satisfaction (IG only, t3) will be quantified by participants of the IG with a four point Likert scale using a modified and shortened version of the ZUF-8 [30, 31]. Modifications were made in order to adapt the eight item instrument to the outpatient setting.
- Patient characteristics
- Body Mass Index (BMI), site (s) of OA joint diagnosis, artificial joint replacement (hip or knee and time of event).
- Safety evaluation (IG only, t3)
- Description of adverse events within the previous 11-week intervention period including frequency, intensity and duration of exercise related pain and possible reasons. Participants get a training log for home-based exercises where they are asked to report adherence as well as exercise related adverse events. In this way summarized data can be provided by the participants at the end of the intervention period by reviewing the training logs. Aside from this study-related safety report, patients are urged to contact their personal trainers or physicians in case of adverse events and side-effects.
- Adherence to exercise (IG only, t3)
- Number of attended training sessions (group and home-based exercises). In case of omitted sessions: Reasons for non-compliance.
- Socio-demographic data (insurance data base)
- The following socio-demographic data will be readout of the data base: date of birth, gender and labor situation (working, retired, unemployed, in rehabilitation status), complexity of work (from 1 = low to 4 = high), level of education (1 = no graduation to 4 = High School), highest level of educational attainment (from 1 = no qualification to 6 = doctoral degree), contractual form (permanent/fixed term contract, full time/part time).
- Economic data (insurance data base)
- Patient-specific economic data comprise (1) unspecific and (2) specific osteoarthritis related health care costs for the diagnosis of hip and/or knee OA, (3) Specific and unspecific days of disability and (4) intervention related costs for the exercise program:
- (1) Unspecific health care costs (overall costs): Sick-pay, hospital costs, out-patient costs, rehabilitation costs and costs related to drugs, physical modalities and adjuvants.
- (2) Specific diagnosis (hip and knee OA) related health care costs: Sick-pay, hospital costs, out-patient costs, rehabilitation costs and costs related to disease related drugs, physical modalities and adjuvants.
- (3) Specific (hip and knee OA related) and unspecific days of disability.
- (4) Intervention related costs include human and physical resources that are required for the institutional exercise sessions. Intervention related costs will be added to the above mentioned diagnosis related costs for all participants of the intervention groups according to the number of their scheduled training session (8 units).
Statistics
Sample size
Below are given details on the sample size estimation for primary clinical outcomes. However, aside from statistical power, a sufficiently large sample is necessary to obtain representative results for the general population and to allow sustainable statements on the effectiveness and efficiency of the intervention for secondary clinical as well as economic outcomes. We therefore plan to include all eligible patients willing to participate in the study and finally assume a number of n = 700 in each group. This number takes into consideration the degree of capacity utilization of hip and knee training courses as well as the number of subjects non eligible due to inclusion- and exclusion criteria as well as subjects refusing consent to participate in the study (Fig. 1).
The empirical basis of the sample size estimation for primary outcomes was retrieved from the paper by Krauss et al. [17]. In this randomized controlled trial (RCT), intra-individual differences of WOMAC subscale pain and WOMAC subscale physical function identically showed an effect size of about 0.5 between the intervention and the control group (pain, intervention: −8.5 ± 13.9, control: −1.3 ± 15.3, physical function, intervention: −8.4 ± 13.4, control: −2.1 ± 12.9).
Based on the previous results of the RCT and the so-called efficacy-effectiveness gap between RCTs and studies under real life conditions [32] we finally assume an effect size of ES = 0.3.
Due to the fact that we want to test the two endpoints (pain and physical function) simultaneously and without hierarchical order, we choose a level of significance of 0.025 (two-sided, Bonferroni correction) and a power of 0.90. This leads to a sample size of 278 subjects per group in the parallel group design without cluster effects (nquery release 7.0). The drop-out rate in the intervention group of the above mentioned RCT was 8 % [17]. As this value is quite low, we assume a somewhat higher number of non-finishers and estimate the rate to be 20 %. Thus 350 subjects should be allocated to each treatment arm.
However, in our study, cluster effects may appear for subjects within identical training groups: We expect 700 included subjects participating in 170 different training courses. Thus four participants of each training group will averagely be subjects of the study (whereas the rest of the group participants are no study subjects). With a rather high intra class correlation of 0.33 we obtain a variance inflation factor of 2 for the intervention. Therefore, with 700 subjects per study arm the aimed power should be reached.
We refrain from analyzing our data using a matched-pair design as propensity score matching does not guarantee that individual pairs will be well-matched on the full set of covariates, only that groups of individuals with similar propensity scores will have similar covariate distributions [33]. Despite this fact, propensity score matching including among other variables baseline WOMAC scores as matching factors may reduce variance of outcome measures and therefore probably increase the power of the study further if the propensity score is included as covariate in the statistical evaluation model. However, it is difficult to quantify this effect and thus we conservatively calculated the sample size obtained for the parallel group design with cluster effect as described in the preceding paragraph.
Matching procedures and statistical analysis of clinical endpoints
The matching procedure for the statistical twins of the control group (CO) will be conducted as followed: ten statistical controls for each participant of the intervention group will be selected from the insurance data base according to the matching criteria displayed in Table 5. If it is not possible to select ten matches for a subject of the intervention group according to the criteria and tolerances displayed in Table 5, the tolerance will be extended in an iterative manner (steps of 100 Euro or 1 day respectively) until ten controls can be extracted from the data base.
Table 5.
Matching criteria to extract 10 controls for each participant of the intervention
| Criteria | Tolerance |
|---|---|
| Co-morbidity: Quantity of hierarchical ordered morbidity groups in the previous year | 0 |
| Osteoarthritis of the hip or knee joint in the previous year: yes/no | 0 |
| Participation in a special general practitioner care program (“Hausarztzentrierte Versorgung”): yes/no | 0 |
| Routine data: Age (years), gender, type of insurance (compulsorily insured, family insured, pensioner, unemployed), complexity of work (from 1 = low to 4 = high), level of education (1 = no graduation to 4 = High School), highest level of educational attainment (from 1 = no qualification to 6 = doctoral degree), contractual form (permanent/fixed term contract, full time/part time) | 0 |
| Joint replacement in the last 24 month prior t0 at the hip and/or knee joint(s): yes/no | 0 |
| Sum of unspecific health care costs (overall costs) in the last 24 month prior t0: Sick-pay, hospital costs, out-patient costs, costs related to periods of disability and costs related to drugs, physical modalities and adjuvants. | +/− 100 EUR |
| Sum of unspecific health care costs (overall costs) in the last 6 month prior t0 (tm6): Sick-pay, hospital costs, out-patient costs, costs related to periods of disability and costs related to drugs, physical modalities and adjuvants. | +/− 100 EUR |
| Sum of specific diagnosis (hip/knee OA) related health care costs in the last 24 month prior t0: Sick-pay, hospital costs, out-patient costs, costs related to periods of disability and costs related to disease related drugs, physical modalities and adjuvants such as walkers, cranks or orthotics. | +/− 100 EUR |
| Sum of specific diagnosis (hip/knee OA) related health care costs in the last 6 month prior t0 (tm6): Sick-pay, hospital costs, out-patient costs, costs related to periods of disability and costs related to disease related drugs, physical modalities and adjuvants such as walkers, cranks or orthotics. | +/− 100 EUR |
| Disability days in the last 24 month prior t0 (tm24) | +/− 1 Day |
| Disability days in the last 6 month prior t0 (tm6) | +/− 1 Day |
| Specific disability days (hip/knee OA) days in the last 24 month prior t0 (tm24) | +/− 1 Day |
| Specific disability days (hip/knee OA) in the last 6 month prior t0 (tm6) | +/− 1 Day |
Legend: a tolerance of “0” is equal to complete agreement, i.e. for age in years. Matching criteria are derived from the insurance data base
The matching process for the identification of statistical controls (CO) for each subject of the intervention group will be permuted in blocks: The matching procedure will be conducted quarterly until the number of needed subjects is reached.
Selected subjects will be asked for study participation (see section recruiting). The final statistical twins for each participant (1:1 matching) will be selected from all eligible responders. As a primary criterion, twins must be identical with regard to site(s) of OA (hip, knee or both). The following independent variables of the self-administered questionnaires (SAQ) and insurance data base (IDB) will then be used as input variables for the logistic regression of the PSM:
Socio-demographic data at t0: age and gender (SAQ)
Co-morbidity: Quantity of hierarchical ordered morbidity groups in the previous year (IDB)
Osteoarthritis of the hip or knee in the previous year (IDB)
WOMAC Index subscale pain at t0 (SAQ).
WOMAC Index subscale physical functioning at t0 (SAQ).
Quality Adjusted Life Years at t0 via VR-6D (SAQ).
Body mass index at t0 (SAQ).
Joint replacement hip and/or knee joint(s) at t0 (SAQ).
General Self-Efficacy scale GSE at t0 (SAQ)
Habitual physical and sports activity status at t0
Participation in health activity programs (i.e. walking courses) at t0
If there are any cost/disability day categories, in which the standard mean differences after matching are above 0.05, a new matching will be conducted with these cost/disability day categories as matching variables in addition. No follow-up data will be analyzed prior to the final definition of the statistical twin of the CO for a given IG patient.
The finally defined controls and cases will be included into a linear model with the propensity score as covariate. Additionally, we will use models for longitudinal data using each measurement point (instead of only t0 and t3 for the primary analysis). The level of significance will be 0.025 for primary endpoints. Secondary endpoints will be analyzed analogously without claiming confirmatory interpretation of p-values.
Descriptive analysis will include absolute and percentage frequencies for categorical variables, means, medians, standard deviations, quartiles and ranges for quantitative variables and medians, quartiles and ranges for ordinal variables. For the main results, two-sided 95 % confidence limits will be given additionally to significance tests. For percentages exact confidence interval for proportions based on the binomial distribution will be given.
Exploratory, prognostic factors will be analyzed using multiple regression models (linear regression) to identify potential responders to the training.
Correlation analyses will use Pearson product moment correlation coefficient (normally distributed variables) and Spearman correlation (non-normally distributed variables).
The primary analysis will be done on the full set of patients recruited in consideration of the exclusion criteria “limited pain and impairment in physical function” (WOMAC Index subscales pain and physical function with values below 15) except for patients refusing consent during the study and obviously wrongly diagnosed patients. A secondary analysis will be done using the full set of eligible and included patients irrespective of their limitations in pain and physical function at baseline (t0). This secondary analysis will only claim confirmatory interpretation of p-values if the primary analysis is able to detect group differences in a statistical manner. Otherwise the secondary analysis will be done in an explorative manner.
Missing values will be imputed using multiple imputation approaches, complete case and last observation forward analysis will be performed as a sensitivity analysis. No interim analysis, except for administrative purposes will be performed. All statistical analysis will be done using the software SPSS and R in the newest release.
Economic evaluation
Cost-efficiency of the intervention will be quantified on the expanded perspective of the payer. If a dominant strategy doesn’t exist (i.e.: lower costs and higher health related effects in the intervention group), the costs will be related to the effects. The costs (including the supplementary costs of the pilot offer for participants) will be related to the differences between the groups (Double-Difference Method, two years pre and two years post intervention) in Quality Adjusted Life Years (QALYs, equation 1) and health related effects (WOMAC Index: equation 2). The costs of the intervention will further be related to the differences in costs (Double-Difference Method of the health care costs (unspecific health care costs (overall costs), specific health care costs and the costs for days of disability (human capital approach): equation 3). The time to surgery will be estimated using the Kaplan-Meier method. For the economic evaluation all participants have to be members of the insurance the period from 24 months before baseline (tm24) up to 24 month after baseline (t24).
Equation 1: Cost-Utility Analysis (CUA) = Incremental Cost Utility Ratio (ICUR)
Equation 2: Cost-Effectiveness Analysis (CEA) = Incremental Cost-Effectiveness Ratio
Equation 3: Cost-Benefit Analysis (CBA) = Incremental Cost-Benefit-Ratio
Timelines
Ethical approval was obtained in August 2015. Training of exercise instructors was undertaken in August and September 2014 and July 2015. Recruitment of participants for the study started in September 2015 for the first intervention period (first patient in) and will be continued until March 2016 (last patient in). All participants are expected to have completed the intervention period in July 2016. All participants are expected to have completed the study by February 2018 (last patient out).
Expert report
The study will be assessed by an independent organization (XCENDA, http://www.xcenda.de/index.php/willkommen.html).
Discussion
Despite the strong evidence for the short-term effects of physical exercises on symptoms in knee and hip OA [8, 15, 16], many open questions remain with respect to (I) the incorporation of interventions into patient care in a regular health care setting, (II) long-term effects of the intervention as well as its impact on disease progression, (III) dose–response-relationship according to FITT principles (frequency, intensity, type and time of training) and (IV) knowledge on variables that act as confounders for treatment response. Open questions are also related to (V) the economic efficiency of exercise interventions in the treatment of hip and knee OA and to (VI) safety aspects of the intervention [8, 11, 15].
This study proceeds in a real health care setting and has the potential to answer questions in the aforementioned research areas.
(I) This study allows us to determine whether the efficacy of an exercise intervention proofed in a randomized controlled trial can be transferred into effectiveness into a real life situation with less standardized and controlled conditions. The intervention described in this study protocol has been derived from a previously tested exercise intervention in patients with hip OA [17, 20]. Yet some adaptations to the intervention had to be made to give consideration to the needs and organizational framework of the health care provider: the formerly 12-week intervention with one supervised session/week and two additional home-exercise sessions/week was reduced to 8 supervised sessions. Patients are instructed to perform the accompanying home-exercise sessions for at least 11 weeks according to the exercise manual every participant receives [21]. Aside from this change that is related to the frequency and duration of the exercise intervention, the population under consideration has been extended: the RCT was conducted on subjects with hip OA only. As prevalence for knee OA in comparison to hip OA is even higher, the program will be offered to patients with hip and/or knee OA. Subjects with knee OA will therefore substitute some of the hip-exercises with knee-specific exercises. On this note, subjects with knee OA receive an amendment for the exercise manual with knee-specific exercises. Results of this study are therefore very valuable as they allow us to verify whether an “evidence-based exercise intervention” can successfully be implemented into everyday routine or not.
(II) Our follow-up data will be gathered up to 2 years after the initiation of the supervised exercise intervention and will therefore give information on clinical and economic outcomes in the long-term.
(III) The intervention program of this study is predefined and we do not differentiate between different dosages. Nonetheless data on adherence to exercise can be related to effect size and may allow an interpretation on continuity of an exercise intervention in relation to response, whereby confounders for exercise adherence such as pain or dissatisfaction with the intervention etc. have to be considered as well. Another aspect that is related to exercise dosage is the possibility to compare results of this study to other interventions with different exercise frequencies, intensities, times and types of intervention (FITT). This comparison may therefore contribute to the knowledge gained with respect to dose–response relationships of exercise interventions in OA.
(IV) Aside from the comparison of different interventions in general, an individual perspective may gather new knowledge in terms of personal contextual factors that are related to intervention outcome. Identification of possible predictors of treatment responsiveness is therefore another important issue of this research. For example data of previous studies report a gender-specific response to exercise [34, 35]. Additional confounders may be related to baseline pain level, self-efficacy, socio-demographic data etc. In the context of this study, a responder analysis will be conducted according to the OMERACT-OARSI Set of responder criteria [25]. A sub-analysis comparing personal contextual factors of responders versus non-responders may allow a better understanding of relevant prerequisites for successful exercise participation and therefore facilitate individualized treatment recommendations in OA.
(V) Considering published reports on cost-effectiveness of non-pharmacologic, non-surgical interventions for hip and/or knee OA, Pinto and colleagues concluded in their review in 2012 that there is only limited evidence for the cost-effectiveness of conservative treatments for hip and knee OA. In this context they highlighted the need for more high-quality economic evaluations [36]. Since then studies have been published that indicate no cost-saving but cost-effectiveness of exercise interventions in knee or hip OA [37, 38]. In addition, an increasing number of study protocols include economic outcomes and underline the need for the evaluation of economic outcomes aside from clinical measures [39–41]. The aforementioned studies as well as the trial of this protocol will allow a better understanding of cost-utility measures in consideration of different exercise programs and populations under study.
(VI) Although non-pharmaceutical trials are not obligated by law to report on adverse events, safety should be evaluated in clinical trials. Knowledge on adverse events may allow a more precise determination of contra-indications for a given exercise setting and/or population group. Report of adverse events is also important as exercise interventions can initially result in a slight increase in pain [42]. Knowledge on potential short-term side-effects in the primary phase of the intervention period is important as it allows health care providers to inform patients about potential harms with no significant lasting effect on health outcomes. Another important safety aspect is related to frequency and severity of adverse events according to the mode of delivery: Reports on adverse events related to exercises may differ between group sessions and home-based exercises. Safety in the home setting may be hampered as exercises are not controlled by a health care professional and the environment may be less appropriate for the execution of exercises (i.e. tripping hazards are more likely in a home-based setting). Information on adverse events is requested in the self-administered questionnaires at the end of the intervention period. This study may therefore gain knowledge on safety aspects and subsequently allow recommendations to improve safety of supervised as well as independently undertaken exercises. However it has to be mentioned that a reporting bias-especially in the context of home-based exercises - cannot be ruled out, as no immediate adverse event reporting is implemented in this study.
There are further limitations to our study which are—from our perspective—mainly related to concomitant health care interventions, limited long—term follow—up data and the statistical power in terms of economic evaluations.
Referring to the first point it has to be mentioned that all participants of the study are allowed to take part in additional health care offers. This holds true for participants of the control group as well. They may also attend the hip and knee training under study within the follow-up phase. This cannot be prohibited as all insurance holders have the same rights to participate in health care services offered. The exclusive participation in the assigned intervention (supervised exercise versus matched pair control group) can therefore only be guaranteed for the first 3 month of the study. This timeframe is also relevant for the evaluation of the primary outcomes. To control for potential confounders related to additional health care interventions, all follow-up questionnaires ask for participation in any course or training offer.
Although follow-up data for two years will be gathered in the context of this trial, statements on the effect of the intervention in the long run such as time to surgery and disease specific health care costs may be hampered. If 24-months evaluations are positive in terms of study outcomes, a protocol amendment for a 5-year follow up may be considered.
This study has 80 % power for unfavorably high cluster effects, in realty probably more than 90 % power to detect clinical relevant changes in the primary outcomes of the trial. Aside from clinical outcomes, cost-utility measures are of special interest in this study. Yet economic outcomes are expected to have much smaller effect sizes. We therefore decided to include all eligible patients, willing to participate in the trial, to increase power for the economic evaluation.
In due consideration of the burden of OA from an individual as well as economic perspective, the importance to evaluate the effectiveness and cost-efficiency of conservative treatment strategies is obvious. This applies in particular for lifestyle interventions such as physical exercises as they can be carried out by the patient himself and are therefore of special importance in the treatment of chronic diseases such as OA. Results of this trial will document accurately the effects of exercises on clinical and economic outcomes as well as safety aspects in a health care setting on the basis of a large sample size. As such, results of this trial should provide externally valid exercise recommendations in hip or knee osteoarthritis.
Data privacy, ethics approval and consent to participate
Data privacy is warranted by including different parties to guarantee pseudonymization of patient data derived from different data sources. Each party has a predefined right of access to personal and study data of the participants. Only one independent party has access to personal insurance data as well as study outcomes. All other parties only have access to personal data or study data. Data privacy was approved by the data protection officer of the insurance company.
All participants of the exercise program receive a postal mailing prior to the first training session. This mailing includes information on the aims and the content of the study as well as on data privacy: in case of study participation, consolidation, analysis and utilization of data is carried out with random names only. The mailing further includes the informed consent form and the outcome questionnaire. The selected subjects from the insurance data base for the control group receive a similar mailing. Interested persons of the intervention as well as control group are informed that they confirm their informed consent by returning the informed consent form for data privacy and the pseudomyzed outcome questionnaires by post. Patients are informed about the voluntariness of study participation at all times. Participants of the exercise program are further informed that study participation is not a requirement for participation in this health care offer.
Ethical approval has been obtained from the Ethics Committee of the University of Tuebingen (Vote number 421/2015BO1).
Study data are erased after the legal retention period according to privacy policy apart from data that were already analyzed and recorded as study report and/or publication.
Acknowledgements
We want to thank Marco Giurgiu for the preparation of the ready-to-scan case report forms.
Funding
This trial is funded by the health insurance company AOK Baden-Wuerttemberg.
Abbreviations
- BMI
Body Mass Index
- CBA
Cost-Benefit Analysis
- CEA
Cost-Effectiveness Analysis
- CEA
Incremental Cost-Effectiveness Ratio
- CO
Control group
- CUA
Cost-Utility Analysis
- FITT
Frequency, Intensity, Time and Type (of exercise interventions)
- GSE
General Self-Efficacy scale
- HKT
Group-based hip and knee training in combination with a structured home-exercise program
- ICUR
Incremental Cost Utility Ratio
- IDB
Insurance data base
- IG
Intervention group
- MM
Exercises for motor learning/mobilization
- OA
Osteoarthritis
- PC
Exercises to improve postural control
- PSM
Propensity score matching
- QALY
Quality adjusted life years
- S
Exercises for strength training
- SAQ
Self-administerd questionnaire
- VR-12
8-scale questionnaire to measure health related quality of life
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
Additional files
Description hip training. (PDF 97 kb)
Description knee training. (PDF 96 kb)
Excerpt of the German-language exercise manual. (PDF 2202 kb)
Footnotes
Competing interests
The non-profit health insurance company AOK Baden Wuerttemberg is involved in the trial set-up, data acquisition, conduction of the intervention and data analysis. IK, GH, BS and PJ get royalties for the exercise book that every patient of the intervention group receives. The Department of Sports Medicine of the Medical Clinic of the University Hospital Tuebingen offers a fee required 2-days education program for exercise instructors for hip training programs. The education program refers to a similar exercise intervention as outlined in this study (“Das Tübinger Hüftkonzept”).
Authors’ contributions
IK, GM, BS, PM and GH conceived and designed the trial protocol. GH, BS, IK, PJ and NJ developed and evaluated the exercise intervention. PM reviewed and complemented the statistical design and statistical analysis. IK and GM initiated the research project. IK and NJ drafted the manuscript. All authors read and revised the manuscript critically for important intellectual content. All authors approved the final manuscript.
References
- 1.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Prevalence and burden of osteoarthritis: results from a population survey in Norway24. J Rheumatol. 2008;35(4):677–684. [PubMed] [Google Scholar]
- 2.Rabenberg M. Arthrose. Berlin: Robert Koch-Institut; 2013. [Google Scholar]
- 3.Symmons D, Mathers C, Pfleger B. Global Burden of Osteoarthritis in the Year 2000. http://www.who.int/healthinfo/statistics/bod_osteoarthritis.pdf. Accessed date 28 Apr 2016.
- 4.Conaghan PG, Kloppenburg M, Schett G, Bijlsma JW. Osteoarthritis research priorities: a report from a EULAR ad hoc expert committee. Ann Rheum Dis. 2014;73(8):1442–1445. doi: 10.1136/annrheumdis-2013-204660. [DOI] [PubMed] [Google Scholar]
- 5.Sharif B, Kopec J, Bansback N, Rahman MM, Flanagan WM, Wong H, Fines P, Anis A. Projecting the direct cost burden of osteoarthritis in Canada using a microsimulation model. Osteoarthritis Cartilage. 2015;23:1654–1663. doi: 10.1016/j.joca.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 6.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Roddy E, Zhang W, Doherty M, Arden NK, Barlow J, Birrell F, Carr A, Chakravarty K, Dickson J, Hay E, Hosie G, Hurley M, Jordan KM, McCarthy C, McMurdo M, Mockett S, O'Reilly S, Peat G, Pendleton A, Richards S. Evidence-based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee—the MOVE consensus. Rheumatology (Oxford) 2005;44(1):67–73. doi: 10.1093/rheumatology/keh399. [DOI] [PubMed] [Google Scholar]
- 8.Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;4 doi: 10.1002/14651858.CD007912.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennell KL, Dobson F, Hinman RS. Exercise in osteoarthritis: moving from prescription to adherence. Best Pract Res Clin Rheumatol. 2014;28(1):93–117. doi: 10.1016/j.berh.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis (OA) of the Knee. http://www.aaos.org/Research/guidelines/GuidelineOAKnee.asp. Accessed date 28 Apr 2016.
- 11.Fernandes L, Hagen KB, Bijlsma JW, Andreassen O, Christensen P, Conaghan PG, Doherty M, Geenen R, Hammond A, Kjeken I, Lohmander LS, Lund H, Mallen CD, Nava T, Oliver S, Pavelka K, Pitsillidou I, da Silva JA, de la TJ, Zanoli G, Vliet Vlieland TP. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125–1135. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken ) 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Conaghan PG, Dickson J, Grant RL. Care and management of osteoarthritis in adults: summary of NICE guidance. BMJ. 2008;336(7642):502–503. doi: 10.1136/bmj.39490.608009.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2009;4 doi: 10.1002/14651858.CD004376.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman R, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18(2):476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Krauss I, Steinhilber B, Haupt G, Miller R, Martus P, Janssen P. Exercise therapy in hip osteoarthritis—a randomized controlled trial. Dtsch Arztebl Int. 2014;111(35/36):592–599. doi: 10.3238/arztebl.2014.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller G. Bericht zur Evaluationsstudie AOK-Rückenkonzept. 2015. Unpublished work.
- 19.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krauss I, Steinhilber B, Haupt G, Miller R, Grau S, Janssen P. Efficacy of conservative treatment regimes for hip osteoarthritis—evaluation of the therapeutic exercise regime "Hip School": a protocol for a randomised, controlled trial. BMC Musculoskelet Disord. 2011;12:270. doi: 10.1186/1471-2474-12-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haupt G, Janßen P, Krauß I, Steinhilber B. Das Tübinger Hüftkonzept. 1. Auflage ed. Essen: Verlag hellblau; 2014.
- 22.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Froböse I, Fiehn R. Muskeltraining (Fünf-Stufen-Modell) In: Froböse I, Nellessen G, Wilke C, editors. Training in der Therapie. 2. München, Jena: Urban & Fischer; 2003. pp. 57–79. [Google Scholar]
- 24.Bellamy N. The WOMAC Knee and Hip Osteoarthritis Indices: development, validation, globalization and influence on the development of the AUSCAN Hand Osteoarthritis Indices. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S148–S153. [PubMed] [Google Scholar]
- 25.Pham T, van der Heijde D, Lassere M, Altman RD, Anderson JJ, Bellamy N, Hochberg M, Simon L, Strand V, Woodworth T, Dougados M. Outcome variables for osteoarthritis clinical trials: The OMERACT-OARSI set of responder criteria. J Rheumatol. 2003;30(7):1648–1654. [PubMed] [Google Scholar]
- 26.Usman Iqbal S, Rogers W, Selim A, Qian S, Lee A, Xinhua SR, Rothendler J, Miller D, Kazis LE. The Veterans Rand 12 Item Health Survey (Vr-12): What It Is and How It Is Used. http://www.rand.org/health/surveys_tools/mos/mos_core_12item.html. Accessed date 28 Apr 2016.
- 27.Schwarzer R, Jerusalem M. Generalized Self-Efficacy scale. In: Weinman J, Wright S, Johnston M, editors. Measures in health psychology: A user's portfolio. Causal and control beliefs. Windsor: NFER-NELSON; 1995. pp. 35–37. [Google Scholar]
- 28.Robert K-I, editor. Sportliche Aktivität. Faktenblatt zu GEDA 2012: Ergebnisse der Studie »Gesundheit in Deutschland aktuell 2012. Berlin: Robert Koch Institut; 2014. [Google Scholar]
- 29.Robert K-I, editor. Körperliche Aktivität. Faktenblatt zu GEDA 2012: Ergebnisse der Studie »Gesundheit in Deutschland aktuell 2012. Berlin: Robert Koch Institut; 2014. [Google Scholar]
- 30.Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann. 1982;5(3):233–237. doi: 10.1016/0149-7189(82)90074-X. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt J, Lamprecht F, Wittmann WW. Satisfaction with inpatient management. Development of a questionnaire and initial validity studies. Psychother Psychosom Med Psychol. 1989;39(7):248–255. [PubMed] [Google Scholar]
- 32.Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118(12):1294–1303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 33.Stuart EA. Matching Methods for Causal Inference: A Review and a Look Forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikesky AE, Mazzuca SA, Brandt KD, Perkins SM, Damush T, Lane KA. Effects of strength training on the incidence and progression of knee osteoarthritis. Arthritis Rheum. 2006;55(5):690–699. doi: 10.1002/art.22245. [DOI] [PubMed] [Google Scholar]
- 35.Steinhilber B, Haupt G, Miller R, Janssen P, Krauss I. Home-based strengthening exercise therapy in male and female patients with hip osteoarthritis: effects on hip muscle strength and it's relation to physical disability and pain - results a randomized, controlled trial. 2015. Unpublished Work.
- 36.Pinto D, Robertson MC, Hansen P, Abbott JH. Cost-effectiveness of nonpharmacologic, nonsurgical interventions for hip and/or knee osteoarthritis: systematic review. Value Health. 2012;15(1):1–12. doi: 10.1016/j.jval.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Marra CA, Grubisic M, Cibere J, Grindrod KA, Woolcott JC, Gastonguay L, Esdaile JM. Cost-utility analysis of a multidisciplinary strategy to manage osteoarthritis of the knee: economic evaluation of a cluster randomized controlled trial study. Arthritis Care Res (Hoboken ) 2014;66(6):810–816. doi: 10.1002/acr.22232. [DOI] [PubMed] [Google Scholar]
- 38.Pinto D, Robertson MC, Abbott JH, Hansen P, Campbell AJ. Manual therapy, exercise therapy, or both, in addition to usual care, for osteoarthritis of the hip or knee. 2: economic evaluation alongside a randomized controlled trial. Osteoarthritis Cartilage. 2013;21(10):1504–1513. doi: 10.1016/j.joca.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Bennell KL, Rini C, Keefe F, French S, Nelligan R, Kasza J, Forbes A, Dobson F, Haxby AJ, Dalwood A, Vicenzino B, Harris A, Hinman RS. Effects of Adding an Internet-Based Pain Coping Skills Training Protocol to a Standardized Education and Exercise Program for People With Persistent Hip Pain (HOPE Trial): Randomized Controlled Trial Protocol. Phys Ther. 2015;95(10):1408–422. doi: 10.2522/ptj.20150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Iversen MD, McAlindon T, Harvey WF, Wong JB, Fielding RA, Driban JB, Price LL, Rones R, Gamache T, Schmid CH. Assessing the comparative effectiveness of Tai Chi versus physical therapy for knee osteoarthritis: design and rationale for a randomized trial. BMC Complement Altern Med. 2014;14:333. doi: 10.1186/1472-6882-14-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foster NE, Healey EL, Holden MA, Nicholls E, Whitehurst DG, Jowett S, Jinks C, Roddy E, Hay EM. A multicentre, pragmatic, parallel group, randomised controlled trial to compare the clinical and cost-effectiveness of three physiotherapy-led exercise interventions for knee osteoarthritis in older adults: the BEEP trial protocol (ISRCTN: 93634563) BMC Musculoskelet Disord. 2014;15:254. doi: 10.1186/1471-2474-15-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes L, Storheim K, Nordsletten L, Risberg MA. Development of a therapeutic exercise program for patients with osteoarthritis of the hip. Phys Ther. 2010;90(4):592–601. doi: 10.2522/ptj.20090083. [DOI] [PubMed] [Google Scholar]


