Abstract
Importance
Ophthalmic artery chemosurgery (OAC) has emerged as a primary treatment for advanced stage retinoblastoma. The incidence of orbital recurrence in eyes treated with OAC has not been described.
Objective
To determine the incidence of orbital recurrence following enucleation or OAC as primary treatments for advanced stage retinoblastoma.
Design
Retrospective chart review.
Setting
Academic ophthalmic oncology practice.
Participants
Single center study of 140 eyes in 135 patients classified as Reese-Ellsworth group V, or International Classification of Retinoblastoma (Children's Oncology Group-COG) group D or E: 63 patients (63 eyes) were primarily treated with enucleation and 72 patients (77 eyes) were primarily treated with OAC.
Main Outcome Measures
Incidence of and time to orbital recurrence, metastasis, and death.
Results
There were 5 orbital recurrences (incidence 7.9%) in the primary enucleation group and 1 orbital recurrence (incidence 1.3%) in the primary OAC group during median follow up times of 42.6 months (range 6.2-97.1) and 38.7 months (range 9.0-104.3) respectively. The 24-month Kaplan Meier (KM) estimate for orbital recurrence free survival was worse for the enucleation group 92.1% (95% C.I. 82.0-96.7) than for the OAC group 100%, Log-Rank p-value = 0.049. The enucleation group had 5 cases of metastatic disease and 2 deaths representing 7.9% and 3.2% of patients respectively. In the OAC group, there were 3 (4.2%) cases of metastatic disease and no deaths. KM analysis of metastasis free survival and overall survival yielded no differences between the two treatment groups. Analysis of a number of features of the two groups revealed more eyes with iris neovascularization in the enucleation group (25.4%) than in the OAC group (5.2%) and more group E eyes in the enucleation group (87.3%) than in the OAC group (29.9%) though neither of these factors was an independent predictor of orbital relapse in a Cox proportional hazards model.
Conclusions and Relevance
In this single institution retrospective study of advanced intraocular retinoblastoma, there were more orbital recurrences in the primarily enucleated group. This study could not identify that OAC for advanced intraocular retinoblastoma increases the chance of orbital recurrence, metastatic disease, or death compared to primary enucleation.
Introduction
Over the past several years, ophthalmic artery chemosurgery (OAC) has emerged as a safe and effective treatment for advanced retinoblastoma. A recent survey revealed that 74% of retinoblastoma treatment centers worldwide are using OAC as first line therapy for advanced unilateral disease.1 Eyes treated initially with OAC have been shown to have ocular event-free survival rates at 2 years of greater than 80% despite their advanced stage. 2 These findings have been replicated at multiple centers, demonstrating that OAC as a primary therapy,3, 4, 5 or with intravenous chemotherapy as a bridge,6 may achieve globe salvage and tumor control in a high percentage of advanced group D and E eyes. Further, OAC has been shown to be a useful in the treatment of vitreous seeds7 and to prevent the development of new intraocular tumors, suggesting that it may affect ophthalmoscopically undetectable tumors present at initial diagnosis.8
While the multiple benefits and favorable risk profile of OAC have been documented in the literature, the incidence of orbital recurrence in patients treated with this modality has not been previously described. Several prior studies have documented rates of orbital recurrence after primary enucleation ranging from 5%9 to 7.6%,10 though these series included eyes that presented with extraocular disease. The one study that was limited to patients who presented with intraocular disease only revealed an incidence of 4.2% over the course of almost 100 years and ranged between 3.7% and 5.1% when calculated using twenty year periods.11
Since orbital involvement carries a poor prognosis and is commonly associated with metastatic disease both outside and within the central nervous system,12, 13, 14 the characterization of orbital recurrence in the context of OAC is of significant interest. Eighty-five percent of patients with orbital recurrence have been reported to develop metastatic disease and 75% to eventually die, although mortality rates have decreased in the modern era.11 The purpose of this study was to reexamine orbital recurrence in advanced retinoblastoma after treatment with OAC and to compare these rates to eyes treated with enucleation during the same period in the same institution.
Methods
Data Collection
This is a retrospective chart review of patients primarily treated at Memorial Sloan-Kettering Cancer Center (MSKCC) who presented between February 2006 and March 2014 with advanced intraocular retinoblastoma that fulfilled criteria for Reese-Ellsworth group V or International Classification of Retinoblastoma (Children's Oncology Group-COG) group D or E. Reese-Ellsworth group V eyes are defined as having massive tumor involving over 50 percent of the retina and vitreous seeding. International Classification of Retinoblastoma COG group D eyes are defined as having diffuse disease with significant vitreous or subretinal seeding, with or without subretinal fluid or up to total retinal detachment. In contrast, group E eyes have one or more poor prognostic features including, tumor touching the lens, tumor anterior to the anterior vitreous face or involving the ciliary body or anterior segment, diffuse infiltrating retinoblastoma, neovascular glaucoma, opaque media from hemorrhage, tumor necrosis with aseptic orbital cellulitis, or phthisis bulbi.
Patients were treated initially with either enucleation at MSKCC or OAC at New York-Presbyterian Hospital (Weill Cornell Medical College) and then followed at MSKCC. The start date of this series represents the advent of OAC at our institutions. Clinical characteristics including sex, age at presentation, laterality of disease, treatment history, the presence of iris neovascularization on clinical examination at initial presentation, and post-enucleation pathology were collected via the electronic medical record.
Orbital recurrence was initially assessed by clinical examination and magnetic resonance imaging (MRI). When there was suspicion for orbital disease, cases were confirmed with biopsy and histopathology. Metastatic disease was assessed by computed tomography (CT), bone scan, bone marrow biopsy/aspirate, and MRI. Eyes that were treated primarily with OAC but eventually enucleated were considered in the OAC and not the enucleation group. Eyes that had received systemic, intraarterial, or intravitreal chemotherapy or external beam or plaque radiotherapy prior to enucleation were excluded from the analysis. Other exclusion criteria were follow-up time less than 6 months, orbital or extraocular retinoblastoma at initial presentation, or trilateral retinoblastoma. The study was approved by the MSKCC institutional review board.
Statistical Analysis
SPSS version 21 (IBM Corporation, Armonk, New York, USA) and Prism (GraphPad Software, La Jolla, California, USA) were used to construct Kaplan Meier (KM) curves of orbital recurrence free survival, metastasis free survival, and overall survival. KM curves were then compared using a Log-Rank test. A multivariate Cox-regression proportional hazards model was used to assess for associations between treatment (OAC versus enucleation), presence of iris neovascularization, and ICRB group E classification and the outcome measure of orbital recurrence free survival using Wald stepwise backward elimination.
Results
Baseline Characteristics
Clinical characteristics of each group are depicted in Table 1. There were 72 patients (77 eyes) in the OAC group and 63 patients (63 eyes) in the enucleation group. There were more eyes from patients with bilateral retinoblastoma in the OAC group (36.4%) than the enucleation group (11.1%). The mean age at diagnosis was 19.3 months in the OAC group compared to 23.2 months in the enucleation group. The mean age at first treatment was 20.7 months in the OAC group versus 25.2 months in the enucleation group. Only 5.2% of eyes in the OAC group had iris neovascularization on clinical examination at initial presentation, while 25.4% of the eyes in the enucleation group had iris neovascularization. There were more group E eyes in the enucleationgroup (87.3%) than the OAC group (29.9%).
Table 1. Clinical Characteristics of Naïve Eyes with Advanced Stage Retinoblastoma Treated with Ophthalmic Artery Chemosurgery or Enucleation.
| Ophthalmic Artery Chemosurgery | Enucleation | |
|---|---|---|
| Patients | 72 | 63 |
| Boys | 33 (46%) | 28 (44%) |
| Girls | 38 (53%) | 35 (56%) |
| Eyes | 77 | 63 |
| Unilateral | 49 (64%) | 56 (89%) |
| Bilateral | 28 (36%) | 7 (11%) |
| Mean Length of Follow Up (months) | 44.9 | 44.4 |
| Mean Age of Onset (months) | 19.3 | 23.2 |
| Eyes with Iris Neovascularization at First Physical Exam | 4 (5%) | 16 (25%) |
| Mean Age at Initial Treatment (months) | 20.7 | 25.2 |
| Eyes with Orbital Disease | 1 (1%) | 5 (8%) |
| Mean Time to Orbital Disease from Initial Treatment (months) | 24.2 | 4.1 |
| Patients with Metastatic Disease | 3 (4%) | 5 (8%) |
| Mean Time to Metastatic Disease from Initial Treatment (months) | 25.5 | 4.6 |
| Deaths | 0 (0%) | 2 (3%) |
| Mean Time to Death from Initial Treatment (months) | - | 30.2 |
| Enucleated eyes with Pathology Available | 10 (100%) | 63 (100%) |
| Optic Nerve Invasion | 2 (20%) | 21 (33%) |
| Negative Optic Nerve Margin | 10 (100%) | 63 (100%) |
| Optic Nerve Invasion Past the Lamina Cribosa | 1 (10%) | 11 (17%) |
| Ciliary Body Invasion | 2 (20%) | 3 (5%) |
| Choroidal Invasion | 1 (10%) | 10 (16%) |
| Massive Choroidal Invasion | 1 (10%) | 0 (0%) |
| Scleral Invasion | 0 (0%) | 0 (0%) |
| Neovascular Glaucoma | 4 (40%) | 19 (30%) |
| Reese-Ellsworth Group | ||
| 5A | 23 (30%) | 5 (8%) |
| 5B | 54 (70%) | 58 (92%) |
| ICRB (COG) Group | ||
| C | 2 (2%) | 0 (0%) |
| D | 52 (68%) | 8 (13%) |
| E | 23 (30%) | 55 (87%) |
Eighty-four percent of eyes treated primarily with OAC received additional subsequent treatments including thermal laser (58%), cryotherapy (44%), external beam radiotherapy (1%), plaque radiotherapy (16%), retinal surgery (4%), periocular chemotherapy (4%), sub-tenons Kenalog (3%), intravitreal chemotherapy (9%), and intravenous chemotherapy (6%). Thirteen percent of eyes treated with OAC were eventually enucleated. In the enucleation group, 11% of eyes received additional subsequent therapy including external beam radiotherapy (2%) and intravenous chemotherapy (10%).
All eyes for which pathology was available in the OAC and enucleation group had negative optic nerve margins. Overall, 1/10 (10%) enucleated eyes in the OAC group and 11/63 (17.5%) of eyes in the primary enucleation group had higher-risk features as defined by optic nerve invasion past the lamina cribosa, massive choroidal invasion, or scleral invasion.
Orbital Recurrences
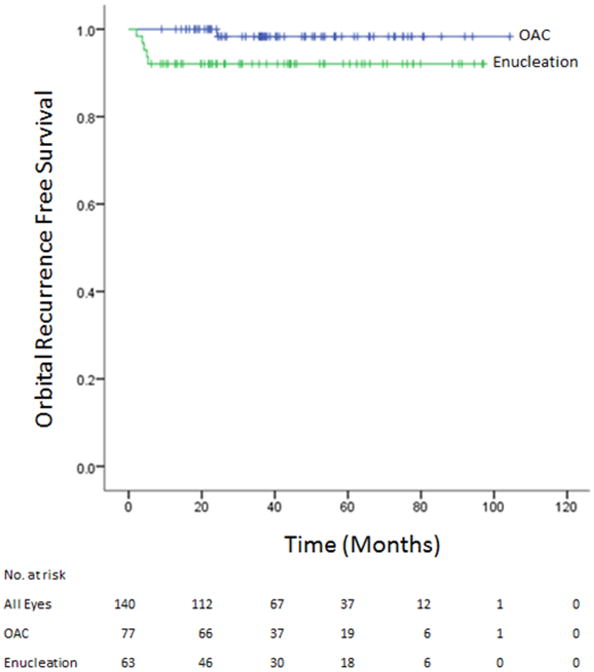
There was 1 orbital recurrence (1.3%) in the primary OAC group and 5 orbital recurrences (7.9%) in the primary enucleation group during median follow up times of 38.7 months (range 9.0-104.3) and 42.6 months (range 6.2-97.1) respectively. KM analysis of orbital free survival is depicted in Figures 1. The 24-month Kaplan Meier (KM) estimate for orbital recurrence free survival was worse for the enucleation group 92.1% (95% C.I. 82.0-96.7) than for the OAC group 100%, Log-Rank p-value = 0.049.
Figure 1.
Kaplan Meier analysis of orbital recurrence free survival of eyes treated with either OAC or enucleation. The Log-Rank p-value was 0.049.
The lone orbital recurrence in the OAC group occurred in a boy who presented at 88.5 months with group 5/D unilateral retinoblastoma. The eye was normotensive on initial presentation without evidence of iris neovascularization. The patient was first treated with three cycles of OAC with melphalan and later received laser, cryotherapy, intravenous chemotherapy, and eventually enucleation where pathology was notable for ciliary body invasion and neovascular glaucoma. Orbital recurrence occurred at 24.2 months after initial treatment with OAC, and a subsequent workup revealed metastatic disease. This patient was later treated for his metastatic disease and is still alive without signs of metastases, 84.2 months after initial presentation.
In the enucleation group, the 5 cases of orbital recurrence occurred in 3 boys and 2 girls. All cases were group 5/E at presentation and were unilateral. Only 1 of the 5 patients in this group had iris neovascularization on initial clinical examination or subsequently on pathology. The mean age at enucleation was 24.0 months, and 1 patient eventually received external beam radiotherapy to the orbit while all 5 eventually received systemic chemotherapy. Pathology revealed that 2 of the 5 eyes had higher-risk features after enucleation, and each of these patients was offered intravenous chemotherapy. One of these patients received chemotherapy directly after enucleation, but the other family elected for observation and this patient was not treated with chemotherapy until he developed orbital disease. Mean time to orbital recurrence was 4.1 months. Of the 5 patients with an orbital recurrence, 1 never developed disease outside the orbit and 4 were found to have metastatic disease outside the orbit on subsequent workup directly after the orbital recurrence. One of the patients with metastatic disease eventually died 38.6 months after initial treatment.
Metastatic Disease and Death
In the OAC group, there were 3 (4.2%) cases of metastatic disease and no deaths. The enucleation group had 5 cases of metastatic disease and 2 deaths representing 7.9% and 3.2% of patients respectively. The 24-month KM estimate of metastasis free survival was 92.0% (95% C.I. 81.9-96.6) for the enucleation group and 98.6% for the OAC group (95% C.I. 90.3-99.8), Log-Rank p-value = 0.32. With respect to overall survival, the 24-month KM estimate was 97.9% (95% C.I. 85.9-99.7) for the enucleation group and 100% for the OAC group, Log-Rank p-value = 0.13.
Cox Multivariate Regression Analysis
A Cox proportional hazards model was used to assess for associations between three covariates (treatment with OAC versus enucleation, presence of iris neovascularization, and ICRB group E classification) and the main outcome measure of orbital recurrence. Using a Wald stepwise backward elimination method, both iris neovascularization and group E classification were eliminated from the model. Only treatment with OAC remained as an independent predictor of orbital recurrence with a hazard ratio of 0.153 (95% CI, 0.018-1.314; p-value = 0.087).
Discussion
In this single institution retrospective study of advanced intraocular retinoblastoma there were more orbital recurrences in the primarily enucleated group (7.9%) than in the OAC treated group (1.3%). We also detected more metastases in the enucleation group (7.9% versus 4.2%) and deaths only in the primarily enucleated group. Eyes treated with OAC were associated with a lower rate of orbital recurrence in the KM univariate analysis (p-value 0.049). In our Cox proportional hazards model, treatment with OAC remained in the model as an independent predictor of orbital recurrence, but the p-value was 0.087, likely because there was insufficient statistical power to show a meaningful difference.
When combining both the primary OAC and primary enucleated cohorts, the total rate of recurrence in this series was 4.3% which aligns with the incidence of orbital disease (4.2%) in a previously reported larger cohort from our institution.11 However, it is notable that these historical estimates were based on enucleated eyes of multiple stages, not just those that were classified as RE stage V, or ICRB stage D, or E. Since prior estimates of orbital disease might be diluted by a lower rate of orbital recurrence within eyes that were not as advanced as the cohort in this study, our overall recurrence rate of 4.2% may in fact represent a decrease in the total number of orbital recurrences in advanced eyes driven by a lower incidence within the OAC group.
The main difference between the patients receiving OAC and those receiving enucleation in this series is the presence of iris neovascularization and the distribution of group E eyes, which were both higher in the enucleation group. Iris neovascularization15,16 has previously been found to be a poor prognostic feature in advanced retinoblastoma. Further, it has been associated with higher-risk histopathology and metastatic disease.17, 18
However, within our entire cohort, we did not identify an association between iris neovascularization or ICRB group E classification and orbital recurrence survival in our Cox proportional hazards model. This is driven by the fact that only 1 of the total 6 patients in the entire cohort with an orbital recurrence had iris neovascularization. Therefore the difference in incidence of orbital recurrence cannot be explained by the selection bias (of iris neovascularization or ICRB group classification) within the enucleation group.
The question of whether OAC as a primary treatment for advanced intraocular disease increases the chance of metastatic disease has been raised in the literature.19, 20 However, our study shows that there is no increased risk of metastatic disease or death in patients treated with OAC. In fact, in the OAC group, there were fewer cases of metastatic disease and no metastatic deaths though the study was underpowered to demonstrate statistical differences.
Orbital disease, in much of the world, represents direct extension of disease through the sclera into the orbit. In the United States and other more developed countries, however, it is a marker for metastatic disease. Eighty percent of patients in the United States who present with orbital disease are found to have concurrent widespread metastases,21 and the orbital disease can be considered a surrogate of metastatic disease. Therefore, it is plausible that orbital disease, as well as metastatic disease, is present microscopically at the time of treatment with enucleation or first OAC. A possible mechanism by which OAC reduces the incidence of orbital disease but not of metastases is that OAC, with its high ocular concentration of drug, effectively obliterates microscopic orbital foci, but because the systemic dose is so low, it has no impact on distant metastatic disease.
A limitation of our study is its retrospective design. Our center typically recommends primary enucleation for eyes with poor prognostic features such as iris neovascularization, neovascular glaucoma, or phthisis bulbi. This explains the larger percentage of advanced group E eyes and iris neovascularization in the enucleated group. However, these differences were addressed in our regression analysis.
In conclusion, this study demonstrates that treatment naïve eyes with advanced retinoblastoma treated in our institutions with OAC had fewer orbital recurrences and that no differences in metastatic disease could be identified compared with enucleation. Additional studies on orbital and metastatic disease in eyes treated with OAC could help to support or refute the findings provided in this report.
Acknowledgments
Dr. Nicolas Yannuzzi and Dr. David Abramson had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The manuscript represents valid work and neither this manuscript nor one with substantially similar content under their authorship has been published or is being considered for publication elsewhere.
Financial Support: The research presented here is supported in part by The Fund for Ophthalmic Knowledge, Inc. The source of our funding played no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Design and conduct of the study (NAY, JHF, DHA), collection (NAY), management, analysis, and interpretation of the data (NAY, JHF, DHA), preparation, review and approval of the manuscript (NAY, JHF, BPM, IB, IJD, YPG, DHA), decision to submit the manuscript for publication (DHA).
Disclosure of Potential Conflicts of Interest: NAY: None
JHF: None
BPM: None
IB: None
IJD: None
YPG: None
DHA: None
References
- 1.Grigorovski N, Lucena E, Mattosinho C, et al. Use of intra-arterial chemotherapy for retinoblastoma: results of a survey. Int J Ophthalmol. 2014 Aug 18;7(4):726–30. doi: 10.3980/j.issn.2222-3959.2014.04.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gobin YP1, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011 Jun;129(6):732–7. doi: 10.1001/archophthalmol.2011.5. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki S, Yamane T, Mohri M, Kaneko A. Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: the long-term prognosis. Ophthalmology. 2011 Oct;118(10):2081–7. doi: 10.1016/j.ophtha.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Shields CL, Manjandavida FP, Lally SE, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014 Jul;121(7):1453–60. doi: 10.1016/j.ophtha.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Peterson EC, Elhammady MS, Quintero-Wolfe S, Murray TG, Aziz-Sultan MA. Selective ophthalmic artery infusion of chemotherapy for advanced intraocular retinoblastoma: initial experience with 17 tumors. J Neurosurg. 2011 Jun;114(6):1603–8. doi: 10.3171/2011.1.JNS10466. [DOI] [PubMed] [Google Scholar]
- 6.Gobin YP, Dunkel IJ, Marr BP, Francis JH, Brodie SE, Abramson DH. Combined, sequential intravenous and intra-arterial chemotherapy (bridge chemotherapy) for young infants with retinoblastoma. PLoS One. 2012;7(9):e44322. doi: 10.1371/journal.pone.0044322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramson DH1, Marr BP, Dunkel IJ, et al. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br J Ophthalmol. 2012 Apr;96(4):499–502. doi: 10.1136/bjophthalmol-2011-300498. [DOI] [PubMed] [Google Scholar]
- 8.Abramson DH, Francis JH, Dunkel IJ, Marr BP, Brodie SE, Gobin YP. Ophthalmic artery chemosurgery for retinoblastoma prevents new intraocular tumors. Ophthalmology. 2013 Mar;120(3):560–5. doi: 10.1016/j.ophtha.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Hungerford J, Kingston J, Plowman N. Orbital recurrence of retinoblastoma. Ophthalmic Paediatr Genet. 1987;8:63–8. doi: 10.3109/13816818709028518. [DOI] [PubMed] [Google Scholar]
- 10.Khelfaoui F, Validire P, Auperin A, et al. Histopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single institution. Cancer. 1996;77:1206–13. [PubMed] [Google Scholar]
- 11.Kim JW, Kathpalia V, Dunkel IJ, Wong RK, Riedel E, Abramson DH. Orbital recurrence of retinoblastoma following enucleation. Br J Ophthalmol. 2009 Apr;93(4):463–7. doi: 10.1136/bjo.2008.138453. [DOI] [PubMed] [Google Scholar]
- 12.Doz F, Khelfaoui F, Mosseri V, et al. The role of chemotherapy in orbital involvement of retinoblastoma. The experience of a single institution with 33 patients. Cancer. 1994 Jul 15;74(2):722–32. doi: 10.1002/1097-0142(19940715)74:2<722::aid-cncr2820740228>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Singh AD, Shields CL, Shields JA. Prognostic factors in retinoblastoma. J Pediatr Ophthalmol Strabismus. 2000;37(3):134–41. doi: 10.3928/0191-3913-20000501-04. [DOI] [PubMed] [Google Scholar]
- 14.Kopelman JE, McLean IW, Rosenberg SH. Multivariate analysis of risk factors for metastasis in retinoblastoma treated by enucleation. Ophthalmology. 1987;94(4):371–77. doi: 10.1016/s0161-6420(87)33436-0. [DOI] [PubMed] [Google Scholar]
- 15.Rubin CM, Robison LL, Cameron JD, et al. Intraocular retinoblastoma group V: an analysis of prognostic factors. J Clin Oncol. 1985 May;3(5):680–5. doi: 10.1200/JCO.1985.3.5.680. [DOI] [PubMed] [Google Scholar]
- 16.Baez KA, Ulbig MW, Cater J, Shields CL, Shields JA. Iris neovascularization, increased intraocular pressure and vitreous hemorrhage as risk factors for invasion of the optic nerve and choroid in children with retinoblastoma. Ophthalmologe. 1994 Dec;91(6):796–800. [PubMed] [Google Scholar]
- 17.Shields CL, Shields JA, Baez KA, Cater J, De Potter PV. Choroidal invasion of retinoblastoma: metastatic potential and clinical risk factors. Br J Ophthalmol. 1993 Sep;77(9):544–8. doi: 10.1136/bjo.77.9.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chantada GL, Gonzalez A, Fandino A, et al. Some clinical findings at presentation can predict higher-risk pathology features in unilateral retinoblastoma. J Pediatr Hematol Oncol. 2009 May;31(5):325–9. doi: 10.1097/MPH.0b013e3181923cc5. [DOI] [PubMed] [Google Scholar]
- 19.Shields CL, Shields JA. Intra-arterial chemotherapy for retinoblastoma: the beginning of a long journey. Clin Experiment Ophthalmol. 2010;38:638–643. doi: 10.1111/j.1442-9071.2010.02297.x. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour P, Chalouhi N, Tjoumakaris S, et al. Pearls and pitfalls of intraarterial chemotherapy for retinoblastoma. J Neurosurg Pediatr. 2012 Sep;10(3):175–81. doi: 10.3171/2012.5.PEDS1277. [DOI] [PubMed] [Google Scholar]
- 21.Ellsworth RM. Orbital retinoblastoma. Trans Am Ophthalmol Soc. 1974;72:79–88. [PMC free article] [PubMed] [Google Scholar]