Abstract
Morphine may alter the permeability of Blood-Brain Barrier (BBB), enhancing the access of molecules normally unable to cross it, as Doxorubicin (Dox). In addition, morphine seems to mediate the uptake of Dox into the brain by its reduced efflux mediated by P-glycoprotein (P-gp). We evaluated the antitumor efficacy of Dox plus morphine treatment by an orthotopic glioblastoma xenograft model. Foxn1 mice were injected with U87MG-luc cells in the left lobe of the brain and treated with Dox (5 mg/kg and 2.5 mg/kg, weekly) with or without morphine pretreatment (10 mg/kg, weekly). Bioluminescence imaging (BLI) was used to monitoring tumor growth and response to therapy. Additionally, we investigated the role of morphine on the uptake of Dox by MDCKII cells transfected with human MDR1 gene encoding for P-gp. The data demonstrate that only Dox 5 mg/kg determined a significant tumor regression while the lower dose (2.5 mg/kg) was not effective. However, if combined with morphine, the group treated with Dox 2.5 mg/kg showed a decreasing tumor growth. The average BLI for Dox 2.5 mg/kg plus morphine was 5 fold lower than Dox 2.5 mg/kg alone (P=0.0053) and 8 fold lower than vehicle (P=0.0004). Additionally, Dox increased in MDCKII-P-gp transfected cells only in the presence of morphine with a significantly higher level comparing control group (3.84) vs Dox plus morphine group (12.29, P<0.05). Our results indicate that Dox alone and in combination with morphine appear to be effective in controlling the growth of glioblastoma in a xenograft mouse model.
Keywords: Glioblastoma, doxorubicin, morphine, blood-brain barrier, P-glycoprotein
Introduction
For over 30 years, anthracyclines, and specially Doxorubicin (Dox), have been used as frontline agents for treating cancer. Unfortunately, Central Nervous System (CNS) tumors seem to be resistant to their use, being these molecules incapable of crossing the Blood-Brain Barrier (BBB).
Clinical trials using new agents for targeted therapy of primary malignant gliomas have often shown no significant results in term of overall survival and progression-free survival [1]. Preclinical studies and some phase I/II clinical trials considering different formulations of Dox, have proved their activity and safety in treatment of brain tumors [2,3].
Intriguingly, Dox, when delivered locally, is an effective monotherapeutic agent against experimental intracranial glioma: it significantly prolongs survival of rodents bearing malignant brain tumors [4]. Moreover, the prolonged exposure to anthracyclines (96-hours) seems to induce a significant apoptosis rate in resistant glioblastoma stem cells [5].
Interestingly, our group has recently demonstrated the therapeutic efficacy of some agents as morphine and ondansetron in faciliting Dox penetration inside the rat brain, without increased acute toxicity [6,7].
Morphine is the most frequently employed analgesic in pain therapy and its use is well documented during antineoplastic agents administration. Conflicting data on the effect of morphine on tumor growth have been published but little is known about its impact on the chemotherapy [8]. In addition, morphine or other psychostimulant drugs are able to alter the neuronal and glial microenvironment, leading to the stroke of the BBB [9-11].
Based on these observations, morphine may act as a “doorkeeper”, increasing the access of molecules normally unable to cross the BBB, as chemotherapy drugs.
BBB also regulates drug uptake into the brain by a broad range of transport proteins. Among these, P-glycoprotein (P-gp) plays the major role in the failure of cancer therapy [12]. P-gp belongs to the ATP binding cassette (ABC) transporters and exhibits a broad substrate specificity interacting with a wide range of molecules, as Dox [13] and morphine [14].
In the present study we have developed a xenograft mice model of brain tumor in order to verify the effectiveness of Dox alone or in association with morphine against glioblastoma cells.
Thus, we have supposed an involvement of morphine in the regulation of Dox efflux mediated by P-gp at the BBB level. Therefore, we have investigated the effect of morphine on the cellular uptake of Dox by MDCKII cells transfected with human MDR1 gene encoding for P-gp as in vitro BBB model.
Materials and methods
Cell line and animals
U87MG-luc2, a luciferase expressing glioblastoma (GBM) cell line stably transfected with firefly luciferase gene (luc2 vector), was obtained by PerkinElmer (PerkinElmer Italia S.P.A., Monza, Italy) and used in vivo to establish an orthotopic brain tumor model.
Parental and P-gp transfected Madin-Darby canine kidney epithelial cells (MDCKII) were obtained from the Netherlands Cancer Institute (Amsterdam, The Netherlands). Both cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 10% fetal bovine serum (FBS). The MDCKII model shows restrictive paracellular pathway and BBB-like discriminative passive permeability which makes it a commonly used tool to study the P-gp-mediated drug transport [15].
51 Female Foxn1 mice (Charles River), 6 weeks old, divided in two phases of the study (31 mice in the phase 1 and 20 mice in the phase 2) have been injected with 3 × 105 U87MG-luc cells in 5 μl of M199 medium into the left lobe of the brain through an infusion of 1 μl/min (Hamilton syringe and microinjector). Intracranial tumor growth was quantified by Bioluminescence imaging (BLI) using an IVIS SPECTRUM 200 system (Perkin Elmer). Mice were housed inside cages of polisulfone (33.2 × 15 × 13 cm) (4 mice/cage) with stainless steel cover-feed, sterilized and dust-free bedding cobs, under a light-dark cycle, keeping temperature and humidity constant. Parameters of the animal rooms were assessed as follows: 22±2°C temperature, 55±10% relative humidity, about 15-20 filtered air changes/hour and 12 hour circadian cycle of artificial light (7 a.m., 7 p.m.). Food and bedding were sterilized. Drinking water was supplied ad libitum. The care and husbandry of animals were in accordance with European Directives and the Italian Regulatory system.
Drug administration and time-treatment
Dox (Doxorubicina Teva, 50 mg) and morphine (Morfina Cloridrato Molteni, 10 mg/ml, solution for injection) were obtained from commercial sources (Teva and Molteni & C s.p.a.) and prepared on each day of injection in physiological saline solution at a concentration of 50 mg/25 ml and 10 mg/4 ml, respectively.
Dox was administered intravenously (IV, tail vein) in a volume of 5 ml/kg to achieve a dose level of 5 mg/kg and 2.5 mg/kg per injection; morphine was administered subcutaneously (SC) in a volume of 5 ml/kg to achieve a dose level of 10 mg/kg per injection.
The drug administration started 7 days after intracranial implantation of GBM cells and it was as follow: a weekly morphine dose by SC injection (i.e., 7, 14, 21, 28, 35 days after cell implantation) followed by (1 hour after the morphine administration) a weekly dose of Dox IV (i.e., 7, 14, 21, 28, 35 days after cell implantation). Control mice received an equivalent volume of physiologic solution (IV, tail vein) once a week for 5 weeks.
The phase 1 study consisted of 8 physiologic solution-treated control mice (Group 1), 7 morphine 10 mg/kg-treated mice (Group 2), 7 Dox 5 mg/kg-treated mice (Group 3) and 7 morphine plus Dox 5 mg/kg-treated mice (Group 4). The treatment was performed from day + 7 (start) to day + 35 (end).
At day 39, all animals were sacrificed by CO2 inhalation.
The phase 2 study consisted of 4 physiologic solution-treated control mice (Group 1), 4 morphine 10 mg/kg-treated mice (Group 2), 4 Dox 2.5 mg/kg-treated mice (Group 3), 4 Dox 5 mg/kg (Group 4), and 4 morphine plus Dox 2.5 mg/kg-treated mice (Group 5). The treatment was performed from day + 7 (start) to day + 35 (end). One week after (day + 42), all animals were sacrificed by CO2 inhalation.
Bioluminescence measurement
BLI was used to monitoring tumor growth and response to therapy by IVIS spectrum image system. Bioluminescence IVIS acquisitions were performed at day 0, 3, 7 and then weekly until the end of experiment. Animals received 150 mg/kg/10 mL D-luciferin (D-luciferin potassium salt 1G, PerkinElmer) by intraperitoneal (i.p.) injection and 30 minutes after the luciferin administration were anesthetized by gas anesthesia (3% isoflurane) and placed into black paper in the IVIS Imaging System box to be imaged.
BLI was expressed as a total radiance in photons per sec/cm2 per steradian.
Clinical signs and mortality
All animals were weighed 3 times/week during the whole treatment. The body weight loss (BWL) was determined as follows: body weight loss percent (% BWL max) = 100 - (mean BW day x/mean BW day 1 × 100), where BWx is the mean BW at the day of maximal loss during the experiment and BW1 is the mean BW on the 1st day of experimental period.
Animal welfare was daily monitored and animals were sacrificed by CO2 inhalation one week after the last administration (before if observed states of suffering and/or a severe weight loss (BWL>15% BW). Physical appearance, behavior and general and local clinical signs of the animals were observed throughout experiment. Any deviation from normality was recorded.
Cellular uptake experiments
Uptake experiments of fluorescent dyes were performed by using a previously reported method [16].
Briefly, parental and P-gp transfected MDCKII cells were seeded on 6-well plates (1 × 105/well) two days before the experiments. Culture medium was removed; cells were washed twice with 2 ml of pre-warmed PBS and then pre-incubated in Opti MEM medium for 15 min at 37°. After pre-incubation, morphine and Dox (suitable for fluorescence, Sigma) were added at the final concentration of 20 µM and 0.5 µg/mL, respectively.
The drug uptake was allowed for 2 h at 37° on dark and arrested by prompt cooling on ice and removal of medium. Each well was washed twice with ice-cold PBS and trypsinized; cells were suspended in PBS supplemented with 2.5% of FBS. Dox fluorescence was determined in 8,000 events for each sample by using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA), equipped with a 15-mW argon-ion laser for excitation.
Statistical analysis
Data were expressed as mean ± SD. Radiance data for experimental groups were assessed by Dixon’s test. Statistical analysis was done by 2-way ANOVA followed by the post hoc Bonferroni-corrected t-test for BLI and by 1-way ANOVA for fluorescence data, using GraphPad Prism 5. P ≤ 0.05 was considered statistically significant.
Results
Effect of Dox on glioblastoma growth in mouse xenograft model
In order to evaluate the effectiveness of Dox, with or without morphine pre-treatment, on growth performances of GBM cells, 31 female Foxn1 mice have received intracranial injection of 3 × 105 U87MG-luc cells. One week after the cells implant, 29 mice were randomized, on the basis of single tumor bioluminescence value, in 4 experimental groups.
On the same day of the randomization the treatments started according to the following schedule:
GP 1 (n=8): physiological solution (IV), q7dx5w; GP 2 (n=7): morphine 10 mg/kg (SC), q7dx5w; GP 3 (n=7): Dox 5 mg/kg (IV), q7dx5w; GP 4 (n=7): morphine 10 mg/kg (SC), q7dx5w, plus Dox 5 mg/kg (IV, 1 hour after morphine administration), q7dx5w.
Tumor growth (IVIS) and weight were carried out weekly.
The images of BLI acquisition of brain tumors on days + 7 (start of treatment) and day + 39 (sacrifice) are shown in Figure 1.
Figure 1.
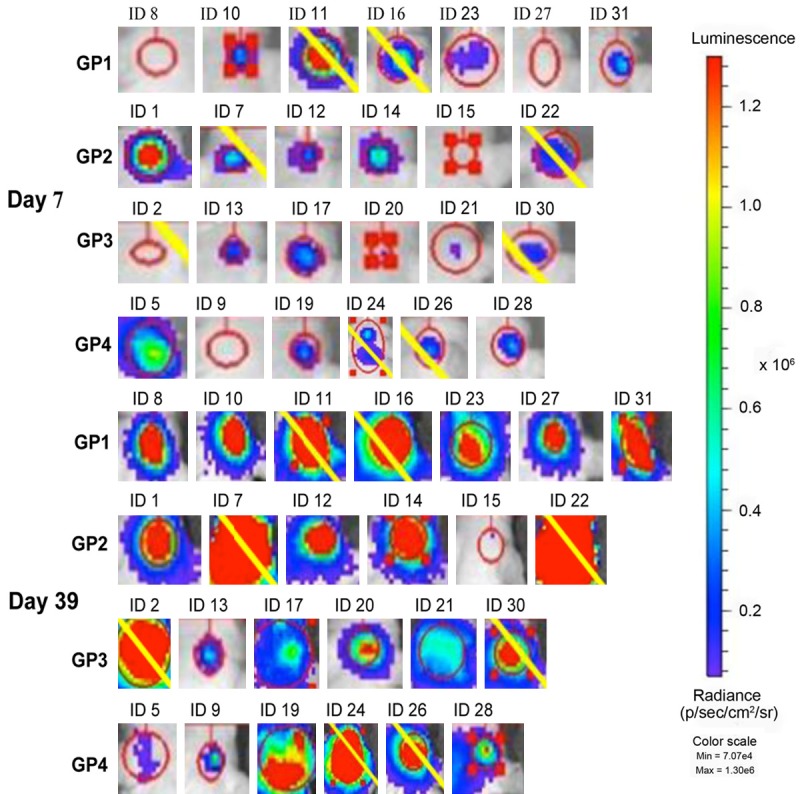
Images of BLI acquisition of brain tumor on day 7 (start of treatment) and day 39 (sacrifice). The colorimetric scale represents the range of radiance values (red=highest value; blu=lowest value) which translates to tumor growth. For each group the yellow bar indicates the animals excluded from the analysis by Dixon’s test (2 higher values of each group).
Two mice (one for GP2 and GP4 groups, respectively) had no detectable tumors with low BLI level during the whole experiment. At the 39 time-point, one mouse for GP1 and GP3 groups died before the image process. Small tumors were detected on day 7 in all animals.
On day 39, BLI showed increasing radiance values corresponding to increasing tumor growth in control and morphine-treated mice. In contrast, tumor-bearing mice treated with Dox or Dox plus morphine exhibited a significant reduced signal intensity compared with untreated and morphine-treated animals.
At the end of experiment, significant differences were observed between GP1 and GP3-4 groups. On day 35, the mean of IVIS data in Dox-treated mice was 3.62E + 05 (P<0.001), in those treated with Dox plus morphine was 2.36E + 05 (P<0.001); on day 39, the mean of IVIS data in Dox-treated mice was 3.99E + 05 (P<0.001), in those treated with Dox plus morphine was 4.84E + 05 (P<0.001). In both cases, the tumor growth was substantially reduced when compared to untreated mice (2.37E + 06 and 2.43E + 06, respectively) (Figure 2).
Figure 2.
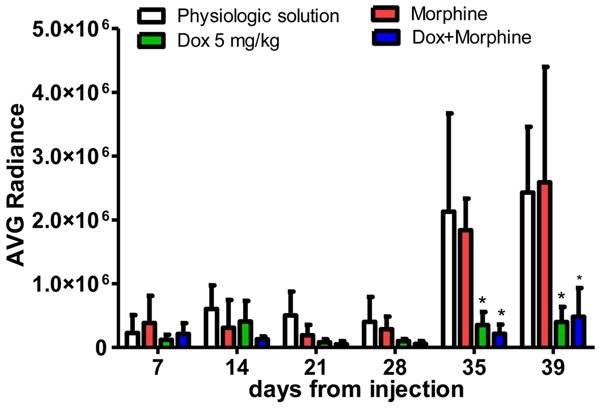
Average IVIS values of mice, distinctly for group of treatment and day of injection. Data are expressed as mean ± SD. *Dox 5 mg/kg and Dox + Morphine treatments vs physiologic solution after 35 days and 39 days (P<0.001, 2-way ANOVA, Bonferroni post-hoc t-test).
Additionally, the TVI (tumor volume inhibition) which represents the rate (%) of signal decreasing compared to the control group (from day + 7 to day + 39) was 87.36% and 87.71% in Dox and Dox plus morphine-treated mice respectively, with no statistical significant difference among these groups. On the other hand, the combined morphine-Dox treatment caused a moderate body weight loss (-11%), demonstrating its severity (Figure 3).
Figure 3.
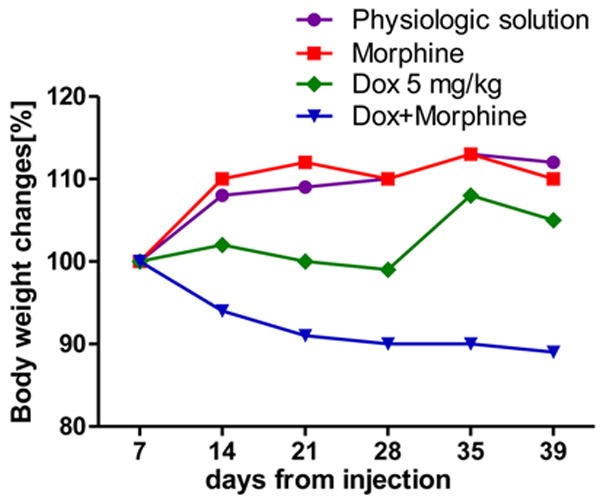
Body weight changes, distinctly for group, from first (day + 7) to the last day of experiment (day + 39).
Effect of morphine-low Dox regime on mice carrying glioblastoma xenograft
Following the same experimental design described above, 3 × 105 U87MG-luc cells were injected in 20 female Foxn1 mice which were randomized, on the basis of single tumor bioluminescence value, in 5 experimental groups as follow:
GP 1 (n=4): physiological solution (IV), q7dx5w; GP 2 (n=4): morphine 10 mg/kg (SC), q7dx5w; GP 3 (n=4): Dox 2.5 mg/kg (IV), q7dx5w; GP 4 (n=4): Dox 5 mg/kg (IV), q7dx5w; GP 5 (n=4): morphine 10 mg/kg (SC), q7dx5w, plus Dox 2.5 mg/kg (IV, 1 hour after the morphine administration), q7dx5w.
Bioluminescence IVIS acquisitions were performed at day 0, 3, 7 and then weekly until the sacrifice (day + 42) in all animals of GP4 and GP5 groups. Two mice (one for GP1 and one for GP2 groups) had a missing value at the 42 time point, dying before the last acquisition; one mouse for GP3 was excluded due to a poor tumor engraftment.
In the first 14 days following the starting treatment, all animals had developed tumors of varying size without significant difference between treated- and untreated mice. At the 35 and 42 time points, the effect of Dox alone or combined to morphine on tumor growth appeared clear.
As showed in Figure 4, only Dox 5 mg/kg determined a significant regression of BLI in a xenograft mouse model of brain glioma while the lower dose of 2.5 mg/kg was not effective, confirming the results of previous experiment. The average BLI for Dox 5 mg/kg was 5 fold lower than that measured for Dox 2.5 mg/kg (P=0.0238) and morphine 10 mg/kg (P=0.0098) and 8 fold lower than vehicle (P=0.0012).
Figure 4.
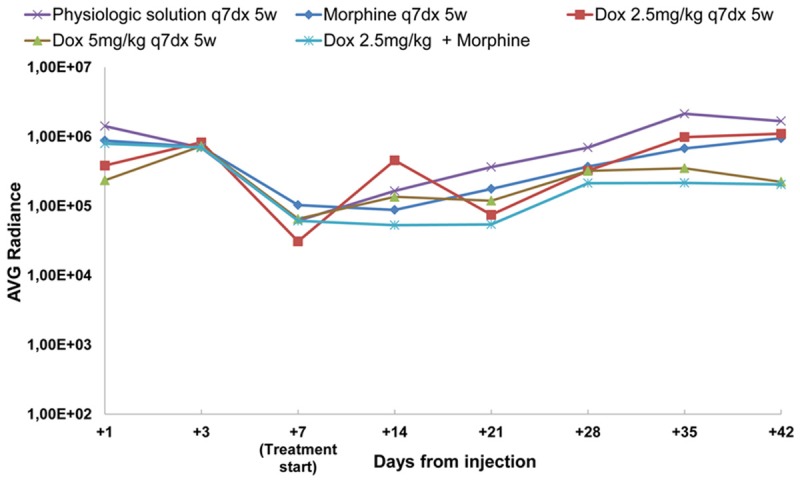
Average radiance curves distinctly for group of treatment and days from injection.
However, if combined with morphine, Dox 2.5 mg/kg showed a decreasing radiance values corresponding to decreasing tumor growth (Figures 4 and 5A). The average BLI for Dox 2.5 mg/kg plus morphine was 5 fold lower (P=0.0053) than Dox 2.5 mg/kg alone and 8-fold lower than vehicle (P=0.0004). On day 42, the mean of IVIS data in Dox 2.5 mg/kg-treated mice was 1.10E + 06 (no significant difference compared to 1.67E + 06 value of vehicle and 9.47E + 05 value of morphine) while in those pre-treated with morphine was 2.04E + 05, likewise to Dox 5 mg/kg-treated mice (2.23E + 05) (P<0.05).
Figure 5.
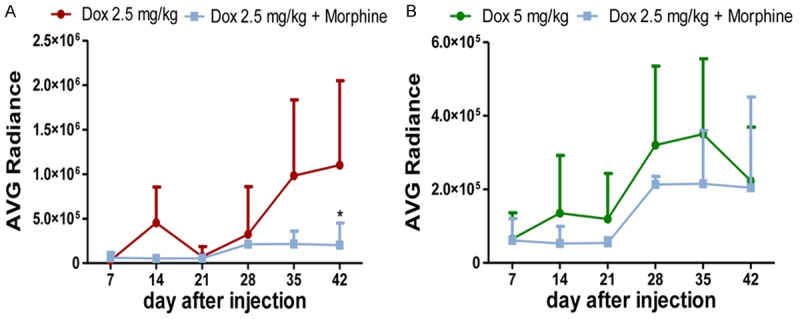
A. Tumor-bearing mice treated with Dox 2.5 mg/kg plus morphine showed a decreased tumor growth when compared to Dox 2.5 mg/kg-treated group. *Dox 2.5 mg/kg+Morphine vs Dox 2.5 mg/kg after 42 days (P<0.05, 2-way ANOVA, Bonferroni post-hoc t-test). B. Average radiance curve for Dox 2.5 mg/kg plus morphine and Dox 5 mg/kg during the whole experiment. No significant difference among these treatments (2-way ANOVA, Bonferroni post-hoc test).
Even more interesting, the average BLI for Dox 2.5 mg/kg plus morphine was lower than Dox 5 mg/kg during the whole experiment (Figure 5B), indicating the effectiveness of low Dox doses combined to morphine as proper dosage to treat poor prognosis brain tumors as high grade gliomas.
In addition, a weekly schedule (up to 5 weeks) with a combination of 10 mg/kg morphine (1 h before Dox administration) and 2.5 mg/kg Dox did not cause a body weight loss of animals; rather, it induced a body weight gain of + 7.98% (on day 42) as a clear signal of minimal toxicity (data not shown).
Uptake of Dox in a BBB in vitro model
We investigated the molecular mechanism underlying the interaction between Dox and morphine. We focused on the role of morphine in the regulation of Dox efflux mediated by P-gp.
Parental and P-gp transfected MDCKII cell lines were used in order to study the Dox uptake when its administration was performed in presence or absence of morphine.
The data showed a Dox accumulation in MDCKII parental cells, without statistically significant difference between Dox and Dox plus morphine groups. The Dox level was significantly increased comparing control group (4.88) vs Dox group (24.4, P<0.01) and Dox plus morphine group (19.82, P<0.05) (Figure 6A).
Figure 6.
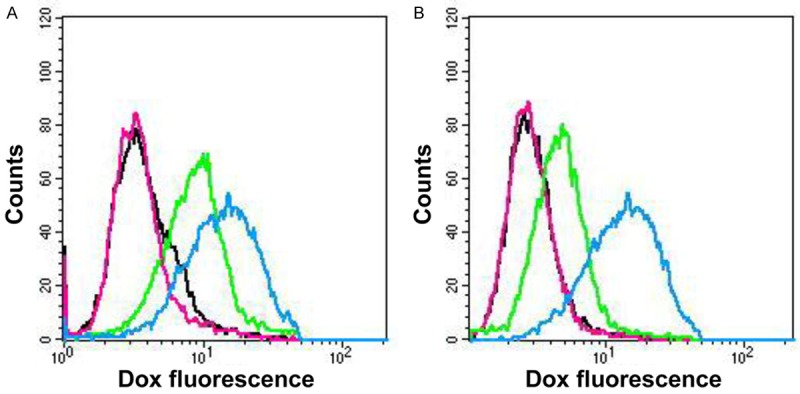
Cellular uptake of Dox in parental (A) and P-gp (B) transfected MDCKII cells by flow cytometry. Each peack represents the mean fluorescence intensity of Dox (0.5 µg/mL): control group (black line), morphine group (pink line), Dox group (green line) and Dox plus morphine group (blu line). Results representative of three indipendent experiments.
On the contrary, fluorescence data on MDCKII P-gp transfected cells indicated no Dox accumulation in both control and Dox groups. Dox increased in MDCKII P-gp transfected cells when its administration was performed in the presence of morphine. The Dox level was significantly higher comparing control group (3.84) vs Dox plus morphine group (12.29, P<0.05) (Figure 6B).
Discussion
Most of the antineoplastic agents either do not penetrate into the CNS or do not arrive in appropriate amounts, so high doses of drugs are required systemically to obtain therapeutic concentrations. The inability to cross the BBB is the major hurdle for using Dox whose effectiveness against tumor cells is well documented in primary cultures [17]. In order to find a safe and reliable method to enhance drug delivery into the brain, several innovative strategies have been proposed, but most of these involve invasive procedures.
Morphine induces a transient alteration of BBB permeability to large molecules in a rat model [9]. Our recent preclinical model has documented a 3-fold increase of brain Dox levels when its administration is performed in the presence of therapeutic plasma levels of morphine [6,7]. On an observation that the enhanced toxicity of dimethyl sulfoxide in patients receiving morphine during hematopoietic stem cell transplantation seemed to be due to a BBB interference [18], we have developed an orthotopic xenograft mice model of glioblastoma with the aim to evaluate the Dox activity after morphine pretreatment.
In the first experiment, 5 weekly doses of 5 mg/kg Dox (a cumulative dose of 25 mg/kg body weight with or without morphine pre-treatment) on brain tumor seemed too high; at day 39 from starting treatment tumor volumes in Dox-treated mice were 6-fold smaller than the vehicle (Figure 1), with no statistical significant difference between Dox alone or combined to morphine (Figure 2).
Based on literature and our previous observation on a rat model, it was indeed very surprising that Dox alone produced a significant delay in the growth of xenografted glioma.
Yet, its anti-glioma activity has been evaluated by U87MG-luc cells that on the one hand are considered an efficient and accurate model for assessing early tumor development and response to therapy [19], on the other they described to have a leaky BBB that makes them accessible to drug treatments [20]. Therefore, it possible to assume that the dose regime and schedule described above are themselves effective on U87MG-luc cells and the combined treatment with morphine does not enhance the effect on tumor inhibition. It has only caused a moderate body weight loss (Figure 3), demonstrating its toxicity.
In contrast, the use of half the dose of Dox (2.5 mg/kg) did not lead to tumor regression (Figure 4). This observation agrees with a number of studies on human tumor xenograft models that describe a not active Dox when it is administered in small doses [21-23].
Interestingly, Dox 2.5 mg/kg when combined to morphine showed a decreasing radiance values corresponding to decreasing tumor growth (Figure 5A), with an average BLI lower than Dox 5 mg/kg (Figure 5B).
Moreover, a weekly schedule (up to 5 weeks) with a pretreatment of 10 mg/kg morphine (1 h before Dox administration) and 2.5 mg/kg Dox did not cause a body weight loss of animals; rather, it induced a body weight gain of + 7.98% (on day 42) as a clear signal of minimal toxicity (data not shown). Finally, it is noteworthy that a cumulative Dox dose of 12.5 mg/kg (37 mg/m2) [24] is clinically achievable and it is 2 times less than the therapeutic dose of 60 to 75 mg/m2 commonly used in cancer treatment [3,25].
It is therefore conceivable that morphine may induce a transient alteration of the permeability of BBB, enhancing the spread of drugs normally unable to cross the BBB, even if administered to a low dose regimen.
In addition, since morphine and Dox use the same efflux channel on the BBB, morphine may likely act as an agonist of Dox efflux, allowing the access of drug into brain parenchyma [26].
We showed a similar uptake of Dox, with or without morphine, in MDCKII parental cells (Figure 6A); in contrast, compared to MDCKII P-gp transfected cells treated with Dox alone, the same cells treated with Dox plus morphine contained higher amount of intracellular Dox (Figure 6B), indicating the effect of morphine on drug efflux pump and suggesting the possibility that morphine enhances the effect of Dox through negative regulation of the ATP binding cassette transporter.
In conclusion, based on our preliminary in vivo and in vitro experience and literature results, morphine seems to facilitate the passage of Dox into the brain parenchyma through the interference with efflux pumps on BBB. These data on a rodent model will enable us to novel therapeutic approaches for glioblastoma and other refractory brain tumors where anticancer drugs are usually cleared by the BBB.
Acknowledgements
This work was supported by: Associazione Italiana per la Ricerca sul Cancro (AIRC), grant IG-12799; Associazione “Amicodivalerio”, Onlus. We are grateful to Prof. ssa E. Baldi, University of Florence, Italy, for her skillful technical assistance and to Dr. J.H.M Schellens, (Netherlands Cancer Institute, Amsterdam) for generously providing MDCKII parental and MDCK-P-gp cell lines.
Disclosure of conflict of interest
None.
References
- 1.Omuro AM, Faivre S, Raymond E. Lessons learned in the development of targeted therapy for malignant gliomas. Mol Cancer Ther. 2007;6:1909–1919. doi: 10.1158/1535-7163.MCT-07-0047. [DOI] [PubMed] [Google Scholar]
- 2.Chastagner P, Devictor B, Geoerger B, Aerts I, Leblond P, Frappaz D, Gentet JC, Bracard S, André N. Phase I study of non-pegylated liposomal doxorubicin in children with recurrent/refractory high-grade glioma. Cancer Chemother Pharmacol. 2015;76:425–32. doi: 10.1007/s00280-015-2781-0. [DOI] [PubMed] [Google Scholar]
- 3.Marrero L, Wyczechowska D, Musto AE, Wilk A, Vashistha H, Zapata A, Walker C, Velasco-Gonzalez C, Parsons C, Wieland S, Levitt D, Reiss K, Prakash O. Therapeutic efficacy of aldoxorubicin in an intracranial xenograft mouse model of human glioblastoma. Neoplasia. 2014;16:874–82. doi: 10.1016/j.neo.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesniak MS, Upadhyay U, Goodwin R, Tyler B, Brem H. Local delivery of doxorubicin for the treatment of malignant brain tumors in rats. Anticancer Res. 2005;25:3825–31. [PMC free article] [PubMed] [Google Scholar]
- 5.Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C, De Maria R. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–41. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 6.Sardi I. Morphine facilitates doxorubicin penetration in the central nervous system: a new prospect for therapy of brain tumors. J Neurooncol. 2011;104:619–20. doi: 10.1007/s11060-010-0518-9. [DOI] [PubMed] [Google Scholar]
- 7.Sardi I, la Marca G, Cardellicchio S, Giunti L, Malvagia S, Genitori L, Massimino M, de Martino M, Giovannini MG. Pharmacological modulation of blood-brain barrier increases permeability of doxorubicin into the rat brain. Am J Cancer Res. 2013;3:424–32. [PMC free article] [PubMed] [Google Scholar]
- 8.Bimonte S, Barbieri A, Palma G, Arra C. The role of morphine in animal models of human cancer: does morphine promote or inhibit the tumor growth? Biomed Res Int. 2013;2013:258141. doi: 10.1155/2013/258141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma HS, Ali SF. Alterations in blood-brain barrier function by morphine and methamphetamine. Ann N Y Acad Sci. 2006;1074:198–224. doi: 10.1196/annals.1369.020. [DOI] [PubMed] [Google Scholar]
- 10.Mercadante S. Intravenous morphine for management of cancer pain. Lancet Oncol. 2010;11:484–9. doi: 10.1016/S1470-2045(09)70350-X. [DOI] [PubMed] [Google Scholar]
- 11.Kast RE. Using blood brain barrier disruption by methamphetamine for drug delivery. J Neurooncol. 2007;85:109–110. doi: 10.1007/s11060-007-9389-0. [DOI] [PubMed] [Google Scholar]
- 12.Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Dagenais C, Graff CL, Pollack GM. Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem. Pharmacol. 2004;67:269–76. doi: 10.1016/j.bcp.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Hellinger E, Veszelka S, Tóth AE, Walter F, Kittel A, Bakk ML, Tihanyi K, Háda V, Nakagawa S, Duy TD, Niwa M, Deli MA, Vastag M. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VBCaco-2) cell-based surrogate blood-brain barrier penetration models. Eur J Pharm Biopharm. 2012;82:340–51. doi: 10.1016/j.ejpb.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Solazzo M, Fantappiè O, D’Amico M, Sassoli C, Tani A, Cipriani G, Bogani C, Formigli L, Mazzanti R. Mitochondrial expression and functional activity of breast cancer resistance protein in different multiple drug-resistant cell lines. Cancer Res. 2009;69:7235–42. doi: 10.1158/0008-5472.CAN-08-4315. [DOI] [PubMed] [Google Scholar]
- 17.Veringa SJ, Biesmans D, van Vuurden DG, Jansen MH, Wedekind LE, Horsman I, Wesseling P, Vandertop WP, Noske DP, Kaspers GJ, Hulleman E. In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. PLoS One. 2013;8:e61512. doi: 10.1371/journal.pone.0061512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caselli D, Tintori V, Messeri A, Frenos S, Bambi F, Aricò M. Respiratory depression and somnolence in children receiving dimethyl sulfoxide and morphine during hematopoietic stem cells transplantation. Haematologica. 2009;94:152–3. doi: 10.3324/haematol.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashizume R, Ozawa T, Dinca EB, Banerjee A, Prados MD, James CD, Gupta N. A human brainstem glioma xenograft model enabled for bioluminescence imaging. J Neurooncol. 2010;96:151–9. doi: 10.1007/s11060-009-9954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemper EM, Leenders W, Küsters B, Lyons S, Buckle T, Heerschap A, Boogerd W, Beijnen JH, van Tellingen O. Development of luciferase tagged brain tumour models in mice for chemotherapy intervention studies. Eur J Cancer. 2006;42:3294–303. doi: 10.1016/j.ejca.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Trail PA, Willner D, Lasch SJ, Henderson AJ, Greenfield RS, King D, Zoeckler ME, Braslawsky GR. Antigen-specific activity of carcinoma-reactive BR64-doxorubicin conjugates evaluated in vitro and in human tumor xenograft models. Cancer Res. 1992;52:5693–700. [PubMed] [Google Scholar]
- 22.Kratz F, Fichtner I, Graeser R. Combination therapy with the albumin binding prodrug of doxorubicin (INNO-206) and doxorubicin achieves complete remissions and improves tolerability in an ovarian A2780 xenograft model. Invest New Drugs. 2012;30:1743–1749. doi: 10.1007/s10637-011-9686-5. [DOI] [PubMed] [Google Scholar]
- 23.Kratz F, Azab S, Zeisig R, Fichtner I, Warnecke A. Evaluation of combination therapy schedules of doxorubicin and an acid-sensitive albumin-binding prodrug of doxorubicin in the MIA PaCa-2 pancreatic xenograft model. Int J Pharm. 2013;441:499–506. doi: 10.1016/j.ijpharm.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Center for Drug Evaluation, Research (CDER) Guidance for Industry. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. 2005 [Google Scholar]
- 25.Desai VG, Herman EH, Moland CL, Branham WS, Lewis SM, Davis KJ, George NI, Lee T, Kerr S, Fuscoe JC. Development of doxorubicin-induced chronic cardiotoxicity in the B6C3F1 mouse model. Toxicol Appl Pharmacol. 2013;266:109–21. doi: 10.1016/j.taap.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Cisternino S, Rousselle C, Dagenais C, Scherrmann JM. Screening of multidrug-resistance sensitive drugs by in situ brain perfusion in Pglycoprotein-deficient mice. Pharm Res. 2001;18:183–90. doi: 10.1023/a:1011080418027. [DOI] [PubMed] [Google Scholar]


