Abstract
Osteosarcoma, the most frequent malignant primary bone tumor in pediatric patients is characterized by osteolysis promoting tumor growth. Lung metastasis is the major bad prognosis factor of this disease. Zoledronic Acid (ZA), a potent inhibitor of bone resorption is currently evaluated in phase III randomized studies in Europe for the treatment of osteosarcoma and Ewing sarcoma. The beneficial effect of the liposomal form of Muramyl-TriPeptide-Phosphatidyl Ethanolamine (L-mifamurtide, MEPACT®), an activator of macrophage populations has been demonstrated to eradicate lung metastatic foci in osteosarcoma. The objective of this study was to evaluate the potential therapeutic benefit and the safety of the ZA and L-mifamurtide combination in preclinical models of osteosarcoma, as a prerequisite before translation to patients. The effects of ZA (100 μg/kg) and L-mifamurtide (1 mg/kg) were investigated in vivo in xenogeneic and syngeneic mice models of osteosarcoma, at clinical (tumor proliferation, spontaneous lung metastases development), radiological (bone microarchitecture by microCT analysis), biological and histological levels. No interference between the two drugs could be observed on ZA-induced bone protection and on L-mifamurtide-induced inhibition of lung metastasis development. Unexpectedly, ZA and L-mifamurtide association induced an additional and in some cases synergistic inhibition of primary tumor progression. L-mifamurtide has no effect on tumor proliferation in vitro or in vivo, and macrophage population was not affected at the tumor site whatever the treatment. This study evidenced for the first time a significant inhibition of primary osteosarcoma progression when both drugs are combined. This result constitutes a first proof-of-principle for clinical application in osteosarcoma patients.
Keywords: Osteosarcoma, liposomal mifamurtide, zoledronic acid, inhibition of lung metastases development, bone protection
Introduction
Osteosarcoma is the most frequent malignant primary bone tumor in young people with around 1000 new cases per year in Europe [1,2]. It arises mainly in adolescents and young adults (median age 18 years), with a preferential location in the metaphysis of long bones. The standard treatment of osteosarcoma consists in multi-drug chemotherapy as neo-adjuvant and adjuvant settings associated with surgical local control [3,4]. Despite recent advances in limb-salvage surgery and poly-chemotherapy combinations, event-free survival and overall survival have only slightly improved during the last decades remaining around 65-70% at 5 years for localized forms and only 25% for patients who present pulmonary metastases at diagnosis. The current drug regimens used for osteosarcoma pediatric patients associate MTX-VP-IFO (High dose methotrexate, Vincristin, platinium and ifosfamide) or API-AI for adults (adriamycin-platinium-ifosfamide). However, patients who do not respond to chemotherapy have an overall survival of only 20-25% at 5 years [5,6]. Therefore, new therapeutic targets are urgently needed for these patients at high risk.
Recently, L-mifamurtide (Liposomal-Muramyl TriPeptide-PhosphatidylEthanolamine: L-MTP-PE) has been proposed as adjuvant therapy for osteosarcoma patients in the United States. It is a synthetic analog of the muramyl dipeptide (MDP), resulting from the covalent addition of alanin and dipalmitoylphosphatidyl ethanolamine to MDP, a peptidoglycan found in bacterial cell wall [7]. L-mifamurtide acts as a nonspecific immunomodulator by activating macrophages and monocytes related to the upregulation of tumoricidal activity and secretion of pro-inflammatory cytokines including tumor necrosis factor (TNF)-a, interleukin (IL)-1, IL-6, IL-8, IL-12, nitric oxide (NO), prostaglandin E2 (PGE2) and PGD2 [8-10]. The lipid composition of mifamurtide has been developed to target delivery of the drug selectively to monocytes and macrophages in liver, spleen and lungs [7,11,12]. It also facilitates signaling via phosphatidyl serine recognition by macrophages [13]. Moreover, the liposomal formulation can improve the safety profile of several drugs by modifying parent drug or solubilization agent toxicity [14]. In 2011, Buddingh and al. reported that tumor-associated-macrophages (TAMs) are associated with good prognosis in high grade osteosarcoma [15]. In contrast to most other tumor types, TAMs are associated with reduced metastasis and improved survival for those patients. A phase III randomized clinical trial was conducted by the Children‘s oncology group (COG) from 1993 to 1997 [16]. Significant improvement in EFS and overall survival were observed in patients randomized to receive L-mifamurtide [7,17,18]. In the European Union, except in France, L-mifamurtide is indicated in patients aged between 2 and 30 years with high-grade, resectable, nonmetastatic osteosarcoma after the complete surgical wide resection.
In France, OS2006 is the current clinical protocol for pediatric and adult osteosarcoma patients, a phase III randomized study associating Zometa® (ZA, zoledronic acid) with conventional poly-chemotherapy and surgery. This protocol is based on preclinical studies from our group demonstrating the advantage of combination of antitumor therapy with drugs that target the bone microenvironment [19,20]. Indeed, interactions between tumor cells and bone cells are closely regulated in the so called “vicious cycle” as initially proposed by Paget [21]. Tumor cells proliferating in bone produce osteoclast activating factors such as PTH-rP, IL-11, IL-6 or the main regulator of osteoclast function: Receptor Activator of NF-кB Ligand (RANKL). When activated, osteoclasts are able to degrade bone, and allow the release of growth factors stored in the bone matrix such as Transforming Growth Factor-b, Insulin-like Growth Factor-1, Fibroblast Growth Factor… that in turn activate tumor cell proliferation [22].
The third generation nitrogen-containing Bisphosphonates (N-BPs) and among them zoledronic acid are potent inhibitors of osteoclast functions resulting in inhibition of bone resorption [23,24]. In addition, N-BPs slow down tumor growth in bone, both indirectly by inhibiting osteoclast activation and directly by inhibition of tumor cell proliferation, angiogenesis, migration and activation of γδT lymphocytes [25,26]. Therefore, these drugs are currently used as therapeutic agents in pathologies associated with bone degradation from tumor origin or not (osteoporosis, multiple myeloma, bone metastases and primary bone tumors) [27,28].
Considering that osteolysis promoting tumor growth and lung metastasis dissemination are the two key points in osteosarcoma progression, it is tempting to propose ZA and L-mifamurtide combination in osteosarcoma treatment, each of these drugs already proving their efficacy. Before clinical application, preclinical studies are needed to see whether L-mifamurtide could interfere with ZA induced prevention of associated bone lesions, and conversely whether ZA may interfere with L-mifamurtide induced inhibition of lung metastasis dissemination.
Material & methods
Cell culture
Human KHOS and murine K7M2 osteosarcoma cell lines were purchased from the American Type Culture Collection (ATCC, LGC Promochem, Molsheim, France) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Lonza, Switzerland). Murine MOS-J (Jackson Laboratory) osteosarcoma cell line was cultured in Roosevelt Park Memorial Institute (RPMI) medium (Lonza). Media were supplemented with 5% fetal calf serum (Hyclone, USA). All cell lines were cultured in a humidified 5% CO2/air atmosphere at 37°C.
Drugs
L-mifamurtide was provided by TAKEDA (IDM PHARMA SAS). Each vial containing 4 mg of mifamurtide powder in 1 g excipient was divided into 8 tubes and stored at 4°C. Powder was reconstituted extemporaneously in sodium chloride 0.9% and used within 6 hrs. ZA, 1-hydroxy-2-(1H-imidazole-1-yl) ethylidene-bisphosphonic acid supplied as the disodium salt by Novartis Pharma AG (Basel, Switzerland), was dissolved in PBS as 10 mM stock solution and stored at -20°C. Liposome-PBS (clodronateliposome.com, Netherlands) was used as control.
Cell proliferation
Osteosarcoma cell lines were plated in respective media and treated with L-mifamurtide or zoledronic acid at indicated concentration for 48 hours, cell growth being measured using crystal violet assay as described previously [29].
Quantitative reverse transcription-PCR
Total RNA was extracted from mice osteosarcoma biopsies (24 h after the last drug injection) using Direct-zol RNA MiniPrep (Zymo Research) with DNase treatment to remove residual genomic DNA. RNA quantity and quality was evaluated by determining A260/A280 ratio using NanoDrop. Complementary DNA was synthesized from isolated RNA using ThermoScript real-time polymerase chain reaction (RT-PCR) System (Invitrogen, Carlsbad, CA, USA). Quantitative-PCR (qPCR) was performed using primers (Table 1) on Chromo4 instrument (Biorad, Richmond, CA, USA) using SYBR Green Supermix reagents (Biorad). Target gene expression was normalized to GAPDH levels in respective samples as an internal standard, and the comparative cycle threshold (Ct) method was used to calculate relative quantification of target mRNAs. Each assay was performed in triplicate.
Table 1.
Primer sequences used in quantitative real-time PCR analysis
| Gene | Forward primer | Reverse primer |
|---|---|---|
| hGAPDH | TGGGTGTGAACCATGAGATATG | GGTGCAGGAGGCATTGCT |
| mHPRT | TCCTCCTCAGACCGCTTTT | CCTGGTTCATCATCGCTAATC |
| mF4/80 | TCCTCCTTGCCTGGACACT | GCCTTGAAGGTCAGCAACC |
| mFas | TGAATGGGGGTACACCAACC | TGCTCTTCATCGCAGAGTGT |
| hFas | GAAGGGAAGGAGTACACAGACA | TGGTATTCTGGGTCCGGGTG |
| CD64 | AGGGCGGAAAGAGAAGATGC | ATGACTGGGGACCAAGCACT |
Animal models of osteosarcoma
Five week-old female Rj: NMRI nude mice for xenogeneic models or C57BL/6, BALB/c mice for syngeneic models were purchased from Janvier Breedings (Le Genest Saint Isle, France). Mice were anesthetized by inhalation of an isoflurane/air mixture (2%, 1 L/min). Primitive osteosarcoma was induced by intramuscular paratibial injection of 106 human KHOS osteosarcoma cells (xenogeneic model) or 3.106 MOS-J or K7M2 mice osteosarcoma cells respectively in C57BL/6 and BALB/c mice (syngeneic models). Tumor development is associated to osteolytic lesions and with dissemination of spontaneous lung metastasis mimicking the human pathology. The day after tumor cell injection, mice were randomly assigned (n=8-10) to vehicle (NaCl 0.9%), ZA, L-mifamurtide or ZA+L-mifamurtide (bitherapy) groups. ZA (100 µg/kg; s.c.) or L-mifamurtide (1 mg/kg; i.v.) was injected twice per week. Tumor volume was measured three times weekly (length*width*depth*0.5). Data points were expressed as mean tumor volume ± SEM.
Mice were sacrificed when the tumor volume reached 10% of body weight for ethical reasons. Primary bone tumor was harvested for immunohistochemistry analysis, lung metastases were macroscopically counted under a binocular loupe. Animal care and experimental protocols were approved by the French Ministry of Agriculture (agreement n°D-44015 for the Experimental Therapy Unit located at the Medical School of Nantes) and were realized in accordance with the institutional guidelines of the regional ethical committee (CREEA Pays de la Loire, PdL06, France), with the protocol agreement n°1280.01 and 1281.01, and under the supervision of authorized investigators.
Immunohistochemistry
Immunohistochemical stainings were performed on formalin-fixed and paraffin-embedded 3 µm sections of tumor samples using adequate primary antibody. Immunodetection performed using secondary biotinylated antibodies and streptavidin HRP-complex was revealed with 3,3’-diaminobenyidine (DAB-Dako) followed by counterstaining with hematoxylin. Negative control was analyzed using a similar procedure without primary antibody. Same slides were used to estimate the percentage of necrosis area among the total tumor.
Micro-CT
Analyses of the bone microarchitecture were performed using a SkyScan 1076 in vivo micro-CT scanner (SkyScan, Kontich, Belgium). Tests were performed at early time (d21, tumor volume around 500 mm3) or at necropsy (tumor volume around 2000 mm3). All tibiae/fibulae were scanned using the same parameters (pixel size 18 µm, 50 kV, 0.5-mm Al filter and 0.7 degree of rotation step). Three-dimensional reconstructions and analysis of bone parameters were performed using NRecon and CTan softwares (SkyScan). Calculation of cortical bone volume (BV) following 3D morphometric parameters (bone ASBMR nomenclature) was performed on 5.5-7.2 mm of tibia length (depending on mice model) from the fibula insertion. This area corresponds to bone in close contact with osteosarcoma and excludes trabecular bone. Cortical thickness (Ct.Th) was defined as the mean cortical volume divided by the outer bone surface as previously described [30]. Trabecular bone parameters were also analyzed.
Statistical analysis
GraphPad InStat v3.02 software (La Jolla, CA, USA) was used. In vivo experimentation results were analyzed with the unpaired nonparametric method and Dunn’s multiple comparisons following the Kruskal-Wallis test. A p value of less than 0.05 was considered statistically significant.
Results
L-mifamurtide does not interfere with ZA-induced bone protection
The first objective of the study was to investigate the potential interference of L-mifamurtide treatment with the protective zoledronic acid effects on bone during osteosarcoma progression. Two syngeneic and one xenogeneic models of osteosarcoma were used: respectively K7M2 and MOS-J induced in the BALB/c and C57BL/6 mouse strains, and KHOS induced in NMRI-nude mice. The day after tumor cell injection, mice were treated with ZA (100 µg/kg) and/or L-mifamurtide (1 mg/kg) twice a week. The bone microarchitecture parameters of the tumor-bearing tibia have been measured in all models and treatment conditions using X-ray, micro-CT and 3D reconstruction analysis (Figure 1A). Data revealed a decrease of the tumor-associated osteolysis in the ZA-treated mice as compared to the untreated control group. Addition of L-mifamurtide did not modulate ZA induced bone protection in all models tested (Figure 1). An extensive analysis of multiple bone parameters revealed an increase of tumor-associated bone quality in the ZA treated groups as compared to control group. In the xenogeneic KHOS mouse model, ZA treatment alone and combined with L-mifamurtide increased the cortical bone volume (BV) (from 4.88 to 7.79 and 7.62 mm3 respectively as compared to control group, p<0.05; Figure 1B), the cortical thickness (Co.Th) (from 0.14 to 0.21 and 0.18 mm; Figure 1C), and trabecular parameters (not shown). Similar observations were made with the syngeneic mouse model of osteosarcoma MOS-J (Figure 1A-C lower panel), in which L-mifamurtide treatment did not affect the tumor-associated bone preservation as compared to control group.
Figure 1.
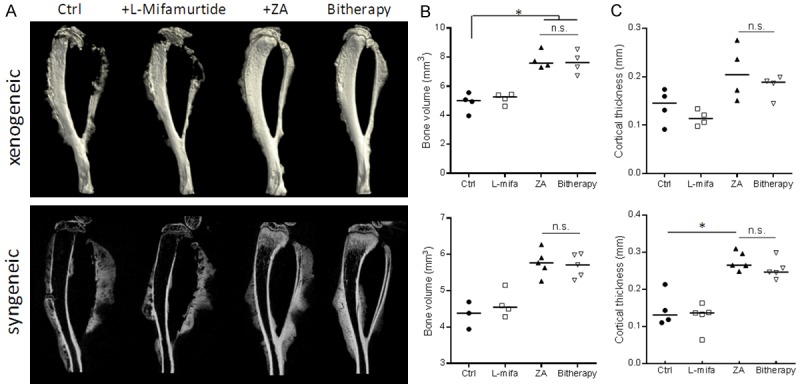
L-mifamurtide does not interfere with ZA-induced bone protection. A. Representative microCT and 3D reconstruction of the tumor-bearing tibia taken ex-vivo, from KHOS xenogeneic (upper panel) and MOS-J syngeneic (lower panel) models treated or not (Ctrl) with L-mifamurtide (1 mg/kg), ZA (100 µg/kg), or both (bitherapy) at day 1, twice a week, until week 4-5. B. MicroCT quantification of tibia bone volume (BV, mm3) and C. Tibia cortical thickness (Co.Th, mm) were calculated on the tibia of tumor bearing mice of the different groups. Median were represented for each group (n=4). *p<0.05.
Zoledronic acid does not interfere with L-mifamurtide-induced inhibition of lung metastasis dissemination and development
The second objective was to investigate the effect of L-mifamurtide and ZA on spontaneous metastasis dissemination from primary bone tumors induced in xenogeneic and syngeneic osteosarcoma models. Mice were treated by ZA and L-mifamurtide during ~5 weeks (Figure 2A) and metastases were counted at equivalent tumor volume. Whatever the model, a trend of diminished spontaneous lung metastasis dissemination was observed in L-mifamurtide treated groups (Figure 2B), and still when animals were treated with ZA combined with L-mifamurtide. No significant differences could be observed probably because of the high variability and the small animal number in each group. No difference was observed between control and zoledronic acid treated groups.
Figure 2.

Treatment of osteosarcoma bearing mice with L-Mifamurtide alone or in association with ZA inhibits lung metastases development in both xenogeneic (KHOS) and syngeneic (MOS-J) models. A. NMRI-nude or C57BL/6 mice were injected respectively with KHOS (xenogeneic) or MOS-J (syngeneic) osteosarcoma cells and treated with vehicle (Ctrl), L-mifamurtide, ZA or both (bitherapy) as described in Figure 1. B. Graphs indicate individual (dots) and median (lines) numbers of lung metastases macroscopically counted in mice lungs from each group at equivalent primary bone tumor volume (2000 mm3).
The combination of ZA and L-mifamurtide shows no interference on ZA induced bone protection effects and L-mifamurtide induced inhibition of lung metastasis development both in syngeneic and xenogeneic models of osteosarcoma in mice. ZA is known to have direct anti-cancer effects in vitro and in vivo in primary and secondary bone tumors [20]. To go further, we investigated whether L-mifamurtide would affect the antitumor effect of ZA on osteosarcoma primary bone tumor.
In vivo significant inhibitory effect of L-mifamurtide associated with zoledronic acid on primary bone tumor progression in syngeneic and xenogeneic models of osteosarcoma
The effect of L-mifamurtide and ZA therapeutic association was analyzed on primary tumor growth in syngeneic (MOS-J) and xenogeneic (KHOS) osteosarcoma models. In both cases, the tumor cells were injected in close contact to the tibia, after periosteum activation. The tumors were apparent within 10 days, and develop for 4-5 weeks. In each model, L-mifamurtide or ZA alone did not induce any significant effect on primary tumor development (Figure 3A, 3B). However, the combination of both drugs significantly inhibited tumor growth in xenogeneic and syngeneic models. The mean tumor size at 22 days for the xenogeneic KHOS model was 1285 ± 143 mm3 in the control group, compared to 872 ± 128 mm3 in mice treated with bitherapy (means ± SEM, p<0.001; Figure 3A). The mean tumor size at 35 days for the syngeneic MOS-J model was 2284 ± 176 mm3 in the control group, compared to 1359 ± 177 mm3 in the group treated with bitherapy (means ± SEM, p<0.001; Figure 3B). Similar results were obtained in the K7M2 syngeneic mice model (not shown). In summary, combinatory therapy induced 41% and 55% inhibition of mean tumor volume in syngeneic models (MOS-J and K7M2 respectively) and 37% in the xenogeneic model (KHOS) at final time point.
Figure 3.

L-mifamurtide acts with ZA to reduce tumor growth both in syngeneic (MOS-J) and xenogeneic (KHOS) mouse models of osteosarcoma. Mice were treated with L-mifamurtide (1 mg/kg) and ZA (100 µg/kg) or both starting at day 1 after tumor cell injection as described in Figure 1. The mean tumor volume from (A) xenogeneic KHOS and (B) syngeneic MOS-J mice models, and individual tumor volumes (C) from xenogeneic KHOS model were compared between the 4 groups ± SEM (n=8). *p<0.001.
In both syngeneic models, the mean decrease in tumor volume of mice treated with ZA and L-mifamurtide reflects a slowing tumor growth in some mice, and no response in others (Figure 3C). These unexpected results demonstrate that L-mifamurtide acts with ZA to reduce primary tumor growth in vivo.
L-mifamurtide does not affect osteosarcoma cell proliferation in vivo or in vitro
In order to analyze the mechanisms involved in the tumor growth inhibition observed in vivo with the L-mifamurtide + ZA therapeutic association, the direct effect of each drug alone and in combination was studied on osteosarcoma cell proliferation in vitro, and in vivo by Ki67 staining on tumor biopsies. Results show that L-mifamurtide alone has no direct effect on the proliferation rate of the two osteosarcoma cell lines MOS-J and KHOS in vitro (Figure 4A). In addition, L-mifamurtide does not significantly modify the ZA-induced inhibition of osteosarcoma cell proliferation (Figure 4A). Considering the in vivo mechanisms of action, no significant difference could be observed by Ki67 immunohistochemical (IHC) staining of KHOS osteosarcoma in situ cell proliferation between treated and untreated mice (Figure 4B, 4C). Moreover, necrosis area percentage is not affected by any treatment and similar observations were obtained for xenogeneic models (not shown). It should be mentioned that osteosarcoma presents high heterogeneity inter- and intra-groups leading to difficult interpretation of IHC results at significant level.
Figure 4.

L-mifamurtide alone or associated with ZA does not directly affect tumor cell proliferation in vivo and in vitro. A. Osteosarcoma cells were treated with ZA, L-mifamurtide or both at indicated doses for 48 h. Cell growth was determined by crystal violet analysis and compared to control untreated cells. B. KHOS tumor biopsies were collected and Ki67 staining was evaluated by immunohistochemical analysis. Specimens were scored and estimated in percentage mean of Ki67+-cells compare to the control group, which corresponds to the mice bearing tumor that did not receive any treatment but vehicle. C. Representative picture of Ki67 staining for control and bitherapy treated groups.
Therefore, based on these in vitro results, the additive effect of both therapies cannot be explained by a direct anti-tumor effect of L-mifamurtide.
Mechanisms of action of L-mifamurtide and ZA combination in vivo
Because L-mifamurtide is known to activate macrophage- and monocyte-based immune response, the significant inhibitory effect of bitherapy that was observed on primary tumor progression may be linked to the modulation of these immune cell populations by the association of both drugs. Therefore, monocyte/macrophage populations were analyzed at primary site by the relative proportion of their markers at transcript (qPCR) and protein levels (IHC) on mouse osteosarcoma biopsies of each group.
IHC analyses showed no differences in the macrophages F4/80+ infiltrates in primary bone tumors of the different groups in the syngeneic MOS-J mouse model (Figure 5A). QPCR analyses from tumor biopsies confirmed that any treatment affected the F4/80 (macrophages) or CD64 (monocytes/macrophages) mRNA expression (Figure 5B). Similar observations were made with the KHOS xenogeneic mouse model (not shown). Moreover, IHC scoring of iNOS (M1 macrophages) and ArgI (M2 macrophages) did not show any differences between groups (not shown). Therefore, Fas mRNA expression was evaluated at the primary tumor site in osteosarcoma models under ZA and L-mifamurtide treatments. Results showed no significant modification of Fas mRNA expression in syngeneic MOS-J (Figure 5B) or xenogeneic KHOS (not shown) bone tumor biopsies.
Figure 5.

L-mifamurtide and/or zoledronic acid treatments did not affect monocyte/macrophage markers in osteosarcoma syngeneic tumor biopsies. A. Staining of osteosarcoma tissues from MOS-J syngeneic mouse model for F4/80 22 days after tumor induction. B. Expression of F4/80, CD64, Fas mRNA by MOS-J tumor biopsies. NS: no statistic differences between groups. Mean ± SD.
In summary, neither ZA nor L-mifamurtide significantly alters the monocyte/macrophage populations in the osteosarcoma models used in this study. Furthermore, our results suggest that L-mifamurtide does not affect macrophage activation in the primary tumor site as analyzed by iNOS/ArgI IHC staining.
Discussion
The absence of overall survival improvement for more than 40 years since the application of Rosen poly-chemotherapy protocols [31] is associated with the lack of relevant therapeutic options for high-grade osteosarcoma patients (especially patients with lung metastases). In addition to conventional chemotherapy, multiple investigational agents have being studied. Among them, zoledronic acid has already demonstrated its therapeutic interest for bone malignancies including osteosarcoma [19,20]. L-mifamurtide is also one of the more promising drugs for osteosarcoma acting as immune stimulatory agent. At clinical level, Meyer’s study was designed to assess whether the addition of L-MTP-PE and/or ifosfamide to a standard chemotherapeutic regimen (doxorubicin-cisplatin-high dose methotrexate) would increase both EFS and overall survival in newly diagnosed patients with high-grade osteosarcoma. ZA is currently proposed in clinical trials for osteosarcoma (OS2006 - NCT00470223) and Ewing sarcoma (EWING2008 - NCT00987636, EuroEWING2012 - ISRCTN92192408) patients, and L-mifamurtide therapy has been conducted in phase II (MEMOS - ISRCTN82138287) and phase III trials in patients with newly diagnosed OS [18,32]. Therefore, the combination of both drugs could represent a promising therapeutic option as both drugs target different complementary pathways: L-mifamurtide activates macrophages and monocytes which help to eradicate lung metastasis development, and ZA inhibits osteoclast function by inducing their apoptosis [33], and therefore indirectly induced tumor growth inhibition.
In the present study, we described for the first time a strong trend for L-mifamurtide to inhibit lung metastasis dissemination in both syngeneic and xenogeneic osteosarcoma models in mice. Moreover, its combination with ZA showed no interference of both drugs on each other. Relevant complementary orthotopic models of osteosarcoma with spontaneous pulmonary metastasis development were used to better reproduce the human clinical features and to demonstrate the value of this therapeutic approach [34]. Preclinical studies using xenogeneic and syngeneic models of osteosarcoma demonstrated that ZA did not interfere with L-mifamurtide-inducing lung metastases reduction, and in parallel, L-mifamurtide did not modulate the ZA-induced bone protection. These results in murine models are in agreement with previous preclinical data reported by only one team who described the benefit of L-mifamurtide on lung metastasis formation in dog models of osteosarcoma [35].
Even if a strong trend (but not significant) toward an anti-metastatic activity of L-mifamurtide was shown in our mouse models, we could not observe any increase of the overall survival in these groups, as it has been previously described in dogs [35] and patients [18]. Overall, analyses of tumor behavior are rendered complicated due to the high tumor heterogeneity in osteosarcoma. The general source of heterogeneity is caused by specific tumor microenvironment, composed of stromal cells, the availability of vascular network, and the host’s immune system [36]. Primary osteosarcoma progression was so rapid in our models that pulmonary metastasis dissemination was systematically observed, prompting us to sacrifice the mice early. In these cases, it could not be possible to observe any significant effect of L-mifamurtide, but only harm trends. The efficacy of the L-mifamurtide should be investigated in models with slower metastasis development.
In the second part of our study, we wanted to understand why the L-mifamurtide and ZA combination induced a synergistic and significant inhibition of primary osteosarcoma progression in our mice models whereas each drug showed no significant effect. This unexpected result was observed both in xenogeneic and syngeneic models with 32% and 41% inhibition of primary tumor growth respectively. Previous results from the literature showed a direct and indirect anti-tumor activity of L-mifamurtide on osteosarcoma cells in vitro or in metastases development after resection of the primary tumor [8,37]. Moreover, this inhibitory effect on primary tumor growth is even more unexpected as ZA and L-mifamurtide were not associated with conventional chemotherapy as usually in clinics. Several hypotheses could be proposed to explain those results.
First, both drugs act synergistically by directly inhibiting tumor proliferation. However, when we studied the potential direct anti-tumor activity of L-mifamurtide alone and associated with ZA in vitro, no inhibition of osteosarcoma cell growth could be observed. In addition, tumor biopsies were collected in mice for histological analysis 24 hours after treatment at early (week 3) or end time point (week 5). We were unable to show a decreased proliferation rate as analyzed by Ki67 staining in the bitherapy group in our different models. However, we may wonder whether the effects of ZA and L-mifamurtide are transient as a consequence of fever and pro-inflammatory cytokines up regulation for example. In that case, these parameters have to be analyzed earlier. In fact, the synergic interaction of ZA and L-mifamurtide on tumor progression may be due to a transient but sufficient effect on pro-inflammatory cytokine upregulation.
Various studies have reported the stimulation of macrophages by L-mifamurtide + IFN-γ in vitro [38,39]. ‘Priming’ of macrophages by IFN-γ may enhance liposome up-take and improve the response to bacterial components such as MDP or presumably mifamurtide. We therefore hypothesized that ZA treatment may increase the IFN-γ level at plasma or local location high enough to ‘prime’ macrophages. It has been reported by clinicians that zoledronic acid therapy leads to fever symptoms in patients for 24-48 h, probably due to transient TNF-α and IL-6 increases, but not IFN-γ [40]. We indeed confirm these data, as we were unable to show a detectable IFN-γ plasma level in the mice 24 hours after zoledronic acid treatment (not shown). It is known that L-mifamurtide binds to the cytosolic NOD2 “receptor” to stimulate NF-ĸB signaling pathways, activating the expression of inflammatory cytokines including IL-12 [41]. It is admitted that plasma IL-12 increases after administration of L-mifamurtide, and that IL-12 upregulates FAS expression in human osteosarcoma lung metastases. It also known that Fas expression inversely correlates with metastatic activity in osteosarcoma [42]. However, we were unable to observe any enhancement of Fas mRNA expression (following L-mifamurtide treatment) in primary bone tumor in both xenogeneic and syngeneic mouse models of osteosarcoma (not shown).
Because L-mifamurtide acts as a potent stimulator of macrophage activity preferentially at lung site [43], the question raised about its action at the primary osteosarcoma site. In parallel, the role of ZA in tumor macrophages population is controversial. Because ZA induced osteoclast apoptosis (via the inhibition of the small G protein prenylation), it could be suggested that ZA also affects macrophages, as previously described in breast cancer [44], especially those present in the bone tumor microenvironment. We hypothesize that L-mifamurtide may counterbalance the potential side effect of ZA on TAMs. Different approaches by IHC, qPCR and flow cytometry were used to study macrophage sub-populations in mice tumor biopsies from xenogeneic and syngeneic osteosarcoma models. However, no differences in macrophage sub-populations infiltrating primary bone tumor could be observed whatever the methodological approaches used.
In conclusion, no interference between zoledronic acid and the liposomal form of mifamurtide was observed for the studied parameters. Combination of both drugs showed no significant difference on ZA induced bone protection effects and on L-mifamurtide induced inhibition of lung metastasis development. Unexpectedly, an additive and in some cases synergistic inhibitory effect was observed on primary osteosarcoma progression that could not be explained by a direct synergy between drugs on osteosarcoma cell proliferation, neither by a modification of TAMs population at the primary bone site. Even if the mechanism remains unclear up to now, those results constitutes a promising demonstration for clinical application in osteosarcoma patients.
Acknowledgements
This work was supported by a grant from Takeda France (Paris, France) and by Region Centre-Val de Loire (MeTaPulm-R; Tours, France). The authors wish thanks G. Hamery, J. Pajot and P. Monmousseau for their kind assistance at the animal facility care platform (Faculté de Médecine, Nantes, France).
Declaration of conflict of interest
None.
References
- 1.Meyers PA, Gorlick R. Osteosarcoma. Pediatric Clinics of North America. 1997;44:973–989. doi: 10.1016/s0031-3955(05)70540-x. [DOI] [PubMed] [Google Scholar]
- 2.Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E. Bone tumours in European children and adolescents, 1978-1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2124–2135. doi: 10.1016/j.ejca.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick JA, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–6. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 4.Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, Applewhite A, Vlamis V, Rosen G. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J. Clin. Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 5.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002;20:776–90. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins DS, Arndt CA. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. 2003;98:2447–2456. doi: 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- 7.Nardin A, Lefebvre ML, Labroquère K, Faure O, Abastado JP. Liposomal muramyl tripeptide phosphatidylethanolamine: Targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr Cancer Drug Targets. 2006;6:123–33. doi: 10.2174/156800906776056473. [DOI] [PubMed] [Google Scholar]
- 8.Kleinerman ES, Erickson KL, Schroit AJ, Fogler WE, Fidler IJ. Activation of Tumoricidal Properties in Human Blood Monocytes by Liposomes Containing Lipophilic Muramyl Tripeptide. Cancer Res. 1983;43:2010–2014. [PubMed] [Google Scholar]
- 9.Fogler WE, Fidler IJ. Nonselective destruction of murine neoplastic cells by syngeneic tumoricidal macrophages. Cancer Res. 1985;45:14–18. [PubMed] [Google Scholar]
- 10.Asano T, McWatters A, An T, Matsushima K, Kleinerman ES. Liposomal muramyl tripeptide up-regulates interleukin-1 alpha, interleukin-1 beta, tumor necrosis factor-alpha, interleukin-6 and interleukin-8 gene expression in human monocytes. J Pharmacol Exp Ther. 1994;268:1032–9. [PubMed] [Google Scholar]
- 11.Anderson P. Liposomal muramyl tripeptide phosphatidyl ethanolamine: ifosfamide-containing chemotherapy in osteosarcoma. Future Oncol. 2006;2:333–343. doi: 10.2217/14796694.2.3.333. [DOI] [PubMed] [Google Scholar]
- 12.Meyers PA. Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma. Expert Rev Anticancer Ther. 2009;9:1035–49. doi: 10.1586/era.09.69. [DOI] [PubMed] [Google Scholar]
- 13.Cryan SA. Carrier-based strategies for targeting protein and peptide drugs to the lungs. AAPS J. 2005;7:E20–41. doi: 10.1208/aapsj070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamboni WC. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin Cancer Res. 2005;11:8230–4. doi: 10.1158/1078-0432.CCR-05-1895. [DOI] [PubMed] [Google Scholar]
- 15.Buddingh EP, Kuijjer ML, Duim RAJ, Bürger H, Agelopoulos K, Myklebost O, Serra M, Mertens F, Hogendoorn PCW, Lankester AC, Cleton-Jansen AM. Tumor-Infiltrating Macrophages Are Associated with Metastasis Suppression in High-Grade Osteosarcoma: A Rationale for Treatment with Macrophage Activating Agents. Clin Cancer Res. 2011;17:2110–2119. doi: 10.1158/1078-0432.CCR-10-2047. [DOI] [PubMed] [Google Scholar]
- 16.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W, Gebhardt M, Goorin AM, Harris MB, Healey J, Huvos A, Link M, Montebello J, Nadel H, Nieder M, Sato J, Siegal G, Weiner M, Wells R, Wold L, Womer R, Grier H. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J. Clin. Oncol. 2005;23:2004–11. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, Yunis EJ, Huvos AG, Betcher DL, Baum ES, Kisker CT, Miser JS. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children’s Cancer Group. J. Clin. Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 18.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE Children’s Oncology Group. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J. Clin. Oncol. 2008;26:633–8. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 19.Heymann D, Ory B, Blanchard F, Heymann MF, Coipeau P, Charrier C, Couillaud S, Thiery JP, Gouin F, Redini F. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone. 2005;37:74–86. doi: 10.1016/j.bone.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Ory B, Heymann MF, Kamijo A, Gouin F, Heymann D, Redini F. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer. 2005;104:2522–2529. doi: 10.1002/cncr.21530. [DOI] [PubMed] [Google Scholar]
- 21.Ribatti D, Mangialardi G, Vacca A. Stephen Paget and the ‘seed and soil’ theory of metastatic dissemination. Clin Exp Med. 2006;6:145–9. doi: 10.1007/s10238-006-0117-4. [DOI] [PubMed] [Google Scholar]
- 22.Chirgwin JM, Guise TA. Molecular mechanisms of tumor-bone interactions in osteolytic metastases. Crit Rev Eukaryot Gene Expr. 2000;10:159–78. [PubMed] [Google Scholar]
- 23.Heymann D, Ory B, Gouin F, Green JR, Rédini F. Bisphosphonates: new therapeutic agents for the treatment of bone tumors. Trends Mol Med. 2004;10:337–43. doi: 10.1016/j.molmed.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, Dreicer R, Kuross SA, Lipton A, Seaman JJ. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaissé JM, Clézardin P. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60:2949–2954. [PubMed] [Google Scholar]
- 26.Gober HJ, Kistowska M, Angman L, Jenö P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–68. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 28.Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, de Souza P, Zheng M, Urbanowitz G, Reitsma D, Seaman JJ. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial--the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J. Clin. Oncol. 2003;21:3150–7. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 29.Leung SY, Jackson J, Miyake H, Burt H, Gleave ME. Polymeric micellar paclitaxel phosphorylates Bcl-2 and induces apoptotic regression of androgen-independent LNCaP prostate tumors. Prostate. 2000;44:156–163. doi: 10.1002/1097-0045(20000701)44:2<156::aid-pros8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Chapurlat RD, Laroche M, Thomas T, Rouanet S, Delmas PD, de Vernejoul MC. Effect of oral monthly ibandronate on bone microarchitecture in women with osteopenia-a randomized placebo-controlled trial. Osteoporos Int. 2013;24:311–20. doi: 10.1007/s00198-012-1947-4. [DOI] [PubMed] [Google Scholar]
- 31.Rosen G, Marcove RC, Caparros B, Nirenberg A, Kosloff C, Huvos AG. Primary osteogenic sarcoma: the rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–2177. doi: 10.1002/1097-0142(197906)43:6<2163::aid-cncr2820430602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 32.Chou AJ, Kleinerman ES, Krailo MD, Chen Z, Betcher DL, Healey JH, Conrad EU, Nieder ML, Weiner MA, Wells RJ, Womer RB, Meyers PA Children’s Oncology Group. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children’s Oncology Group. Cancer. 2009;115:5339–5348. doi: 10.1002/cncr.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriceau G, Ory B, Gobin B, Verrecchia F, Gouin F, Blanchard F, Redini F, Heymann D. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010;16:2981–2987. doi: 10.2174/138161210793563554. [DOI] [PubMed] [Google Scholar]
- 34.Khanna C, Fan TM, Gorlick R, Helman LJ, Kleinerman ES, Adamson PC, Houghton PJ, Tap WD, Welch DR, Steeg PS, Merlino G, Sorensen PHB, Meltzer P, Kirsch DG, Janeway KA, Weigel B, Randall L, Withrow SJ, Paoloni M, Kaplan R, Teicher BA, Seibel NL, Smith M, Uren A, Patel SR, Trent J, Savage SA, Mirabello L, Reinke D, Barkaukas DA, Krailo M, Bernstein M. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res. 2014;20:4200–9. doi: 10.1158/1078-0432.CCR-13-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacEwen EG, Kurzman ID, Rosenthal RC, Smith BW, Manley PA, Roush JK, Howard PE. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst. 1989;81:935–8. doi: 10.1093/jnci/81.12.935. [DOI] [PubMed] [Google Scholar]
- 36.Botter SM, Neri D, Fuchs B. Recent advances in osteosarcoma. Curr Opin Pharmacol. 2014;16:15–23. doi: 10.1016/j.coph.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Sone S, Utsugi T, Tandon P, Ogawara M. A dried preparation of liposomes containing muramyl tripeptide phosphatidylethanolamine as a potent activator of human blood monocytes to the antitumor state. Cancer Immunol Immunother. 1986;22:191–6. doi: 10.1007/BF00200032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pahl JH, Kwappenberg KM, Varypataki EM, Santos SJ, Kuijjer ML, Mohamed S, Wijnen JT, van Tol MJ, Cleton-Jansen AM, Egeler RM, Jiskoot W, Lankester AC, Schilham MW. Macrophages inhibit human osteosarcoma cell growth after activation with the bacterial cell wall derivative liposomal muramyl tripeptide in combination with interferon-γ. J Exp Clin Cancer Res. 2014;33:27. doi: 10.1186/1756-9966-33-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saiki I, Sone S, Fogler WE, Kleinerman ES, Lopez-Berestein G, Fidler IJ. Synergism between Human Recombinant γ-Interferon and Muramyl Dipeptide Encapsulated in Liposomes for Activation of Antitumor Properties in Human Blood Monocytes. Cancer Res. 1985;45:6188–6193. [PubMed] [Google Scholar]
- 40.Dicuonzo G, Vincenzi B, Santini D, Avvisati G, Rocci L, Battistoni F, Gavasci M, Borzomati D, Coppola R, Tonini G. Fever after zoledronic acid administration is due to increase in TNFalpha and IL-6. J Interferon Cytokine Res. 2003;23:649–54. doi: 10.1089/107999003322558782. [DOI] [PubMed] [Google Scholar]
- 41.Kager L, Pötschger U, Bielack S. Review of mifamurtide in the treatment of patients with osteosarcoma. Ther Clin Risk Manag. 2010;6:279–86. doi: 10.2147/tcrm.s5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endo-Munoz L, Evdokiou A, Saunders NA. The role of osteoclasts and tumour-associated macrophages in osteosarcoma metastasis. Biochimica Et Biophysica Acta. 2012;1826:434–442. doi: 10.1016/j.bbcan.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Rietkötter E, Menck K, Bleckmann A, Farhat K, Schaffrinski M, Schulz M, Hanisch UK, Binder C, Pukrop T. Zoledronic acid inhibits macrophage/microglia-assisted breast cancer cell invasion. Oncotarget. 2013;4:1449–1460. doi: 10.18632/oncotarget.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]


