Abstract
Background
The pathogenesis of osteoarthritis following anterior cruciate ligament (ACL) reconstruction is currently unknown. The study purpose was to leverage recent advances in quantitative and dynamic MRI to test the hypothesis that abnormal joint mechanics within four years of reconstruction is accompanied by evidence of early compositional changes in cartilage.
Methods
Static MR imaging was performed bilaterally on eleven subjects with an ACL reconstruction (1-4 years post-surgery) and on twelve healthy subjects to obtain tibial cartilage thickness maps. Quantitative imaging (mcDESPOT) was performed unilaterally on all subjects to assess the fraction of bound water in the tibial plateau cartilage. Finally, volumetric dynamic imaging was performed to assess cartilage contact patterns during an active knee flexion-extension task. A repeated-measures ANOVA was used to test for the effect of surgical reconstruction and location on cartilage thickness, bound water fractions, and contact across the medial and lateral tibia plateau.
Findings
No significant differences in cartilage thickness were found between groups. However, there was a significant reduction in the fraction of water bound by proteoglycan in the ACL reconstructed knees, most notably along the anterior portion of the medial plateau and the weight-bearing lateral plateau. During movement, reconstructed knees exhibited greater contact along the medial spine in the medial plateau and along the posterior aspect of the lateral plateau, when compared with their healthy contralateral knees and healthy controls.
Interpretation
This study provides evidence that abnormal mechanics in anterior cruciate ligament reconstructed knees are present coincidently with early biomarkers of cartilage degeneration.
Keywords: biomechanics, cartilage, anterior cruciate ligament reconstruction, dynamic MRI, thickness, biomarkers
1. INTRODUCTION
Early onset osteoarthritis (OA) is common in ACL-reconstructed (ACLR) knees, with greater than 50% of patients displaying some signs of radiographic OA within 20 years post-surgery (Liden et al., 2008). The underlying etiology of early OA in this patient population remains unknown, but identifying potential causes is important for establishing clinical management approaches that can best mitigate OA risk. However, investigating the pathogenesis of post-traumatic OA is challenging given the long time periods typically needed to detect clinical and radiographic manifestations of the disease. (Haughom et al., 2012).
Recent developments in quantitative magnetic resonance imaging (MRI) have enabled noninvasive evaluation of cartilage composition and ultra-structure. For example, T1rho relaxation rates have been correlated with proteoglycan content (Duvvuri et al., 2002), while T2 relaxation rates have been shown to be sensitive to changes in the collagen fiber network and water content (Mosher et al., 2000). A more recent bi-component T2 mapping technique, mcDESPOT (Deoni et al., 2008; Liu et al., 2014; Liu et al., 2015), can provide relative measures of the fractions of the fast and slow relaxing water components of cartilage which are thought to respectively represent water bound to proteoglycan (FPG) and bulk water loosely associated with the cartilage macromolecular matrix (Reiter et al., 2009). Thus, quantitative MRI can potentially detect compositional changes in cartilage that occur early on in the development in OA (Haughom et al., 2012; Li et al., 2011). Indeed, abnormal T1rho and T2 relaxation rates have been detected in specific regions of the tibiofemoral cartilage within 1-2 years of ACL reconstructive surgery (Li et al., 2011). However, it remains unclear whether biomechanical factors contribute to the changes in MRI biomarkers of early cartilage degeneration.
There is ample evidence that ACLR knees often exhibit subtle abnormalities in knee motion when compared to the contralateral uninjured knee. For example, a small, but significant, shift toward external tibia rotation and medial tibia translation has been observed during locomotion in ACLR knees (Carpenter et al., 2009; Scanlan et al., 2010; Tashman, 2004), along with a potential progressive increase in anterior tibia translation (Hofbauer et al., 2014). It has been theorized that these abnormal kinematic patterns may alter cartilage loading patterns and thereby initiate a cyclic catabolic response that eventually leads to OA (Andriacchi and Mündermann, 2006; Chaudhari et al., 2008). New dynamic MRI sequences can be coupled with high resolution cartilage imaging to investigate whether abnormal kinematics influence cartilage contact patterns during motion (Borotikar and Sheehan, 2013; Kaiser et al., 2013). Further, quantitative MRI can then be used to investigate the association between changes in cartilage contact patterns and the onset of early cartilage degeneration in ACLR knees.
The goal of this study was to use static, dynamic and quantitative MRI to investigate whether abnormal knee mechanics is linked to the pathogenesis of early post-traumatic cartilage degeneration. To do this, we compared images of tibial cartilage morphology, composition, and contact patterns between healthy and ACLR knees within four years of reconstructive surgery. We hypothesized that ACLR knees would display different cartilage contact patterns than their contralateral knees and healthy control knees. Further, we hypothesized ACLR knees would exhibit no change in cartilage morphology but would exhibit region specific reductions in bound water.
2. METHODS
2.1. Subjects
The bilateral knees of eleven subjects with a primary unilateral, isolated ACL-reconstruction (6 F, 24.7 (SD: 4.7) yrs, 83.9 (SD: 17.8) kg, 2.1 (SD: 0.7) years since surgery, 6 patellar tendon grafts, 5 hamstrings grafts, 1 partial lateral meniscectomy, 1 subject with small, stable medial and lateral meniscal tears) and the dominant knees of twelve healthy controls (5F, 24.5 (SD: 4.7) years, 74.9 (SD: 10.0) kg) were tested after obtaining informed consent according to an IRB-approved protocol. Control subjects and the contralateral knees of ACLR subjects had no history of knee pain, injury or surgery and no history of septic, inflammatory or crystalline induced arthritis. ACLR subjects had no history of septic, inflammatory or crystalline induced arthritis, and no post-operative complications. Leg dominance of the control subjects was determined by a self-report of the leg with which they would kick a ball.
2.2. Static and Quantitative MRI
Subjects underwent a static MR protocol consisting of an axial fat-suppressed three-dimensional spoiled gradient recall-echo (3D SPGR) sequence (TR/TE = 10.48/2.24 ms, in-plane resolution = 0.37×0.37 mm, slice thickness = 0.90 mm resolution, image matrix size = 512×512×304 pixels) and a sagittal three-dimensional fast spin-echo (3D FSE Cube) sequence (TR/TE = 2066.7/19.8 ms, in-plane resolution = 0.39×0.39 mm, slice thickness = 1.0 mm resolution, acquisition matrix size = 384×384 pixels) (Fig. 1). These sequences were performed bilaterally for patient subjects and unilaterally for control subjects. A mcDESPOT sequence, consisting of twenty five (8 spoiled gradient echo SPGR, 1 inversion recovery SPGR and 16 balanced steady-state free precession bSSFP) steady-state image sequences with varying flip angles, was performed on the reconstructed knee of the patient subjects and the dominant knee of the control subjects (in-plane resolution = 0.62×0.62 mm, slice thickness = 3.0 mm, image matrix size = 256×256 pixels, (Liu et al., 2014). The mcDESPOT sequence was only performed unilaterally due to limitations in scanning time. All static scans were performed in a 3.0T clinical MR scanner (Discovery MR750, GE Healthcare, Waukesha, WI, USA) using an 8-channel phased array extremity coil (InVivo, Orlando, FL, USA). Foam padding was used to firmly secure the knee within the coil to minimize subject motion during the static MR examination.
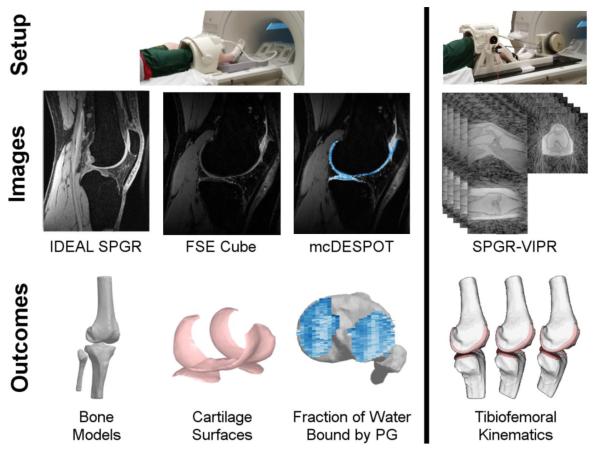
Figure 1. Experimental Protocol.
Subjects underwent a MR protocol consisting of two static sequences (IDEAL SPGR, FSE Cube), a quantitative sequence (mcDESPOT) and dynamic imaging (SPGR-VIPR) of a knee flexion-extension task. The static images were used to create subject-specific models of the bone and cartilage geometries. mcDESPOT was used to compute maps of the fraction of water bound by proteoglycan (FPG). Finally, the bone and undeformed cartilage models were registered to the dynamic images, providing a quantitative characterization of the tibiofemoral contact patterns.
Distal femur and proximal tibia bone geometries were manually segmented from the 3D SPGR images. Femoral and tibial articular cartilage surfaces were manually segmented (MIMICS, Materialise Group, Leuven, Belgium) from the 3D FSE Cube images (Fig 1), smoothed and then described by polygon meshes (approximately 0.33 mm2/triangles) that were registered to the bone models. Anatomical coordinate systems were determined for each bone independently using the bones’ inertial and geometric properties (Miranda et al., 2010). Cartilage thickness was defined as the normal distance from each face of the cartilage mesh to the underlying bone.
The mcDESPOT series was used to reconstruct maps of FPG using a custom Matlab routine (MathWorks, Natick, MA, USA) (Liu et al., 2014). Image registration (FLIRT, Functional Magnetic Resonance Imaging of the Brain Analysis Group, Oxford University, UK) was used to align the static and mcDESPOT image sequences. Cartilage masks, segmented from the 3D FSE Cube images, were then interpolated to the mcDESPOT images to separate the articular cartilage from the surrounding tissues. The FPG of three ACLR and one control subjects were omitted from analysis due to substantial motion artifacts.
2.3. Dynamic MRI
Immediately following the static MRI protocol, subjects were positioned supine with their lower leg secured on a MRI-compatible loading device. Cyclic knee flexion and extension was performed at 0.5 Hz with the rate maintained via an audible metronome. An inertial loading device induced eccentric quadriceps contraction with knee flexion, similar to the load-acceptance phase of gait (Kaiser et al., 2013). During this open-chained task, subjects reached ~35° of knee flexion with a peak extension moment of ~0.5 Nm/kg. A 3D SPGR sequence with vastly-undersampled isotropic projections (SPGR-VIPR, 1.5 mm isotropic resolution, pulse repetition time/echo time = 4 ms/1.4 ms, flip angle = 8°, receiver bandwidth = 32.5 kHz, unique radial lines = 93,922, field of view = 48 cm, scan time = 5 min) was used to continuously collect volumetric image data over five minutes of continuous motion (Fig. 1). A MR-compatible rotary encoder (MR310, Micronor, Newbury Park, CA, USA) mounted on the device was used to monitor knee flexion angle. The encoder data (collected at 50 Hz) was used retrospectively to bin the SPGRVIPR projections into 60 equal duration intervals over the motion cycle. Sixty SPGR-VIPR images were then reconstructed offline with no view-sharing between frames. We dynamically imaged both the reconstructed and contralateral knees in the subjects who previously underwent ACL-reconstructive surgery. Dynamic imaging was only performed on the dominant knee of healthy control subjectss.
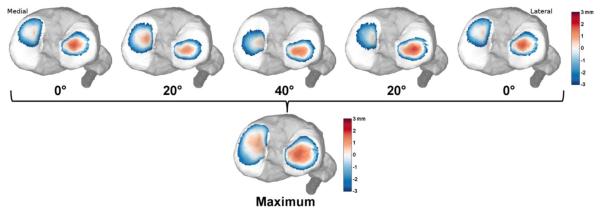
Femoral and tibial bone segments and cartilage surfaces were registered to each frame of the dynamic images. Registration was achieved by using numerical optimization to position and orient the bone segments in a way that minimized the sum squared intensities of the dynamic images at the outer bone model vertices (Powell, 1964). This numerical routine drives the bone models to the dark bone outlines in the dynamic images, and yields the bone 3D translations and rotations in space. Tibiofemoral kinematics were defined as the position and body fixed rotations (flexion-adduction-internal rotation) of the tibia relative to the femur (Grood and Suntay, 1983). Tibiofemoral kinematics were low pass filtered with a third-order bidirectional Butterworth filter with a cut-off frequency of 5 Hz. Contact patterns were characterized by the proximity between the undeformed tibial and femoral cartilage surfaces. Proximity was calculated at each face of the tibial mesh by projecting along the normal direction to determine the closest femoral mesh face. Positive proximity was indicative of cartilage contact at that location. Tibial cartilage proximity through flexion and extension was re-zeroed such that at least one mesh triangle remained in contact in both the medial and lateral compartments at each frame (Borotikar and Sheehan, 2013). Tibial plateau proximity maps were presented for those faces in contact or within 3 mm of the femoral cartilage surface. A tibial plateau proximity map was then defined as the closest proximity of each face throughout the motion cycle (Fig 2).
Figure 2. Dynamic cartilage contact.
Tibiofemoral kinematics were used to characterize regions of proximity between the tibial and femoral cartilage. Contact maps for each subject were created by identifying the greatest proximity of each face of the cartilage mesh over a flexion-extension motion cycle.
2.6 Statistical Analysis
Our primary metrics were cartilage thickness, FPG and proximity over the tibial plateau surface. Regional analysis was performed by equally dividing a bounding box of the medial and lateral compartments of the tibial plateau cartilage into 20 rectangular regions of interest (ROIs). The anterior-posterior and medial-lateral directions were determined from the tibial anatomical coordinate systems, described above. Each of the primary metrics were averaged for all cartilage surface pixels within the ROI. FPG measures were averaged through the cartilage thickness. The most lateral and medial ROIs were excluded for the FPG comparisons due to significant partial-volume fractioning at these locations.
A repeated measures ANOVA tested the effect of surgery (between-group factor) and location (within-group factor) on thickness, FPG, and contact between the ACLR knees and the control subject knees. A repeated measures ANOVA was used to compare the cartilage thickness and contact of the ACLR knees to the contralateral knees of the same subjects. If a significant difference was found (P<0.05), a post-hoc Tukey test was performed to identify the location of group differences (P<0.05).
3. RESULTS
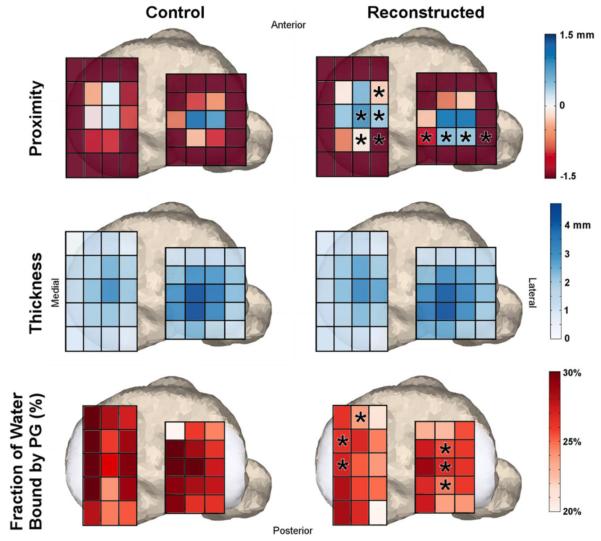
The thickest cartilage regions were found in the middle regions of the medial (ACLR: 3.4 (SD: 0.9) mm, contralateral: 3.4 (SD: 0.8) mm, control: 3.1 (SD: 0.7) mm) and lateral (ACLR: 4.5 (SD: 1.0) mm, contralateral: 4.6 (SD: 0.8) mm, control: 4.6 (SD: 0.7) mm) compartments of the tibial plateau (Fig. 3). There were no significant differences in cartilage thickness between any groups (Fig. 4, Fig. 5).
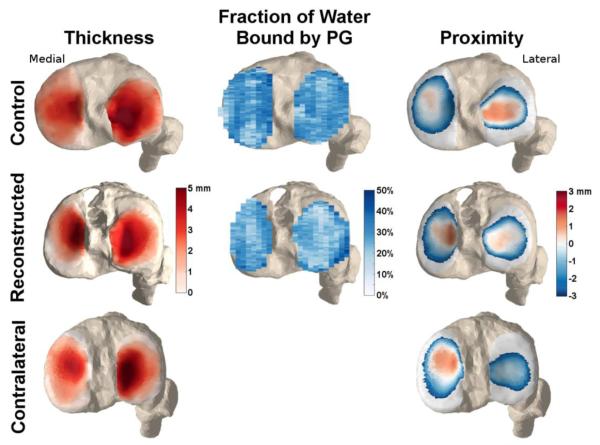
Figure 3. Example maps of cartilage thickness, FPG, and thickness.
Representative thickness, fraction of water bound by proteoglycan (FPG), and proximity maps for the tibial plateau of one control subject, an ACL-reconstructed knee and their healthy contralateral knee. Note the greater contact along the medial spine of the medial plateau, and the posteriolateral tibia of the reconstructed knee, when compared to the contralateral and control knees. The subject also exhibits lower fraction of bound water relative to the control, particularly in the lateral tibia plateau.
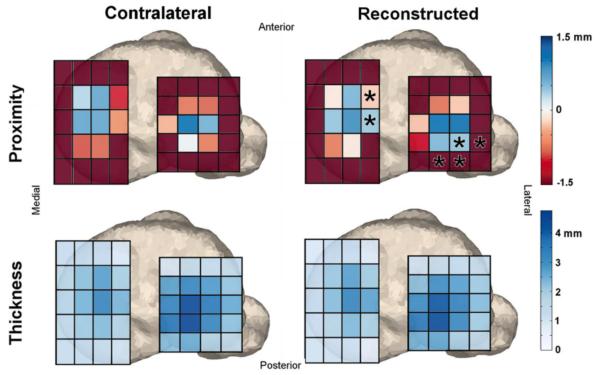
Figure 4. ROI comparison with contralateral knees.
Region of interest comparisons of average cartilage proximity and thickness between reconstructed and contralateral knees of the subjects who underwent unilateral ACL reconstructive surgery. Asterisks denote areas of significantly larger contact along the medial spine and on the posterior portion of the lateral tibial plateau.
Figure 5. ROI comparison with control knees.
Region of interest comparisons of average cartilage proximity, thickness and fraction of water bound by proteoglycan (FPG) between reconstructed knees and healthy control knees. Asterisks denote areas of significant difference between groups. There were no significant differences in cartilage thickness, but there were regions of significantly greater magnitudes of cartilage contact and lower fractions of bound water on both the medial and lateral tibia plateaus.
The average FPG in the tibial plateau cartilage ranged from 15.3%-38.5% and 11.3%-37.8% in the control and ACLR knees, respectively. There was a significant decrease in the FPG in the ACLR knees (25.5% (SD: 1.5%)) when compared with the control knees (27.5% (SD: 1.6%)). These differences were apparent in the anterior portion of the medial tibia plateau and the medial portion of the lateral tibial plateau (Fig 5).
Cartilage proximity over the tibial plateau cartilage was significantly greater in the ACLR knee when compared to both the control (−0.4 mm average difference) and contralateral (−0.3 mm) knees. In the post-hoc analyses, significantly greater contact in the ACLR knees was observed along the medial ridge of the medial tibial plateau and the posterior aspect of the lateral tibial plateau (Fig. 4, Fig. 5).
4. DISCUSSION
It has been speculated that abnormal knee mechanics can contribute to the development of early OA in ACL-reconstructed knees (Andriacchi et al., 2009). However, direct links between abnormal mechanics and early manifestations of OA are lacking. In this study, we leveraged advances in dynamic and quantitative MRI techniques to test the hypotheses that ACLR knees will exhibit evidence of abnormal cartilage loading and cartilage composition within four years of surgery. Our hypotheses were supported, with ACLR knees exhibiting greater contact in characteristic regions of both the medial and lateral tibial plateau. Further, despite no evidence of cartilage thinning, we noted a decrease in the fraction of bound water in the ACLR knees. Thus, this study provides evidence that early MRI biomarkers of cartilage degeneration coincide with the time point at which abnormal knee mechanics can be detected in ACL reconstructed knees.
To our knowledge, this is the first study to show that abnormal tibiofemoral cartilage contact patterns can be detected in reconstructed knees with dynamic MRI. Prior studies using biplanar fluoroscopy have identified greater joint sliding in the medial tibiofemoral compartment during downhill running (Hoshino et al., 2013) A posteriolateral shift in contact in the medial plateau and a posteriomedial shift in the lateral plateau were also found during a single leg quasi-static lunge (Hosseini et al., 2012). Similarly, we observed a significant lateral shift in contact on the medial compartment and a posterior shift on the lateral compartment in the ACLR knees. The similarity of these results suggests that there may be systematic differences in the passive restraint provided by an ACL graft, relative to the native ACL. It is noteworthy that we observed such systematic shifts despite the study population having mixed clinical presentation, graft types and meniscal conditions. This would suggest that other surgical factors, such as graft placement (Abebe et al., 2011; Bedi et al., 2011) and pre-tensioning (Brady et al., 2007; Melby et al., 1991), may contribute to the altered knee mechanics. While greater subject numbers are needed to better test the effects of surgical factors, ultimately computational knee models are important to establish causal relationships between surgical factors and functional knee behavior (Lenhart et al., 2015; Pena et al., 2006; Salehghaffari and Dhaher, 2014; Smith et al., 2015)
The absence of changes in cartilage thickness in the ACLR knees within 4 years is consistent with prior studies suggesting that longer time frames are needed for cartilage thinning to be visible. For example, (Andreisek et al., 2009) found a very small amount of cartilage thinning (on average <0.1 mm) in the lateral aspect of the lateral tibial compartment seven years following ACLR surgery. Additional studies have shown either longitudinal increases in cartilage thickness in ACLR knee over a 2 year follow-up period (Frobell, 2011) or no changes in cartilage thickness in ACLR knees when compared to the contralateral healthy knees at 7 year follow-up (Andreisek et al., 2009).
In contrast, quantitative MRI is able to detect potential evidence of altered cartilage composition within 1-2 years of reconstruction (Li et al., 2011; Tiderius et al., 2005). Quantitative MRI also has the benefit of providing a laminar analysis of cartilage (Li et al., 2011), potentially providing additional information on the depth-dependent changes of early post-traumatic cartilage degeneration. In this study, we found a decrease in the fraction of water bound by proteoglycan. Proteoglycan is necessary to produce the hydrostatic compressive stiffness of cartilage (Buschmann and Grodzinsky, 1995) and the loss of proteoglycan has been identified as a critical event in OA (Rizkalla et al., 1992; Sandy et al., 1992). While quantitative MRI has been directly linked to histological changes evident in OA (Regatte et al., 2006), further study is still needed to see whether changes in these MRI parameters are associated with in vivo cartilage loss over time and the development of joint pain and radiographic OA.
Interestingly, we found no direct correspondence between the regions of abnormal cartilage contact and lower FPG. While reductions in bound water were evident in the reconstructed knees, significant differences were found in cartilage adjoining the higher contact regions. This could be due to several factors. First, all metrics were averaged within ROIs on the order of 60 mm2. While the ROIs simplify the analysis, it also significantly reduces the quantitative detail available with our methodology. Other studies have shown interesting information exists not only in the magnitude of individual voxels of relaxation rates maps, but also in the spatial distribution of relaxation rates (Blumenkrantz et al., 2008; Li et al., 2009). A voxel-based analysis therefore may further elucidate the relationship between contact, morphology, and composition in the ACLR knees. Furthermore, changes in knee biomechanics may not be the only cause of early cartilage degeneration in ACLR knees. Other factors not investigated in our study including meniscus tears (Li et al., 2011; Neuman et al., 2011), acute cartilage injury sustained at the time of joint trauma (Bolbos et al., 2008) and post-traumatic synovial inflammation (Elsaid et al., 2008; Marks and Donaldson, 2005), all of which may have played a role in reduction in bound water seen in our patient population.
We also recognize that our cartilage contact metric may not fully capture the abnormal mechanical environment of the cartilage tissue. Cellular mechano-transduction is one potential mechanism controlling the catabolic response of cartilage post-ACLR and is influenced by changes in fluid flows and pressures (Mizuno et al., 2002), osmotic levels (Hopewell and Urban, 2002), and changes in pH (Halloran et al., 2012). At a tissue scale, these signals are altered by differing compressive and shear strains (Guilak and Hung, 2005), which are further influenced by cartilage thickness, stiffness, and sliding friction. Our proximity metric likely best reflects compressive pressure and perhaps could be used within a computational model of the cartilage tissue to more fully characterize factors that can regulate mechano-biological responses. Further, emerging imaging techniques for measuring in vivo cartilage tissue strain (Coleman et al., 2013; Neu and Walton, 2008) may provide greater insights into functional cartilage tissue behavior.
Our study was limited to an ROI analysis of only the tibial plateau, though early OA has been found in all articular cartilages of the tibiofemoral (Kessler et al., 2008; Meunier et al., 2007; Sward et al., 2010) and patellofemoral (Neuman et al., 2009) joints in ACLR knees. SPGR-VIPR and the mcDESPOT sequences provide a large enough field of view (40×40×40 cm) to enable kinematic and compositional information of all three joints, allowing us to perform a similar analysis of the femoral and patellar cartilages in the future. Additionally, the complete mcDESPOT analysis was available for only a subset of subjects (8/11 patient subjects, 11/12 control subjects). While the flexion-extension task performed used in this study is not weight-bearing, the loading paradigm was designed to induce eccentric quadriceps loads of comparable magnitudes to that seen in walking (Kaiser et al., 2013). It is notable that the abnormalities in cartilage contact in the ACLR knees are comparable to that seen during lunges while using biplane fluoroscopy (Hosseini et al., 2012). This result is important since it means that morphology measures, functional behavior and biomarkers of cartilage degeneration can all potentially be assessed with clinical MRI scanners. Finally, our dynamic MRI methodology acquires image data over many repeat motion cycles, which necessitates the repeatability of the task. Our prior study shows that subjects can closely replicate the desired cycle period when undergoing an inertial loading (Kaiser et al., 2013).
In summary, we found evidence of a shift in the location of contact in the medial and lateral tibial plateaus following ACL-reconstruction surgery. We also identified significantly lower fractions of bound water in the tibial cartilage, which may reflect early cartilage degeneration. Future work will try to explore potential links between surgical factors, cartilage contact and MRI biomarkers following ACLR.
HIGHLIGHTS.
MRI was used to image cartilage composition and contact in ACL-reconstructed knees
Reconstructed knees exhibited abnormal contact on the tibia plateau during movement
There were lower fractions of water bound by proteoglycan in reconstructed knees
No cartilage thickness difference was found between reconstructed and control knees
Results consistent with theorized link between mechanics and cartilage degeneration
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the funding provided by the NIH (EB015410, AR062733) and the contributions of Oliver Wieben, Kevin Johnson, Kelli Hellenbrand, Jan Yakey, Rachel Lenhart, Colin Smith, James Hermus, and Arezu Monawer.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abebe ES, Kim JP, Utturkar GM, Taylor DC, Spritzer CE, Moorman CT, 3rd, Garrett WE, DeFrate LE. The effect of femoral tunnel placement on ACL graft orientation and length during in vivo knee flexion. J Biomech. 2011;44:1914–1920. doi: 10.1016/j.jbiomech.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreisek G, White LM, Sussman MS, Kunz M, Hurtig M, Weller I, Essue J, Marks P, Eckstein F. Quantitative MR imaging evaluation of the cartilage thickness and subchondral bone area in patients with ACL-reconstructions 7 years after surgery. Osteoarthritis Cartilage. 2009;17:871–878. doi: 10.1016/j.joca.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91(Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriacchi TP, Mündermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Current opinion in rheumatology. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- Bedi A, Maak T, Musahl V, Citak M, O'Loughlin PF, Choi D, Pearle AD. Effect of tibial tunnel position on stability of the knee after anterior cruciate ligament reconstruction: is the tibial tunnel position most important? Am J Sports Med. 2011;39:366–373. doi: 10.1177/0363546510388157. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz G, Stahl R, Carballido-Gamio J, Zhao S, Lu Y, Munoz T, Le Graverand-Gastineau M-PH, Jain S, Link T, Majumdar S. The feasibility of characterizing the spatial distribution of cartilage T 2 using texture analysis. Osteoarthritis and Cartilage. 2008;16:584–590. doi: 10.1016/j.joca.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolbos RI, Ma CB, Link TM, Majumdar S, Li X. In vivo T1ρ quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance imaging. Investigative radiology. 2008;43:782. doi: 10.1097/RLI.0b013e318184a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borotikar BS, Sheehan FT. In vivo patellofemoral contact mechanics during active extension using a novel dynamic MRI-based methodology. Osteoarthritis and Cartilage. 2013:9. doi: 10.1016/j.joca.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady MF, Bradley MP, Fleming BC, Fadale PD, Hulstyn MJ, Banerjee R. Effects of initial graft tension on the tibiofemoral compressive forces and joint position after anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35:395. doi: 10.1177/0363546506294363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann M, Grodzinsky A. A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J Biomech Eng. 1995;117:179–192. doi: 10.1115/1.2796000. [DOI] [PubMed] [Google Scholar]
- Carpenter RD, Majumdar S, Ma CB. Magnetic resonance imaging of 3-dimensional in vivo tibiofemoral kinematics in anterior cruciate ligament-reconstructed knees. Arthroscopy. 2009;25:760–766. doi: 10.1016/j.arthro.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Chaudhari AMW, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee Kinematics, Cartilage Morphology, and Osteoarthritis after ACL Injury. Med Sci Sports Exerc. 2008;40:8. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- Coleman JL, Widmyer MR, Leddy HA, Utturkar GM, Spritzer CE, Moorman CT, III, Guilak F, DeFrate LE. Diurnal variations in articular cartilage thickness and strain in the human knee. J Biomech. 2013;46:541–547. doi: 10.1016/j.jbiomech.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magnetic Resonance in Medicine. 2008;60:1372–1387. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- Duvvuri U, Kudchodkar S, Reddy R, Leigh JS. T1ρ relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthritis and Cartilage. 2002;10:838–844. doi: 10.1053/joca.2002.0826. [DOI] [PubMed] [Google Scholar]
- Elsaid K, Fleming B, Oksendahl H, Machan J, Fadale P, Hulstyn M, Shalvoy R, Jay G. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis & Rheumatism. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frobell RB. Change in cartilage thickness, posttraumatic bone marrow lesions, and joint fluid volumes after acute ACL disruption. The Journal of Bone & Joint Surgery. 2011;93:1096–1103. doi: 10.2106/JBJS.J.00929. [DOI] [PubMed] [Google Scholar]
- Grood E, Suntay W. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:9. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- Guilak F, Hung C. Physical regulation of cartilage metabolism. Lippincott Williams & Wilkins; Philadephia: 2005. [Google Scholar]
- Halloran J, Sibole S, van Donkelaar C, van Turnhout M, Oomens C, Weiss J, Guilak F, Erdemir A. Multiscale mechanics of articular cartilage: potentials and challenges of coupling musculoskeletal, joint, and microscale computational models. Ann Biomed Eng. 2012;40:2456–2474. doi: 10.1007/s10439-012-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughom B, Schairer W, Souza RB, Carpenter D, Ma CB, Li X. Abnormal tibiofemoral kinematics following ACL reconstruction are associated with early cartilage matrix degeneration measured by MRI T1rho. Knee. 2012;19:482–487. doi: 10.1016/j.knee.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer M, Thorhauer ED, Abebe E, Bey M, Tashman S. Altered Tibiofemoral Kinematics in the Affected Knee and Compensatory Changes in the Contralateral Knee After Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2014 doi: 10.1177/0363546514549444. 0363546514549444. [DOI] [PubMed] [Google Scholar]
- Hopewell B, Urban J. Adaptation of articular chondrocytes to changes in osmolality. Biorheology. 2002;40:73–77. [PubMed] [Google Scholar]
- Hoshino Y, Fu F, Irrgang J, Tashman S. Can Joint Contact Dynamics Be Restored by Anterior Cruciate Ligament Reconstruction? Clin Orthop Relat Res. 2013 doi: 10.1007/s11999-012-2761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A, Van de Velde S, Gill TJ, Li G. Tibiofemoral cartilage contact biomechanics in patients after reconstruction of a ruptured anterior cruciate ligament. J Ortho Research. 2012;30:1781–1788. doi: 10.1002/jor.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Bradford R, Johnson K, Wieben O, Thelen DG. Measurement of tibiofemoral kinematics using highly accelerated 3D radial sampling. Magnetic Resonance in Medicine. 2013;69:1310–1316. doi: 10.1002/mrm.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler MA, Behrend H, Henz S, Stutz G, Rukavina A, Kuster MS. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16:442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- Lenhart RL, Kaiser J, Smith CR, Thelen DG. Prediction and Validation of Load- Dependent Behavior of the Tibiofemoral and Patellofemoral Joints During Movement. Ann Biomed Eng. 2015:1–11. doi: 10.1007/s10439-015-1326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, Ma CB, Majumdar S. Cartilage in anterior cruciate ligament-reconstructed knees: MR T1rho and T2-initial experience with 1-year follow-up. Radiology. 2011;258:10. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, Ries M, Majumdar S. Spatial distribution and relationship of T1ρ and T2 relaxation times in knee cartilage with osteoarthritis. Magnetic Resonance in Medicine. 2009;61:1310–1318. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liden M, Sernert N, Rostgard-Christensen L, Kartus C, Ejerhed L. Osteoarthritic changes after anterior cruciate ligament reconstruction using bone-patellar tendon-bone or hamstring tendon autografts: a retrospective, 7-year radiographic and clinical follow-up study. Arthroscopy. 2008;24:10. doi: 10.1016/j.arthro.2008.04.066. [DOI] [PubMed] [Google Scholar]
- Liu F, Chaudhary R, Hurley SA, Rio A, Alexander AL, Samsonov A, Block WF, Kijowski R. Rapid multicomponent T2 analysis of the articular cartilage of the human knee joint at 3.0 T. Journal of Magnetic Resonance Imaging. 2014;39:1191–1197. doi: 10.1002/jmri.24290. [DOI] [PubMed] [Google Scholar]
- Liu F, Choi KW, Samsonov A, Spencer RG, Wilson JJ, Block WF, Kijowski R. Articular Cartilage of the Human Knee Joint: In Vivo Multicomponent T2 Analysis at 3.0 T. Radiology. 2015:142201. doi: 10.1148/radiol.2015142201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks PH, Donaldson MLC. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate Ligament–Deficient knee. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2005;21:1342–1347. doi: 10.1016/j.arthro.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Melby A, Noble JS, Askew MJ, Boom AA, Hurst FW. The effects of graft tensioning on the laxity and kinematics of the anterior cruciate ligament reconstructed knee. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 1991;7:257–266. doi: 10.1016/0749-8063(91)90123-f. [DOI] [PubMed] [Google Scholar]
- Meunier A, Odensten M, Good L. Long-term results after primary repair or non-surgical treatment of anterior cruciate ligament rupture: a randomized study with a 15-year follow-up. Scand J Med Sci Sports. 2007;17:230–237. doi: 10.1111/j.1600-0838.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- Miranda DL, Rainbow MJ, Leventhal EL, Crisco JJ, Fleming BC. Automatic determination of anatomical coordinate systems for three-dimensional bone models of the isolated human knee. J Biomech. 2010;43:4. doi: 10.1016/j.jbiomech.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno S, Tateishi T, Ushida T, Glowacki J. Hydrostatic fluid pressure enhances matrix synthesis and accumulation by bovine chondrocytes in three-dimensional culture. Journal of Cellular Physiology. 2002;193:319–327. doi: 10.1002/jcp.10180. [DOI] [PubMed] [Google Scholar]
- Mosher TJ, Dardzinski BJ, Smith MB. Human Articular Cartilage: Influence of Aging and Early Symptomatic Degeneration on the Spatial Variation of T2—Preliminary Findings at 3 T 1. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- Neu CP, Walton JH. Displacement encoding for the measurement of cartilage deformation. Magnetic Resonance in Medicine. 2008;59:149–155. doi: 10.1002/mrm.21464. [DOI] [PubMed] [Google Scholar]
- Neuman P, Kostogiannis I, Fridén T, Roos H, Dahlberg L, Englund M. Patellofemoral osteoarthritis 15 years after anterior cruciate ligament injury–a prospective cohort study. Osteoarthritis and Cartilage. 2009;17:284–290. doi: 10.1016/j.joca.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Neuman P, Tjörnstrand J, Svensson J, Ragnarsson C, Roos H, Englund M, Tiderius CJ, Dahlberg L. Longitudinal assessment of femoral knee cartilage quality using contrast enhanced MRI (dGEMRIC) in patients with anterior cruciate ligament injury–comparison with asymptomatic volunteers. Osteoarthritis and Cartilage. 2011;19:977–983. doi: 10.1016/j.joca.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Pena E, Calvo B, Martinez MA, Palanca D, Doblare M. Influence of the tunnel angle in ACL reconstructions on the biomechanics of the knee joint. Clin Biomech. 2006;21:508–516. doi: 10.1016/j.clinbiomech.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Powell MJD. An efficient method for finding the minimum of a function of several variables without calculating derivatives. The Computer Journal. 1964;7:155. [Google Scholar]
- Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23:547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- Reiter DA, Lin PC, Fishbein KW, Spencer RG. Multicomponent T2 relaxation analysis in cartilage. Magnetic Resonance in Medicine. 2009;61:803–809. doi: 10.1002/mrm.21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkalla G, Reiner A, Bogoch E, Poole A. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. Journal of Clinical Investigation. 1992;90:2268. doi: 10.1172/JCI116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehghaffari S, Dhaher YY. A model of articular cruciate ligament reconstructive surgery: A validation construct and computational insights. J Biomech. 2014;47:1609–1617. doi: 10.1016/j.jbiomech.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. Journal of Clinical Investigation. 1992;89:1512. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43:1817–1822. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CR, Lenhart RL, Kaiser J, Vignos MF, Thelen DG. Influence of Ligament Properties on Tibiofemoral Mechanics in Walking. The journal of knee surgery. 2015 doi: 10.1055/s-0035-1558858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sward P, Kostogiannis I, Neuman P, Von Porat A, Boegard T, Roos H. Differences in the radiological characteristics between post-traumatic and non-traumatic knee osteoarthritis. Scand J Med Sci Sports. 2010;20:731–739. doi: 10.1111/j.1600-0838.2009.01000.x. [DOI] [PubMed] [Google Scholar]
- Tashman S. Abnormal Rotational Knee Motion During Running After Anterior Cruciate Ligament Reconstruction. American Journal of Sports Medicine. 2004;32:975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- Tiderius CJ, Olsson LE, Nyquist F, Dahlberg L. Cartilage glycosaminoglycan loss in the acute phase after an anterior cruciate ligament injury: Delayed gadolinium-enhanced magnetic resonance imaging of cartilage and synovial fluid analysis. Arthritis & Rheumatism. 2005;52:120–127. doi: 10.1002/art.20795. [DOI] [PubMed] [Google Scholar]