Abstract
Herein, we describe a regioselective Rh-catalyzed decarboxylative cross-coupling of β–keto acids and alkynes to access branched γ,δ–unsaturated ketones. Rh-hydride catalysis enables the isomerization of an alkyne to generate a metal-allyl species that can undergo carbon-carbon bond formation. Ketones are generated under mild conditions, without the need for base or activated electrophiles.
A range of natural processes are driven by the loss of carbon dioxide, from polyketide synthesis to γ-aminobutyric acid (GABA) production.1 Various synthetic strategies have emerged using the formation of CO2 gas as the driving force. Tsuji and Saegusa independently reported decarboxylative allylation of β-keto allyl esters.2,3 Shair developed a decarboxylative aldol using malonic acid half thioesters,4 while Gooßen pioneered decarboxylative biaryl cross-couplings.5 More recently, MacMillan and Doyle have used CO2 gas extrusion and photoredox catalysis to generate a wide range of cross-couplings, including those that generate Csp2–Csp3 bonds.6 Most relevant to our study, Breit has developed a bioinspired coupling of β-keto acids with allenes under Rh-hydride catalysis.7,8 It occurred to us that by using tandem Rh-catalysis, we could achieve a complementary cross-coupling of β-keto acids with alkynes. We chose alkynes as allyl electrophiles because they are a common and readily accessible functional group. Our approach would enable unique access to ketones under mild conditions, without the need for generating enolates or the use of activated allylating agents.9–13
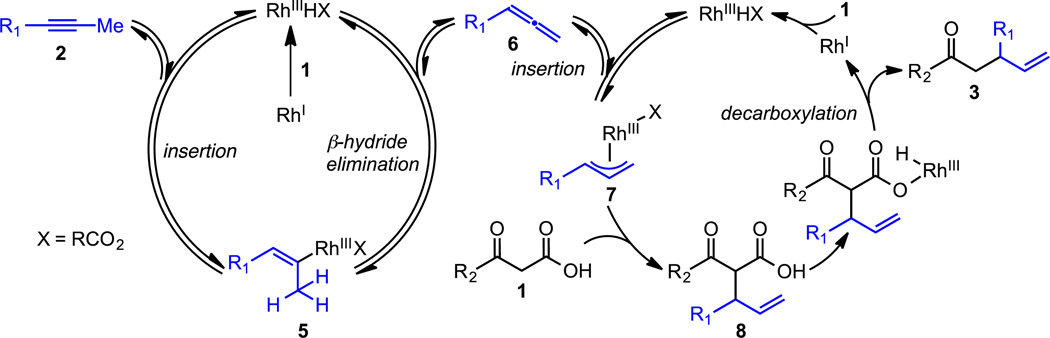
On the basis of previous studies from Yamamoto,14 Breit,15 and our laboratory,16 we proposed a pathway involving tandem Rh-catalysis to enable decarboxylative coupling between β-keto acids 1 and alkynes 2 (Scheme 1).17 First, β-keto acid 1 and a Rh(I) species combine to generate a Rh(III)-hydride intermediate.18 Insertion of alkyne 2 into the Rh(III)–H bond gives Rh-vinyl species 5. Subsequent β-hydride elimination generates allene 6 and regenerates the Rh(III)-hydride species. Insertion of allene 6 into the Rh(III)-H bond then forms Rh(III)-allyl species 7 that can be trapped with a carbon-based nucleophile.19 Indeed, Breit recently reported the coupling of 1,3-diketones with terminal alkynes.20 In the presence of β-keto acid 1, C–C bond formation yields allylated β-keto acid 8.21 Finally, decarboxylation affords the desired ketone 3.
Scheme 1.
Proposed decarboxylative β-keto acid and alkyne coupling via tandem Rh-Catalysis.
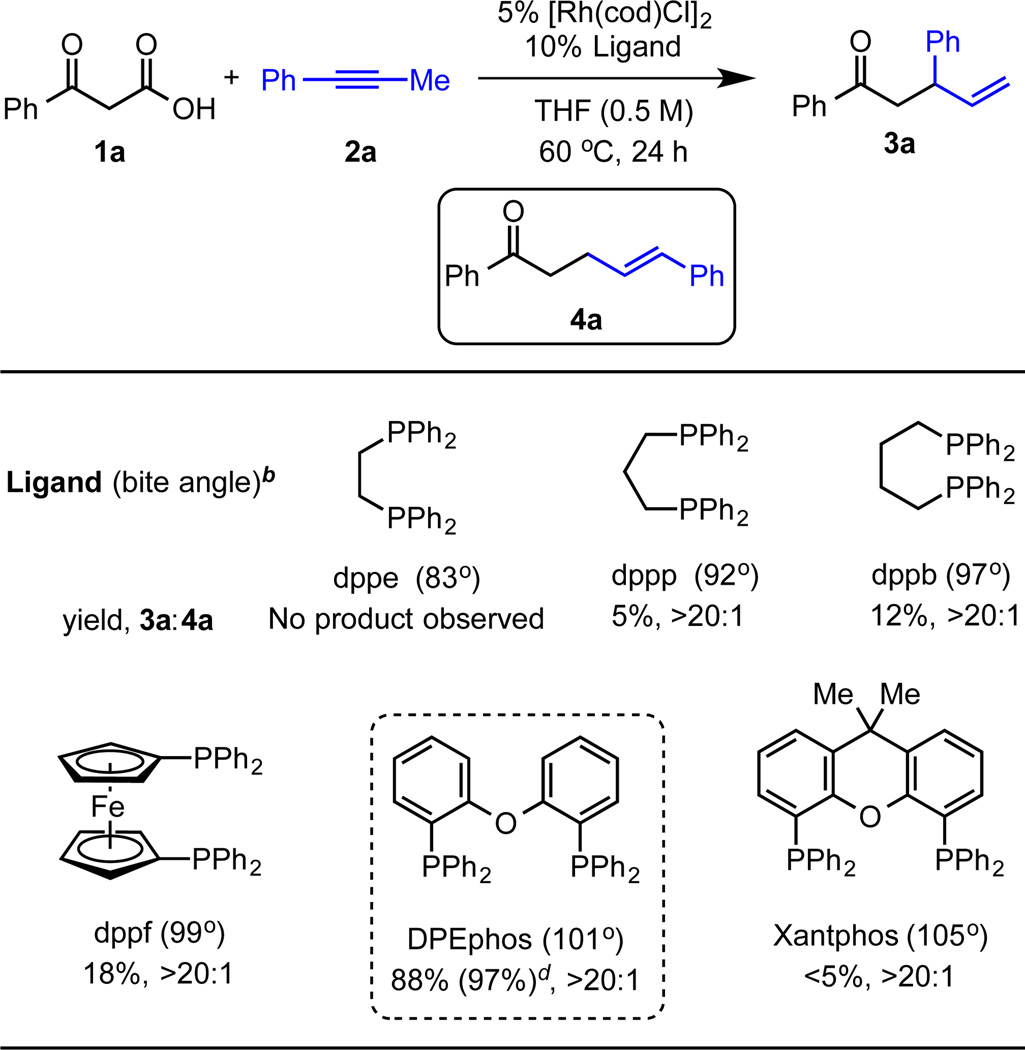
To test our mechanistic proposal, we investigated the cross-coupling of benzoylacetic acid (1a) and 1-phenyl-1-propyne (2a). In the presence of 5 mol% of [Rh(cod)Cl]2 and 10 mol% 1,3-bis(diphenylphosphino)propane (dppp), the desired branched γ, δ–unsaturated ketone 3a was observed in 5% yield with >20:1 branched to linear regioselectivity (Figure 1). Notably, no allyl ester formation was observed despite the precedence for C–O bond formation between carboxylic acids and alkynes.22 The major by-product observed was acetophenone arising from decarboxylation of benzoylacetic acid (1a). From further evaluation of bidentate phosphine ligands, we observed a relationship between ligand bite angle and reactivity. Bisphosphine ligands with larger bite angles than dppp, such as 1,4-bis(diphenylphosphino)butane (dppb) and 1, 1’-bis(diphenylphosphino)ferrocene (dppf), resulted in increased reactivity. Further increasing the bite angle by use of Xantphos as a ligand resulted in a dramatic decrease in reactivity. Using DPEphos provided an optimal bite angle of approximately 101° for promoting the desired transformation.23 By switching from THF to 2-MeTHF and increasing the equivalents of benzoylacetic acid (1a), the catalyst loading can be decreased while increasing the yield to 97%.
Figure 1.
Ligand Effects on Decarboxylative β-keto Acid and Alkyne Coupling.a
aReaction conditions: 0.1 mmol 1a, 0.1 mmol 2a, 5 mol% [Rh(cod)Cl]2, 10 mol% ligand, 0.2 mL THF (0.5 M), 60 °C, 24 hours. bSee ref 23. cDetermined by GC-FID analysis using mesitylene as internal standard. dUsing 0.2 mmol 1a, 4 mol% [Rh(cod)Cl]2, 8 mol% DPEphos, and 2-MeTHF instead.
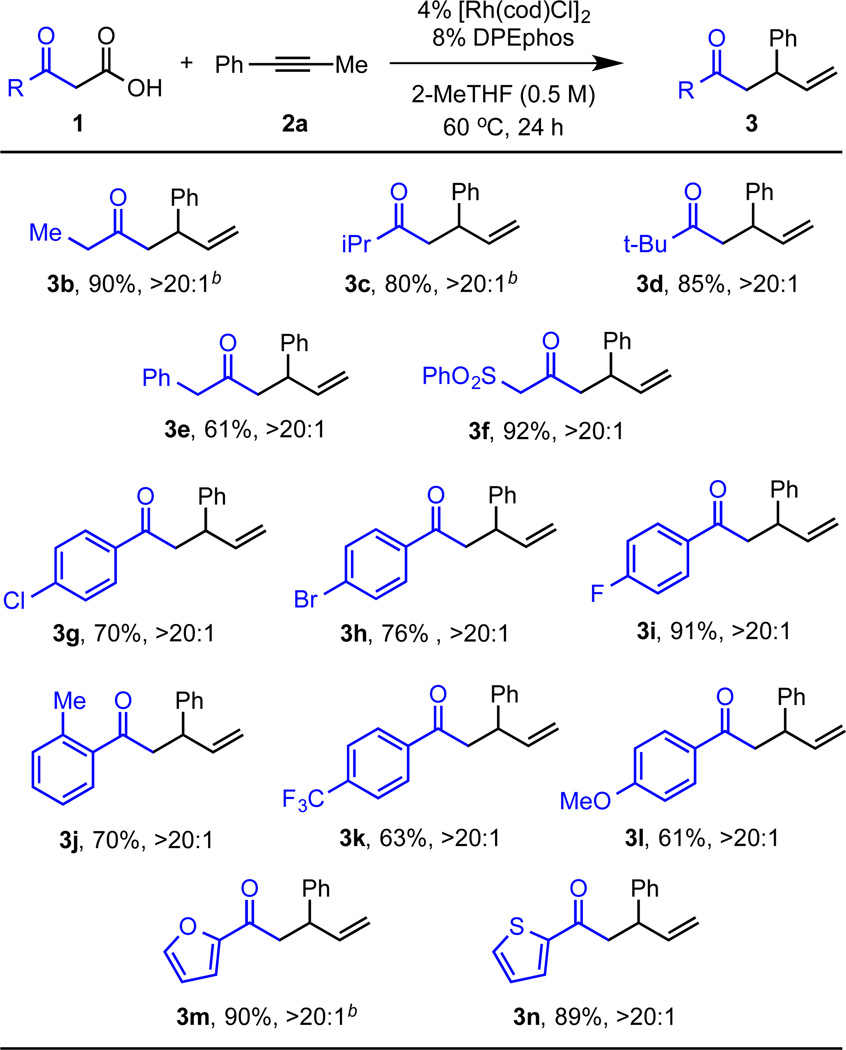
With this protocol in hand, we explored the coupling of various β–keto acids 1 with 1-phenyl-1-propyne (2a). Aliphatic β–keto acids, bearing multiple acidic α-hydrogens, were alkylated with >20:1 regioselectivity (Figure 2). Primary (3b, 3e, and 3f), secondary (3c), and tertiary (3d) substitution are all tolerated (61–92%). Notably, β–keto acids with electron-withdrawing groups (phenyl and phenylsulfonyl) can be used to give ketones formally derived from the methyl-ketone dianions (highlighted in blue, 3e and 3f). β–keto acids bearing aromatic rings with a variety of substituents underwent alkylation with high branched to linear regioselectivity. Halogenated aromatic rings are well tolerated (3g–3i, 70–91%). Regioselective coupling still occurs when the aromatic ring has an ortho-methyl group (3j). In addition, electron-deficient para-trifluoromethyl and electron-rich para-methoxy substituted rings are tolerated (3k and 3l, 63% and 61%, respectively). Finally, β–keto acids with heterocycles (e.g., furan and thiophene) can be used as carbon pronucleophiles to yield 3m and 3n (90% and 89%, respectively).
Figure 2.
Branched Selective Decarboxylative Coupling of Alkyne 2a with Various β –keto Acids.a
aReaction conditions: 0.4 mmol 1, 0.2 mmol 2a, 4 mol% [Rh(cod)Cl]2, 8 mol% DPEphos, 0.4 mL 2-MeTHF, 60 °C, 24 hours. >20:1 denotes the ratio of 3:4. bReaction ran with 50 mol% benzoic acid.
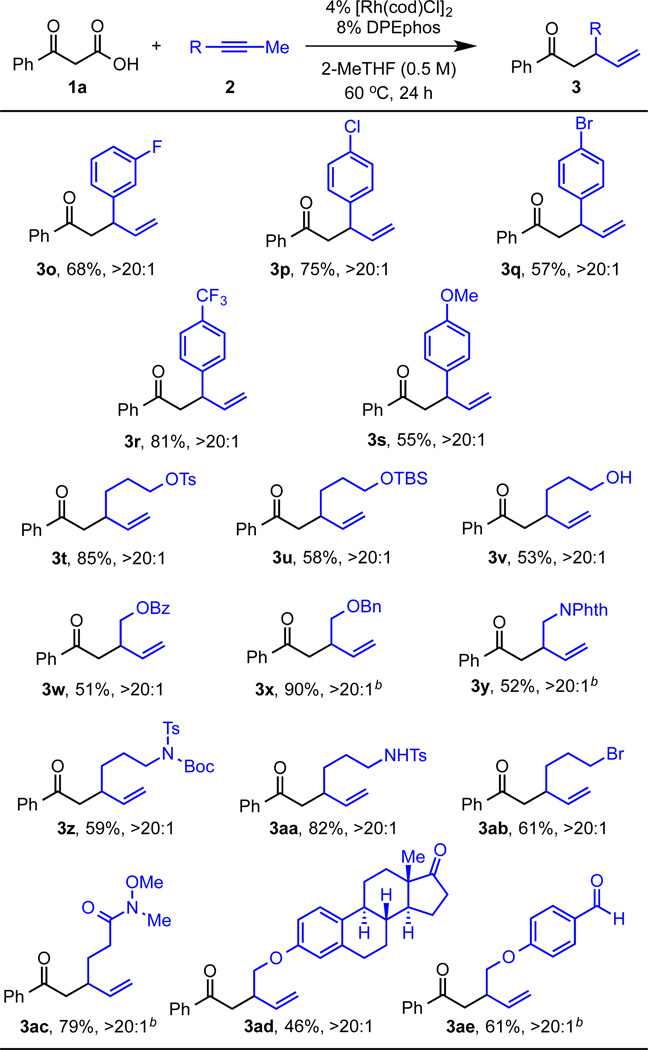
Next, we examined the coupling benzoylacetic acid (1a) with various alkynes 2 (Figure 3). Halogenated 1-aryl-1-propynes were used to alkylate benzoylacetic acid (1a) with >20:1 regioselectivity (3o–3q, 57–75%). In addition, alkynes with electron-deficient para-trifluoromethyl and electron-rich para-methoxy phenyl rings are amenable to alkylating 1a to afford ketones 3r and 3s (81% and 55%, respectively). Benzoylacetic acid (1a) can be alkylated using alkynes with aliphatic substitution in place of aromatic. Aliphatic alkynes present a challenge as a result of having more than one possible site for β-hydride elimination for allene formation. Given this challenge, we were pleased to find that using alkynes bearing aliphatic substituents gave the branched ketone product bearing a terminal olefin. Both free and protected alcohols are tolerated. A sensitive functional group handle (e.g., the tosyl group) remains intact under these alkylating conditions (3t, 85%). Silylated, benzoylated, and benzylated alcohols are all also well-tolerated (3u, 3w, and 3x, 51–90%). Phthalimide protected amines, as well as Boc and Ts protected amines can be installed on the alkyne coupling partner (3y and 3z, 52% and 59%, respectively). Acidic N-H bonds are tolerated as shown by the formation of ketone 3aa in 82% yield. Notably, using alkynes with free alcohols or amines, as in 3v and 3aa, does not result in intramolecular cyclization to form the corresponding tetrahydrofuran or pyrrolidine. These results highlight the high chemoselectivity of this protocol. Finally, electrophilic functionalities can be tolerated as evidenced by the formation of ketones 3ab–3ae bearing an alkyl bromide, Weinreb amide, ketone, and aldehyde, respectively (46–79%).
Figure 3.
Branched Selective Decarboxylative Coupling of β–keto acids 1a with Various Alkynes.a
aReaction conditions: 0.4 mmol 1a, 0.2 mmol 2, 4 mol% [Rh(cod)Cl]1, 8 mol% DPEphos, 0.4 mL 2-MeTHF, 60 °C, 24 hours. >20:1 denotes the ratio of 3:4. bReaction ran with 50 mol% benzoic acid.
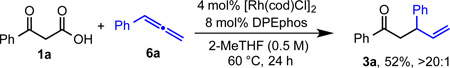
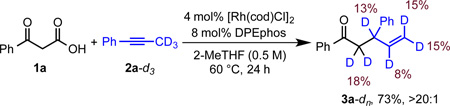
To provide evidence for the proposed allene intermediate, we used allene 6a as a substitute for alkyne 2a under standard reaction conditions. Ketone 3a was obtained in 52% yield with >20:1 regioselectivity (eq 1). This result suggests the feasibility of an allene intermediate in the catalytic cycle. To better understand the proposed β-hydride elimination, we performed an experiment with deuterated 1-phenyl-1-propyne 2a-d3 (eq 2). Ketone 3a-dn was obtained in 73% yield with high-branched regioselectivity. We observed deuterium scrambling which suggests reversible β-hydride elimination during allene formation. Initial studies with chiral ligands resulted in moderate enantioselectivities (up to 54% ee) using a MeOBIPHEP-based ligand.24 These results support the proposed role of the Rh-phosphine complex in the key C-C bond formation, however, developing highly enantioselective variants warrants further efforts.
 |
(1) |
 |
(2) |
Conclusions
This Rh-catalyzed decarboxylative coupling between β-keto acids and alkynes provides a complementary approach to generate ketones, without need for enolate generation and activated allylic electrophiles. In addition, alkylation at specific sites can be performed in the presence of multiple reactive sites due to the directing effect of the carboxylic acid. Our study contributes to the emerging use of alkynes in various cross-couplings to generate C–O,25 C–N,26 C–S,27 and C–C bonds.28 Further studies are underway to expand the scope of carbon pronucleophiles and identify more enantioselective variants for tandem Rh-catalysis.
Supplementary Material
Acknowledgments
Funding provided by UC Irvine and the National Institutes of Health (GM105938). We are grateful to Eli Lilly for a Grantee Award. F.A.C. is grateful for an NSF Graduate Research Fellowship.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.van Poelje PD, Snell EE. Annu. Rev. Biochem. 1990;59:29. doi: 10.1146/annurev.bi.59.070190.000333. [DOI] [PubMed] [Google Scholar]
- 2.(a) Shimizu I, Yamada T, Tsuji J. Tetrahedron Lett. 1980;21:3199. [Google Scholar]; (b) Tsuda T, Chujo Y, Nishi S, Tawara K, Saegusa T. J Am. Chem. Soc. 1980;102:6381. [Google Scholar]
- 3.For a review on transition metal-catalyzed decarboxylative allylations, see: Weaver JD, Recio A, Grenning AJ, Tunge JA. Chem. Rev. 2011;111:1846. doi: 10.1021/cr1002744.
- 4.(a) Lalic G, Aloise AD, Shair MD. J Am. Chem. Soc. 2003;125:2852. doi: 10.1021/ja029452x. [DOI] [PubMed] [Google Scholar]; (b) Magdziak D, Lalic G, Lee HM, Fortner KC, Aloise AD, Shair MD. J Am. Chem. Soc. 2005;127:7284. doi: 10.1021/ja051759j. [DOI] [PubMed] [Google Scholar]; (c) Fortner KC, Shair MD. J Am. Chem. Soc. 2007;129:1032. doi: 10.1021/ja0673682. [DOI] [PubMed] [Google Scholar]
- 5.(a) Gooßen LJ, Deng G, Levy LM. Science. 2006;313:662. doi: 10.1126/science.1128684. [DOI] [PubMed] [Google Scholar]; (b) Gooßen LJ, Rodríguez N, Melzer B, Linder C, Deng G, Levy LM. J Am. Chem. Soc. 2007;129:4824. doi: 10.1021/ja068993+. [DOI] [PubMed] [Google Scholar]; (c) Gooßen LJ, Zimmermann B, Knauber T. Angew. Chem. Int. Ed. 2008;47:7103. doi: 10.1002/anie.200800728. [DOI] [PubMed] [Google Scholar]; (d) Gooßen LJ, Rudolphi F, Oppel C, Rodríguez N. Angew. Chem. Int. Ed. 2008;47:3043. doi: 10.1002/anie.200705127. [DOI] [PubMed] [Google Scholar]; (e) Gooßen LJ, Rodríguez N, Linder C. J Am. Chem. Soc. 2008;130:15248. doi: 10.1021/ja8050926. [DOI] [PubMed] [Google Scholar]
- 6.(a) Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chu L, Ohta C, Zuo Z, MacMillan DWC. J Am. Chem. Soc. 2014;136:10886. doi: 10.1021/ja505964r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Noble A, MacMillan DWC. J Am. Chem. Soc. 2014;136:11602. doi: 10.1021/ja506094d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Noble A, McCarver SJ, MacMillan DWC. J Am. Chem. Soc. 2015;137:624. doi: 10.1021/ja511913h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ventre S, Petronijevic FR, MacMillan DWC. J Am. Chem. Soc. 2015;137:5654. doi: 10.1021/jacs.5b02244. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chu L, Lipshultz JM, MacMillan DWC. Angew. Chem. Int. Ed. 2015;54:7929. doi: 10.1002/anie.201501908. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Nawrat CC, Jamison CR, Slutskyy Y, MacMillan DWC, Overman LE. J Am. Chem. Soc. 2015;137:11270. doi: 10.1021/jacs.5b07678. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Le C, MacMillan DWC. J Am. Chem. Soc. 2015;137:11938. doi: 10.1021/jacs.5b08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Breit B. J Am. Chem. Soc. 2014;136:862. doi: 10.1021/ja411397g. [DOI] [PubMed] [Google Scholar]
- 8.For an example of the coupling of β-keto acids with allylic alcohols, see: Chen S-J, Lu G-P, Cai C. Chem. Commun. 2015;51:11512. doi: 10.1039/c5cc03591k.
- 9.For selected reviews on transition metal catalyzed allylic substitutions, see: Trost BM. J Org. Chem. 2004;69:5813. doi: 10.1021/jo0491004. Helmchen G. J Organomet. Chem. 1999;576:203. Liu Y, Han S-J, Liu W-B, Stoltz BM. Acc. Chem. Res. 2015;48:740. doi: 10.1021/ar5004658. Zhuo C-X, Zheng C, You S-L. Acc. Chem. Res. 2014;47:2558. doi: 10.1021/ar500167f. Hartwig JF, Stanley LM. Acc. Chem. Res. 2010;43:1461. doi: 10.1021/ar100047x. Trost BM, Van Vranken DL. Chem. Rev. 1996;96:395. doi: 10.1021/cr9409804. Trost BM, Crawley ML. Chem. Rev. 2003;103:2921. doi: 10.1021/cr020027w. Tsuji J, Minami I. Acc. Chem. Res. 1987;20:140. Lu Z, Ma S. Angew. Chem. Int. Ed. 2008;47:258. doi: 10.1002/anie.200605113. Helmchen G, Dahnz A, Dübon P, Schelwies M, Weihofen R. Chem. Commun. 2007:675. doi: 10.1039/b614169b.
- 10.For selected examples of branched selective Pd-catalyzed allylic alkylations, see: Trost BM, Maholtra S, Chan WH. J Am. Chem. Soc. 2011;133:7328. doi: 10.1021/ja2020873. Chen J-P, Peng Q, Lei B-L, Hou X-L, Wu Y-D. J Am. Chem. Soc. 2011;133:14180. doi: 10.1021/ja2039503. Chen J-P, Ding C-H, Liu W, Hou X-L, Dai L-X. J Am. Chem. Soc. 2010;132:15493. doi: 10.1021/ja106703y. Zhang P, Brozek LA, Morken JP. J Am. Chem. Soc. 2010;132:10686. doi: 10.1021/ja105161f.
- 11.For selected examples of branched selective Ir-catalyzed allylic alkylations, see: Chen W, Hartwig JF. J Am. Chem. Soc. 2013;135:2068. doi: 10.1021/ja311363a. Krautwald S, Sarlah D, Schafroth MA, Carreira EM. Science. 2013;340:1065. doi: 10.1126/science.1237068. Hamilton JY, Sarlah D, Carreira EM. Angew. Chem. Int. Ed. 2013;52:7532. doi: 10.1002/anie.201302731. Lipowsky G, Miller N, Helmchen G. Angew. Chem. Int. Ed. 2004;43:4595. doi: 10.1002/anie.200460016.
- 12.For selected examples of branched selective Rh-catalyzed allylic alkylations, see: Tsuji J, Minami I, Shimizu I. Tetrahedron Lett. 1984;25:5157. Hayashi T, Okada A, Suzuka T, Kawatsura M. Org. Lett. 2003;5:1713. doi: 10.1021/ol0343562. Kazmaier U, Stolz D. Angew. Chem. Int. Ed. 2006;45:3072. doi: 10.1002/anie.200600100. Evans PA, Nelson JD. J Am. Chem. Soc. 1998;120:5581. Ashfield BL, Miller KA, Martin SF. Org. Lett. 2004;6:1321. doi: 10.1021/ol0496529. Evans PA, Oliver S, Chae J. J Am. Chem. Soc. 2012;134:19314. doi: 10.1021/ja306602g.
- 13.For selected examples of branched selective allylic alkylations catalyzed by other metals, see: (a) Fe: Plietker B. Angew. Chem. Int. Ed. 2006;45:1469. doi: 10.1002/anie.200503274. (b) Co: Bhatia B, Reddy MM, Iqbal J. Tetrahedron Lett. 1993;34:6301. (c) Mo: Trost BM, Miller JR, Hoffman CM. J Am. Chem. Soc. 2011;133:8165. doi: 10.1021/ja2029602. (d) Ru: Sundararaju B, Achard M, Demerseman B, Toupet L, Sharma GVM, Bruneau C. Angew. Chem. Int. Ed. 2010;49:2782. doi: 10.1002/anie.200907034. (e) W: Lloyd-Jones GC, Pflalz A. Angew. Chem. Int. Ed. Engl. 1995;34:462.
- 14.(a) Narsireddy M, Yamamoto Y. J Org. Chem. 2008;73:9698. doi: 10.1021/jo801785r. [DOI] [PubMed] [Google Scholar]; (b) Patil NT, Wu H, Yamamoto Y. J Org. Chem. 2007;72:6577. doi: 10.1021/jo0708137. [DOI] [PubMed] [Google Scholar]; (c) Patil NT, Lutete LM, Wu H, Pahadi NK, Gridnev ID, Yamamoto Y. J Org. Chem. 2006;71:4270. doi: 10.1021/jo0603835. [DOI] [PubMed] [Google Scholar]; (d) Patil N, Huo Z, Bajracharya GB, Yamamoto Y. J Org. Chem. 2006;71:3612. doi: 10.1021/jo060142x. [DOI] [PubMed] [Google Scholar]; (e) Bajracharya GB, Huo Z, Yamamoto Y. J Org. Chem. 2005;70:4883. doi: 10.1021/jo050412w. [DOI] [PubMed] [Google Scholar]; (f) Patil NT, Wu H, Kadota I, Yamamoto Y. J Org. Chem. 2004;69:8745. doi: 10.1021/jo0485684. [DOI] [PubMed] [Google Scholar]; (g) Patil NT, Yamamoto Y. J Org. Chem. 2004;69:6478. doi: 10.1021/jo0490144. [DOI] [PubMed] [Google Scholar]; (h) Lutete LM, Kadota I, Yamamoto Y. J Am. Chem. Soc. 2004;126:1622. doi: 10.1021/ja039774g. [DOI] [PubMed] [Google Scholar]; (i) Kadota I, Shibuya A, Lutete LM, Yamamoto Y. J Org. Chem. 1999;64:4570. doi: 10.1021/jo990498r. [DOI] [PubMed] [Google Scholar]; (j) Kadota I, Shibuya A, Gyoung YS, Yamamoto Y. J Am. Chem. Soc. 1998;120:10262. [Google Scholar]; (k) Patil NT, Kadota I, Shibuya A, Gyoung YS, Yamamoto Y. Adv. Synth. Catal. 2004;346:800. [Google Scholar]
- 15.(a) Gellrich U, Meißner A, Steffani A, Kähny M, Drexler HJ, Heller D, Plattner DA, Breit B. J Am. Chem. Soc. 2014;136:1097. doi: 10.1021/ja411204d. [DOI] [PubMed] [Google Scholar]; (b) Lumbroso A, Koschker P, Vautravers NR, Breit B. J Am. Chem. Soc. 2011;133:2386. doi: 10.1021/ja1108613. [DOI] [PubMed] [Google Scholar]; (c) Xu K, Khakyzadeh V, Bury T, Breit B. J Am. Chem. Soc. 2014;136:16124. doi: 10.1021/ja509383r. [DOI] [PubMed] [Google Scholar]; (d) Koschker P, Kähny M, Breit B. J Am. Chem. Soc. 2015;137:3131. doi: 10.1021/jacs.5b01131. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q-A, Chen Z, Dong VM. J Am. Chem. Soc. 2015;137:8392. doi: 10.1021/jacs.5b05200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.For selected reviews on tandem catalysis, see: Fogg DE, dos Santos EN. Coord. Chem Rev. 2004;248:2365. Chapman CJ, Frost CG. Synthesis. 2007;1:1. Shindoh N, Takemoto Y, Takasu K. Chem. Eur. J. 2009;15:12168. doi: 10.1002/chem.200901486.
- 18.Oxidative addition into the β-keto acid O-H bond may occur to generate a Rh(III)-hydride. Alternatively, a pathway involving protonation is possible, see: reference 15a.
- 19.For selected examples of transition metal catalyzed alkyne to allene isomerization followed by trapping with electrophiles, see: Obora Y, Hatanaka S, Ishii Y. Org. Lett. 2009;11:3510. doi: 10.1021/ol901366q. Park BY, Nguyen KD, Chaulagain MR, Komanduri V, Krische MJ. J Am. Chem. Soc. 2014;136:11902. doi: 10.1021/ja505962w. Liang T, Nguyen KD, Zhang W, Krische MJ. J Am. Chem. Soc. 2015;137:3161. doi: 10.1021/jacs.5b00747. Chen Q-A, Cruz FA, Dong VM. J Am. Chem. Soc. 2015;137:3157. doi: 10.1021/ja512015w.
- 20.For a recent example of Rh-catalyzed alkyne isomerization followed by trapping with 1,3-diketones as a carbon pronucleophile, see: Beck TM, Breit B. Org. Lett. 2016;18:124. doi: 10.1021/acs.orglett.5b03391.
- 21.For related examples where C-C bond formation precedes decarboxylation, see: references 7 and 8.
- 22.See references 15b and 15d.
- 23.(a) Dierkes P, van Leeuwen PWNM. J Chem. Soc., Dalton Trans. 1999:1519. [Google Scholar]; (b) Kamer PCJ, van Leeuwen PWNM, Reek JNH. Acc. Chem. Res. 2001;34:895. doi: 10.1021/ar000060+. [DOI] [PubMed] [Google Scholar]; (c) van Leeuwen PWNM, Kamer PCJ, Reek JNH, Dierkes P. Chem. Rev. 2000;100:2741. doi: 10.1021/cr9902704. [DOI] [PubMed] [Google Scholar]
- 24.See ESI.
- 25.For select examples of C–O bond formation from alkynes, see: references 14c, 15b and 15d.
- 26.For select examples of C–N bond formation from alkynes, see: references 14a, 14b, 14c, 14d, 14e, 14f, 14h, 14i and 16.
- 27.For a select example of C–S bond formation from alkynes, see: reference 15c.
- 28.For select examples of C–C bond formation from alkynes, see: references 14c, 14f, 14g, 14j, 14k and 19.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.