Abstract
Most host-parasite systems exhibit remarkable heterogeneity in the contribution to transmission of certain individuals, locations, host infectious states or parasite strains. While significant advancements have been made in the understanding of the impact of transmission heterogeneity in epidemic dynamics and parasite persistence and evolution, the knowledge base of the factors contributing to transmission heterogeneity is limited. We argue that research efforts should move beyond considering the impact of single sources of heterogeneity and account for complex couplings between conditions with potential synergistic impacts on parasite transmission. Using theoretical approaches and empiric evidence from various host-parasite systems, we investigate the ecological and epidemiological significance of couplings between heterogeneities and discuss their potential role in transmission dynamics and the impact of control.
Keywords: transmission heterogeneity, risk heterogeneity, super-spreader, within-host dynamics, vector-borne pathogen, dengue
Transmission Heterogeneity in Host-Parasite Systems
Heterogeneity, broadly defined as the variability of a property of a system across space, time, and/or the system’s individual constituents [1], is a pervasive feature of all host-parasite transmission systems. Empirical evidence shows that individual hosts can vary in their susceptibility to infection and parasite infectiousness or shedding rates (mediated by immunological factors or complex host-pathogen interactions); contacts between hosts or hosts and vectors tend to be highly variable in space and time and dependent on social, behavioral or environmental conditions; and pathogen strains can vary in their level of virulence and transmissibility. One of the properties emerging from such individual, temporal and spatial variability is the consistent finding of transmission heterogeneity (TH), in which certain individuals, locations, age or social groups, host species, or pathogen strains are responsible for a high proportion of overall transmission events [2–6]. Superspreading is an extreme case of TH in which a disproportionately large amount of transmission events are driven by very few individuals [7, 8]. Theoretical and empirical studies indicate that interventions that account for TH can have a disproportionately high impact on pathogen transmission in comparison to blanket or random implementations [3, 6, 7, 9]. While the public health impacts of TH have been extensively evaluated theoretically (e.g., [6, 7, 10–12]) and manifested in recent infectious disease outbreaks (e.g., the recent Ebola outbreak in West Africa [13]), the causal drivers leading to TH are not well understood. In order to better account for those extremely important yet rare contributors to transmission and improve disease prevention programs, two key questions will first need to be addressed: (1) Is TH the result of identifiable traits inherent to specific individuals and/or locations? 2) Can we use such traits to predict TH across different epidemiological settings and time points?
Given that TH can arise from a wide array of putative factors, a major challenge infectious disease researchers face when addressing these questions is the integration of available parasite-related information into a mechanistic framework that allows identification of the most epidemiologically relevant sources of heterogeneity [2, 14, 15]. When confronting mechanistic models of parasite transmission with epidemiological data, it also becomes apparent that there are often multiple factors that could potentially contribute to TH. The ways in which these factors interact to determine overall TH is a largely unexplored topic. Here, we introduce the concept of “coupled heterogeneities” to capture the interrelated and complex interactions among conditions contributing to TH. We apply this concept to dengue virus (a multi-strain, vector-borne viral pathogen with well-identified heterogeneities at the virus, mosquito vector, and human host levels) and expand it to other vector-borne and parasitic diseases to support the notion that accounting for the couplings between key heterogeneities could lead to a more effective mechanistic interpretation of parasite transmission dynamics and programs designed to prevent disease.
From Individual to Coupled Heterogeneities
Initial quantifications of TH by Woolhouse et al. [6] and Lloyd-Smith et al. [7] focusing on the role of individual heterogeneities, primarily contact rates and infectiousness, provide a foundation for understanding the role of functional heterogeneities (see Box 1 for a definition) in disease systems. Extensions of these seminal studies have led to the development of novel approaches for accounting for functional heterogeneities, including the explicit simulation of pathogen transmission within heterogeneous contact networks [16–18], the consideration of individual- and population-level variability in infectiousness [7, 19–21], the evaluation of the role of spatial heterogeneity in the emergence of disease hot-spots [5, 22, 23], and the evaluation of disease severity (or the inclusion of asymptomatic infections) in forecasts of pathogen transmission [24–26]. The magnitude of such effect is, however, compounded by correlations between functional heterogeneities. As Woolhouse et al. [6] note, “The magnitude of the effect of these other heterogeneities at the population level is unknown; but they will not decrease R0 unless negatively correlated with the variables analyzed here. There may also be effects of ‘higher order’ heterogeneities, all of which may further increase R0.”. This observation underscores a key, but poorly explored, aspect relevant for the identification of the drivers leading to TH; i.e., the coupled nature of functional heterogeneities. Specifically, system properties may be strongly coupled with one another for a number of reasons, including multiple symptoms associated with disease manifestation, behavioral syndromes, or other phenotypic suites [27], or because of trade-offs in pathogen fitness [28]. As intimated by Woolhouse et al. [8], the sign of this coupling (positive or negative) between functional heterogeneities can significantly influence estimates of the basic reproduction number (R0 see Box 1 for a definition), or other measures of pathogen transmission.
Box 1. System Properties and Functional Heterogeneities Relevant for Dengue Virus Transmission.
All individual-level, temporal or spatial attributes of potential epidemiological interest (e.g., parasitemia, duration of infectiousness, spatial distribution of people and households) can be referred to as ‘system properties’ of a particular disease system [1]. For dengue virus (DENV), the most important mosquito-borne viral infection of humans [70], a wide array of system properties at the human, virus, vector and environmental levels have been identified as crucial for persistent virus transmission (Figure I). At the virus level, each of the four DENV serotypes elicits different immune responses in humans [71, 72] as well as variable effects on vector competence [20, 73]. Spatial and temporal variability in productivity of larval habitats and abundance of the primary worldwide vector, Aedes aegypti [74], together with variations in adult Ae. aegypti longevity, impact vector survival, virus’ extrinsic incubation period, and, consequently, transmission probability [75]. Temperature fluctuations impact Ae. aegypti vectorial capacity [76], and immune and/or serotype-specific interactions within human hosts can impact disease severity and, likely, the individual infectiousness of humans to mosquitoes [35, 77]. Mosquito dispersal and human movement and other behavioral responses to infection are key determinants of effective human-mosquito contacts and thus virus transmission [78, 79].
If system properties vary from individual to individual, location to location, or over time in a way that impact an epidemiological or ecological measure such as vectorial capacity or transmission potential (e.g., the pathogen’s basic reproduction number, R0, defined as the expected number of secondary cases produced by a single infection in a completely susceptible population), then we refer to this variability as ‘functional heterogeneity’ [1]. For dengue, several functional heterogeneities have been identified (black bold font words in Figure I). They involve heterogeneous biting, variability in human infectiousness to mosquitoes, disease-related behavioral response, herd immunity, vector competence and incubation periods in humans and mosquitoes and human daily movement patterns Some system properties cannot be predicted exactly but their variability may be accurately described through probability distributions. Such properties can be said to exhibit ‘stochastic variability’. Stochastic variability can emerge from environmental stochasticity (e.g., increase in mosquito larval habitats due to a scattered rain event), measurement error (which can increase the variability in estimates of a system property) or sampling bias (which can lead to an incomplete characterization of a system property). Thus, TH is determined by a combination of functional heterogeneities specific to a given transmission system and stochastic variability that obscures and potentially alters the relationship between functional heterogeneities and TH. We further posit that focusing research efforts on the quantification of couplings between functional heterogeneities with strong influence in DENV transmission could increase our ability to develop realistic estimates of pathogen transmission and innovative concepts for disease prevention.
Figure I. System properties responsible for dengue virus transmission.
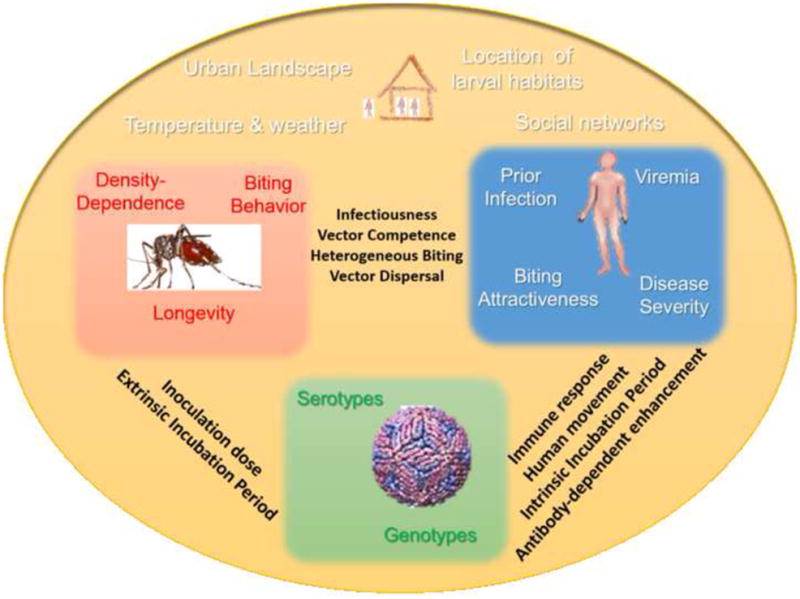
Colored boxes identify specific traits known to vary in space (yellow), time or among individual people (blue), mosquitoes (red), or viruses (green). Black bold text identify functional heterogeneities known to have a direct impact in virus pathogen transmission and propagation.
One of the earliest theoretical explorations of coupled heterogeneities was provided by Dietz [29] in a model for schistosomiasis transmission. Specifically, he extended Barbour’s [30] formulation of the classic Ross-Macdonald model for malaria transmission to include a correlation structure between two functional heterogeneities: times for which different individuals are exposed to parasites at water ponds (heterogeneity in susceptibility) and rates at which different hosts contaminated ponds (parasite shedding rates, i.e. heterogeneity in infectiousness). Dietz’s findings, summarized in the following formula:
concluded that the impact of the modeled heterogeneities on R0 depends on their magnitudes (quantified by their standard deviations, SD) and the correlation between them (ρhet1,het2). Later, Koella [31] followed Dietz’s approach by extending Dye and Hasibeder’s [32] formulation of R0 for malaria transmission (developed to better account for heterogeneous biting) to include a covariance structure between three functional heterogeneities: biting rate, host susceptibility, and duration of infectiousness [31]. Both theoretical approaches arrived to a similar conclusion: positive correlations between two heterogeneous system properties increase R0 relative to the situation in which the properties vary independently or do not exhibit heterogeneity, whereas negative correlations have the opposite effect. Here, we build on these finding by calling attention to these and other effects of coupling between heterogeneities, and we use dengue as a case study to investigate the empirical plausibility and epidemiological significance of coupled heterogeneities.
Figure 1 is a conceptual summary of known or hypothesized relationships between functional heterogeneities and TH that are typical of dengue transmission systems. The distribution of the number of secondary infections generated by any one individual in a population could be a function of a specific confluence of functional heterogeneities that lead to some individuals contributing more to transmission, infecting more mosquitoes, and thus generating a higher number of secondary infections (Figure 1A). For instance, a negative coupling between out-of-home human mobility and disease severity [33] may result in severely ill individuals reducing their movement because of fever, fatigue, and other symptoms, and, perhaps, reducing the overall number of mosquito encounters within their infectious period across their activity space (Figure 1B). Dengue disease severity may be positively coupled with viral titer [34, 35] and thus overall infectiousness to mosquitoes. Due to increases in body temperature and lethargy, we may also expect disease severity to be positively correlated with attractiveness to mosquitoes and mosquito feeding success (Figure 1B). Figure 1C summarizes all known couplings between the four functional heterogeneities considered here.
Figure 1. Coupled heterogeneities in dengue virus transmission.
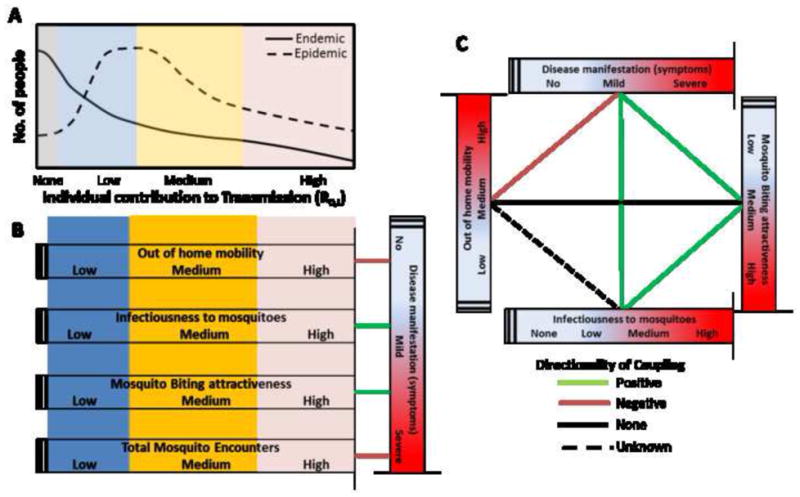
(A) The contribution of each individual in a population to dengue virus (DENV) transmission (expressed as the individual-level R0, R0,i, defined as the espected number of secondary infections produced by each individual in a fully susceptible population) may differ between endemic and epidemic settings due to the levels of virus circulation and the proportion of individuals that are immune to the circulating serotype(s). With endemic transmission, the distribution of R0,i is significantly biased towards zero (due to the high proportion of immune individuals) whereas during epidemics a much larger proportion of the population contributes to transmission. Despite this difference, the expected distribution of R0,i allows grouping the individual contribution of individuals to transmission as poor (blue box), intermediate (yellow box) and high (red box). (B) Multiple functional heterogeneities can lead to this uneven distribution of R0,i. As an extreme example, individuals with high out of home mobility, high infectiousness to mosquitoes, and high mosquito attractiveness will infect more mosquitoes and contribute more to transmission compared to individuals in the low end of the spectrum for these characteristics. Such heterogeneities are not independent from each other. For instance, disease manifestations (severity of dengue infection) may be correlated with multiple conditions, positively (green lines) or negatively (orange lines). (C) Diagram outlining what is known about the correlation structure among various sources of heterogeneity in DENV transmission.
To focus on a specific example with plausible couplings between heterogeneities, in Box 2 we consider a simplified model wherein either positive or negative coupling between infectiousness and host-vector contact clearly impacts R0. Even in this basic example, different couplings can lead to differing conclusions regarding transmission potential and the difficulty associated with parasite elimination; increasing it in one case and decreasing it in another. Another implication of this simple model is that if heterogeneities are negatively coupled, targeting control efforts on individuals who are at the upper extreme with respect to one heterogeneity may have no net effect if those individuals are simultaneously at the lower extreme with respect to another epidemiologically relevant heterogeneity. Similarly, benefits of targeted control on a single heterogeneity that is positively coupled with another should yield greater marginal returns on control than if the heterogeneities were uncoupled. Without a mechanistic understanding of couplings, incorrect predictions of control impacts and biased inferences about drivers of transmission could be commonplace (particularly if, after the consideration of couplings, transmission systems respond much differently to perturbation than we think). Given that multiple functional heterogeneities are present in dengue transmission systems (Box 1), it is critical that future research address the population-level effect of coupled heterogeneities so that interventions can be designed and deployed to take better advantage of the inner workings of this complex transmission system.
Box 2. Moving beyond single heterogeneities.
Consider a host population with four distinct types representing a two-by-two combination of high and low states of infectiousness to vectors (qh, ql) and high and low contact with vectors when sick (γh, γl) (Figure IA). Heterogeneity in the latter is assumed to derive from heterogeneity in the proportion of time that individuals spend at home versus elsewhere. If we consider only a single type of vector and assume that encounters between vectors and hosts are well mixed, then R0 is
where parameters are defined in Table I ([32, 80]).
We explored two aspects of coupling between heterogeneities in infectiousness and contact with vectors: (1) the direction of coupling (three scenarios in Figure IA, with shaded boxes denoting which types are present), and (2) the overall extent of heterogeneity (from left to right in Figure IB). Colors in Figure IA and IB are coordinated across the three scenarios (i.e., positive, blue; none, black; negative, red), with Figure IA indicating the proportion of individuals of each type that are present in a population under each scenario. For example, under the positive coupling scenario, the only individuals present are those who either have high contact and high infectiousness or low contact and low infectiousness. Positive coupling increases R0, negative coupling decreases it, and no coupling leads to no change in R0 (Figure IB). As the overall extent of heterogeneity increases (i.e., as lows become lower and highs become higher), differences due to either positive or negative coupling lead to increasingly large departures in R0 relative to the situation in which the heterogeneities are uncoupled (from left to right in Figure IB).
Intuitively, a decrease in R0 in the presence of negatively coupled heterogeneities can be thought of as a consequence of the two heterogeneities canceling each other out. In an extreme example, if highly infectious hosts are never bitten by vectors, then transmission cannot occur, despite the presence of highly infectious hosts. Applying similar intuition, positive coupling leads to ‘superspreaders’ that are capable of individually contributing far more to transmission than individuals who possess either high infectiousness or high contact, but not both. Although infectiousness and contact may be much more continuous than ‘low’ and ‘high’, the qualitative results about the effects of negative and positive couplings on R0 are general. Coupling between other heterogeneities, such as contact rates when sick versus healthy, may lead to different results and merit further theoretical investigation.
Figure I. Epidemiologic impact of coupling contact rates and infectiousness.
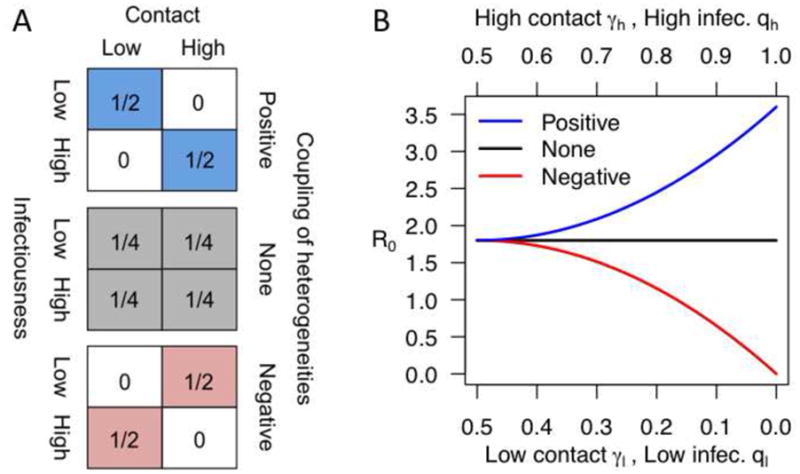
(A) The proportion of the population assigned to each of four types (i.e., low-low, low-high, high-low, high-high) under different scenarios about coupling. (B) Effects of positive (blue), negative (red), or no (black) coupling on R0 given different levels of heterogeneity in contact when sick and infectiousness. The extent of heterogeneity in contact and infectiousness are assumed to be equal under a given scenario (i.e., at a given point on the x-axis in (B)). The overall extent of heterogeneity—i.e., how low is low and how high is high—is increased from left to right on the x-axis in (B).
Table I.
Parameter definitions and specific values for the model in Box 2.
| Symbol | Definition | Value |
|---|---|---|
| K | Vector biting rate | 0.3 |
| V | Vector density | 1.0 |
| Δ | Vector death rate | 0.15 |
| P | Vector-to-host infectiousness | 0.5 |
| Q | Host-to-vector infectiousness | 0–1 |
| γ | Host-vector contact rate | 0–1 |
| Ξ | Host recovery rate | 0.33 |
| H | Host density | 1.0 |
Empirical Support for Coupled Heterogeneities
The perspectives emerging from the study of within host-dynamics and ecological immunology are beginning to shed light on the complexities associated with the process of host infection and parasite transmission [14, 36], providing fertile ground for the study of couplings among functional heterogeneities across a range of disease systems. Such insights are also changing the field of epidemiological modeling by providing a higher level of granularity in the elaboration of model parameters contributing to TH, the evolution of virulence, and the impact of interventions [2, 14, 37–39]. For instance, the recognition that pathogen transmission is dependent on trade-offs between individual-level factors affecting infectiousness or parasite load (driven by disease severity, immune response, genetic attributes of the host and/or pathogen, etc.) and host behavioral changes exerted by those conditions (e.g., changes in mobility or frequency of contacts, behavior change due to vaccination, etc.) is beginning to gain traction as an important factor driving parasite transmission dynamics and evolution [14, 37].
Evidence from multiple host-parasite systems emphasize the epidemiological impact of couplings between disease severity, infectiousness, parasite load, and host behavior. A recent empirical evaluation of TH in amphibian-parasitizing trematodes used mechanistic experiments manipulating host size, behavior, and immunity to evaluate their impact in parasite burden. Aggregation of parasites within hosts was dependent on the occurrence of particular dyads, of which those including reductions in host behavior (inactivity simulating presence of a predator) had the highest influence on patterns of parasite aggregation [40]. For malaria, expanding mathematical models by compartmentalizing the human reservoir into multiple sub-classes with different infectiousness and exposure profiles (e.g., uninfected, subpatent, asymptomatic, and symptomatic) may allow for better estimates of disease burden and the impact of interventions [24, 25]. Submicroscopic gametocyte carriers (individuals that transmit the sexual stage of the Plasmodium parasite infectious to mosquitoes) are primarily asymptomatic and have a lower infectiousness to mosquitoes compared to symptomatic individuals with microscopically detectable malaria infections [41]. Coupling disease severity status, infectiousness and localized exposure to mosquito bites is thus an important new frontier for malaria modeling [5, 42], as is the identification of parasite transmission hotspots [22, 25]. A survey performed during the 2009 influenza pandemic showed that sick individuals had significantly fewer contacts compared with healthy individuals, leading to the general notion of a negative coupling between contact rates and disease severity [43].
Due to the role of multiple dynamic, interacting populations of different kinds of hosts (i.e., insect and human), many vector-borne and zoonotic systems are more complex than those in which transmission is direct and restricted solely to human-human contact networks [44]. In particular, reservoir host heterogeneity is a significant functional heterogeneity for zoonotic systems. The aggregation of ticks in specific rodent species, age groups, and individuals leads to heterogeneous bacteremia of Borrelia burgdorferi, Babesia microti, and Anaplasma phagocytophilum, and these heterogeneities appear to be explained by system properties such as high population density, small body size, and short generation time [45–47]. In the US, American robins (Turdus migratorius) were identified as potential superspreading species for West Nile virus (WNV) transmission in the East and Midwest [48, 49], but not in the Southeast [50]. This difference in epidemiological role of robins may be due to intrinsic (heterogeneity in infectiousness, e.g., [51, 52]) and extrinsic (avian community composition and bird-mosquito encounters, e.g., [49]) factors. Thus, in order to understand how much host heterogeneity matters for the dynamics of zoonotic parasites, more information will be needed on the structure and magnitude of couplings between heterogeneities with an influential role connecting individual reservoir host traits to reservoir species (and potentially vector species) community assemblages [44].
These examples point to the value of explicitly understanding how pathogen load, host immunity, symptom severity, and host behavior combine to drive TH. Whether such information could lead to improved disease management strategies (e.g., by identifying and targeting those individuals that disproportionately contribute to TH [3, 6, 7]) will depend on the impact of such couplings at the population or community levels. In an elegant assessment of the role of anthelmintic treatment on the regulation of the host immune response system by parasitic worms on Mycobacterium bovis (bovine tuberculosis) infected African buffalo, Ezenwa and Jolles [53] showed that treatment significantly increased buffalo survival (i.e., a positive individual-level impact), leading to an increase in the duration of bovine tuberculosis infectiousness and an eight- fold increase in M. bovis’s R0; i.e., a negative population-level impact. Thus, defining the quantitative link between functional heterogeneities and pathogen transmission will depend on carefully collected data, proper statistical and quantitative approaches, and the evaluation of the individual- and population/community-level implications of couplings for pathogen transmission and disease management.
Data and Quantitative Challenges to Reveal Coupled Heterogeneities
The empiric quantification of the structure of couplings between functional heterogeneities will require complex study designs focusing on the within-host dynamics, but also capturing the inter-individual variability embedded in host populations. Such data needs can be best met if studies track (either experimentally or observationally) traits responsible for pathogen infection dynamics, the structure of contacts leading to pathogen propagation, the host behavioral attributes that may be influenced by infection, and the environmental context within which transmission occurred. Experimental infection studies have provided evidence of couplings among heterogeneities in non-human infectious diseases and parasites (e.g., [40, 51, 54]). For human infectious diseases, however, experimental infection studies are unethical, rarely capture disease-related behavioral attributes of individual hosts, and often involve small sample sizes. Longitudinal cohort studies examining naturally infected individuals to quantify infection-related quantities (e.g., pathogen load, immune response, symptoms), together with the structure of their contact networks and behavioral responses to infection represent a more amenable design to the study of coupled heterogeneities. Datasets meeting such criteria are now being generated for malaria and dengue, as well as other infectious agents (e.g., [20, 41, 55, 56]).
As adequate data become available, the quantification of the ecological and epidemiological role of coupled heterogeneities will strongly rely on quantitative approaches involving both statistical models of observed associations and mathematical models to illustrate the impact of propagating uncertainty through the dynamic system [57]. Here we highlight three classes of analytic approaches and the potential strengths and challenges each brings to the quantification and understanding of the epidemiological role of couplings (Table 1). Importantly, each class of quantitative analysis brings different strengths and challenges, and all three will provide valuable insight into the nature and impact of coupled heterogeneities.
Table 1.
Quantitative approaches to unveil the epidemic role of coupled heterogeneities.
| Analytic approach | Analytic goals and challenges. | Example approaches and potential insights into coupled heterogeneities |
|---|---|---|
| Theoretical (Mathematical) |
GOALS:
|
Patch and metapopulation models
|
| Hybrid (Simulation) |
GOALS:
|
Individual based models.
|
| Empirical (Statistical) |
GOALS:
|
Structural equation models.
|
Within empiric statistical models, extensions to consider complex correlation structures between covariates are technically straightforward but often computationally intensive (e.g., structural equation models [58], hierarchical and measurement error models [59]). Such approaches could provide information on both the epidemiologic role of couplings as well as the functional relationship between heterogeneities after accounting for stochastic effects embedded within a models’ random effects [33, 60]. Unfortunately, applications of such methodologies to identify coupled heterogeneities in real datasets are rare. One exception is the use of time- varying frailty models to explore the effect of host heterogeneities in the force of infection of infectious diseases occurring sequentially on the same individual [61, 62]. The basic assumption of this approach is that high correlation in the occurrence of two infections within the same individual should be found for parasites that share a similar route of transmission (due to shared conditions associated with exposure such as social contacts, mobility, etc.). Such correlations can be equivalent to couplings between functional heterogeneities, and frailty models allow embedding individual-level covariates in the estimation of force of infection [61, 62]. The observational nature of the data and the non-unique links between pattern and process mentioned above, however, can create challenges in both the statistical identifiability of parameters and the interpretation of the output of dynamic models. Coupling empiric quantifications with simulation or modeling could help overcome some of the challenges outlined for statistical approaches (e.g., [63]).
Simulation-based analyses (especially with individual-based models [64] or mechanistic Bayesian approaches) allow for a high level of flexibility in the consideration of couplings. Such approaches can play a crucial and complementary role relative to other quantitative approaches, because they allow for the impacts of a nearly endless variety of couplings to be explored. A strength of simulation models is their ability to facilitate the development of biological intuition and to assess the quantitative plausibility of the impacts of coupled heterogeneities on epidemiological outcomes. Confronting these models with data can be challenging, however, and development of simulation models always involves a cost associated with obtaining sufficient data to parameterize them. Estimation of covariance structure between heterogeneities (e.g., disease severity and infectiousness) by fitting simulation models to empirical data will be crucial before these or any other models can be used to accurately and reliably predict dynamics. Simulations evaluating the role of uncertainty in estimation of couplings [65] could, however, provide critical insights about their role in overall model performance, as well as, to identify data needs to improve the measurement of heterogeneities.
Approaches that utilize somewhat simpler, but nonetheless mechanistic, mathematical models will encounter significant analytical challenges in terms of mathematical tractability once multiple heterogeneities and their couplings are accounted for. Nonetheless, they also represent a crucial tool in the theoretical exploration of the role of couplings, as outlined in Box 2. Extensions of classic modeling frameworks to incorporate couplings (e.g, metapopulation, household, or network models) are possible [66, 67]. As we point out in Table 1, there is considerable room for improvement in the thoughtful design of epidemiological, statistical, and theoretical studies and, importantly, the interactions of the three toolsets in order to focus insight on these challenging problems.
Concluding Remarks
The time is ripe for more explicit consideration of the ecological and epidemiological role of coupling between functional heterogeneities in host-parasite transmission systems. As disease eco-epidemiologists searching for traits responsible of TH and researchers focusing on understanding within-host dynamics converge in their questions, data, and analytic methodologies, there is an emerging need for a framework that enables them to consider the role of multiple, overlapping, and correlated sources of heterogeneity. Given the potential synergistic, antagonistic, or additive role of such couplings, the empirical quantification of the functional relationship among heterogeneities and the influence they may have on parasite transmission metrics (e.g., R0, outbreak size distribution, vectorial capacity) should become a focus for future research. Hypothetically, intervention strategies that simultaneously target couplings that act synergistically could have a higher impact than if only one source of heterogeneity is targeted in isolation (e.g., individuals who engage in risky sexual behaviors may have sex with more partners and also be less likely to use condoms). Similarly, couplings that contribute disproportionately to transmission could be exploited by evolving parasites that are constantly exploring new opportunities for maximizing fitness [68, 69]. Such possibilities will not come about though without carefully developed field studies and advances in quantitative methods that allow for a comprehensive mechanistic understanding of the epidemiological and evolutionary mechanics of coupling between functional heterogeneities. Forthcoming endeavors along these lines have the potential to contribute to a range of timely questions (see Outstanding Questions Box) that must be addressed before the promise of leveraging knowledge of heterogeneities for improved control and evolutionary management can be realized.
Outstanding Questions.
To what extent is TH a function of individual-level characteristics, coupled heterogeneities or stochastic variation?
Within a host species, how do within-individual and (meta) population level interactions couple?
If significant couplings are identified and predicted to have a disproportionate role in the emergence of TH: How will such information be used to develop rational disease mitigation strategies that take such information into consideration to identify those who disproportionately contribute to transmission?
Can disease mitigation strategies that target coupled heterogeneities be more efficacious than those focused on individual traits?
Can couplings among specific heterogeneities modulate rates of pathogen evolution?
Trends Box.
The uneven contribution of certain individuals, locations, parasite strains or reservoir host species to transmission --termed transmission heterogeneity-- is a widespread attribute of most host-parasite systems.
Multiple conditions contributing to transmission heterogeneity can be correlated with each other, leading to non-linear impacts on parasite transmission potential (R0).
Targeting epidemiologically relevant couplings can lead to more impactful control interventions.
Acknowledgments
We would like to thank Valerie Paz-Soldan, John Elder, Alan Rothman, Louis Lambrechts, and Amy Morrison for helpful comments during the development of our ideas. We also appreciate the feedback provided by two anonymous reviewers. This research was funded by a grant from the U.S. National Institutes of Health - National Institute of Allergy and Infectious Diseases (NIH/NIAID) award number P01AI098670 (TWS PI, GVP co-PL) and by the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Science and Technology Directory, Department of Homeland Security, and Fogarty International Center, National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li H, Reynolds JF. On Definition and Quantification of Heterogeneity. Oikos. 1995;73:280–284. [Google Scholar]
- 2.Paull SH, et al. From superspreaders to disease hotspots: linking transmission across hosts and space. Frontiers in Ecology and the Environment. 2012;10:75–82. doi: 10.1890/110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Streicker DG, et al. Differential sources of host species heterogeneity influence the transmission and control of multihost parasites. Ecology letters. 2013;16:975–984. doi: 10.1111/ele.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poulin R. Explaining variability in parasite aggregation levels among host samples. Parasitology. 2013;140:541–546. doi: 10.1017/S0031182012002053. [DOI] [PubMed] [Google Scholar]
- 5.Perkins TA, et al. Heterogeneity, mixing, and the spatial scales of mosquito-borne pathogen transmission. PLoS Computational Biology. 2013;9:e1003327. doi: 10.1371/journal.pcbi.1003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolhouse ME, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd-Smith JO, et al. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuang S, et al. Superspreading SARS Events, Beijing, 2003. Emerging Infectious Disease journal. 2004;10:256. doi: 10.3201/eid1002.030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins SE, et al. Empirical evidence for key hosts in persistence of a tick-borne disease. Int J Parasitol. 2003;33:909–917. doi: 10.1016/s0020-7519(03)00128-0. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RM, May RM. Population biology of infectious diseases. Springer-Verlag; 1982. [Google Scholar]
- 11.Hethcote HW, Yorke J. Gonorrhea Transmission Dynamics and Control. Springer; Berlin Heidelberg: 2014. [Google Scholar]
- 12.Grenfell BT, Dobson AP. Ecology of infectious diseases in natural populations. Cambridge University Press; 1995. [Google Scholar]
- 13.Pandey A, et al. Strategies for containing Ebola in West Africa. Science. 2014;346:991–995. doi: 10.1126/science.1260612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handel A, Rohani P. Crossing the scale from within-host infection dynamics to between- host transmission fitness: a discussion of current assumptions and knowledge. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2015:370. doi: 10.1098/rstb.2014.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koopman JS. Modeling infection transmission- the pursuit of complexities that matter. Epidemiology. 2002;13:622–624. doi: 10.1097/00001648-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Bansal S, et al. When individual behaviour matters: homogeneous and network models in epidemiology. Journal of the Royal Society, Interface/the Royal Society. 2007;4:879–891. doi: 10.1098/rsif.2007.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eames KT, Keeling MJ. Modeling dynamic and network heterogeneities in the spread of sexually transmitted diseases. Proc Natl Acad Sci U S A. 2002;99:13330–13335. doi: 10.1073/pnas.202244299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keeling MJ, Eames KTD. Networks and epidemic models. Journal of the Royal Society Interface. 2005;2:295–307. doi: 10.1098/rsif.2005.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lloyd AL. Realistic distributions of infectious periods in epidemic models: Changing patterns of persistence and dynamics. Theoretical Population Biology. 2001;60:59–71. doi: 10.1006/tpbi.2001.1525. [DOI] [PubMed] [Google Scholar]
- 20.Nguyet MN, et al. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2013;110:9072–9077. doi: 10.1073/pnas.1303395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd AL. Destabilization of epidemic models with the inclusion of realistic distributions of infectious periods. Proceedings of the Royal Society of London Series B-Biological Sciences. 2001;268:985–993. doi: 10.1098/rspb.2001.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bousema T, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS medicine. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitron U. Landscape ecology and epidemiology of vector-borne diseases: tools for spatial analysis. Journal of medical entomology. 1998;35:435–445. doi: 10.1093/jmedent/35.4.435. [DOI] [PubMed] [Google Scholar]
- 24.Hansen E, Buckee CO. Modeling the human infectious reservoir for malaria control: does heterogeneity matter? Trends in parasitology. 2013;29:270–275. doi: 10.1016/j.pt.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bousema T, et al. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Micro. 2014;12:833–840. doi: 10.1038/nrmicro3364. [DOI] [PubMed] [Google Scholar]
- 26.King AA, et al. Inapparent infections and cholera dynamics. Nature. 2008;454:877–880. doi: 10.1038/nature07084. [DOI] [PubMed] [Google Scholar]
- 27.Sih A, et al. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Ewald P. Evolution of Infectious Disease. Oxford University Press; USA: 1993. [Google Scholar]
- 29.Dietz K. Models for Vector-Borne Parasitic Diseases. In: Barigozzi C, editor. Vito Volterra Symposium on Mathematical Models in Biology. Springer; Berlin Heidelberg: 1980. pp. 264–277. [Google Scholar]
- 30.Barbour AD. Macdonald’s model and the transmission of bilharzia. Trans R Soc Trop Med Hyg. 1978;72:6–15. doi: 10.1016/0035-9203(78)90290-0. [DOI] [PubMed] [Google Scholar]
- 31.Koella JC. On the use of mathematical models of malaria transmission. Acta tropica. 1991;49:1–25. doi: 10.1016/0001-706x(91)90026-g. [DOI] [PubMed] [Google Scholar]
- 32.Dye C, Hasibeder G. Population dynamics of mosquito-borne disease: effects of flies which bite some people more frequently than others. Trans R Soc Trop Med Hyg. 1986;80:69–77. doi: 10.1016/0035-9203(86)90199-9. [DOI] [PubMed] [Google Scholar]
- 33.Hobbs NT, Hooten MB. Bayesian Models: A Statistical Primer for Ecologists. Princeton University Press; 2015. [Google Scholar]
- 34.Libraty DH, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis. 2002;185:1213–1221. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 35.Vaughn DW, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 36.Schulenburg H, et al. Introduction. Ecological immunology. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2009;364:3–14. doi: 10.1098/rstb.2008.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manfredi P, D’Onofrio A. Modeling the Interplay Between Human Behavior and the Spread of Infectious Diseases. Springer; 2013. [Google Scholar]
- 38.Heesterbeek H, et al. Modeling infectious disease dynamics in the complex landscape of global health. Science. 2015;347:6227. doi: 10.1126/science.aaa4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith DL, et al. Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Trans R Soc Trop Med Hyg. 2014;108:185–197. doi: 10.1093/trstmh/tru026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson PT, Hoverman JT. Heterogeneous hosts: how variation in host size, behaviour and immunity affects parasite aggregation. The Journal of animal ecology. 2014;83:1103–1112. doi: 10.1111/1365-2656.12215. [DOI] [PubMed] [Google Scholar]
- 41.Bousema T, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PloS one. 2012;7:e42821. doi: 10.1371/journal.pone.0042821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith DL, et al. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS biology. 2007;5:e42. doi: 10.1371/journal.pbio.0050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eames KT, et al. The impact of illness and the impact of school closure on social contact patterns. Health Technol Assess. 2010;14:267–312. doi: 10.3310/hta14340-04. [DOI] [PubMed] [Google Scholar]
- 44.Johnson PT, et al. Why infectious disease research needs community ecology. Science. 2015;349:1259504. doi: 10.1126/science.1259504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostfeld RS, et al. Life history and demographic drivers of reservoir competence for three tick-borne zoonotic pathogens. PloS one. 2014;9:e107387. doi: 10.1371/journal.pone.0107387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perkins SE, et al. Empirical evidence for key hosts in persistence of a tick-borne disease. International journal for parasitology. 2003;33:909–917. doi: 10.1016/s0020-7519(03)00128-0. [DOI] [PubMed] [Google Scholar]
- 47.Calabrese JM, et al. Partitioning the aggregation of parasites on hosts into intrinsic and extrinsic components via an extended Poisson-gamma mixture model. PloS one. 2011;6:e29215. doi: 10.1371/journal.pone.0029215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kilpatrick AM, et al. Host heterogeneity dominates West Nile virus transmission. Proceedings. Biological sciences/The Royal Society. 2006;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamer GL, et al. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg. 2009;80:268–278. [PubMed] [Google Scholar]
- 50.Levine RS, et al. Limited spillover to humans from West Nile Virus viremic birds in Atlanta, Georgia. Vector Borne Zoonotic Dis. 2013;13:812–817. doi: 10.1089/vbz.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komar N, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerging infectious diseases. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.VanDalen KK, et al. West Nile virus infection in American Robins: new insights on dose response. PloS one. 2013;8:e68537. doi: 10.1371/journal.pone.0068537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ezenwa VO, Jolles AE. Epidemiology. Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales. Science. 2015;347:175–177. doi: 10.1126/science.1261714. [DOI] [PubMed] [Google Scholar]
- 54.Fleming-Davies AE, et al. Effects of host heterogeneity on pathogen diversity and evolution. Ecology letters. 2015 doi: 10.1111/ele.12506. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Handel A, et al. Trade-offs between and within scales: environmental persistence and within-host fitness of avian influenza viruses. Proceedings. Biological sciences/The Royal Society. 2014:281. doi: 10.1098/rspb.2013.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Killingley B, et al. Use of a human influenza challenge model to assess person-to-person transmission: proof-of-concept study. The Journal of infectious diseases. 2012;205:35–43. doi: 10.1093/infdis/jir701. [DOI] [PubMed] [Google Scholar]
- 57.Waller LA. Bridging gaps between statistical and mathematical modeling in ecology. Ecology. 2010;91:3500–3502. doi: 10.1890/10-0432.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Little TD, Card NA. Longitudinal Structural Equation Modeling. Guilford Press; 2013. [Google Scholar]
- 59.Clark JS. Models for Ecological Data: An Introduction. Princeton University Press; 2007. [Google Scholar]
- 60.Zuur AF, et al. Mixed effects models and extensions in ecology with R. Springer; 2009. [Google Scholar]
- 61.Unkel S, et al. Time varying frailty models and the estimation of heterogeneities in transmission of infectious diseases. Journal of the Royal Statistical Society: Series C (Applied Statistics) 2014;63:141–158. [Google Scholar]
- 62.Farrington CP, et al. Correlated Infections: Quantifying Individual Heterogeneity in the Spread of Infectious Diseases. American Journal of Epidemiology. 2013 doi: 10.1093/aje/kws260. [DOI] [PubMed] [Google Scholar]
- 63.Waller LA, et al. Monte Carlo assessments of goodness-of-fit for ecological simulation models. Ecol Model. 2003;164:49–63. [Google Scholar]
- 64.Railsback SF, Grimm V. Agent-Based and Individual-Based Modeling: A Practical Introduction. Princeton University Press; 2011. [Google Scholar]
- 65.Xu C, et al. Understanding uncertainties in model-based predictions of Aedes aegypti population dynamics. PLoS Negl Trop Dis. 2010;4:e830. doi: 10.1371/journal.pntd.0000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ball F, et al. Seven challenges for metapopulation models of epidemics, including households models. Epidemics. 2015;10:63–67. doi: 10.1016/j.epidem.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Pellis L, et al. Eight challenges for network epidemic models. Epidemics. 2015;10:58–62. doi: 10.1016/j.epidem.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Frank SA. Models of parasite virulence. Q Rev Biol. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- 69.Day T. Virulence evolution and the timing of disease life-history events. Trends in ecology & evolution. 2003;18:113–118. [Google Scholar]
- 70.Brady OJ, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rico-Hesse R. Dengue virus virulence and transmission determinants. In: Rothman AL, editor. Dengue virus. Springer; 2010. pp. 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rothman AL. Cellular immunology of sequential dengue virus infection and its role in disease pathogenesis. Curr Top Microbiol Immunol. 2010;338:83–98. doi: 10.1007/978-3-642-02215-9_7. [DOI] [PubMed] [Google Scholar]
- 73.Lambrechts L. Quantitative genetics of Aedes aegypti vector competence for dengue viruses: towards a new paradigm? Trends in parasitology. 2011;27:111–114. doi: 10.1016/j.pt.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 74.Getis A, et al. Characteristics of the spatial pattern of the dengue vector, Aedes aegypti, in Iquitos, Peru. Am J Trop Med Hyg. 2003;69:494–505. [PubMed] [Google Scholar]
- 75.Smith CE. The significance of mosquito longevity and blood-feeding behaviour in the dynamics of arbovirus infections. Med Biol. 1975;53:288–294. [PubMed] [Google Scholar]
- 76.Lambrechts L, et al. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishiura H, Halstead SB. Natural history of dengue virus (DENV)-1 and DENV-4 infections: reanalysis of classic studies. J Infect Dis. 2007;195:1007–1013. doi: 10.1086/511825. [DOI] [PubMed] [Google Scholar]
- 78.Stoddard S, et al. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl Trop Dis. 2009:e481. doi: 10.1371/journal.pntd.0000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harrington LC, et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- 80.Lloyd AL, et al. Stochasticity and heterogeneity in host-vector models. J R Soc Interface. 2007;4:851–863. doi: 10.1098/rsif.2007.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]


