Abstract
Central sensitization (CS), nociceptive hyper-excitability known to amplify and maintain clinical pain, has been identified as a leading culprit responsible for maintaining pain in several chronic pain conditions. Recent evidence suggests that it may explain differences in the symptom experience of individuals with sickle cell disease (SCD). Quantitative sensory testing (QST) can be used to examine CS and identify individuals that may have a heightened CS profile. The present study categorized patients with SCD based on QST responses into a high or low CS phenotype and compared these groups on measures of clinical pain, vaso-occlusive crises, psychosocial factors, and sleep continuity. Eighty-three adult patients with SCD completed QST, questionnaires, daily sleep and pain diaries over a three-month period, weekly phone calls for three months, and monthly phone calls for 12 months. Patients were divided into CS groups (i.e. No/Low CS [n=17] vs. High CS [n=21]), based on thermal and mechanical temporal summation and aftersensations, which were norm-referenced to 47 healthy controls. High CS subjects reported more clinical pain, vaso-occlusive crises, catastrophizing, and negative mood, and poorer sleep continuity (p’s<0.05) over the 18-month follow-up period. Future analyses should investigate whether psychosocial disturbances and sleep mediate the relationship between central sensitization and pain outcomes.
Perspective
In general, sickle cell disease patients with greater central sensitization had higher clinical pain, more crises, worse sleep, and more psychosocial disturbances compared to the low CS group.
Keywords: Sickle cell disease, clinical pain, laboratory pain, QST, central sensitization, sleep, catastrophizing
Introduction
Sickle cell disease (SCD), an inherited blood disorder, is associated with significant morbidity including severe episodic pain and, in a sizeable subset of patients, chronic pain [47]. While the mechanisms of SCD pain remain poorly understood, recent reports have implicated a process of central sensitization (CS). CS is a process whereby nociceptive signals coming from the periphery assault the central nervous system and alter the spinal cord and brain producing a chronic amplification of pain sensations [57]. This manifests clinically in a number of ways, including hyperalgesia and allodynia, enlarged area of hyperalgesia beyond the initial area of injury, and after sensations following cessation of the initial insult [57]. Rewiring of pain transmission occurs in patients with CS and a growing body of literature documents augmented central nervous system (CNS) processing in SCD. Transgenic mice models of SCD (expressing sickle hemoglobin) suggest that CS occurs through aberrant heightened spinal and supraspinal processing that is demonstrated by increased sensitivity to cold, heat, and mechanical stimuli as well as musculoskeletal pain behavior compared with control animals [15]. Ballas and colleagues [3] note that the best example of this phenomenon observed in patients with SCD is the continuation of severe pain, even following successful bone marrow transplantation, which essentially “cures” the patient of SCD. They note that resetting the aberrant wiring of the brain back to normal may take an extended period of time.
CS in humans can be assessed through the application of quantitative sensory testing (QST) using standardized, calibrated, consistently applied noxious stimuli to measure pain processing. Typical QST measures of CS include the measurement of painful aftersensations or temporal summation, which is heightened perceptual responses to repeated stimulation of identical intensity [22][57]. QST is often used to measure patient characteristics that might be associated with pain-related outcomes [28], and increased sensitivity to painful stimuli has been shown to incur risk for poor outcomes. For example, in non-SCD chronic pain conditions, lower pain tolerance [20;24] and higher levels of temporal summation [2] are associated with more frequent, intense, and disabling episodes of recent pain. The few studies that have examined QST in adults with sickle cell disease generally find enhanced sensitivity of thermal detection and reduced pain thresholds in patients with SCD [5;26;27]. A recent report found evidence of central sensitization in 60% of patients with SCD tested and a combination of central and peripheral sensitization in an additional 32% [23]. The goal of the current study was to use the extant literature to categorize patients with SCD as either showing no/low CS or high CS based on responses derived during QST and examine differences in clinical characteristics between these two groups. We hypothesized that those in the high CS group would endorse greater pain, worse psychosocial/behavioral comorbidities and a more severe symptom experience than those in the no/low CS group.
Methods
The current analyses are part of a larger ongoing project designed to examine pain and crises in patients with SCD and compare them to healthy-matched controls. All subjects were recruited for participation from the Sickle Cell Center for Adults at Johns Hopkins Hospital or through posted advertisements. The current analyses focused on thirty-eight adult patients with SCD, derived from a larger sample of 83 patients with SCD and classifying CS based on QST responses observed in 47 healthy controls (see Table 1 for demographic data and QST used for categorization). Major inclusion criteria for the SCD group included age ≥18 years, formal diagnosis of SCD (by confirmed genotyping or confirmation by study hematologist), no changes in dose of long- and short-acting opioids, NSAIDs or acetaminophen one month prior to pain testing (if on any of these medications) and self-identifying as black or African American (for matching purposes in the larger study). Exclusion criteria included chronic transfusions, active alcohol or substance abuse/dependence; significant cognitive impairment; unstable psychiatric illness; HIV infection, viral hepatitis, or other current infection. While not the focus of the current analyses, additional exclusion criteria for healthy controls included any acute or chronic pain, regular use of anti-inflammatory medication, opioids, or antidepressant medication; and smoking greater than 1 pack/day. While not the specific focus in the current analyses, CS data from healthy controls were used to categorize SCD patients into groups. In brief control participants were healthy African Americans, 65% were women and the group mean age = 33 (SD=9.5) with a BMI of 26 (SD=4.8).
Table 1.
Demographic variables [Mean (SD) / % (n)]
| Demographic Variables | Low CS N=17 | High CS N=21 | p value |
|---|---|---|---|
| Age (mean ± SD) | 35.6 (10.6) | 42.8 (13.1) | 0.08 |
| Female | 70.6% (12) | 76.2% (16) | 0.49 |
|
| |||
| Education Level | |||
|
| |||
| ≤High School/GED | 11.8% (2) | 23.8% (5) | 0.24 |
| Some College/Technical School | 35.3% (6) | 42.8% (9) | |
| ≥Bachelor’s degree | 53.0% (9) | 33.4% (7) | |
|
| |||
| Occupational Status | |||
|
| |||
| Employed (full or part time) | 70.6% (12) | 42.9% (9) | 0.11 |
|
| |||
| Marital Status | |||
|
| |||
| Single/Divorced/Separated | 64.7% (11) | 81.0% (17) | 0.34 |
| Married/Living with partner | 35.3% (6) | 19.0% (4) | |
Procedures
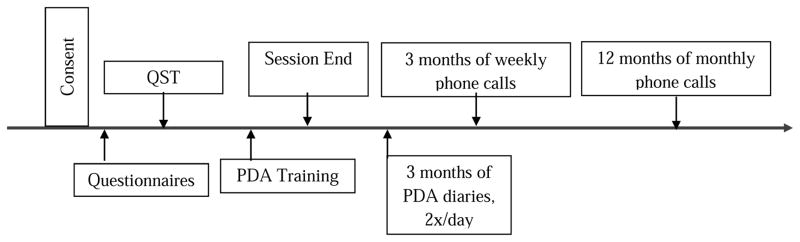
Following initial telephone screening to ensure eligibility criteria were met, participants attended an in-person visit. Participants were asked to attend only when their pain was typical of their SCD pain and at no greater intensity than 5 /10 and they had not experienced a vaso-occlusive crisis in at least the previous 3 weeks. Following informed consent procedures, participants completed a standardized laboratory pain testing protocol between 9 and 11am; upon completion they were instructed in the use of electronic diary monitoring via personal digital assistant (PDA) and informed they would receive follow-up calls for a total of 18 months (see Figure 1 for a timeline). Participants were allowed to stop or refuse any procedure at any time and all study-related procedures were approved by the Johns Hopkins University School of Medicine Institutional Review Board. Additionally, clinical characteristics (e.g. history of acute chest syndrome, presence of avascular necrosis) were obtained from the medical record.
Figure 1.
Timeline
Quantitative Sensory Testing (QST)
Pain threshold/tolerance
Heat pain threshold (HPTh) was assessed via a Peltier element-based stimulator (Medoc, Pathway, Advanced Thermal Stimulator (ATS) thermode), on the dominant ventral forearm, using an ascending method of limits paradigm with a 9 cm2 probe and 0.5°C/sec rate of rise. Subjects underwent two trials and indicated when they first felt painful (HPTh) via button press which turned the device off. Heat Pain Tolerance (HPTo) was conducted in a similar manner, with participants indicating when they could no longer tolerate the pain from the thermal device. Pressure pain threshold (PPTh) was assessed via algometer (SBmedic, 1 cm2 hard rubber probe), bilaterally and 2 times each, at the trapezius muscle, interphalangeal joint of the thumb, the proximal third of the brachioradialis muscle (forearm), and middle of the quadriceps insertion point [6]. Mean HPTh, HPTo (in °Celsius) and PPTh (in kilopascals) values were averaged across trials, respectively.
Temporal Summation
Thermal Temporal Summation (TTS) was assessed via response to three randomized series (temperatures: HPTh, HPTh+2, 45°C) of 10 heat pulses with each temperature rated on a 0–100 scale (0=no pain; 100=worst pain imaginable), applied to the dominant ventral forearm by the Medoc, Pathway Contact Heat-Evoked Potential Stimulator (CHEPS) [54]. A 2.5 second inter stimulus interval (ISI) and a 70°C/sec rate of rise was employed. A TTS difference score (maximal rated pulse of the series minus first pulse of the series) was created for each temperature. The thermode was moved slightly between trials to avoid overlapping stimulation sites. One additional pain rating was obtained 15 seconds following the final pulse in each series to characterize after sensations. Mechanical Temporal Summation (MTS) was assessed at two weights via response to an initial single stimulus, and then to a sequence of 10 stimuli of weighted punctate probes applied on a flat contact area of 0.2 mm diameter with a force of 128mN and 256mN, to the middle phalanx, dorsal surface of the dominant middle finger. Each series was delivered with an ISI of 1 second. Participants were instructed to rate the “peak” pain experienced over the train of 10 stimuli. A MTS difference score was calculated (peak rating minus initial stimulus rating) for each probe weight.
Hot Water Immersion Tests
Pain ratings were additionally assessed using hot water. This task is similar to cold pressor testing in terms of eliciting a pain response. Hot water was chosen to avoid the possibility of prompting a vaso-occlusive crisis and avoids baroreflex activation [48]. Participants immersed the dominant hand in a circulating water bath maintained at a tailored temperature designed to be moderately painful. The tailoring process included a series of up to 5 brief hand immersions starting at 42°C and increasing by 1–2°C until a rating of 60/100 was reached or as high as tolerable for 20 seconds. Following the tailoring procedures, a total of 3 immersions were conducted, two in the context of CPM (as described below) and one hot water tolerance test. Participants were permitted to remove their hand prior to the completion of any trial if the pain became intolerable. Pain ratings on a 0–100 scale were obtained at 30-second intervals for up to two minutes, the (uninformed) time limit during the hot water tolerance test.
Conditioned pain modulation (CPM)
Two PPTh readings were obtained on the non-dominant side trapezius muscle immediately prior to commencing the main hot water immersion tests. At the 20-second point during each of the hand immersion trials, a PPTh reading was obtained on the trapezius muscle. A difference value was created for CPM, such that the 2 PPTh values obtained during each of the CPT trials were averaged and the average of the 2 baseline PPTh readings was subtracted from it (during-baseline to yield a positive number if threshold increased during hand immersion).
Questionnaires
Pain
Brief Pain Inventory (BPI) items were used to assess self-reported clinical pain severity based on the patients’ current, average, worst and least pain mean over the preceding week using a 0–10 scale (0 – No Pain to 10 – Pain as bad as it could be). Additionally, participants completed twice daily (daytime and evening) pain ratings using electronic diaries (described in more detail below).
Depression
The Center for Epidemiological Studies Depression Scale (CES-D) [35] was used to measure depressive symptomatology. Participants responded to items based on frequency of feelings/experiences during the last week on a 5-point scale (0 - rarely/less than one day to 4 - most of the time/5–7 days). Total score was calculated as the sum of responses. A score of 16 or higher identifies individuals at risk for clinical depression [29].
Catastrophizing
The Pain Catastrophizing Scale (PCS) [49] assesses exaggerated negative cognitive and affective response to pain. This standard version of the scale assesses trait-like responses to pain in general and consists of 13 items rated on a 5-point scale (0 - not at all to 4 - all the time) with higher scores indicating greater pain catastrophizing. Participants are instructed to indicate the degree to which he/she has specific thoughts and feelings when experiencing pain. The measure assesses three dimensions of catastrophizing: rumination, magnification, and helplessness. In the current study, we asked patients to base their answers specifically on their sickle cell pain. Total score was calculated as the sum of all responses.
The Situational Catastrophizing Questionnaire (SCQ) [19] is a six item adaptation of the PCS. It has been used by our group and others in several studies [12]. Participants completed this questionnaire immediately after each QST procedure, with instruction to reference the pain procedures while answering, these were averaged to create one situational catastrophizing score.
Affect
The Positive and Negative Affect Schedule (PANAS) [55] was used to evaluate mood. It is comprised of two scales, one positive and one negative, each including 10 descriptive words rated on a 5-point scale (not at all to extremely). A total score was calculated for each scale as the sum of all responses within its dimension.
Sleep
Participants completed the Pittsburgh Sleep Quality Index (PSQI [9]), a widely used 19-item self-report questionnaire for the evaluation of sleep quality over the previous month. The PSQI includes 7 component scales, which were examined separately and added together to yield a global sleep quality score. Global scores greater than five indicate clinically significance [9].
The Insomnia Severity Index [ISI] [4] is a seven item questionnaire focused on insomnia. Higher scores indicate greater severity, with a score of less than 10 representing no clinically significant insomnia and over 10 indicating strong likelihood of clinical insomnia [31].
Diary Measures
In addition to the self-report questionnaires, participants completed diary measurement of pain, vaso-occlusive crises (VOC) and sleep for three months, starting at the conclusion of their initial session. These diaries were completed on a Palm Pilot (Tungsten E2, Palm® personal electronic organizer, Sunnyvale, CA) using a customized application. Specifically, immediately upon waking, participants entered the time they went to bed, latency to sleep onset, wake after sleep onset, final awakening, and time out of bed in order to create indices of sleep continuity.
Just prior to bedtime, participants were instructed to record their average pain level for the day. Participants used an electronic slider on the PDA screen between zero (“no pain”) and 100 (“pain as bad as you can imagine”). Participants were also instructed to self-report the degree to which pain interfered with daytime activities and if they experienced a crisis that day. If they did experience a crisis, they were asked to rate their crisis pain (0–100). A proportion of diary days for which a vaso-occlusive crisis was reported [47] was calculated for each participant as an index of sickle cell disease severity.
Participants did not receive reminders to complete entries; however participants were scheduled for a one-week follow-up to check diary use, answer any questions about the diary and to identify any technical issues regarding its use. Participants received financial incentives based on the number of diaries completed. During data cleaning, entries that were not completed within 12 hours of waking or bedtime were removed to decrease recall bias.
Telephone Calls
Following completion of the diary period, participants were then called weekly and queried regarding their pain, mood and sleep for three months. They were then contacted monthly for an additional 12 months. Specifically, participants were asked about their normal level of pain (BPI-like measure) and pain during crises, catastrophizing, positive and negative affect, as well as questions regarding sleep continuity and duration. In total, the follow-up period lasted 18 months from their initial assessment.
Data Analysis
The initial analyses included standardizing pre-identified QST parameters for the patients with SCD to the mean for each parameter derived from the controls. Specifically, thermal temporal summation (tailored and 45°C), mechanical temporal summation and TTS after sensations were standardized by subtracting the healthy control mean from each individual patient with SCD’s value and dividing by the control standard deviation for each task ((X-μ)/s). Z-scores that were greater than one standard deviation above the healthy control mean of each task were noted. Patients with SCD that scored greater than 1 standard deviation on at least two tasks were categorized into the “high CS” group. Those that were within 0.25 standard deviation of the healthy control group on all tasks were categorized into the “low CS” group. Additionally, a summary score was created that combined the remaining QST variables (QST index), to reflect general sensitivity. A series of t-tests and chi-square tests (for categorical data) were conducted to compare SCD CS groups on measures of pain, psychosocial/behavioral and crisis measures. No imputation was conducted for missing variables.
Results
Demographic, Clinical and Clinical Pain Information
Demographic and clinical information for both groups are presented in Tables 1 and 2. The high CS group tended to be older (p=.09), had a significantly higher body mass index (BMI) and were more often taking long and/or short acting opioids (p’s < 0.03). The groups differed on a variety of indices of pain, including pain severity from the BPI (p=0.001), diary pain ratings averaged over the three months (p=0.003), and average number of days reporting a VOC (p=0.044; see Table 3). Non-crisis pain was rated more severe in the high CS group across the three months of weekly (p=0.018) and 12 months of monthly phone calls (p=0.009). In addition to more severe clinical pain, the high CS group reported more calls made to providers and a greater number of medical visits (p=0.003 and p=0.028, respectively).
Table 2.
Clinical characteristics [Mean (SD)]
| Clinical Variables | Low CS N=17 | High CS N=21 | p value |
|---|---|---|---|
| Body Mass Index | 24.5 (3.2) | 27.4 (4.6) | 0.04* |
| Systolic Blood Pressure | 112.6 (11.0) | 116.0 (14.9) | 0.44 |
| Diastolic Blood Pressure | 69.8 (10.2) | 70.5 (10.6) | 0.83 |
| Heart Rate | 80.3 (11.0) | 80.5 (13.1) | 0.95 |
| Nicotine Use (smoking) | 0% (0) | 28.6% (6) | 0.42 |
| Genotype | |||
| SS | 70.6% (12) | 57.1% (12) | 0.47 |
| S-Beta zero | 5.9% (1) | 9.5% (2) | |
| SC | 17.6% (3) | 33.3% (7) | |
| Unknown | 5.9% (1) | 0% (0) | |
| History of Acute Chest Syndrome | 41.2% (7) | 42.9% (9) | 0.59 |
| Presence of Avascular Necrosis | 29.4% (5) | 33.3% (7) | 0.54 |
| Taking Hydroxyurea | 35.3% (6) | 19.0% (4) | - |
| Taking long-acting opioids | 17.6% (3) | 57.1% (12) | 0.013* |
| Taking short-acting opioids | 52.9% (9) | 85.7% (18) | 0.027* |
(p<.05).
Table 3.
Pain variables by group [Mean (SD)]
| Clinical Pain Variables | Low CS (n=17) | High CS (n=21) | p value |
|---|---|---|---|
|
Pain
| |||
| Pain Severity (BPI) | 0.9 (1.3) | 2.8 (1.8) | 0.001*** |
| Interference (BPI–Extended) | 1.5 (2.7) | 3.1 (2.4) | 0.06 |
|
| |||
| Pain from PDA (0–100; average over 3 months) | n=16 | n=20 | |
| Proportion of PDA’s completed (total completed days/total possible days) | 0.78 (0.2) | 0.76 (0.3) | 0.80 |
|
| |||
| Non-Crisis Pain | 8.8 (14.5) | 26.1 (20.5) | 0.008** |
| VOC Pain | 35.6 (23.4) | 52.1 (21.0) | 0.11 |
| Average Number of days reporting VOC | 0.09 (0.1) | 0.23 (0.2) | 0.044* |
| Average length of Crises | 0.8 (1.1) | 1.5 (1.0) | 0.045* |
| Number of calls to providers | 1.8 (3.0) | 4.5 (6.0) | 0.11 |
| Number of medical visits | 1.6 (1.8) | 4.9 (6.1) | 0.05 |
| Number of Crises | 5.4 (9.2) | 12.3 (12.7) | 0.08 |
|
| |||
| From Weekly Calls (0–10; average over 3 months) | n=14 | n=18 | |
| Number of weekly calls completed (of 12) | 10.6 (2.9) | 11.0 (1.6) | 0.66 |
|
| |||
| Non-Crisis Pain | 1.4 (1.4) | 2.9 (1.8) | 0.018* |
| VOC Pain | 5.0 (1.8) | 5.8 (1.7) | 0.31 |
| Average Number of Crises/Week | 0.034 (0.06) | 0.09 (0.1) | 0.13 |
| Number of calls to providers | 0.6 (0.8) | 1.4 (1.5) | 0.23 |
| Number of medical visits | 0.7 (0.8) | 1.1 (1.4) | 0.55 |
|
| |||
| From Monthly Calls (0–10; average over 12 months) | n=13 | n=17 | |
| Number of monthly calls completed (of 12) | 10.5 (2.8) | 9.8 (2.3) | 0.44 |
|
| |||
| Non-Crisis Pain | 1.6 (1.5) | 3.5 (2.1) | 0.009** |
| VOC Pain | 5.3 (1.9) | 6.2 (1.5) | 0.18 |
| Average Number of Crises/Month | 0.9 (0.9) | 1.3 (1.1) | 0.24 |
| Number of calls to providers | 1.0 (1.4) | 4.5 (3.2) | 0.003** |
| Number of medical visits | 1.5 (2.1) | 4.2 (3.5) | 0.028* |
(p<.05),
(p<.01),
(p≤.001).
Psychosocial Variables
Although reports of SCD pain catastrophizing were similar between the high and no/low CS groups, situational catastrophizing in response to pain testing was enhanced among the high CS group (p< 0.001; Table 4). Consistent with this, catastrophizing was also more frequently reported in the high CS group on PDAs and during the weekly and monthly telephone assessments (p’s < 0.01). While negative and positive affect did not differ between groups during QST, the high CS group reported lower positive affect from three to 18 months of follow-up phone calls (p’s < 0.05). This group also reported greater negative affect in the three months of daily diaries following testing (p= 0.026) and during months six through 18 of monthly phone calls (p = 0.026).
Table 4.
Psychological characteristics by group [Mean (SD)]
| Psychosocial Variables | Low CS (n=17) | High CS (n=21) | p value |
|---|---|---|---|
| Depression (CESD) | 11.6 (11.6) | 17.5 (12.8) | 0.15 |
| Catastrophizing (PCS) | 24.6 (15.4) | 26.4 (11.7) | 0.69 |
| Situational Catastrophizing (SCQ) | 0.4 (0.3) | 1.4 (0.9) | <0.001*** |
| Positive Affect (PANAS) | 35.4 (8.6) | 34.1 (8.4) | 0.64 |
| Negative Affect (PANAS) | 11.1 (1.2) | 12.7 (3.6) | 0.09 |
|
| |||
| From PDAs (average over 3 months) | n=16 | n=20 | |
|
| |||
| Catastrophizing | 5.0 (8.2) | 22.1 (20.2) | 0.003** |
| Positive Affect | 63.7 (16.3) | 56.8 (17.4) | 0.230 |
| Negative Affect | 15.5 (14.7) | 27.5 (15.7) | 0.026* |
|
| |||
| From Weekly Calls (average over 3 mo) | n=14 | n=18 | |
|
| |||
| Catastrophizing | 3.8 (3.3) | 12.1 (10.3) | 0.007** |
| Positive Affect | 6.9 (1.3) | 5.7 (1.5) | 0.024* |
| Negative Affect | 2.5 (1.6) | 3.4 (1.7) | 0.12 |
|
| |||
| From Monthly Calls (average over 12 mo) | n=13 | n=18 | |
|
| |||
| Catastrophizing | 4.8 (4.7) | 17.1 (12.5) | 0.002** |
| Positive Affect | 7.2 (1.5) | 5.8 (1.4) | 0.014* |
| Negative Affect | 2.6 (1.7) | 4.1 (1.8) | 0.026* |
(p<.05),
(p<.01),
(p≤.001).
BPI: Brief Pain Inventory, CESD: Center for Epidemiological Studies Depression Scale, PCS: Pain Catastrophizing Scale, SCQ: Situational Catastrophizing Questionnaire, PANAS: Positive and Negative Affect Schedule.
Sleep Characteristics
As shown in Table 5, the CS group reported greater sleep disturbance across a number of indices of sleep. This group reported poorer overall sleep as reflected by the PSQI global score as well as multiple PSQI components (p’s < 0.05) and insomnia (from the ISI, p=0.005). These summary reports of sleep disturbance were consistent with the pattern of sleep disturbance reported by the high CS group during the home monitoring period, including daily PDA diaries and weekly and then monthly phone calls (p’s < 0.05). Although no group differences emerged in sleep duration between groups (p’s > 0.05), sleep fragmentation was two times greater in the high CS group on the PDAs (though only trended towards statistical significance), weekly (p=0.029) and monthly phone calls (p=0.006). These differences remained significant even when controlling for group differences in BMI.
Table 5.
Sleep parameters by group [Mean (SD)]
| Sleep Variables | Low CS (n=17) | High CS (n=21) | p value |
|---|---|---|---|
| PSQI Components | |||
| 1. Subjective Sleep Quality | 0.8 (0.5) | 1.5 (0.8) | 0.007** |
| 2. Sleep Latency | 0.9 (0.9) | 1.8 (1.1) | 0.01* |
| 3. Sleep Duration | 0.5 (0.7) | 1.3 (1.2) | 0.01* |
| 4. Habitual Sleep Efficiency | 2.6 (1.0) | 2.0 (1.3) | 0.10 |
| 5. Sleep Disturbance | 1.4 (0.7) | 2.0 (0.7) | 0.006** |
| 6. Use of Sleep Medications | 0.1 (0.3) | 1.0 (1.2) | 0.007** |
| 7. Daytime Dysfunction | 0.9 (0.7) | 1.3 (0.9) | 0.14 |
| Global Score | 7.1 (3.1) | 10.9 (4.0) | 0.003** |
| ISI | 5.4 (6.1) | 12.5 (8.2) | 0.005** |
|
| |||
| Sleep from PDA† (average over 3 months) | |||
| Sleep Efficiency (%) | 89.6% (6.7) | 77.1% (17.7) | 0.011* |
| Wake After Sleep Onset (in minutes) | 17.5 (24.3) | 35.6 (29.5) | 0.057 |
| Sleep Onset Latency (in minutes) | 16.8 (12.9) | 37.4 (25.1) | 0.005** |
| Sleep Duration (in hours) | 7.2 (1.2) | 7.9 (3.7) | 0.53 |
| From Weekly Calls (average over 3 months) | |||
| Sleep Continuity Disturbance | 0.9 (.9) | 1.9 (1.3) | 0.029* |
| Sleep Duration | 6.7 (0.9) | 6.2 (1.5) | 0.34 |
| From Monthly calls (averaged over 12 months) | |||
| Sleep Continuity Disturbance | 1.1 (0.9) | 2.2 (1.0) | 0.006** |
| Sleep Duration | 6.5 (1.2) | 5.7 (1.1) | 0.068 |
(p<.05),
(p<.01).
PSQI: Pittsburgh Sleep Quality Index, ISI: Insomnia Severity Index.
Does not include VOC nights.
QST Measures
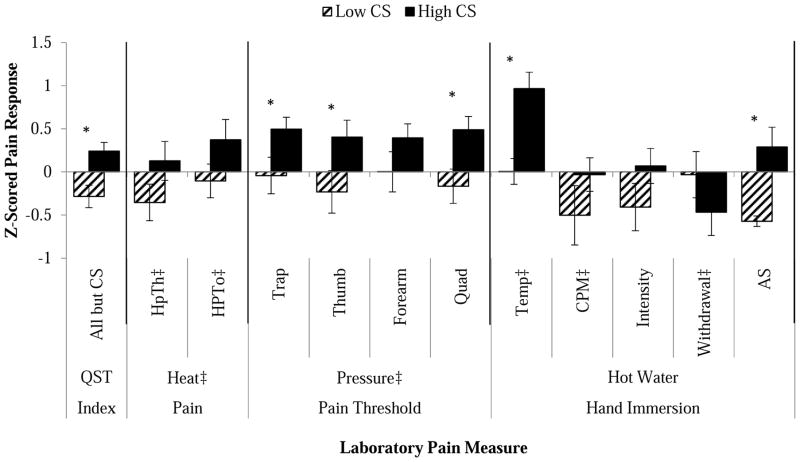
Laboratory pain measures are displayed for each group in Table 6 and normalized and presented graphically in Figure 2. The QST index (composite score of each QST measure, except those that comprised the CS categorization) varied by group (p = 0.002). Significant differences between the low and high CS groups were observed in several QST measures including pressure pain threshold at the trapezius, thumb and quadriceps, and temperature of the hot water task (tailored to a perceptual level; p’s < 0.05). [Insert Table 6 and Figure 2 about here]
Table 6.
Quantitative Sensory Testing (QST) measures by group [Mean (SD)]
| QST Variables | Low CS (n = 17) | High CS (n=21) | p value |
|---|---|---|---|
| QST used to categorize groups | |||
|
| |||
| Thermal Temporal Summation difference scores | |||
| at Heat Pain Threshold | 0.5 (1.3) | 9.7 (10.6) | |
| at Heat Pain Threshold +2 °C | 0.9 (2.6) | 8.7 (13.6) | |
| at 45°C | 0.06 (0.2) | 9.6 (9.8) | |
| After Sensation Ratings (TTS, 0–100) | 0.2 (0.7) | 29.7 (21.6) | |
| Mechanical Temporal Summation difference scores | |||
| 128 mN (Probe 5) | 2.2 (5.2) | 25.0 (18.0) | |
| 256 mN (Probe 6) | 5.5 (6.7) | 30.0 (25.2) | |
|
| |||
| Summary Measure | |||
|
| |||
| QST Index | −0.3 (0.5) | 0.3 (0.3) | 0.007** |
|
| |||
| Thermal Pain in °Celsius | |||
|
| |||
| Threshold (HPTh) | 42.1 (2.5) | 40.7 (3.0) | 0.14 |
| Tolerance (HPTo) | 44.9 (1.9) | 43.8 (22.5) | 0.14 |
|
| |||
| Pressure Pain Threshold in kilopascals (kPa) | |||
|
| |||
| Trapezius | 276.8 (102.2) | 213.8 (74.0) | 0.034* |
| Thumb | 336.4 (108.8) | 268.5 (94.9) | 0.050 |
| Forearm | 261.9 (109.1) | 217.3 (84.8) | 0.17 |
| Quadriceps | 612.9 (204.9) | 448.8 (176.4) | 0.012 |
|
| |||
| Hot Water Hand Immersion Tests | |||
|
| |||
| Temperature of Hot Water (in °Celsius) | 46.2 (1.3) | 44.3 (1.8) | <0.001*** |
| CPM Difference Trapezius (difference score) | 94.4 (91.1) | 64.1 (57.5) | 0.22 |
| Hot Water Pain Ratings (0–100) | 50.6 (29.8) | 63.3 (24.5) | 0.16 |
| Hot Water Tolerance (in seconds) | 43.9 (35.1) | 57.8 (38.4) | 0.26 |
| After Sensation Ratings (hot water; 0–100) | 2.6 (4.0) | 16.0 (16.4) | 0.002** |
(p<.05),
(p<.01),
(p≤.001).
Difference Scores represent the maximal rated pulse (for Thermal Temporal Summation) or following the train of 10 stimuli (for Mechanical Temporal Summation) of the series minus first pulse of the series. CPM: Conditioned Pain Modulation. CPM Difference represents pressure pain thresholds at the trapezius obtained during water immersion of the hand minus baseline trapezius pressure pain thresholds. QST Index: Quantitative sensory testing index z-scores all QST variables (except those used to categorize groups) and combines them into one general sensitivity index.
Figure 2.
Normalized means and standard error for laboratory pain methods displayed by group.
*p < 0.05. Difference between groups. Measures denoted with ‡ were reversed (multiplied by −1) in order to keep directionality consistent. QST Index is a summary of all parameters presented, HPTh = Heat Pain Threshold, HPTo = Heat Pain Tolerance, PPTh = Pressure Pain Threshold, Temp = Water Temperature, CPM = Conditioned Pain Modulation, Intensity = Pain ratings to hot water, AS = After Sensation to hot water.
Discussion
In comparing QST defined low and high central sensitization groups, we observe differences in pain and psychosocial/behavioral patterns between groups. Specifically, the high CS group reported greater clinical pain at the time of testing and on non-crisis days during the daily/weekly/and monthly follow-up, as well as a greater percentage of crisis days during the daily diary monitoring period. Additionally, this group reported enhanced catastrophizing in response to pain testing and worse sleep continuity on a number of parameters and assessment methods. Not surprisingly, other QST measures differed between groups as well, reflecting an overall greater sensitivity in the high CS group. Consistent with this pattern of greater pain sensitivity and disturbed psychosocial/behavioral function, the high CS group reported more contacts with health care providers, either by telephone or in person.
The phenomenon of central sensitization occurs when nociceptive input creates a sustained, reversible increased responsivity of central nociceptive neurons [57]. CS has been hypothesized to affect 17.5–35.3 % of chronic pain patients [41], thus it is not a completely overlapping phenomenon. This sensitization can be quantified in humans using different laboratory pain assessments, including, temporal summation (wind-up like pain) and after sensation, employed here. These indices were chosen, as opposed to others in our QST battery, as they are two of the most commonly assessed QST measures in relation to CS [57], are mediated by different underlying central mechanisms [34;60] and have been documented to have clinical and diagnostic value [21;40]. The method of standardized scores (z-scoring approach) is recommended to evaluate individual patients and patient groups on patterns of QST and determine where they fit in regard to normative ranges [36;37]. Our findings are consistent with the extant literature indicating increased laboratory and clinical pain and greater psychosocial/behavioral sequelae among individuals showing central sensitization. Using fairly stringent criteria (greater than 1 standard deviation above the mean of healthy controls on 2 of the chosen QST tasks), we classified CS in approximately 25% of our sample of SCD patients. This contrasts with a recent study reporting components of CS present in over 90% of patients with SCD [23]. Differences in methodology likely contribute to this discrepancy. We used healthy controls to quantify abnormal responses, whereas Ezenwa et al. [23] used a decision tree based on patient responses to painful vs. non-painful sites. Thus, these different methodologies may have assessed different aspects of CS-ours focused on local summation and theirs on widespread characteristics.
One potential explanation for the differences observed between the low and high CS groups could be that of opioid-induced hyperalgesia (OIH), given that those in the high CS group were far more likely to be on long and/or short-acting opioids (57% vs. 18%). The concept of OIH is an important one that has received much discussion in the pain management literature. We are unable to determine the potential role for OIH in contributing to the increased sensitivity seen in the high CS group, as we do not have data regarding pain levels and pain sensitivity prior to initiation of opioids. This important iatrogenic effect of opioids is a particularly challenging topic to study in sickle cell, as complications of the disease typically require early, and sometimes extensive, exposure to opioid therapy intermittently over years. The use of ketamine, the noncompetitive NMDA receptor antagonist, in recent case reports of acute pain management of sickle cell crises [52] that have successfully reduced opioid dosing while enhancing pain management support the potential importance of OIH in sickle cell patients.
The underlying mechanism(s) and predisposing factors for developing CS are receiving increased attention and include genetic [45;46], neurophysiological [39], psychosocial [33] and behavioral [25;61] conceptualizations. Recent data suggest that targeting individuals with dysfunctional CS pain modulatory mechanisms with specific interventions may be beneficial [7;11;58]. For example, painful diabetic neuropathy patients with reduced CPM (a proxy for endogenous opioid tone) reported enhanced pain reduction from duloxetine, while the drug was less effective in those with more efficient CPM [59]. In another study, post-cesarean pain patients with enhanced CS (quantified via temporal summation) experienced the greatest analgesic effect from ketamine [58]. These findings provide increasing support for using QST-derived CS profiles to identify specific subgroups for which medications may be most effective, as suggested by others [58]. Whether this approach can be used to manage pain in SCD is an important area for future study.
A growing literature documents the bidirectional association between sleep and pain [25] and several reports have documented that experimentally-induced sleep deprivation promotes hyperalgesia [42] and increases spontaneous pain while reducing central pain modulation in healthy participants [43]. The current differentiation between CS groups in sleep disturbance over an extended follow-up period is quite striking. Patients in the high CS group reported overwhelmingly poorer sleep than those in the low CS group and these differences appear stable over time. These findings are consistent with a growing literature documenting a specific relationship between sleep disturbance and CS indices in clinical pain samples [10;18;42]. It is important to note, however, that our analyses are unable to speak to the temporal relationship between these constructs. For example, it is unclear if sleep disturbance predisposes someone to develop central sensitization or whether the process of central sensitization produces downstream effects on sleep continuity. However, reviews of the existing literature infer a stronger impact of sleep on pain sensitivity than vice versa [25] and we have shown in our larger SCD sample that diary reports of sleep fragmentation in particular precedes increases in next-day clinical pain [32]. Given the broad health benefits of good sleep [50] and the potential for improvements in sleep to reduce clinical pain [44], testing whether improved sleep in SCD reduces clinical pain and possibly CS is an important next step in understanding the potential causal contribution of sleep disturbance to central sensitization in SCD.
The high CS group reported greater situational catastrophizing as well as reports of pain catastrophizing in daily life during follow-up. Surprisingly, we did not observe differences in depressive symptoms or SCD-related pain catastrophizing between CS groups. Maladaptive psychological characteristics, including pain catastrophizing, have been previously associated with identifiable CS clusters in OA patients [16], and several reports have specified pain catastrophizing as an important component in central sensitization [1;8;38;53]. Although only tested in healthy volunteers, current data suggest that situational catastrophizing is not an artifact produced by the enhanced pain experienced by the high CS group during laboratory pain testing. We have shown that increases in pain are not predictive of increases in catastrophizing, but increases in catastrophizing are predictive of increases in pain in the laboratory using healthy volunteers [13;14]. Also using healthy volunteers, Salomons and colleagues [38] found that eight sessions of brief cognitive training in healthy volunteers reduced pain unpleasantness and central sensitization, but not pain catastrophizing; however, reductions in secondary hyperalgesia, their index of central sensitization, were correlated with reductions in pain catastrophizing in the experimental treatment group. Their findings suggest that changes in central sensitization may be related to changes in pain catastrophizing and that CS may be modulated by altering pain-related thoughts.
One potential limitation of our work is the method we used to classify high vs. no/low CS, which created extreme groups using a healthy control group for standardization and classification. The reference group was limited to 47 healthy African American volunteers from our local community and may not be fully representative of the African American/black population. One clear indicator of this is the sample age of 33 years which is consistent with our sample of SCD patients, but not representative of the larger community. Other groups using a similar method [36;37;51] standardize patients to samples of hundreds of healthy volunteers. We were able to identify a sample of 17 patients with SCD who essentially looked comparable to these healthy volunteers in terms of pain sensitivity and CS indices. Other clinical groups, such as fibromyalgia have individuals who show little to no central sensitization using QST [17;30;56]. An issue for future research to examine is whether our approach of using extreme groups is replicable and produces continued insight into the factors that contribute to central sensitization and its sequelae. Another limitation may be the small sample identified here to create these extreme groups. While not statistically significant, conditioned pain modulation was higher (in the expected direction) among the low CS group. Enhanced descending pain inhibition could be a protective factor; however we may have had too little power, given our small sample size, to truly elucidate differences in CPM. An additional limitation of the current work is a potential confluence of the temporal relationship between situational catastrophizing and the QST measures used to classify groups. Specifically, situational catastrophizing is measured during the execution of QST and subjects are asked to report thoughts/emotions in response to the pain of testing. As noted above, we have shown that changes in catastrophizing in healthy volunteers precede changes in pain, rather than vice versa, but this needs to be replicated in clinical samples, such as SCD patients.
Despite these limitations, the current data suggest greater clinical pain, health care utilization, pain catastrophizing and sleep disturbance in high CS patients with SCD as compared to no/low CS SCD patients. We present an innovative approach to categorizing patients into CS groups which needs to be replicated and extended to other patient groups for further validation. Application of this method to other pain populations may produce valuable information regarding psychosocial factors associated with central sensitization, either causal or sequelae. Future studies may wish to examine CS differences and combined psychosocial/behavioral influences in healthy participants and chronic pain patients and investigate whether sleep and psychosocial disturbances mediate the relationship between central sensitization and pain outcomes.
Highlights.
Sickle cell patients were split into low vs. high central sensitization groups.
High central sensitization (CS) subjects reported worse SCD outcomes.
The high CS group reported more clinical pain, vaso-occlusive crises, and poorer sleep
The high CS group also reported higher catastrophizing and negative mood
Footnotes
Disclosures
This research was supported by a National Heart, Lung, and Blood Institute R01HL98110 (JAH) and Career Development Award K01HL108832 (CHJR), in addition to a Career Development Award from NINDS K23 NS070933 (CMC). All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Akhter R, Benson J, Svensson P, Nicholas MK, Peck CC, Murray GM. Experimental jaw muscle pain increases pain scores and jaw movement variability in higher pain catastrophizers. J Oral Facial Pain Headache. 2014;28(3):191–204. doi: 10.11607/ofph.1211. [DOI] [PubMed] [Google Scholar]
- 2.Arendt-Nielsen L, Petersen-Felix S. Wind-up and neuroplasticity: is there a correlation to clinical pain? Eur J Anaesthesiol Suppl. 1995;10:1–7. [PubMed] [Google Scholar]
- 3.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120(18):3647–3656. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- 4.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 5.Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol. 2013;88(1):37–43. doi: 10.1002/ajh.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennum J, Kjeldsen M, Jensen K, Jensen TS. Measurement of human pressure-pain thresholds on fingers and toes. Pain. 1989;38:211–217. doi: 10.1016/0304-3959(89)90240-6. [DOI] [PubMed] [Google Scholar]
- 7.Bruehl S, Burns JW, Gupta R, Buvanendran A, Chont M, Kinner E, Schuster E, Passik S, France CR. Endogenous opioid function mediates the association between laboratory-evoked pain sensitivity and morphine analgesic responses. Pain. 2013;154(9):1856–1864. doi: 10.1016/j.pain.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgmer M, Petzke F, Giesecke T, Gaubitz M, Heuft G, Pfleiderer B. Cerebral activation and catastrophizing during pain anticipation in patients with fibromyalgia. Psychosom Med. 2011;73(9):751–759. doi: 10.1097/PSY.0b013e318236588a. [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 10.Campbell CM, Buenaver LF, Finan P, Bounds SC, Redding M, McCauley L, Robinson M, Edwards RR, Smith MT. Sleep, pain catastrophizing and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res (Hoboken ) 2015;67(10):1387–96. doi: 10.1002/acr.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell CM, Buenaver LF, Raja SN, Kiley KB, Swedberg LJ, Wacnik PW, Cohen SP, Erdek MA, Williams KA, Christo PJ. Dynamic Pain Phenotypes are Associated with Spinal Cord Stimulation-Induced Reduction in Pain: A Repeated Measures Observational Pilot Study. Pain Med. 2015;16(7):1349–60. doi: 10.1111/pme.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell CM, Kronfli T, Buenaver LF, Smith MT, Berna C, Haythornthwaite JA, Edwards RR. Situational versus dispositional measurement of catastrophizing: associations with pain responses in multiple samples. J Pain. 2010;11(5):443–453. doi: 10.1016/j.jpain.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell CM, McCauley L, Bounds SC, Mathur VA, Conn L, Simango M, Edwards RR, Fontaine KR. Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: cross-lagged panel analyses of dispositional and situational catastrophizing. Arthritis Res Ther. 2012;14(5):R231. doi: 10.1186/ar4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell CM, Quartana PJ, Buenaver LF, Haythornthwaite JA, Edwards RR. Changes in situation-specific pain catastrophizing precede changes in pain report during capsaicin pain: a cross-lagged panel analysis among healthy, pain-free participants. J Pain. 2010;11(9):876–884. doi: 10.1016/j.jpain.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Cataldo G, Rajput S, Gupta K, Simone DA. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain. 2015;156(4):722–730. doi: 10.1097/j.pain.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz-Almeida Y, King CD, Goodin BR, Sibille KT, Glover TL, Riley JL, Sotolongo A, Herbert MS, Schmidt J, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care Res (Hoboken ) 2013;65(11):1786–1794. doi: 10.1002/acr.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curatolo M, Arendt-Nielsen L. Central Hypersensitivity in Chronic Musculoskeletal Pain. Phys Med Rehabil Clin N Am. 2015;26(2):175–184. doi: 10.1016/j.pmr.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 18.de TM, Delussi M, Vecchio E, Sciruicchio V, Invitto S, Livrea P. Sleep features and central sensitization symptoms in primary headache patients. J Headache Pain. 2014;15:64. doi: 10.1186/1129-2377-15-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards RR, Bingham CO, III, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum. 2006;55(2):325–332. doi: 10.1002/art.21865. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Edwards RR, Fillingim RB. Effects of age on temporal summation of thermal pain: clinical relevance in healthy older and younger adults. Journal of Pain. 2001;2:307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 22.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: Clinical relevance in healthy older and younger adults. The Journal of Pain. 2001;2(6):307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 23.Ezenwa MO, Molokie RE, Wang ZJ, Yao Y, Suarez ML, Pullum C, Schlaeger JM, Fillingim RB, Wilkie DJ. Safety and Utility of Quantitative Sensory Testing among Adults with Sickle Cell Disease: Indicators of Neuropathic Pain? Pain Pract. 2015 Jan 12; doi: 10.1111/papr.12279. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fillingim RB, Edwards RR, Powell T. The relationship of sex and clinical pain to experimental pain responses. Pain. 1999;83:419–425. doi: 10.1016/S0304-3959(99)00128-1. [DOI] [PubMed] [Google Scholar]
- 25.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gil KM, Phillips G, Webster DA, Martin NJ, Abrams M, Grant M, Clark WC, Janal MN. Experimental pain sensitivity and reports of negative thoughts in adults with sickle cell disease. Behav Ther. 1995;26(2):273–293. [Google Scholar]
- 27.Gil KM, Wilson JJ, Edens JL, Webster DA, Abrams MA, Orringer E, Grant M, Clark WC, Janal MN. Effects of Cognitive Coping Skills Training on Coping Strategies and Experimental Pain Sensitivity in African American Adults With Sickle Cell Disease. Health Psychol. 1996;15(1):3–10. doi: 10.1037//0278-6133.15.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Granot M. Can we predict persistent postoperative pain by testing preoperative experimental pain? Curr Opin Anaesthesiol. 2009;22(3):425–430. doi: 10.1097/ACO.0b013e32832a40e1. [DOI] [PubMed] [Google Scholar]
- 29.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 30.Malfliet A, Kregel J, Cagnie B, Kuipers M, Dolphens M, Roussel N, Meeus M, Danneels L, Bramer WM, Nijs J. Lack of evidence for central sensitization in idiopathic, non-traumatic neck pain: a systematic review. Pain Physician. 2015;18(3):223–236. [PubMed] [Google Scholar]
- 31.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moscou-Jackson G, Finan PH, Campbell CM, Smyth JM, Haythornthwaite JA. The Effect of Sleep Continuity on Pain in Adults with Sickle Cell Disease. J Pain. 2015;16(6):587–93. doi: 10.1016/j.jpain.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mourao AF, Blyth FM, Branco JC. Generalised musculoskeletal pain syndromes. Best Pract Res Clin Rheumatol. 2010;24(6):829–840. doi: 10.1016/j.berh.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Price DD, Hayes RL, Ruda M, Dubner R. Neural representation of cutaneous aftersensations by spinothalamic tract neurons. Fed Proc. 1978;37:2237–2239. [PubMed] [Google Scholar]
- 35.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Journal of Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 36.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 37.Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10(1):77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Salomons TV, Moayedi M, Erpelding N, Davis KD. A brief cognitive-behavioural intervention for pain reduces secondary hyperalgesia. Pain. 2014;155(8):1446–1452. doi: 10.1016/j.pain.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Sarzi-Puttini P, Atzeni F, Mease PJ. Chronic widespread pain: from peripheral to central evolution. Best Pract Res Clin Rheumatol. 2011;25(2):133–139. doi: 10.1016/j.berh.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Sato H, Saisu H, Muraoka W, Nakagawa T, Svensson P, Wajima K. Lack of temporal summation but distinct aftersensations to thermal stimulation in patients with combined tension-type headache and myofascial temporomandibular disorder. J Orofac Pain. 2012;26(4):288–295. [PubMed] [Google Scholar]
- 41.Schliessbach J, Siegenthaler A, Streitberger K, Eichenberger U, Nuesch E, Juni P, Arendt-Nielsen L, Curatolo M. The prevalence of widespread central hypersensitivity in chronic pain patients. Eur J Pain. 2013;17(10):1502–1510. doi: 10.1002/j.1532-2149.2013.00332.x. [DOI] [PubMed] [Google Scholar]
- 42.Schuh-Hofer S, Wodarski R, Pfau DB, Caspani O, Magerl W, Kennedy JD, Treede RD. One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. Pain. 2013;154(9):1613–1621. doi: 10.1016/j.pain.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 43.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 44.Smith MT, Finan PH, Buenaver LF, Robinson M, Haque U, Quain A, McInrue E, Han D, Leoutsakis J, Haythornthwaite JA. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: a randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol. 2015;67(5):1221–1233. doi: 10.1002/art.39048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith SB, Maixner DW, Fillingim RB, Slade G, Gracely RH, Ambrose K, Zaykin DV, Hyde C, John S, Tan K, Maixner W, Diatchenko L. Large candidate gene association study reveals genetic risk factors and therapeutic targets for fibromyalgia. Arthritis Rheum. 2012;64(2):584–593. doi: 10.1002/art.33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SB, Maixner DW, Greenspan JD, Dubner R, Fillingim RB, Ohrbach R, Knott C, Slade GD, Bair E, Gibson DG, Zaykin DV, Weir BS, Maixner W, Diatchenko L. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. J Pain. 2011;12(11 Suppl):T92–101. doi: 10.1016/j.jpain.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 48.Streff A, Kuehl LK, Michaux G, Anton F. Differential physiological effects during tonic painful hand immersion tests using hot and ice water. Eur J Pain. 2010;14(3):266–272. doi: 10.1016/j.ejpain.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan MJ, Bishop S, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 50.Toth LA, Jhaveri K. Sleep mechanisms in health and disease. Comp Med. 2003;53(5):473–486. [PubMed] [Google Scholar]
- 51.Treede RD, Baron R. How to detect a sensory abnormality. Eur J Pain. 2008;12(4):395–396. doi: 10.1016/j.ejpain.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Uprety D, Baber A, Foy M. Ketamine infusion for sickle cell pain crisis refractory to opioids: a case report and review of literature. Ann Hematol. 2014;93(5):769–771. doi: 10.1007/s00277-013-1954-3. [DOI] [PubMed] [Google Scholar]
- 53.Vase L, Nikolajsen L, Christensen B, Egsgaard LL, Arendt-Nielsen L, Svensson P, Staehelin JT. Cognitive-emotional sensitization contributes to wind-up-like pain in phantom limb pain patients. Pain. 2011;152(1):157–162. doi: 10.1016/j.pain.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Vierck CJJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78(2):992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 55.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality & Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 56.Wood PB. A reconsideration of the relevance of systemic low-dose ketamine to the pathophysiology of fibromyalgia. J Pain. 2006;7(9):611–614. doi: 10.1016/j.jpain.2006.01.457. [DOI] [PubMed] [Google Scholar]
- 57.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: between pro- and antinociception. Pain. 2014;155(4):663–665. doi: 10.1016/j.pain.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153(6):1193–1198. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 60.You HJ, Colpaert FC, Arendt-Nielsen L. The novel analgesic and high-efficacy 5-HT1A receptor agonist F 13640 inhibits nociceptive responses, wind-up, and after-discharges in spinal neurons and withdrawal reflexes. Exp Neurol. 2005;191(1):174–183. doi: 10.1016/j.expneurol.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 61.Yunus MB. Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Pract Res Clin Rheumatol. 2007;21(3):481–497. doi: 10.1016/j.berh.2007.03.006. [DOI] [PubMed] [Google Scholar]